Antimicrobial resistance (AMR) poses a severe threat to global health. The World Health Organization (WHO) lists AMR as one of the top 10 global health threats (World Health Organization, 2022; Michael et al., 2014). A study published in 2019 revealed that nearly 457,000 deaths were attributed to resistance caused by seven primary pathogens. These include Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus faecium, Streptococcus pneumoniae, and Acinetobacter baumannii, listed in order of decreasing mortality (Antimicrobial Resistance Collaborators, 2022). Carbapenem resistance in K. pneumoniae is crucial to guiding antimicrobial selection, given how serious the threat is to public health (Karampatakis et al., 2023). The pathogenicity of carbapenem-resistant K. pneumoniae infections is enhanced by adhesive fimbriae, lipopolysaccharides, capsules, and siderophores. The formation of biofilms in patients with chronic or recurrent infectious diseases reflects bacterial resistance to antimicrobial drugs. Treatment strategies for this disease include traditional options (colistin and tigecycline) as well as newer alternatives (plazomicin and ceftolozane-tazobactam). Another major AMR concern is with microbes that cause urinary tract infections (UTIs) (Algammal et al., 2023). It is the second most prevalent infectious disease, caused by various gram-negative and gram-positive bacteria in all demographics. Notably, among the gram-negative bacteria, E. coli is a significant contributor to UTIs (Issakhanian and Behzadi, 2019).
With frequent infections and limited treatment options, AMR enforces alternate medication trials, prolonged hospital stays, and increased treatment costs. AMR arises from the overuse and misuse of antimicrobial drugs, inappropriate prescriptions, and insufficient knowledge of infection control. Repetitive antibiotic onslaughts apply genetic evolution pressure on the microbes, allowing them to acquire multidrug resistance (MDR) (Salam et al., 2023). The acquired genetic modification confers bacteria with either of the three main mechanisms: resistance, persistence, and antibiotic tolerance. Knowledge of antibiotic resistance’s exact mechanism and regulation strategies is vital for a suitable treatment strategy. Numerous studies were conducted to understand and control these mechanisms (Pang et al., 2018; Saha and Sarkar, 2021; Uddin et al., 2021). Traditional methods for diagnosing antibiotic resistance are slow, costly, and intricate.
Fortunately, nanotechnology emerged as a boon and a transformative opportunity, enhancing the speed and affordability of rapid point-of-care platforms. Nanoparticles (NPs) have dimensions typically in the 1 to 100 nm range (Boverhof et al., 2015; Teow et al., 2018). Their high surface area-to-volume ratio makes them versatile drug delivery vehicles (Ingle et al., 2013; Khan et al., 2019). NPs come in various categories: membrane-bound, metal-based, metal oxides, carbon, chitosan-based, and mesoporous structures. Additionally, formulations incorporate nanocomposites, nanosheets, nanomesh, hydrocarbons, and solid lipid NPs, each playing a role in enhancing antibiotic effectiveness, specificity, and delivery (Gabrielyan et al., 2020; Mamun et al., 2021; Parra-Ortiz et al., 2022). Recent advancements in NP vehicles have further increased their potency against drug-resistant bacteria (Wang et al., 2018; Brar et al., 2022; Binnebose et al., 2023). NP formulations can interact with cells through various mechanisms, such as adsorption, penetration, generation of reactive oxygen species, and interference with cellular processes (Barua and Mitragotri, 2014; Kim et al., 2015). The advantages include improved drug delivery, enhanced bioavailability, targeted therapy, prolonged drug release, improved drug stability, and minimized toxicity (Eleraky et al., 2020; Yeh et al., 2020). They have also been used as diagnostics and biosensing devices and as combination therapies against drugs in nanomaterials and nanoparticle formulations, biosensors, microfluidic devices, and so on. They have facilitated improved detection and treatment of antibiotic-resistant infections (Mubeen et al., 2021; Saxena et al., 2022). Nanoantibiotics (nAbts), a subfield of nanomedicine, are gaining attention due to their potential to revolutionize bacterial infection treatment (Edson and Kwon, 2016; Masri et al., 2019).
Nature has an abundance of compounds harboring antimicrobial properties. They are less toxic and more effective than synthetic drugs due to their evolution over time (Atanasov et al., 2021). The useful phytochemicals are sourced using ethnopharmacology and traditional medicine knowledge (Bonifácio et al., 2013; Nasim et al., 2022). Around one-third of popular pharmaceutical products are derived from natural sources, reflecting the growing demand for alternative healthcare solutions. Natural compounds are being studied for their medicinal potential in various health issues, including cancer and microbial diseases (Elkordy et al., 2021). Herb-based essential oils and secondary metabolites have antibacterial, antifungal, and antiviral properties, offering potential alternatives to traditional antibiotics (Joshi, 2016; Bhatwalkar et al., 2021; Magryś et al., 2021). Newman and Cragg (2016) reported that between 1981 and 2014, the FDA approved 1,562 pharmaceuticals, with 44% being unaltered natural products, 9.1% being botanical drugs, 21% being natural product derivatives, and 4% being synthetic drugs.
The shift from conventional to plant-based nanoformulations represents a noteworthy change in antimicrobial research and therapy. The abundant pharmacological possibilities in nature’s resources provide optimism for innovative therapies and improved healthcare outcomes. Green synthesis utilizes naturally sourced starting materials and low-energy processes as a sustainable alternative to conventional synthesis methods. This approach relies on a safer, cleaner, and more environmentally friendly nanomaterial manufacturing process (Huston et al., 2021; Chopra et al., 2022). Conjugation of these compounds with NPs shows more effectiveness than traditional antibiotics. They target multiple pathways in the body, reducing side effects such as liver or kidney damage, and are more biocompatible (Anand et al., 2022). This review aims to provide an extensive overview of innovative approaches, including nanocarriers and herbal compounds. It highlights the synergistic potential of combining multiple antibiotics within nanocarriers to maximize efficacy against drug-resistant pathogens. In addition, it emphasizes the importance of the green synthesis of NPs as a revolutionary method for enhancing antimicrobial effectiveness while minimizing the risk of systemic side effects. Unlike earlier reports, this review delves into the intricacies of antimicrobial resistance mechanisms while stressing the ethical utilization of natural resources and nanotechnology to address the challenge of drug resistance.
2 Antimicrobial resistanceThe ability of pathogens to sustain and even counteract antibiotic activity poses the greatest challenge to the administration of drug therapy. The discovery of antibiotics and their extensive use have inadvertently facilitated the emergence of resistant pathogenic strains, which significantly challenge the current healthcare system and pose a serious environmental threat (Boucher et al., 2009; Huh and Kwon, 2011). Drug resistance is defined as the minimum inhibitory concentration (MIC) of antimicrobial agents exceeding the agent’s inhibitory effects, allowing the microorganism to persist and thrive (Andrews, 2001). The antibiotic resistance mechanism is divided into two sorts:
A. Natural (or intrinsic): Cell wall or outer membrane thickening preventing antimicrobial entry (Nikolic and Mudgil, 2023); efflux pump (lipophilic and hydrophilic efflux pump) activation on the cell membrane (Nishino et al., 2021), inactivation of the drug (beta-lactamases hydrolyze the beta-lactam ring in penicillin and cephalosporins) (Bush and Bradford, 2016).
B. Acquired: Comprises genetic material alterations (mutations, transformation, transposition, and conjugation) and biochemical mechanisms (secretion of alternative enzymes to degrade the concerned antibiotics; enzymatic modification such as methylation, adenylation, acetylation, etc., of target molecules; use of alternative pathways and quorum sensing; antibiotic sequestration) (Walsh, 2000; Munita and Arias, 2016; Kapoor et al., 2017; Peterson and Kaur, 2018).
2.1 Antibiotic resistance mechanismsSome of the most important ways (Figure 1) by which bacteria get a survival advantage are as follows:
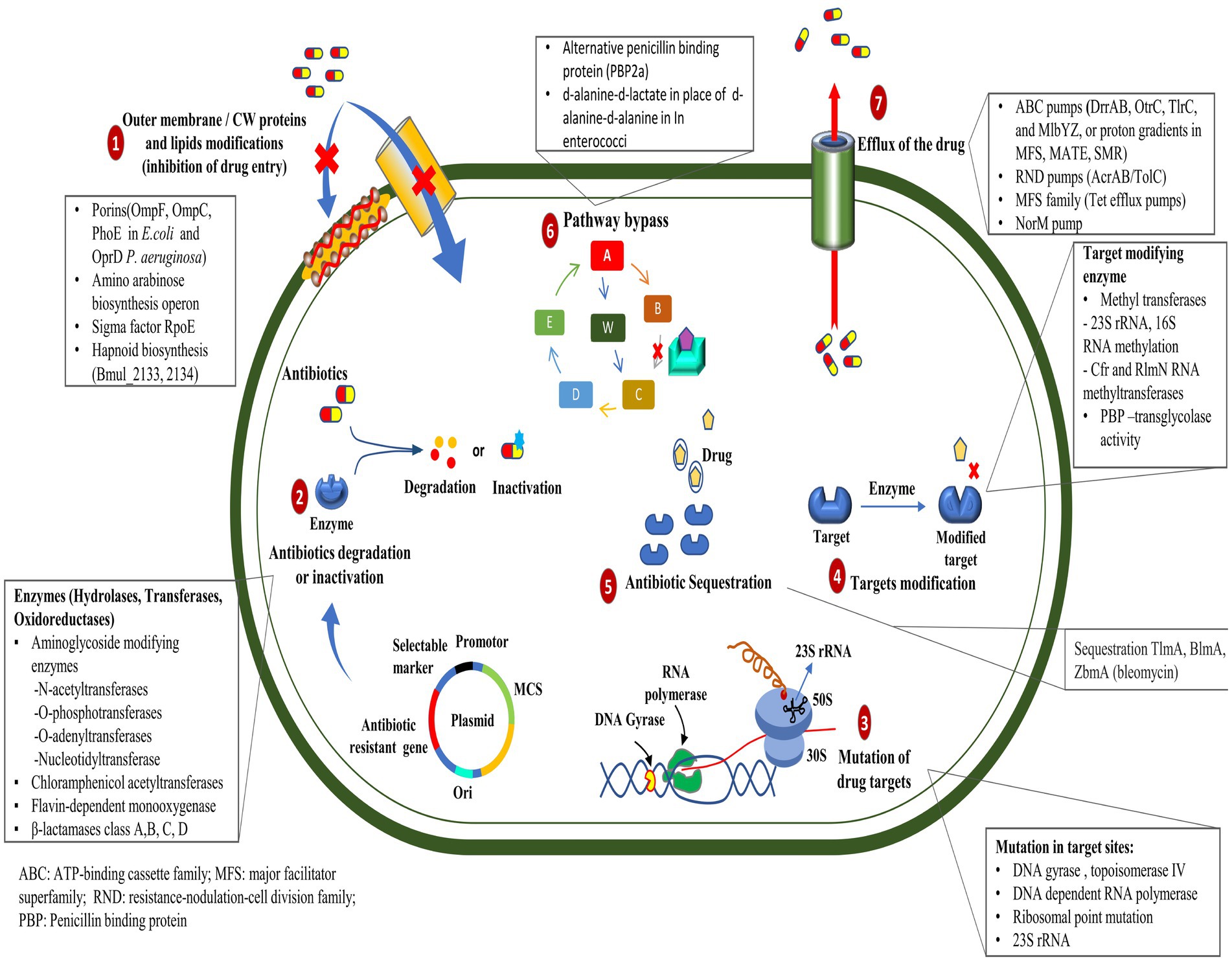
Figure 1. Mechanisms of antibiotic resistance developed by bacteria.
2.1.1 Mutation in target genesMutations in the genes encoding the target proteins of antibiotics can confer resistance in bacteria by changing the structure or function of the target, rendering it less sensitive to the antibiotic’s action (Peterson and Kaur, 2018). The mutations in the gyrA and parC genes play an essential role in resistance to ciprofloxacin in clinical isolates of Pseudomonas aeruginosa (Arabameri et al., 2021). Resistance mechanisms in Acinetobacter baumannii include plasmid-associated resistance genes (qnrA, qnrS, aac (6′)-Ib-cr, oqxA, and oqxB) and chromosomal mutations in the gyrA and parC genes (Mohammed et al., 2021). In a recent study, the AmpC and AmpR high expression was associated with resistance to tazobactam, ampicillin, gentamicin, nitrofurantoin, and cephalosporins, whereas AmpR deletion reduced β-lactam and aminoglycoside resistance in Citrobacter freundii (Tariq et al., 2023).
2.1.2 Efflux pump mutationsThe mutation in efflux pumps enables the active elimination of antibiotics from their cellular environment, which can impede the intracellular accumulation of antibiotics in bacterial cells (Whittle et al., 2021). Recent studies have demonstrated that mutations occurring in the mepA gene can lead to the development of tigecycline resistance in Staphylococcus aureus (Huang et al., 2023). However, mutations and genomic amplifications in the efflux pump gene, SdrM, contribute to delafloxacin resistance in methicillin-resistant S. aureus (MRSA) (Silva et al., 2023).
2.1.3 Enzyme productionEnzymatic drug resistance manifests by two processes: (a) enzymatic modification of antibiotics and (b) modification of drug targets. The chemical modification of antibiotics by bacterial enzymes renders them ineffective. Aminoglycoside antibiotic resistance is caused by aminoglycoside-modifying enzymes (AME), which alter hydroxyl or amino groups in aminoglycosides, causing them to lose their ability to bind 16S rRNA of the 30S ribosomal subunit (Zárate et al., 2018). In mycobacterial infection, enzymatic inactivation of rifamycin is facilitated by many enzymatic modifications, including ADP ribosyltransferases, glycosyltransferases, phosphotransferases, and monooxygenases (Surette et al., 2021). New Delhi metallo-β-lactamase (NDM-1) is a carbapenemase-producing bacterium having a mutated gene, blaNDM-1, which confers resistance to carbapenems in Enterobacteriaceae and various other bacteria (Khan et al., 2017). Antibiotic resistance, primarily caused by β-lactamase, is prevalent within ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species), causing significant economic burden and fatality risks (Mancuso et al., 2021). Given their significant implications for global health care, Behzadi et al. (2020) reviewed the distinctive characteristics of metallo-β-lactamases found in microbial pathogens, particularly within the Enterobacteriaceae family. Bacteria can evolve and adapt to antibiotics through modifications to the molecules or structures that are normally targeted. The Erm methyltransferase family alters a nucleotide in the 23S rRNA of the bacterial 50S ribosomal subunit, causing resistance to the prototypic macrolide erythromycin (Weisblum, 1995). The other drug linezolid target, the 23S rRNA region in the 50S ribosomal subunit, was altered by the inactivation of a methyltransferase (Long and Vester, 2011); likewise, the 16S rRNA methylase (ArmA) in A. baumannii, methylates adenine residues in the bacterial ribosome, reducing the binding affinity of aminoglycosides such as gentamicin and kanamycin (Jouybari et al., 2021).
2.1.4 Altered antibiotic entryMutations in bacterial outer membrane proteins can reduce the permeability of the cell membrane, limiting the entry of antibiotics into the bacterial cell. Increased resistance to penicillin and tetracycline in Neisseria gonorrhoeae is due to mutations in the outer membrane protein porin IB at positions G120D and A121D (Olesky et al., 2002). Physical modification in the membrane creates a physical obstruction that hinders the absorption of drugs into the cellular compartment. The modification in the core oligosaccharide of lipopolysaccharide in the outer membrane of E. coli inhibits vancomycin action (Stokes et al., 2016).
2.1.5 Horizontal gene transferHorizontal gene transfer (HGT) involves organisms transferring genes between themselves, different species or genera, or even across different domains of life in a manner other than traditional reproduction. Many organisms can acquire new genes that can confer advantageous traits in bacteria, such as antibiotic resistance, adaptability, and metabolic versatility, and this mechanism plays a crucial role in their evolution (von Wintersdorff et al., 2016). Bacteria can acquire resistance genes through HGT mechanisms such as conjugation, transformation, or transduction. These resistance genes may be on mobile genetic elements such as plasmids or integrons (Bello-López et al., 2019). HGT occurs more frequently in biofilms than in planktonic cultures, promoting the rapid dissemination of antibiotic-resistance genes (Michaelis and Grohmann, 2023). Outer membrane vesicles can mediate the horizontal transfer of virulence and resistance plasmid phvK2115 between Klebsiella pneumoniae strains and between K. pneumoniae and Escherichia coli strains (Wang et al., 2022). The vancomycin resistance gene, vanP, was presumed to be acquired by HGT from Clostridium scidens and Roseburia sp. Four hundred and ninety-nine in the Enterococcus faecium isolate (Xavier et al., 2021). A. baumannii employs HGT to efficiently acquire and exchange mobile genetic elements, contributing to its adaptability. It utilized outer membrane vesicles and phages as transfer mechanisms, thus aiding in the spread of antibiotic resistance genes. Its diverse virulence factors and flexible genome present a significant challenge to global public health systems (Karampatakis et al., 2024).
2.1.6 Antibiotic sequestrationBacteria can resist antibiotics by sequestering them, a process where drug-binding proteins prevent the antibiotic from reaching its target. These proteins deactivate antibiotics through hydrolysis or chemical modification (Blair et al., 2014). The drug-binding protein AlbA binds to albicidin and confers resistance to Klebsiella oxytoca (Rostock et al., 2018). The bleomycin family of antibiotics exhibits resistance in Streptomyces verticillus and Streptoalloteichus hindustanus strains that produce N-acetyltransferase and a binding protein. The N-acetyltransferase disrupts the antibiotic’s metal-binding domain, while the binding protein sequesters the metal-bound antibiotic and inhibits drug activation (Rudolf et al., 2015).
2.1.7 Biofilm-associated resistanceBiofilms are structured communities of bacteria showing resilience against antibiotics and diverse environmental pressures enclosed within a self-generated matrix composed of polysaccharides, proteins, and DNA (Shree et al., 2023). Biofilms resist antibiotics by utilizing extracellular components such as DNA, enzymes, and regulated genes. This resistance varies depending on the specific antibiotic, making biofilms a major contributor to chronic infections (Bano et al., 2023). Bacteria possess a strong quorum-sensing network system that can respond easily to environmental stress factors (Zhao et al., 2020). The presence of high-density colony populations has been seen to give rise to the production of small molecule signals called autoinducers (Waters and Bassler, 2005; Melke et al., 2010). This network system exhibits successful microbial interaction and physiological processing, constituting one of the best examples of antimicrobial resistance (Prestinaci et al., 2015). The significance of quorum-sensing systems in governing microbial resistance mechanisms, including drug efflux pump regulation and microbial biofilm formation (Zhao et al., 2020). According to statistical data from the National Institute of Health (NIH), biofilm development is observed in roughly 65% of bacterial infections and around 80% of chronic illnesses (Preda and Săndulescu, 2019).
Intraspecies communication regulates cellular functions such as pathogenesis, genetic material transfer, nutrition uptake, and secondary metabolite formation (Kamaruzzaman et al., 2018). This communication is pivotal for the simultaneous development of biofilms in gram-positive bacteria (e.g., S. aureus, S. epidermidis, and L. monocytogenes), which use oligopeptides as signaling molecules (Chen et al., 2016; Zhou et al., 2020). However, gram-negative bacteria (e.g., P. aeruginosa, V. fischeri, S. marcescens, K. pneumoniae) utilize N-acyl homoserine lactones (AHLs) as the signaling molecules in this particular system (Steindler and Venturi, 2007; Galloway et al., 2010).
In a cohort study involving S. aureus, the samples displayed penicillin resistance, with the majority exhibiting MDR. In vitro assessments revealed substantial biofilm production, with approximately one-fourth of the isolates demonstrating these capabilities (Dash et al., 2023). A study revealed that the overexpression of the TaPLA2 constructs in T. asahii resulted in increased resistance to azoles, achieved through drug efflux augmentation and biofilm formation (Ma et al., 2023). PatA facilitates mycolic acid production via an unidentified mechanism in M. tuberculosis, mitigating the inhibitory effects of isoniazid. Furthermore, PatA was shown to influence biofilm formation and the ability of organisms to withstand environmental stress by modulating lipid production (Wang et al., 2023). Naziri and Majlesi (2022) examined the incidence, patterns of antimicrobial resistance, and biofilm development of methicillin-resistant S. pseudintermedius (MRSP) on pets’ skin, exploring the potential for zoonotic transmission. Treating infections caused by these resilient microorganisms can be prolonged and challenging to eradicate. Their presence complicates treatment and management strategies, leading to prolonged illness, increased healthcare costs, and increased patient risk. Additionally, biofilms pose a significant global concern in relation to chronic diseases and medical devices.
2.1.7.1 Association of biofilm with chronic diseasesChronic diseases are associated with the development of biofilms, which are crucial survival strategies for bacteria. There are a variety of chronic illnesses in which bacteria can form complex biofilms, including chronic wounds, cystic fibrosis, otitis, urinary tract infections (UTIs), and others (Mirzaei et al., 2020). Biofilm formation by gram-negative bacteria worsens chronic and nosocomial infections, particularly chronic respiratory infections. Alternative therapies, such as antimicrobial peptides and liposomal formulations, are becoming increasingly important due to antibiotic resistance (Karmakar et al., 2023). According to experimental research, biofilms are present in chronic wounds at rates ranging from 20 to 100%, indicating their importance in healing (Goswami et al., 2023). Using a dynamic system and a chronic wound-like medium, Pouget et al. (2022) examined the formation and evolution of biofilms formed by S. aureus and P. aeruginosa in chronic wounds. These bacteria can form robust biofilms, perpetuate chronic infection, impair wound healing, and increase antibiotic resistance (Roy et al., 2020; Qin et al., 2022). The Lubbock chronic wound biofilm model resembling a chronic wound was developed as an in vitro study tool to investigate wound healing processes, biofilm inhibition, and the antibacterial efficacy of novel compounds (Diban et al., 2023). Meanwhile, El Masry et al. (2023) also established the swine model, enabling the study of wound biofilm infections by involving the host immune system and monitoring iterative changes during biofilm formation. Antiseptic therapy, with a specific focus on povidone-iodine (Alves et al., 2020) and synthetic antimicrobial peptides, noted for their increased efficacy and reduced toxicity (Pfalzgraff et al., 2018), is employed in the management of chronic wounds and biofilms.
Cystic fibrosis (CF) lung disease is predominantly an infectious condition where robust inflammation prevents the effective elimination of pathogens, hampers the lungs’ function, and results in respiratory failure and death (Cantin et al., 2015). CF patients with chronic P. aeruginosa infections produce mucoid alginate and form biofilms, conferring antibiotic resistance and immune responses (Høiby, 2002). Individuals with CF are primarily affected by P. aeruginosa and B. cenocepacia, where low iron concentrations induce free-living forms and motility. In contrast, high iron concentrations promote aggregation and biofilm formation (Berlutti et al., 2005). As an adjunctive therapy, cephalosporin effectively disperses biofilms formed by P. aeruginosa and may benefit patients with CF (Soren et al., 2020).
Urinary tract infections (UTIs) are among humans’ most prevalent bacterial infections, accounting for approximately 40% of all hospital-acquired infections (Haque et al., 2018). Approximately 75% of urinary tract infections acquired in hospital settings are associated with urinary catheters (Al-Qahtani et al., 2019). The pathogenic strains of E. coli cause UTIs and can form biofilms that facilitate the bacteria’s survival and persistence. Moreover, these E. coli strains possess strong biofilm-forming abilities and are resistant to many antimicrobial agents, including ampicillin, cefazolin, cefepime, ampicillin-sulbactam, and ceftazidime (Karigoudar et al., 2019; Katongole et al., 2020; Ramírez Castillo et al., 2023). A study in western Saudi Arabia involved testing urine samples for E. coli prevalence associated with UTIs, with a higher occurrence among females. Among these samples, numerous isolates showed resistance to norfloxacin and ampicillin, with no evidence of biofilm formation detected (Arafa et al., 2022). Another investigation in Ahvaz, Iran, focused on biofilm formation, structural characteristics, and antibiotic resistance of S. saprophyticus strains that cause female UTIs. Most S. saprophyticus isolates were resistant to erythromycin, with 58% exhibiting MDR. Additionally, 65% of these isolates demonstrated biofilm formation, primarily characterized by a polysaccharide matrix (Hashemzadeh et al., 2020).
Otitis media with effusion (OME), a childhood condition attributed to bacterial infection associated with biofilms, has been found to contain coagulase-negative staphylococci in samples (Daniel et al., 2012). Furthermore, other pathogenic bacteria, such as H. influenzae, S. pneumoniae, and M. catarrhalis, have been reported in infections (Van Hoecke et al., 2016; Korona-Glowniak et al., 2020).
2.1.7.2 Formation of biofilm on medical devicesUtilizing biomaterials such as prosthetic heart valves, intravenous central line prosthetics, contact lenses, urinary tract catheters, and prosthetic joints has been associated with the formation of biofilms, leading to potential infections (Zhao et al., 2013; Li P. et al., 2023). Both gram-positive (S. aureus, E. faecalis, S. viridans, and S. epidermidis) and gram-negative bacteria (P. aeruginosa, E. coli, P. mirabilis, and K. pneumoniae) can form biofilms on medical devices (Donlan, 2001). Biofilm-associated infections are primarily caused by S. aureus and S. epidermidis, which are frequently found on cardiovascular devices. Their versatility allows them to transition from single free-floating cells to multicellular biofilms (Schilcher and Horswill, 2020). It is noted that S. aureus and S. epidermidis are responsible for approximately 40–50% of infections associated with prosthetic heart valves and 50–70% with catheter biofilms (Chen et al., 2013).
Microbial colonization of central venous catheters (CVC) can lead to biofilm formation, aiding bacterial survival against antimicrobial agents and the host immune system, potentially causing severe infections, and spreading to other body sites (Gominet et al., 2017). High-dose antibiotics inside the catheter can significantly reduce bloodstream infection (Wolcott, 2021). A systematic review conducted by Cangui-Panchi et al. (2022) reported that biofilm formation was observed in 59 to 100% of clinical isolates, with prevalence rates varying notably among regions. Various microorganisms were identified among the clinical isolates, including gram-positive and gram-negative strains and C. albicans. The findings highlight the association between the high prevalence of biofilm-forming microorganisms and the increased incidence of nosocomial infections among catheterized patients.
The development of mature biofilms on the contact lens surface is associated with severe eye infections such as keratitis. Among different pathogens, S. aureus (including MRSA) and P. aeruginosa are the most commonly encountered in contact lens-related eye infections (Dosler et al., 2020). Additional fungal pathogens such as Candida, Fusarium, and Aspergillus contribute to the development of keratitis in individuals wearing soft contact lenses, playing a role in contact lens-associated fungal keratitis (Yi et al., 2023). Raksha et al. (2020) collected 265 gram-positive and gram-negative isolates from contact lens wearers and confirmed the presence of biofilm by tube and Congo red agar method.
A catheter-associated urinary tract infection poses significant risks to patients and the healthcare system. In a study, biofilms formed by E. coli, P. aeruginosa, and P. mirabilis were detected on three distinct types of commercially available catheters: hydrogel latex, silicone, and silver alloy-coated hydrogel latex (Wilks et al., 2021). As urease-producing species such as P. mirabilis colonize catheter surfaces, they form crystalline biofilms that encrust and block catheter surfaces, resulting in severe clinical complications and necessitating emergency hospital referrals (Pelling et al., 2019). In vitro tests showed that urinary catheters containing a blend of rifampicin, sparfloxacin, and triclosan were effective in preventing colonization by common uropathogens, including S. aureus, P. mirabilis, and E. coli (Fisher et al., 2015). Almalki and Varghese (2020) conducted antibiotic sensitivity tests on clinical samples from catheter-associated urinary tract infections (UTIs). E. coli was identified as MDR to pan-drug resistant (PDR), while Klebsiella and Pseudomonas were categorized as extensively drug-resistant (XDR) organisms. However, other isolates such as E. fecalis, S. aureus, P. mirabilis, and Citrobacter exhibited resistance to a limited range of antibiotics. Using urine samples and urinary catheter segments, Ramadan et al. (2021) assessed biofilm development using the tube method (TM) and scanning electron microscope (SEM). Their findings revealed an 82.85% prevalence of biofilm-dependent catheter-associated urinary tract infections, with K. pneumoniae displaying the highest biofilm-forming capacity.
A significant challenge remains in treating prosthetic joint infections (PJIs), mainly due to the formation of biofilms by infectious bacteria (Gbejuade et al., 2014). During the implantation of a device, an immunologically vulnerable area is created around the device. In this region, the host may be unable to effectively eliminate bacteria, leading to the formation of biofilms on the surface of the biomaterial (Rochford et al., 2012). Sadovskaya et al. (2006) found that biofilm-producing staphylococci isolated from infected orthopedic implants contain two carbohydrate molecules (N-acetyl-D-glucosamine and teichoic acid). Svensson Malchau et al. (2021) characterized the biofilm capabilities and antimicrobial susceptibilities of staphylococci responsible for causing PJIs. They revealed a noteworthy correlation between biofilm formation, increased antimicrobial resistance, and the recurrence of PJIs. Macias-Valcayo et al. (2022) investigated the antimicrobial susceptibility of clinical isolates of gram-negative bacilli from PJIs. Additionally, they examined the possible correlation between antimicrobial resistance and the formation of biofilms.
3 NanoantibioticsnAbts utilize nanoscale vehicles called nanoparticles (NPs) to encapsulate naturally produced and artificially derived compounds. These cutting-edge technologies are at the forefront of medical advancements (Soares et al., 2018). These structured nanomaterials exhibit enhanced antimicrobial activity and play a crucial role in effectively boosting the efficacy of administered antibiotics in combating infectious diseases (Beyth et al., 2015). As compared to traditional antibiotics, nAbts have many advantages. Firstly, encapsulating drugs in NPs improves their solubility and stability, thus increasing their bioavailability and half-life (Yeh et al., 2020). Furthermore, it prevents rapid renal clearance and enzymatic hydrolysis, facilitating a long-term therapeutic effect (Huo et al., 2022). Secondly, nAbts are capable of circumventing bacterial biofilms, thus bypassing the protective barrier that inhibits conventional antibiotic treatment. This results in the effective delivery of drugs to infected tissues (Karnwal et al., 2023). Additionally, they increase membrane permeability, enhancing encapsulated drugs’ therapeutic efficiency (Liu et al., 2022). The targeted delivery reduces the likelihood of side effects, as well as MDR, by minimizing systemic exposure (Wang et al., 2018). The strategies for managing microbial infections are outlined in Figure 2.
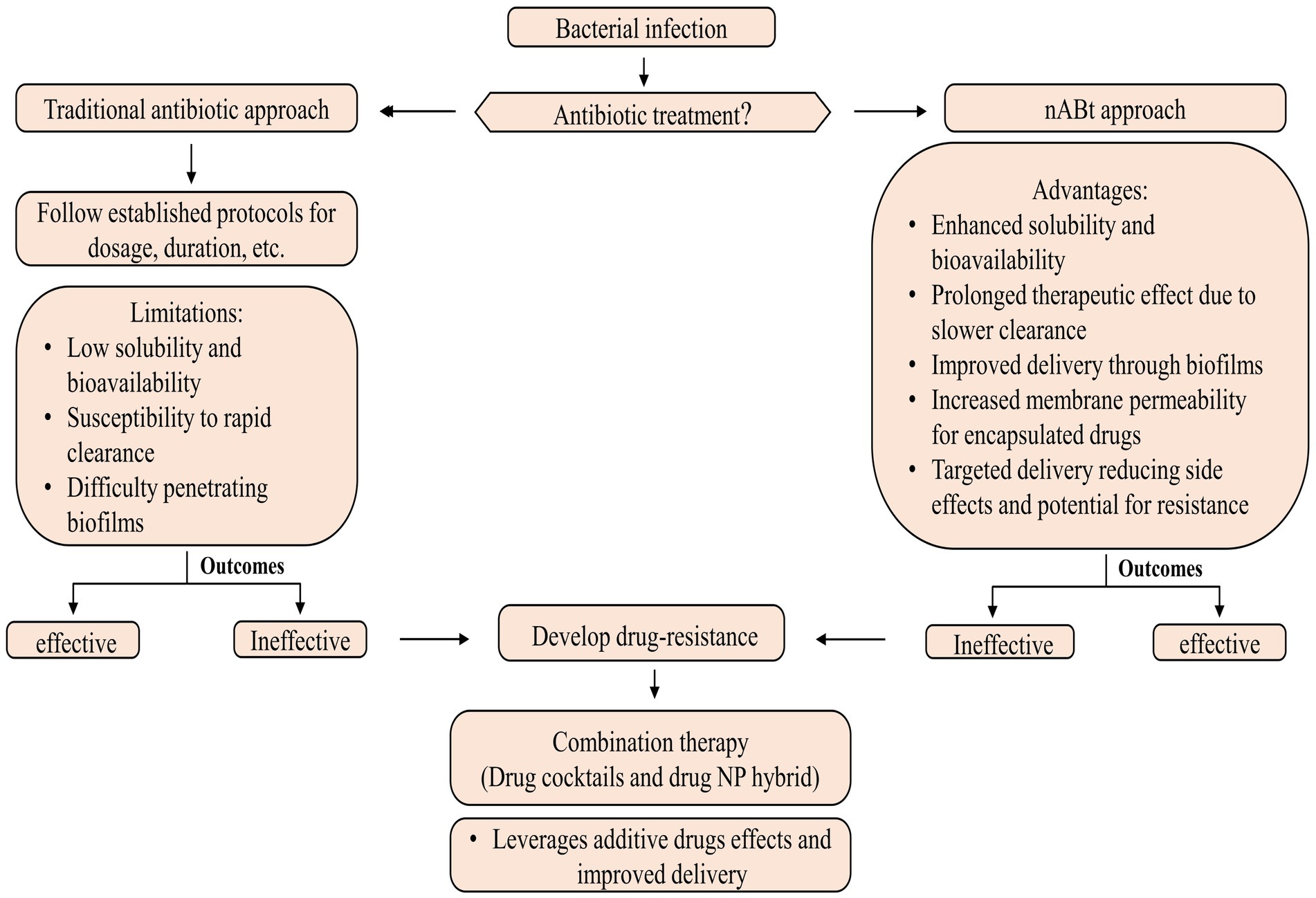
Figure 2. Methods for controlling microbial infections.
Most nAbts are typically smaller than 100 nm in at least one dimension, reflecting their nanoscale nature. Due to their exceptional size and controllability, NPs are suitable for antimicrobial and intracellular bacterial operations (Huh and Kwon, 2011). The size and shape of NPs influence many factors, such as drug delivery efficiency, biodistribution, and interactions with biological systems (Khan et al., 2019). These nanoscale formulations enable enhanced bioavailability and targeted delivery, making them promising candidates for various medical and therapeutic applications (Kirtane et al., 2021). In general, antibiotics target pathogenic bacteria by inhibiting protein synthesis, degrading cell wall components, interfering with energy production and restoration, and disrupting components across a cell membrane (Kohanski et al., 2010; De Maio et al., 2019). Although antibiotics systematically require multiple doses to be effective. In contrast, nAbts may be effective if only a single, target-specific dose is provided (Vallet-Regí et al., 2007; Li et al., 2015). The emergence of antibiotic-resistant pathogens poses a serious health threat, but NPs may provide potential solutions through their properties as antibacterial agents and their ability to deliver customized antibiotics (Ozdal and Gurkok, 2022). Combining therapy, including drug cocktails and drug-NP hybrids, is emerging as a powerful approach for combating bacterial resistance and enhancing antibiotic effectiveness. Through this strategy, both additive drug effects and improved cellular delivery are leveraged, paving the way for more effective and targeted chemotherapy for infection (Adeniji et al., 2022; Brar et al., 2022).
4 NanoparticlesTraditional antibiotic delivery presents challenges such as low solubility, poor permeability, gastrointestinal instability, and limited antibacterial activity, particularly when given orally (Wu et al., 2020). Antibiotic-conjugated NPs have effectively controlled bacterial infections by enhancing antibiotic uptake, local concentration, and other shortcomings of traditional antibiotics (Jelinkova et al., 2019). Nanotechnology introduces two groundbreaking tools, nanobactericides and nanocarriers, revolutionizing antibacterial therapy. These nanostructures, abbreviated as NPs, represent cutting-edge advancements in the field. Nanobactericides, tiny warriors with built-in antibacterial properties, attack and destroy microbes directly. In contrast, nanocarriers, discrete transporters, are capable of delivering conventional antibiotics directly to their targets, allowing them to unleash their potent effects within the core of the microbial threat (Vassallo et al., 2020). Given the potential toxicity of many engineered NPs, it is crucial to investigate methods for creating safe NPs, such as those obtained from plant sources.
NPs derived from natural sources exhibit unique properties that make them suitable for use in the antimicrobial field. The rapidity, safety, and cost-effectiveness of synthesizing NPs using plant extracts are characterized by minimal energy consumption and non-toxic derivatives (Patra et al., 2018; Nguyen et al., 2022). Drug delivery NPs typically range from 10 to 1,000 nm, with at least one dimension falling below 100 nm. The diminutive sizes of NPs and their surface chemistry confer pharmaceutically advantageous characteristics, although they may have associated toxic effects (Yusuf et al., 2023). Moreover, their nanometric size facilitates effective interactions with bacteria, another reason for their nomenclature.
In general, depending upon the biomolecular conjugation of antibiotics, the NPs have been categorized into different classes, viz. membrane-bound, metal-based, carbon-based, chitosan-based, mesoporous, and others (Figure 3). The membrane-bound or lipid-based NP delivery systems encompass liposomes, self-nano emulsifying drug delivery systems (SNEDDS), solid lipid NPs (SLNs), niosomes, nanostructured lipid carriers (NLCs), and polymeric micelles (Gkartziou et al., 2021). Metal-and metal oxide-based NPs display antibacterial properties due to diverse weak, non-covalent interactions with the ligands and host receptors (Shaikh et al., 2019). NPs derived from metals incorporate heavy metals such as silver (Ag), gold (Au), titanium (Ti), zinc (Zn), iron (Fe), and copper (Cu). Metal oxide NPs, on the other hand, include copper oxide (CuO), cobalt oxide (CoO), titanium oxide (TiO2), cerium oxide (CeO2), bismuth oxide (Bi2O3), iron oxide (Fe2O3), zinc oxide (ZnO), magnesium oxide (MgO2), nickel oxide (NiO), etc. (Beyth et al., 2015; Motakef-Kazemi and Yaqoubi, 2020). However, unlike conventional therapies, including radiation or chemotherapy, iron oxide with a hyperthermic effect can be confined to the area containing the magnetic particles, minimizing harm to healthy tissue within the surrounding area (Durmus et al., 2013). Nanocarriers are ubiquitous, but liposomes are the pioneering nanotechnology for this specific application. In addition, dendrimers, cyclodextrins, nanoemulsions, micelles, solid lipid carriers, nanostructured lipid carriers, mesoporous polymeric NPs, hydrogels, fullerenes, and carbon nanotubes are notable nanocarriers (Din et al., 2017; Sultana et al., 2022).
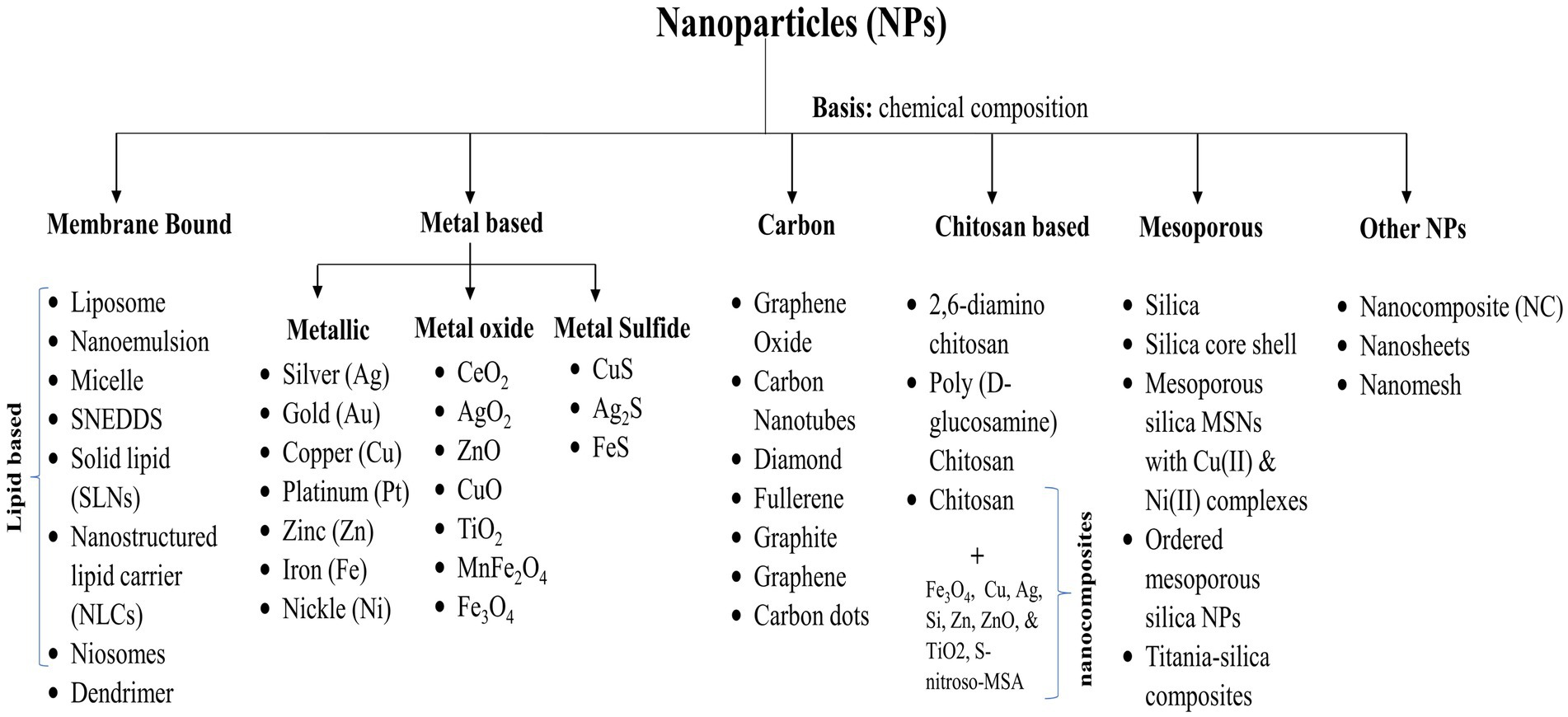
Figure 3. Various categories of nanoparticles utilized for drug delivery.
5 Structural foundations of nanoantibioticsIn the pharmaceutical industry, nanoformulations have been widely utilized to develop nAbts, leveraging advantages such as enhanced drug loading capacity, prolonged release durations, and better binding affinities (Rana et al., 2019). These nanoscale functionalities have demonstrated the ability to restore drug efficacy in various applications. nAbts exhibit distinctive properties, which allow them to target multiple bacteria concurrently, providing a significant advantage in combating microbial infections (Mamun et al., 2021). A nanoscale antibiotic’s interaction with bacteria has profound implications for the delivery of antibiotics, as nanoscale antibiotics act as drug carriers, penetrate cell membranes, and interfere with protein synthesis in bacteria (Baptista et al., 2018). However, nanomaterials’ effectiveness in targeting bacteria depends upon their physiological state (Gao and Zhang, 2021), including nutrition availability, biofilm formation, bacterial growth stages, and environmental conditions, including aeration, pH, and temperature (Chakraborty et al., 2022). Understanding these complex interactions is essential to designing effective antimicrobial strategies.
An integral aspect of the biology of antibiotics and their associated NPs is their linkage, which can display a diverse array of surface charges such as zwitterionic, cationic, anionic, or neutral (Miller et al., 2015). In nets, the structural and physical characteristics of NPs can be controlled; it is possible to modify the structural characteristics of NPs, such as particle size and lattice constant, increasing charge densities within the NPs and, therefore, increasing the contact area with antibiotics (Mamun et al., 2021). Biomolecularly connected NPs, including metal-ion, oxidative, and non-oxidative components, can interact directly with bacteria (Fasting et al., 2012). Furthermore, nAbts may mitigate the adverse effects of conjugated antibiotics within the host cell (Saha et al., 2007; Gupta et al., 2017). Combining nAbts with pure NP or surface functionalization with structural moieties such as citrate or carboxylate results in prolonged stability to novel antibiotics (Hemaiswarya et al., 2008; Mamun et al., 2021).
6 Mechanism of action of nano-bactericidesThe antimicrobial activity of nanomaterials is characterized by physical, chemical, and photo-mediated damage mechanisms. By exploring the interaction between nanomaterials and bacteria, nanotherapeutics may serve as an alternative to traditional antibiotics in treating bacterial infections (Makabenta et al., 2020; Ullah and Khan, 2022). NPs penetrate bacterial envelopes through Van der Waals forces, receptor-ligand interactions, and hydrophobic interactions, damaging the structural integrity of the bacterial membrane. As a result, it interferes with the proton motive force across the cell membrane, limiting the bacteria’s ability to store or produce energy (Erdem et al., 2015). Additionally, it inhibits the enzyme activity of bacteria, suppresses their efflux pumps (Baptista et al., 2018), and increases membrane permeability, which facilitates the accumulation of NPs within membranes and subsequent uptake by cells (Shaikh et al., 2019) (Figure 4). By entering bacterial cells, NPs disrupt microbial pathways, affecting enzymatic proteins, DNA, ribosomes, and lysosomes, resulting in catastrophic outcomes for the bacteria (Karnwal et al., 2023). NPs adhere to the exterior of the biofilm via electrostatic interaction and diffuse throughout the matrix, which is influenced by a variety of factors, including the size, shape, and charge of NPs (Makabenta et al., 2020), the viscosity of exopolysaccharide, cell density, compaction level, liquid flow, and physicochemical interactions with extracellular polymeric substances (Harper et al., 2018; Robino and Scavone, 2020).
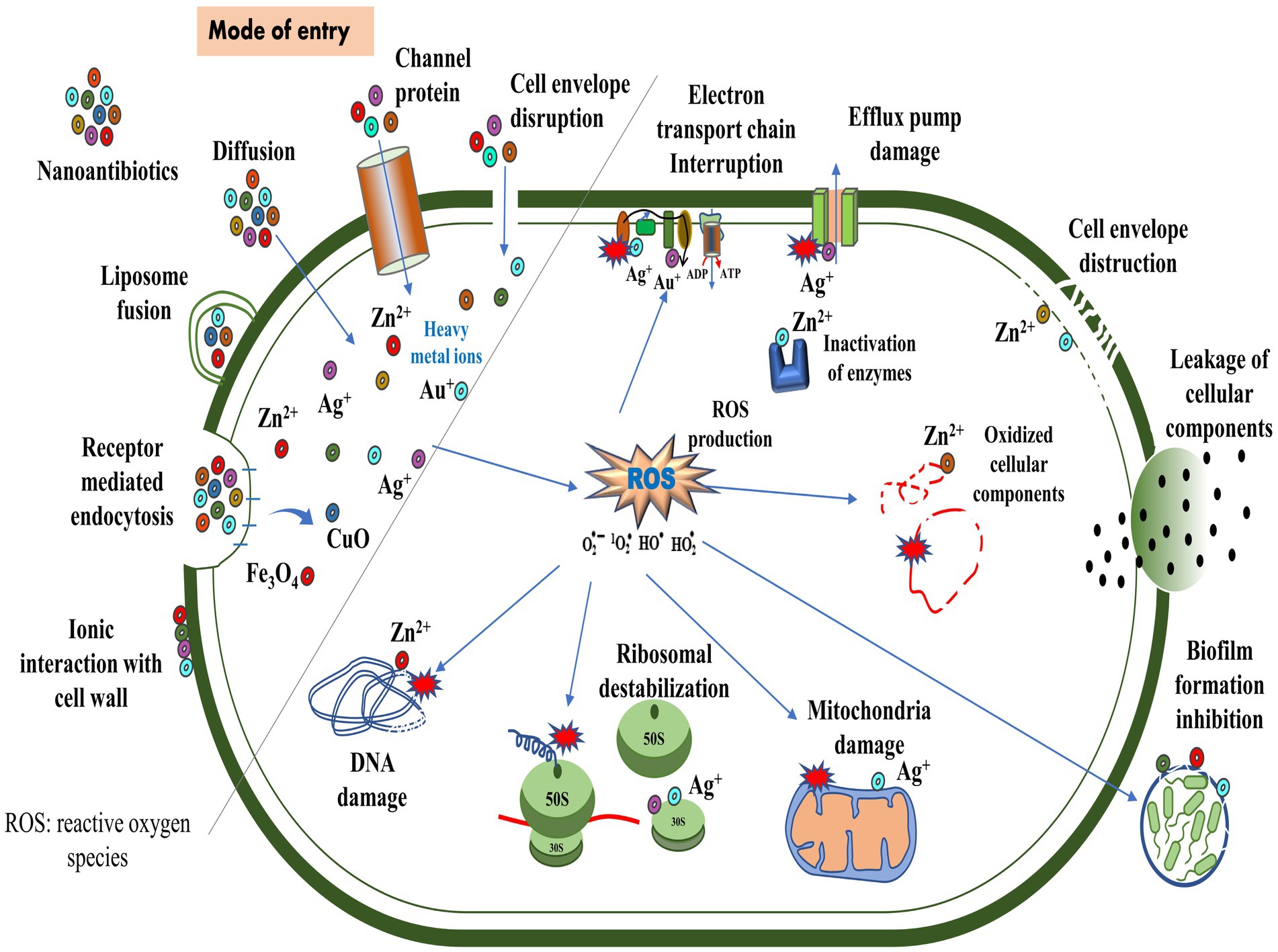
Figure 4. Routes of entry of nanoparticles and their mechanisms of action against bacteria.
According to Liu et al. (2016), titanium alloys infused with copper can effectively eliminate Streptococcus mutans and Porphyromonas gingivalis. In addition, these alloys prevent the formation of biofilms, thereby reducing bacterial infections and implant failures (Junejo et al., 2023). The binding of gold NP to ATP synthase inhibits ATP synthesis, disrupting energy production. Furthermore, its binding to tRNA inhibits its binding to ribosomes (Cui et al., 2011). Researchers examined the biocidal effects of silver (Ag)-NP by scanning and transmission electron microscope in E. coli and observed the pits in the cell wall and accumulation of Ag-NPs (Sondi and Salopek-Sondi, 2004). The copper (Cu)-NP disrupted bacterial membrane integrity, releasing reducing sugars and proteins (Li et al., 2016a). In another study, Cu-NPs strongly inhibited norA efflux pumps by directly binding to the pumps, disrupting efflux kinetics and energy levels (Ashajyothi et al., 2016). Moreover, Au-NP diminished the expression of mexA and mexB genes, reducing active efflux pumps on the cell surface in P. aeruginosa (Dorri et al., 2022). Metal oxide NPs, such as titanium dioxide (TiO2) and zinc oxide (ZnO), are known to exhibit antimicrobial properties. Upon exposure to light or air, these NPs produce reactive oxygen species (ROS), detrimental to bacterial growth. The ROS can induce oxidative stress, damaging the bacterial cell membrane, DNA, and proteins. This disruption of essential cellular components ultimately leads to the death of the bacteria (Malka et al., 2013; Gold et al., 2018; Prakash et al., 2022). The use of antimicrobial polymers may increase the effectiveness of antimicrobial agents. Nanoengineered antibacterial polymers, with increased surface area and reactivity, have great potential for design and biomedical applications. By inhibiting pathogenic bacteria’s growth or destroying their cell membranes, they possess superior antibacterial activity to conventional agents (Borjihan and Dong, 2020).
7 Nanotechnology and nanoparticles in combating drug-resistant strainsNanotechnology offers a transformative solution to combat the escalating menace of MDR, XDR, and PDR, holding immense promise. To develop innovative strategies for targeted drug delivery, biofilm disruption, and overcoming bacterial resistance mechanisms, researchers are harnessing the unique properties of nanomaterials. The use of nanomaterials has the potential to provide an effective means of combating MDR bacteria due to their diverse antibacterial mechanisms and lower propensity to cause resistance (Li M. et al., 2023). Currently, numerous NPs exhibit in vitro antimicrobial efficacy against MDR pathogens, encompassing the ESKAPE pathogen. Moreover, researchers are dedicated to exploring NP pharmacokinetics, pharmacodynamics, and the mechanisms of bacterial resistance (Lee N.-Y. et al., 2019; Adeniji et al., 2022).
Bacterial biofilms contribute to persistent infections by exhibiting increased resistance to antibiotics, disinfectants, and host immune responses (Shree et al., 2023). A promising application of nanotechnology involves the penetration of NPs into biofilms and the exertion of bactericidal effects on those biofilms. Their unique size and characteristics enable these NPs to efficiently target biofilms of drug-resistant pathogens (Hetta et al., 2023). A variety of NPs have been utilized to control microbial biofilm formation. These include metal and metal oxide NPs, solid lipid NPs, liposomes, micro-and nanoemulsions, and polymeric NPs (Mohanta et al., 2023). Metallic NPs represent a promising approach for combating MDR P. aeruginosa (Liao et al., 2019; Abeer Mohammed et al., 2022). The conjugation of Ag-NPs with vancomycin demonstrated potent antimicrobial activity against the MDR pathogen (Esmaeillou et al., 2017). Additionally, Ag-NPs synthesized from Phyllanthus amarus extract exhibited effective antibacterial potential against MDR strains of P. aeruginosa from burn patients (Singh et al., 2014). A study by da Cunha et al. (2023) demonstrated the antibacterial and antibiofilm properties of Ag-NP against MDR Staphylococcus species. Additionally, when conjugated with chitosan, Ag-NP demonstrated inhibitory activity against MDR strains of S. aureus and A. baumannii (Mohammadinejat et al., 2023). The Ag-NPs possess intrinsic antimicrobial properties, whereas the Au-NPs require ampicillin binding to carry out their antimicrobial activity. However, Au-NP and Ag-NP functionalized with ampicillin exhibit broad-spectrum bactericidal activities, especially against MDR bacteria (Brown et al., 2012). Although Ag-NPs have undergone extensive evaluation for their antibacterial properties, there is a scarcity of studies investigating their effectiveness against MDR pathogens, with even fewer addressing XDR or PDR strains.
A nanoantibiotic and a SERS-nanoTag have been created by complexing bi-metallic NPs (Au and Ag) with linezolid and 4-mercaptophenyl boronic acid, respectively. These complexes demonstrated effective antibacterial activity against various microorganisms, including MRSA (Hada et al., 2022). Metal oxide NPs, including both ZnO-NPs and a combination of MgO-NPs and ZnO-NPs, exhibit enhanced bactericidal activity against MDR-TB (Yaghubi Kalurazi and Jafari, 2020). A drug-loaded PLGA-NP containing levofloxacin, linezolid, ethambutol, prothionamide, and pyrazinamide has demonstrated promising efficacy and triggers macrophage innate bactericidal events, offering a promising strategy for treating MDR-TB (Jiang et al., 2023). Graphene oxide (GO) serves as an adjuvant for developing improved anti-TB treatments by trapping mycobacteria in the extracellular compartment, thus inhibiting their entry into macrophages (Salustri et al., 2023). Furthermore, the combination of GO with linezolid has been demonstrated to have a potential anti-TB property that is being explored to combat drug-resistant M. tuberculosis strains (De Maio et al., 2020). Nanoemulsions containing Curcuma longa enhanced ceftazidime’s antibacterial and antibiofilm activity for treating bacterial infections caused by MDR K. pneumoniae (Confessor et al., 2024). In another study, NPs loaded with farnesol (FSL NPs) successfully eradicated S. aureus within a few hours and achieved 100% inhibition of biofilm formation by drug-resistant S. aureus (Maruthapandi et al., 2023). Figure 5 illustrates the contribution of nanotechnology in addressing drug-resistant bacterial infections.
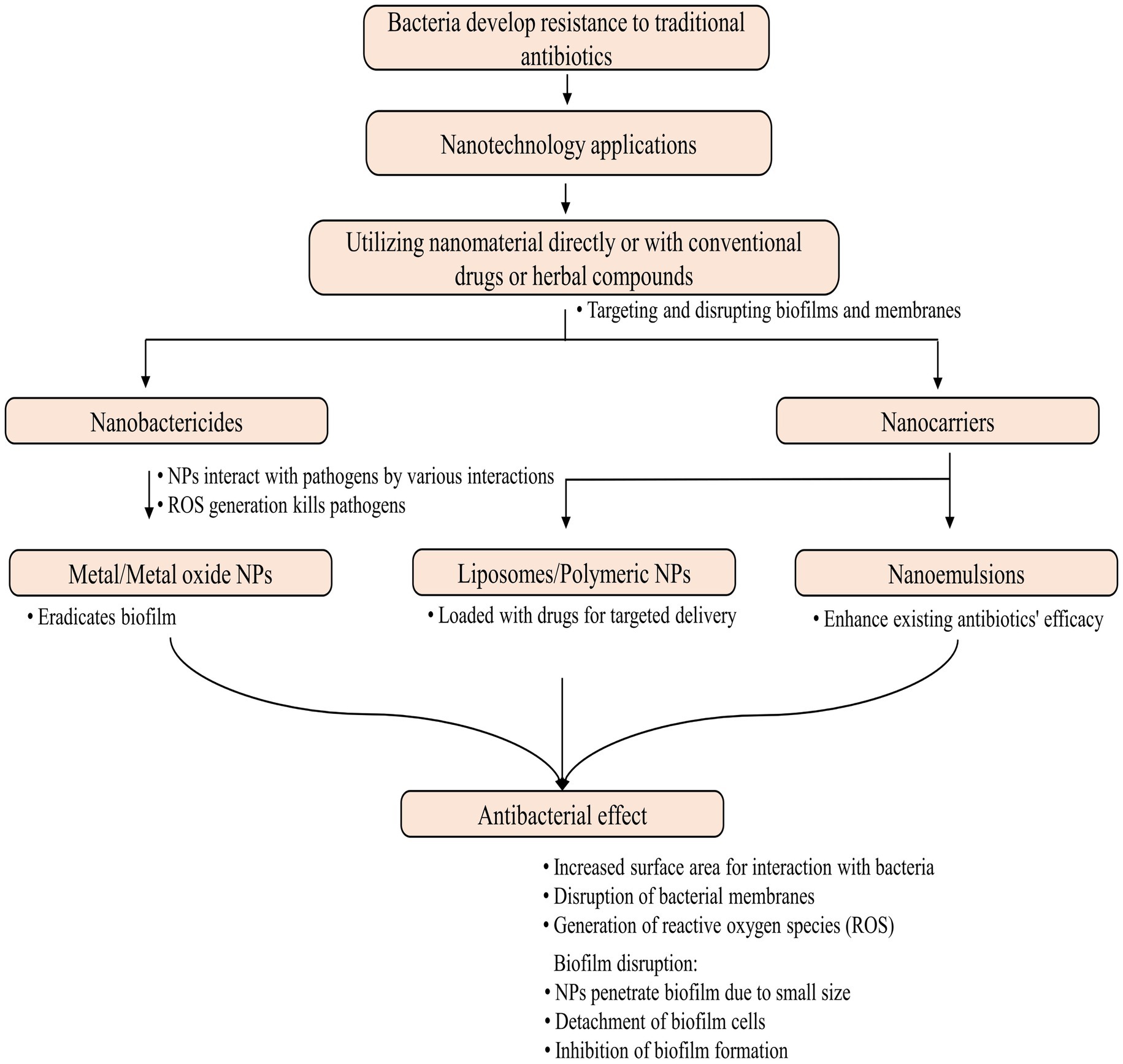
Figure 5. Nanotechnology in combating drug-resistant bacterial infections.
Although nanotechnology has shown promise, its applications against XDRs and PDRs have been addressed in only a few studies where herbal compounds have been explored. The effectiveness of Ag-NPs synthesized from Helicteres isora aqueous fruit was evaluated against XDR strains of P. aeruginosa. The findings suggest that the disruption of membrane permeability induced by Ag-NPs may account for the growth inhibition and death of the XDR pathogen (Mapara et al., 2015). According to Banihashemi et al. (2021), carbon nanotubes coated with an antibacterial compound have demonstrated antibacterial performance against MDR and XDR strains of A. baumannii. However, agar well diffusion and broth microdilution techniques assessed cinnamon oil’s antimicrobial efficacy against XDR and PDR P. aeruginosa isolates (Abdelatti et al., 2023). Moreover, another study showed the highest antimicrobial activity against MDR or PDR H. pylori strains (Ali et al., 2022). These findings underscore the promising role of nanotechnology in addressing the growing challenge of antimicrobial resistance.
8 Plant-derived nanoparticlesGreen NP synthesis involves natural materials and environmentally friendly methods, which eliminate the use of harsh chemicals and solvents. NPs created through this method are known for their biocompatibility, sustainability, minimal environmental impact, and cost-effectiveness (Singh et al., 2023). In general, natural NPs are found to have more stability and compatibility than artificial ones due to the presence of capping layers (Javed et al., 2020). Fe-NPs derived from blueberry leaf extracts possess a natural capping of polyphenols that promote stability (Manquián-Cerda et al., 2017). Additionally, these capping layers provide surface area for biological interactions (Singh et al., 2018) and increase the shelf life of the NPs, as well as enhance their physical and biological properties, making them more effective in treating diseases.
Various studies have explored the synthesis of metal and metal oxide NPs, including silver (Ag), gold (Au), copper (Cu), zinc oxide (ZnO), and others, using plant extracts such as Phyllanthus emblica, Trachyspermum ammi, Clerodendrum inerme, Azadirachta indica, Emblica officinalis, and others. The synthesis of Ag-NP and Au-NP remains challenging due to high energy and chemical requirements, as well as byproduct formation. However, plant-based NPs have medical potential and compatibility for the treatment of drug-resistant microbes (Hammami et al., 2021; Wahab et al., 2021; Balaji et al., 2023). Specifically, Ag-NP derived from Phyllanthus emblica fruit extract exhibited significant antimicrobial activity against Acidovorax oryzae strain RS-2 (Masum et al., 2019), Au and Ag-NPs from Clerodendrum inerme leaf extract (Khan et al., 2020), and Au-NPs synthesized from Trachyspermum ammi seed extract effectively targeted drug-resistant biofilms of Listeria monocytogenes and Serratia marcescens (Perveen et al., 2021). Yadeta Gemachu and Lealem Birhanu (2024) synthesized ZnO, CuO, and NiO-NPs from Azadirachta indica leaf extract, with CuO-NPs displaying excellent photocatalytic activity. ZnO-NPs exhibit various unique mechanical attributes, including high catalytic and photochemical activity, a low melting point as biosensors, and exceptional antibacterial and antifungal properties (Sirelkhatim et al., 2015). ZnO-NP from Emblica officinalis showed antibacterial and anti-biofilm activity (Kaur et al., 2020), whereas those derived from orange fruit peel extract demonstrated bactericidal activity (Doan Thi et al., 2020). Furthermore, ZnO-NP from the aqueous extract of Ocimum lamifolium (Tilahun et al., 2023) and Cocos nucifera leaf (Rahman et al.
留言 (0)