Keloid, also known as “crab claw,” is an overgrown pathological scar tissue that spontaneously forms or is secondary to skin trauma. It usually shows that the lesion exceeds the scope of the original skin lesion, higher than the surface of the surrounding normal skin, and presents a continuous growth of nodular, cord-like, or sheet-like swelling tissue with tough and congestive texture, which is prone to occur in the anterior sternum, upper back, and upper arm triangle (1). Keloids are characterized by several distinct pathological features, including: (1) enhanced fibroblast proliferation, marked by an active mitotic phase; (2) a noticeably thickened and flattened epidermal layer; (3) a distinctive advancing margin within the dermis; (4) disorganized and thick hyalinized collagen bundles, which are a prominent feature of the dermis, resulting in the obliteration of the papillary-reticular junction; (5) elevated cellular density within the dermal compartment; (6) signs indicative of inflammation; (7) fluctuating expression levels of α-smooth muscle actin (α-SMA) (2). These characteristics together define the unique morphological and pathological landscape of keloids. The incidence of keloids is affected by racial ethnicity, higher prevalence of keloids in Asian populations compared to Caucasians (3, 4).
Keloid, a prevalent condition in the field of plastic surgery, exhibits notable resistance to treatment and high rates of recurrence (5). Surgical and non-surgical interventions are the primary therapeutic options for scar treatment. Among the non-surgical treatment methods include the application of silicone, local pressure, local injection of drugs, cryotherapy, laser, etc (6). Previous research has indicated that the recurrence rate of surgical excision alone can range from 45 to 100%, with recurrent scars being significantly larger in volume compared to the initial scar tissue (6). Currently, with the complex formation mechanism of keloids and the diverse treatment means, there is still a lack of uniform standard comprehensive treatment measures for keloids (7). This study aims to provide more clinical evidence for management of keloids by retrospective analysis of the clinical effects of patients with keloids who have received sequentially comprehensive treatment based on surgery.
Materials and methods Patient collectionPatients with keloids who attended the Department of Plastic Surgery of Shandong Provincial Hospital for sequentially comprehensive treatment from January 2018 to August 2022 were collected for this study. Patients who met the following criteria were excluded:(1) patients with postoperative pathological findings showing non-keloid lesions (2) patients who did not undergo surgical excisional treatment (3) women during pregnancy or lactation.
All patients received sequentially comprehensive treatment based on surgery. Prior to treatment initiation, patients underwent preoperative assessments including blood routine examination, coagulation function evaluation, and electrocardiography, among others, to rule out any surgical contraindications. Additionally, patients provided informed consent by signing a consent form for both medical intervention procedure and inclusion in publication.
Treatment methods sequentially comprehensive treatment(1) Surgical resection (workflow diagram shown in Supplementary Figure 1): Different surgical techniques were selected based on the location, size, and distribution of the keloids. In cases where the keloid was located in the ear, the core excision method was performed on the lesion, while the remaining keloids were completely excised. Following excision, the surgical area was rinsed sequentially with glucocorticoids, 5-fluorouracil, and saline. For keloids with lengths ranging from 2.0 to 10.0 cm and widths of ≤5.0 cm, the tension-reducing suture was directly applied after excision. In instances where the keloids exceeded 10.0 cm in length and 5.0 cm in width or where the local tension was high, flap transfer or skin graft was chosen to cover the resulting trauma after complete excision of the keloids in a single procedure.
(2) Intraoperative and post-operative administration of hormonal and antitumor chemotherapeutic agents: In the case of partially resected keloids or patients ineligible for postoperative radiation therapy, immediate injection of triamcinolone and 5-fluorouracil were administered at the incision site following surgery. The injection was prepared by mixing 2 ml of triamcinolone (10 mg/ml) with 0.3 ml of 5- fluorouracil stock solution(25 mg/ml) and 2.0 ml of 2% lidocaine.
(3) Post-operative radiation therapy: Postoperative radiation therapy was administered promptly, within 24 h following the surgical procedure. The treatment involved the application of superficial X-rays, with an irradiation range extending approximately 5 mm outward from the surgical site in all directions. A distance of 15 cm was maintained during irradiation, and lead plates were employed to safeguard the surrounding skin. The prescribed regimen consisted of 5 daily treatments, each delivering a single irradiation dose of 4 Gy. The cumulative irradiation dose did not exceed 20 Gy.
(4) Wound healing stage: The recommended course of action includes the application of topical silicone medication for a minimum of 6 months following wound healing. Additionally, tension-reducing treatment should be implemented involving the gentle pulling of normal skin tissues on both sides of the vertical incision line to alleviate tension in the surrounding skin. Regular monitoring is essential, and if any signs of recurrence such as incision bulging, itching, or pain arise, the administration of triamcinolone and 5-fluorouracil via injection into the edges of the incision and abnormal scar tissues may be considered. The injection was prepared by mixing 5.0 ml of triamcinolone (10 mg/ml) with 0.6 ml of 5- fluorouracil stock solution (25 mg/ml) and 2.0 ml of 2% lidocaine. Treatment intervals for injections in recurrent patients were 1 month until the incision returned to flatness and softness, requiring 3–5 treatments. Intervention in the early stages of recurrence was recommended.
Clinical efficacy evaluationThe clinical data of patients who met the inclusion criteria were collected, including age, gender, site, size (A small-sized keloid is defined as having a diameter less than 2.0 cm, while a medium-sized keloid is characterized by a length ranging from 2.0 to 10.0 cm and a width less than 5.0 cm, a large-sized keloid is identified by a length exceeding 10.0 cm and a width equal to or greater than 5.0 cm.) and thickness of keloid, family history, etiology, type (inflammatory-type or tumor-type, inflammatory-type keloid is distinguished by swift peripheral infiltration and pronounced congestion, on the other hand, tumor-type keloid is characterized by prominent protrusion from the skin surface without significant congestion, typical clinical manifestations are shown in Figure 1) (8), surgical procedure, whether radiotherapy was given after surgery, adverse reactions during treatment, whether anti-scar treatment was given after surgery, incision scar characteristics (vascularity, thickness, pliability and pigmentation), whether recurrence, time of recurrence and clinical manifestations.
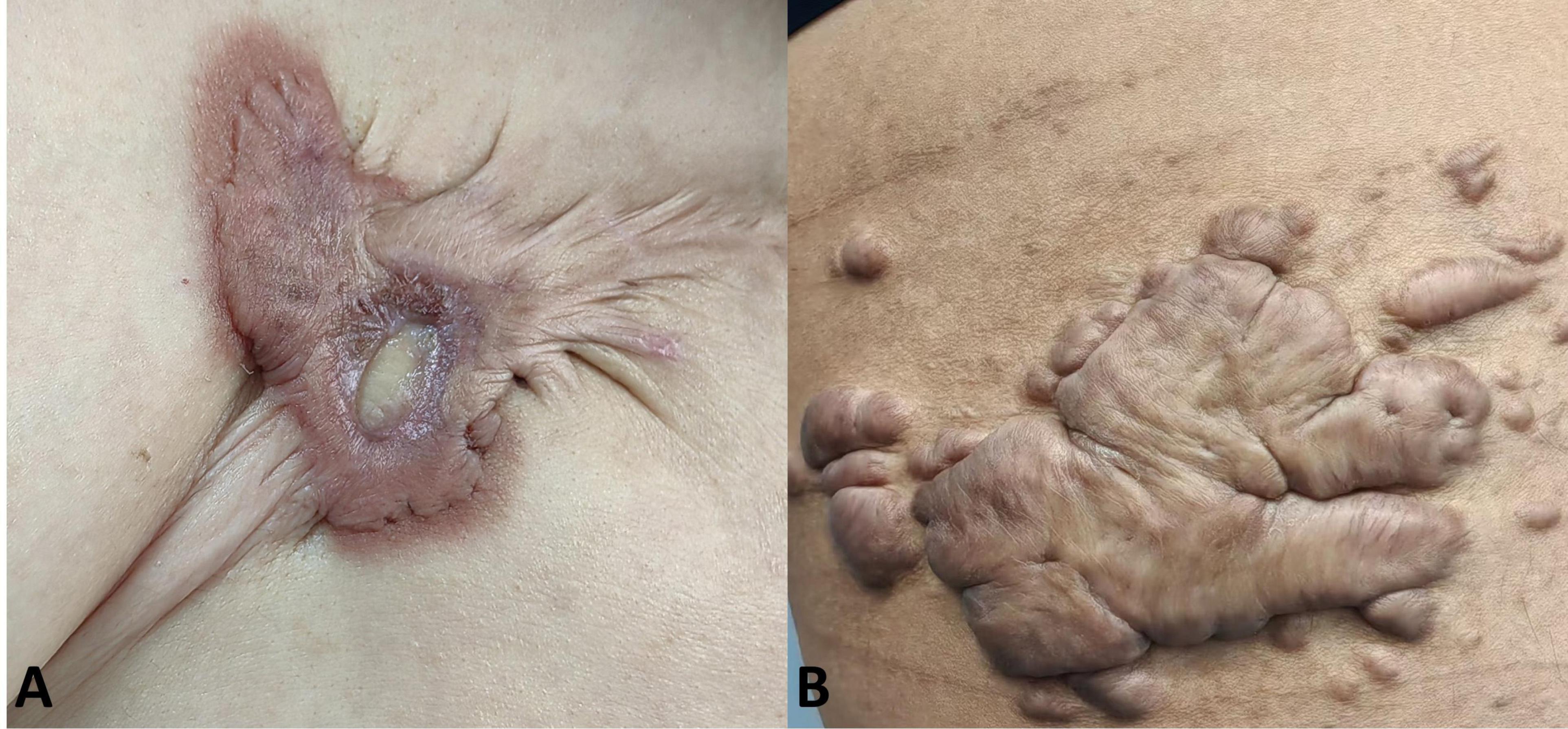
Figure 1. Typical clinical manifestations of two subtypes of keloids. (A) Inflammatory-type keloid, distinguished by swift peripheral infiltration and pronounced congestion; (B) tumor-type keloid, characterized by prominently bulging and appearing less congested or darker.
(1) The clinical effective rate was assessed for patients with a follow-up period exceeding 1 year, with treatment outcomes categorized as cured, markedly effective, and ineffective. The evaluation criteria are presented in Table 1. Total clinical effective rate = (number of cured cases + number of markedly effective cases) / total number of cases × 100%.
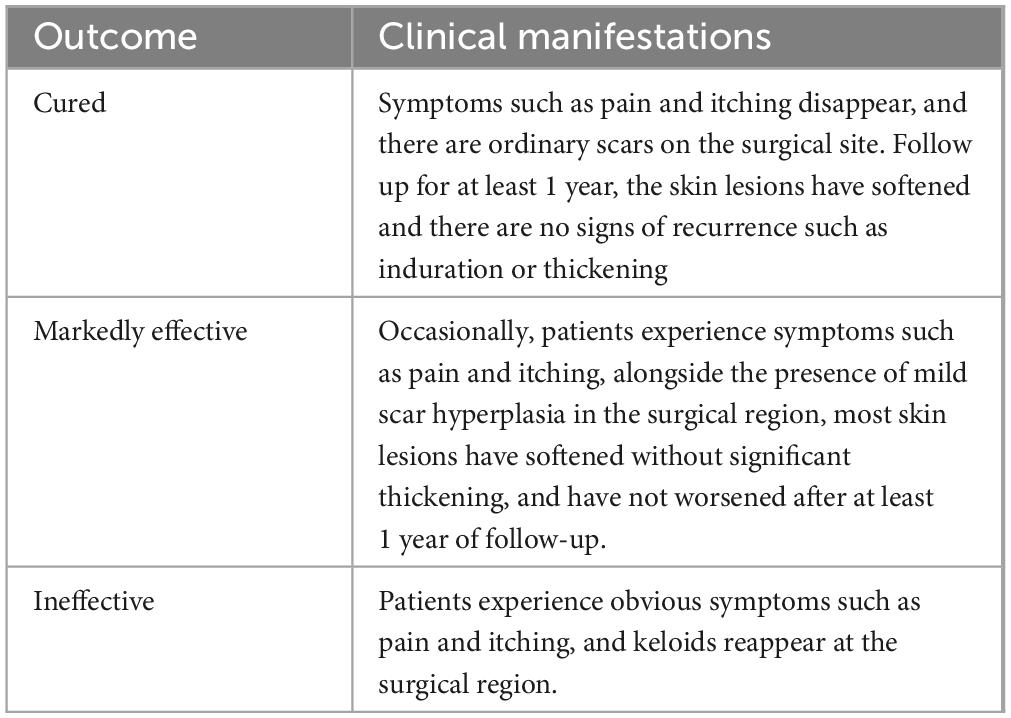
Table 1. Evaluation criteria.
(2) The effectiveness of the treatment was evaluated using the Vancouver Scar Scale (VSS) for patients with a follow-up period of less than 1 year (9). The VSS assigns a total score of 15, to evaluate the vascularity (0 = normal, 1 = pink, 2 = red, and 3 = purple), pigmentation (0 = normal, 1 = hypopigmentation, 2 = hyperpigmentation), pliability (0 = normal, 1 = supple, 2 = yielding, 3 = firm, 4 = banding, 5 = contracture) and height (0 = flat, 1 = 0–2 mm, 2 = 2–5 mm and 3 > 5 mm), with higher values indicating greater severity of scar proliferation.
Statistical methodCounting data were expressed as frequency and percentages (%), and grade data were subjected to analysis using the rank-sum test. The statistical methods utilized for analysis and processing encompassed the chi-square test and paired t-test, with statistical significance defined as P < 0.05. In the aforementioned analysis findings, both the factors exhibiting statistically significant differences and the factors that, although not statistically significant, are deemed clinically relevant to the dependent variable, have been incorporated as independent variables and subjected to multivariate logistic regression analysis.
Results Patient characteristicsA total of 67 cases were included in this study, including 24 male patients and 43 female patients, with a male-to-female ratio of 1:1.79. 80 keloids were found in 67 patients, including 26 (32.50%) in the ear, 15 (18.75%) in the anterior chest, 15 (18.75%) in the back and shoulders, 7 (8.75%) in the abdomen, 7 (8.75%) in the face, 3 (3.75%) in the upper extremities, 3 (3.75%) in the knees, 2 (2.50%) in the perineum, and 2 (2.75%) in feet. The 80 included keloids were classified according to their origins. 38 (47.5%) keloids were the inflammatory-type and 42 (52.5%) keloids were the tumor-type. As shown in Table 2. 67 patients underwent surgical resection of the keloids, 45 patients completed 5 radiation treatments. 20 patients did not undergo radiation therapy, and two patients discontinued after receiving radiation therapy because of discomfort.
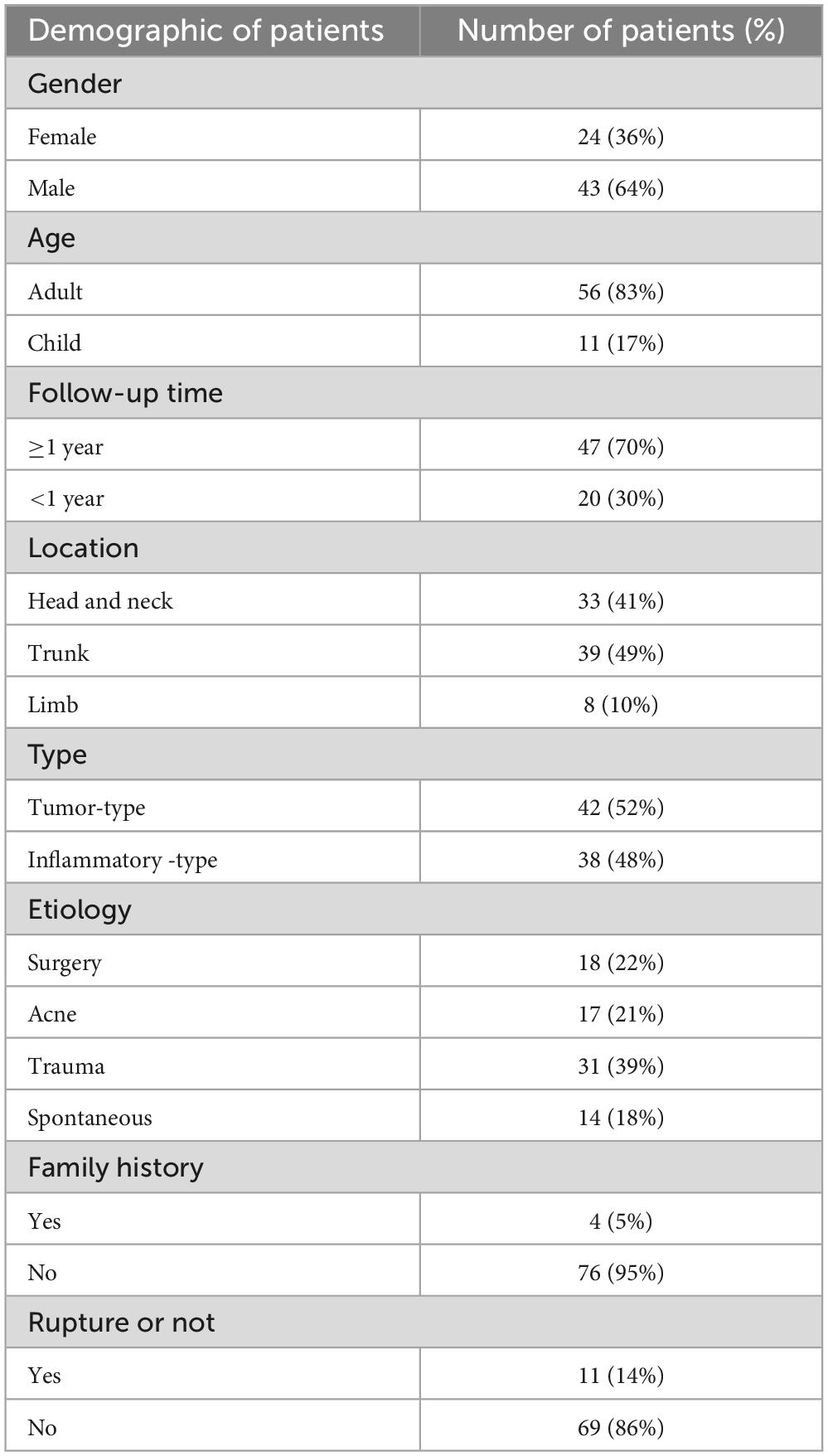
Table 2. Demographic of patients’ keloid characteristics.
Clinical effective rate and analysis of influencing factors Clinical effective rate47 patients were followed up for more than 1 year after surgery, with a total of 54 keloids. Among the total cases observed, 30 (55.6%) were cured, 14 (25.9%) were effective, and 10 (18.5%) were deemed non-responsive. Consequently, the clinical effective rate was determined to be 81.5%.
Analysis of influencing factors(1) The chi-square test, as evidenced in Table 3, revealed that there was no statistically significant difference (P > 0.05) among the influencing factors of gender, site, etiology, family history, preoperative rupture, size, and clinical efficiency. However, there were significant differences (P = 0.040) in the clinical efficiency between different types of keloids. Specifically, the inflammatory-type of keloid exhibited an efficiency of 70.0%, which was notably lower than the efficiency observed in the tumor-type keloid (95.8%). Logistic regression model was used to exclude confounding factors.
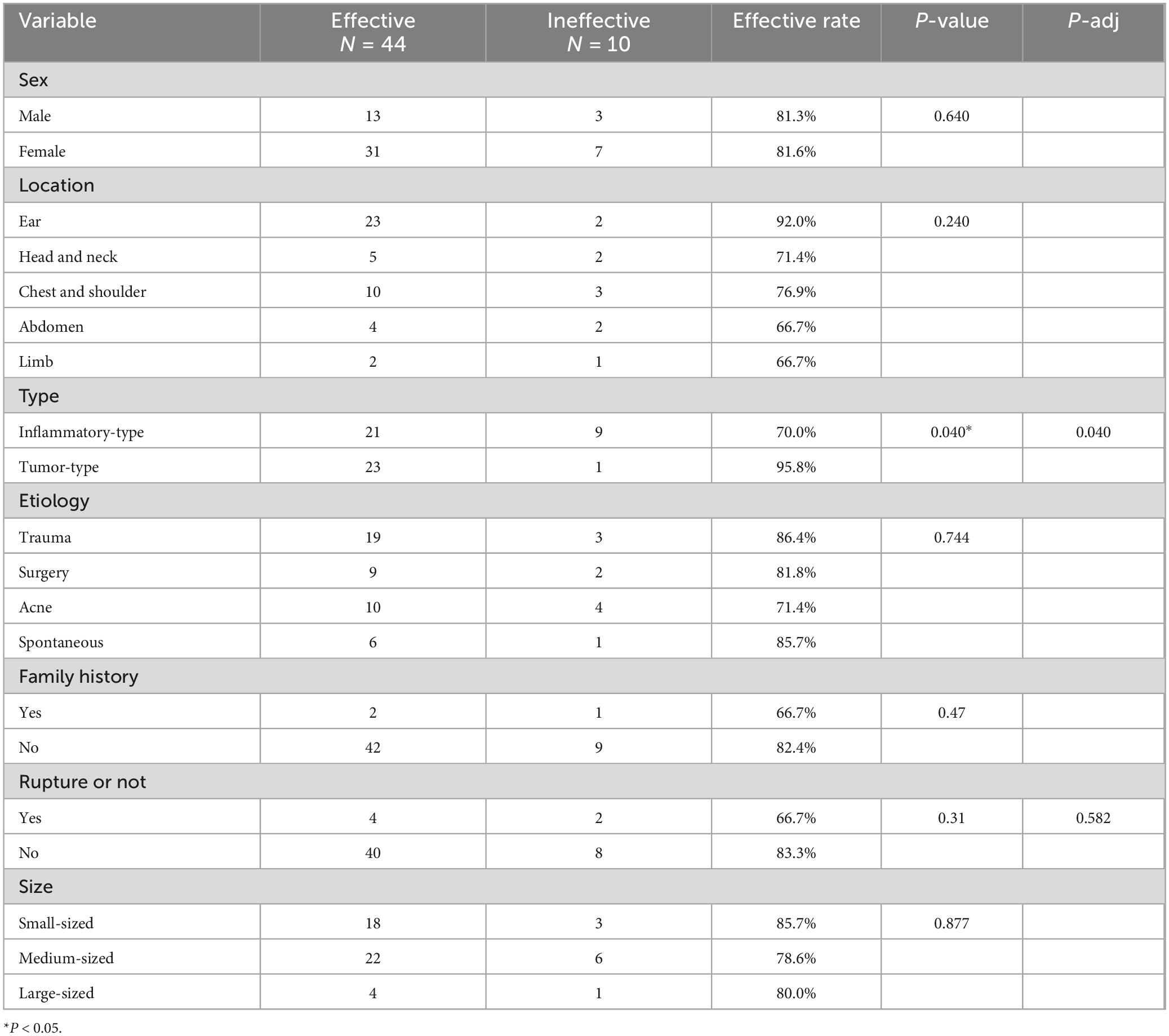
Table 3. Impact factors of clinical effective rate.
(2) The statistical analysis conducted in this study revealed that preoperative rupture did not exhibit a significant association with clinical effectiveness. Nevertheless, it was observed that follow-up time and whether to rupture were influential factors impacting clinical effectiveness. Consequently, the logistic regression model incorporated the following independent variables: type, follow-up time, and whether to rupture, as depicted in Table 4. The logistic model obtained in the final analysis demonstrated statistical significance, χ2 = 8.163, P = 0.043. Notably, among the three independent variables incorporated in the model, a statistically significant distinction in clinical effectiveness was observed between the various types of keloids. Specifically, the tumor-type keloid exhibited a clinical efficiency that was 9.7 times greater than that of the inflammatory-type keloid.
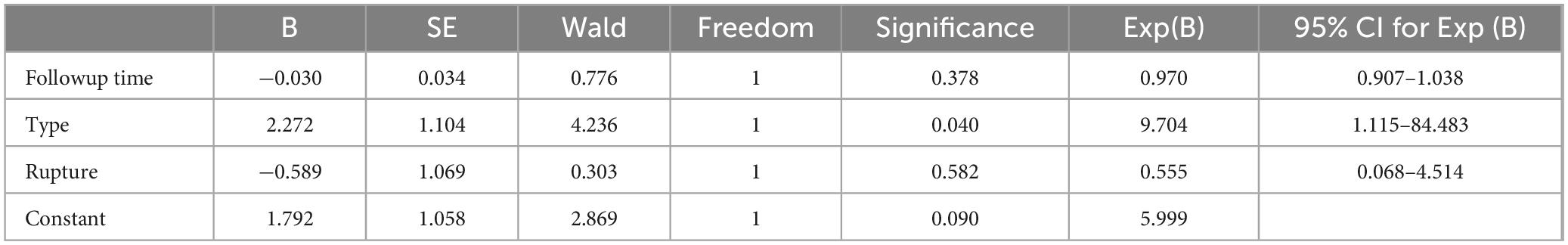
Table 4. The results of the multivariate logistic analysis.
Vancouver scar scale scoreA total of 20 patients, each with less than 1 year of postoperative follow-up, were included in the study. Among these patients, a total of 26 keloids were observed. The paired t-test was employed to analyze the scores obtained before and after treatment. The statistical analysis revealed a reduction in vascularity, thickness, pliability, pigmentation, and VSS scores following treatment and the difference was statistically significant (P < 0.001). These findings are presented in Table 5.

Table 5. Vancouver Scar Scale scores before and after treatment.
Recurrence rateA total of 67 patients, with 80 keloids, were enrolled in the study. Out of these, 12 keloids experienced recurrence, resulting in a recurrence rate of 15.0% (12/80). Specifically, the distribution of these recurrences was as follows: 3 in the chest and back, 3 in the ear, 3 in the abdomen, 2 in the face, and 1 in the elbow joint. For instance, as depicted in Figure 2, a patient diagnosed with an auricular keloid underwent surgical excision followed by postoperative radiotherapy, subsequently experiencing recurrence after a duration of 11 months.
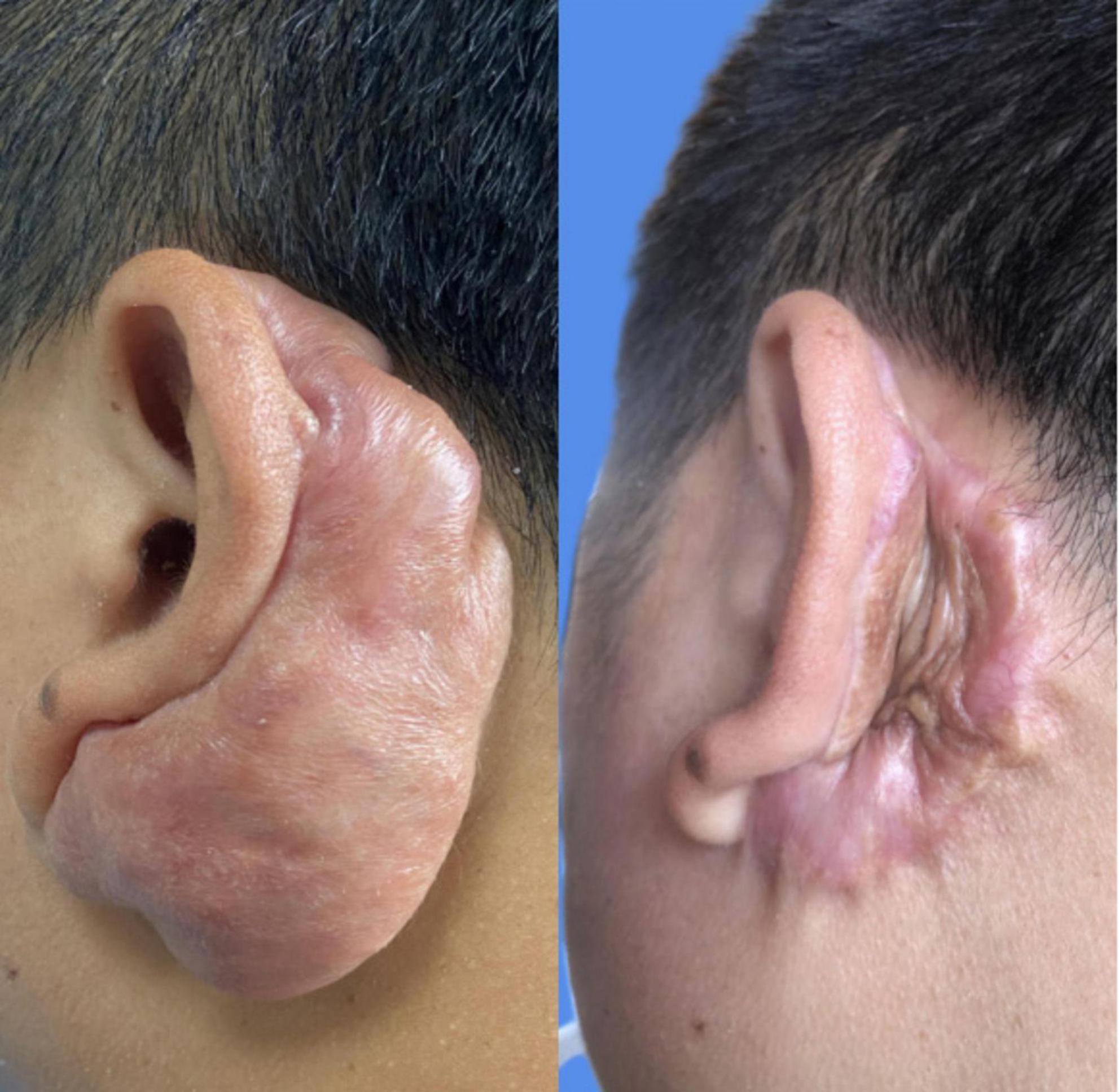
Figure 2. The recurrence of keloid behind the auricle.
Adverse reactionsAmong the 67 patients observed, there were three instances of adverse reactions, resulting in an adverse reaction rate of 4.5% (3/67). One patient experienced ulceration and pus at the incision site following stitch removal, which was successfully treated through active dressing changes. However, the patient subsequently experienced a recurrence of keloid in the operated area. Another patient reported an allergic reaction characterized by generalized itching and rash after the first radiotherapy session. Following the patient’s refusal of further radiotherapy, the allergy improved without needing specific treatment. A patient with bilateral earlobe keloids experienced the onset of craniocervical alopecia over a month following the completion of five radiotherapy sessions, at the 10-month follow-up, the hair in the alopecia region had regrown without any abnormal hair quality, as depicted in Figure 3.

Figure 3. Local alopecia after radiotherapy.
DiscussionKeloids can cause localized impairment in both aesthetic appearance and functional abilities. This progression is frequently accompanied by various symptoms including pain, pruritus, and ulceration (1, 10–12). When solely employing the monotherapy for keloid treatment, a frequent occurrence of relapse is observed, necessitating the utilization of multiple treatment approaches in combination. Consequently, the author advocates for a surgery-based sequentially comprehensive treatment. By allowing doctors to make timely treatment decisions based on individual patient conditions, the dynamic treatment strategy facilitates the achievement of the most favorable expected outcomes in clinical practice (13). Sequential therapy aims to optimize the outcome over time by addressing different stages of the treatment process with specific strategies (14).
The sequentially comprehensive treatment can be categorized into three distinct phases: the initial phase involves clarifying the diagnosis and enhancing patient education on the disease to prevent keloid infection and rupture. the subsequent phase primarily focuses on lesion removal and facilitating wound healing; and the final phase pertains to maintenance, primarily aiming to prevent keloid recurrence. During the surgical procedure, it is imperative for the surgeon to focus on the tension-reduction operation (15, 16). The application of glucocorticoids, 5-fluorouracil, and saline in a sequential manner to rinse the surgical site before wound closure has been shown to be an effective strategy for decreasing the occurrence of postoperative recurrence (17). In cases where partial resection of keloid has been performed or when postoperative radiation therapy is not feasible, immediate administration of triamcinolone acetonide injection and 5-fluorouracil injection at the incision site has been implemented (18, 19). Patients in this study underwent radiation therapy within 24 h after their surgery, as previous research has indicated that the most favorable outcomes are obtained when radiation therapy is administered within this timeframe (20–22). The patients included in this study were treated with superficial X-rays. For children who have experienced a recurrence or have rapidly and severely developed keloids, the decision to utilize radiation therapy can be made in consultation with a radiologist (23). Emphasizing postoperative care, such as regular dressing changes, can effectively minimize inflammatory response and subsequently decrease the likelihood of recurrence (24). Additionally, in cases where postoperative skin exhibits signs of recurrence such as erythema, pruritus, and pain, the injection of glucocorticoids into both sides of the incision margin and abnormal scar tissue can be considered.
A total of 67 patients were included in the study, with a total of 80 keloids. Among them, 47 cases were followed up for more than 1 year, with a total of 54 keloids. 30 (55.6%) were cured, 14 (25.9%) were markedly effective, and 10 (18.5%) were deemed ineffective. The clinical effective rate was 81.5%. A statistically significant distinction in the efficacy of various types of keloids (P = 0.040) persisted, suggesting that tumor-type keloid exhibits superior effectiveness compared to inflammatory-type keloid (25). The findings of this study demonstrate that tumor-type keloid exhibits superior clinical efficacy for a surgery-based sequentially comprehensive treatment. Consequently, in clinical treatment, surgical resection can be regarded as the preferred treatment modality for tumor-type keloid.
Meanwhile, in this study, the effective rate of keloids in the ear was found to be as high as 92%, which aligns with the findings reported in the literature regarding the combination of keloid core excision of the ear and radiotherapy (21, 26). This suggests that the treatment approach focusing on keloid core excision of the ear in conjunction with radiotherapy not only yields a high therapeutic efficacy but also optimizes the restoration of the natural aesthetic morphology of the patient’s ear.
Three patients reported adverse reactions during the treatment process, one patient had ulceration and pus at the incision after suture removal, although the wound healed well after active treatment, the keloid in the operated area recurred more than 2 months after the operation; Two additional adverse reactions were associated with radiotherapy. One patient experienced an allergic reaction characterized by generalized itchiness and rash following the initial radiotherapy session. This symptom spontaneously resolved after the patient declined further radiotherapy. Another patient, who had bilateral earlobe keloids, developed alopecia in the craniocervical region over a month after completing five radiotherapy treatments. However, at the 10-month follow-up, the hair in the alopecia region had regrown without any abnormal hair quality. This particular complication has not been documented in the pertinent academic literature thus far, and it is anticipated that it will garner clinical attention. Adverse reactions associated with radiotherapy are contingent upon therapeutic variables, including the treatment site, radiation modality, radiation dosage, cumulative dosage, fractionation, and overall treatment time (27).
This study reveals that patients with a prior or familial history of keloid should minimize exposure to avoidable trauma or surgery, while promptly addressing skin issues like acne and festering infection to mitigate the likelihood of keloid occurrence. Nevertheless, in clinical practice, it was found that patients’ compliance with glucocorticoids injection was poor, so few patients after discharge completed regular hormone injection treatment. In the subsequent investigation, it is imperative to enhance patient education, secure improved patient cooperation, and delve deeper into the integrated utilization of surgery, radiotherapy, and hormone injection.
This topic exhibits several limitations. The present study adopts a retrospective analysis approach, which introduces a potential recall bias. Despite adhering to rigorous inclusion and exclusion criteria, ensuring the homogeneity and completeness of the follow-up data remains challenging. Consequently, a multicenter randomized controlled study with a larger sample size is needed to explore the clinical value of dynamic sequential therapy based on surgery.
ConclusionThis study indicate that this sequentially comprehensive treatment based on surgery exhibits notable clinical effectiveness, and a significant reduction in the VSS score, while also demonstrating a low recurrence rate and occurrence of adverse reactions. Furthermore, it has been found that the type of keloid has a statistically significant effect on clinical effectiveness. It’s worth noting that the comprehensive treatment process should emphasize thorough patient-doctor communication and shared decision-making. Prospective, controlled studies to systematically compare the safety and efficacy of surgery-based versus non-surgical comprehensive treatments are needed in the future.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Shandong Provincial Hospital. Written informed consent was obtained from all the patients.
Author contributionsKY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft. MS: Data curation, Investigation, Methodology, Software, Writing – review and editing. SL: Methodology, Validation, Writing – review and editing. JS: Validation, Writing – review and editing. RH: Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – review and editing. CF: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (No. 82172227) and the Clinical Medical Science Innovation Program of Jinan (202019076).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1492407/full#supplementary-material
Supplementary Figure 1 | Workflow diagram of sequentially comprehensive treatment based on surgery.
References1. Liu S, Yang H, Song J, Zhang Y, Abualhssain ATH, Yang B. Keloid: genetic susceptibility and contributions of genetics and epigenetics to its pathogenesis. Exp Dermatol. (2022) 31:1665–75. doi: 10.1111/exd.14671
PubMed Abstract | Crossref Full Text | Google Scholar
2. Limandjaja GC, Niessen FB, Scheper RJ, Gibbs S. The keloid disorder: heterogeneity, histopathology, mechanisms and models. Front Cell Dev Biol. (2020) 8:360.
3. Kim S, Choi TH, Liu W, Ogawa R, Suh JS, Mustoe TA. Update on scar management: guidelines for treating Asian patients. Plast Reconstr Surg. (2013) 132:1580–9.
4. Kiprono SK, Chaula BM, Masenga JE, Muchunu JW, Mavura DR, Moehrle M. Epidemiology of keloids in normally pigmented Africans and African people with albinism: population-based cross-sectional survey. Br J Dermatol. (2015) 173:852–4. doi: 10.1111/bjd.13826
PubMed Abstract | Crossref Full Text | Google Scholar
5. Tan S, Khumalo N, Bayat A. Understanding keloid pathobiology from a quasi-neoplastic perspective: less of a scar and more of a chronic inflammatory disease with cancer-like tendencies. Front Immunol. (2019) 10:1810. doi: 10.3389/fimmu.2019.01810
PubMed Abstract | Crossref Full Text | Google Scholar
6. Siotos C, Uzosike AC, Hong H, Seal SM, Rosson GD, Cooney CM, et al. Keloid excision and adjuvant treatments: a network meta-analysis. Ann Plast Surg. (2019) 83:154–62. doi: 10.1097/SAP.0000000000001951
PubMed Abstract | Crossref Full Text | Google Scholar
7. Ekstein SF, Wyles SP, Moran SL, Meves A. Keloids: a review of therapeutic management. Int J Dermatol. (2021) 60:661–71.
8. Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, et al. Updated international clinical recommendations on scar management: part 2–algorithms for scar prevention and treatment. Dermatol Surg. (2014) 40:825–31. doi: 10.1111/dsu.0000000000000050
PubMed Abstract | Crossref Full Text | Google Scholar
9. Deng H, Li-Tsang CWP. Measurement of vascularity in the scar: a systematic review. Burns. (2019) 45:1253–65.
10. Macarak EJ, Wermuth PJ, Rosenbloom J, Uitto J. Keloid disorder: fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp Dermatol. (2021) 30:132–45. doi: 10.1111/exd.14243
PubMed Abstract | Crossref Full Text | Google Scholar
11. Wang Z-C, Zhao W-Y, Cao Y, Liu YQ, Sun Q, Shi P, et al. The roles of inflammation in keloid and hypertrophic scars. Front Immunol. (2020) 11:603187. doi: 10.3389/fimmu.2020.603187
PubMed Abstract | Crossref Full Text | Google Scholar
12. Stevenson AW, Deng Z, Allahham A, Prêle CM, Wood FM, Fear MW. The epigenetics of keloids. Exp Dermatol. (2021) 30:1099–114.
13. Laber EB, Davidian M. Dynamic treatment regimes, past, present, and future: a conversation with experts. Stat Methods Med Res. (2017) 26:1605–10. doi: 10.1177/0962280217708661
PubMed Abstract | Crossref Full Text | Google Scholar
14. Yuan C, Zhang X, Yan Y, Wang B, Li Z, Li L. Sequential therapy with intralesional injections and superficial X-ray therapy in 96 keloids. J Cosmet Dermatol. (2022) 21:5276–8. doi: 10.1111/jocd.14943
PubMed Abstract | Crossref Full Text | Google Scholar
15. Ogawa R, Akaishi S, Huang C, Dohi T, Aoki M, Omori Y, et al. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch. (2011) 78:68–76. doi: 10.1272/jnms.78.68
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tsuge T, Aoki M, Akaishi S, Dohi T, Yamamoto H, Ogawa R. Geometric modeling and a retrospective cohort study on the usefulness of fascial tensile reductions in severe keloid surgery. Surgery. (2020) 167:504–9. doi: 10.1016/j.surg.2019.07.028
PubMed Abstract | Crossref Full Text | Google Scholar
17. Uppal RS, Khan U, Kakar S, Talas G, Chapman P, McGrouther AD. The effects of a single dose of 5-fluorouracil on keloid scars: a clinical trial of timed wound irrigation after extralesional excision. Plast Reconstr Surg. (2001) 108:1218–24. doi: 10.1097/00006534-200110000-00018
PubMed Abstract | Crossref Full Text | Google Scholar
18. Durani P, Bayat A. Levels of evidence for the treatment of keloid disease. J Plast Reconstr Aesthet Surg. (2008) 61:4–17.
19. Choi Y-J, Lee YH, Lee HJ, Lee G-Y, Kim W-S. Auricular keloid management in Asian skin: Clinical outcome of intralesional excision and postoperative triamcinolone acetonide intralesional injection. J Cosmet Dermatol. (2020) 19:3041–7. doi: 10.1111/jocd.13383
PubMed Abstract | Crossref Full Text | Google Scholar
20. Liu EK, Cohen RF, Chiu ES. Radiation therapy modalities for keloid management: A critical review. J Plast Reconstr Aesthet Surg. (2022) 75:2455–65.
22. Wang W, Zhao J, Zhang C, Zhang W, Jin M, Shao Y. Current advances in the selection of adjuvant radiotherapy regimens for keloid. Front Med (Lausanne). (2022) 9:1043840. doi: 10.3389/fmed.2022.1043840
PubMed Abstract | Crossref Full Text | Google Scholar
23. Menchaca AD, Style CC, Olutoye OO. A review of hypertrophic scar and keloid treatment and prevention in the pediatric population: where are we now? Adv Wound Care (New Rochelle). (2022) 11:255–79. doi: 10.1089/wound.2021.0028
PubMed Abstract | Crossref Full Text | Google Scholar
24. Lee T, Bilionis I, Tepole AB. Propagation of uncertainty in the mechanical and biological response of growing tissues using multi-fidelity Gaussian process regression. Comput Methods Appl Mech Eng. (2020):359. doi: 10.1016/j.cma.2019.112724
PubMed Abstract | Crossref Full Text | Google Scholar
25. Chinese Journal of Aesthetic Plastic Surgery. Recommended guidelines for clinical treatment of keloids in China. Chinese J Aesthetic Plastic Surg. (2018) 29:245–56.
26. Ogawa R, Akaishi S, Dohi T, Kuribayashi S, Miyashita T, Hyakusoku H. Analysis of the surgical treatments of 63 keloids on the cartilaginous part of the auricle: effectiveness of the core excision method. Plast Reconstr Surg. (2015) 135:868–75.
27. Xu J, Yang E, Yu N-Z, Long X. Radiation therapy in keloids treatment: history, strategy, effectiveness, and complication. Chin Med J (Engl). (2017) 130:1715–21. doi: 10.4103/0366-6999.209896
留言 (0)