Major Depressive Disorder (MDD) is a prevalent mental health condition affecting approximately 280 million people worldwide, which accounts for about 5% of the adult population (1). Characterized by persistent depressed mood most of the day, nearly every day for at least two weeks, depressive episodes are often accompanied by symptoms such as disrupted appetite and sleep, poor concentration, and feelings of excessively low self-worth. These symptoms significantly impact quality of life and can lead to severe consequences, including an increased risk of suicide, with individuals suffering from MDD being nearly twenty times more likely to commit suicide compared to those without depression (2).
MDD is a heterogeneous disorder with complex origins, including varied genetic and environmental factors (3, 4). Approximately 35%–40% of depression cases are inherited, suggesting that external factors such as adverse life experiences account for the remaining 60%–65% (5, 6). Risk factors for MDD include childhood trauma, substance use disorders, and low socioeconomic status (7).
Current treatments for MDD encompass psychotherapy (e.g., cognitive behavioral therapy, supportive therapy, and psychoeducation), pharmacological treatments (e.g., selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and ketamine), and somatic treatments (e.g., electroconvulsive therapy) (7, 8). Although these interventions are effective for some patients, the Global Burden of Disease Study 2019 indicates that the incidence and burden of depression among young people (ages 10-24) have been increasing annually over the past decade (9). This trend raises questions about the efficacy of the serotonin hypothesis, which has dominated MDD research and treatment for decades, and the widespread use of SSRIs (10).
For 30-40% of patients, antidepressants do not provide adequate responses (3, 5). Moreover, one-third of MDD patients show no response even after four lines of antidepressant treatment (5, 11). A recent systematic review found no consistent evidence linking serotonin with depression, further challenging the serotonin hypothesis (10). Additionally, withdrawal symptoms upon discontinuing antidepressants present another significant issue (12, 13). These findings underscore the urgent need to deepen our understanding of the dynamic and complex mechanisms underlying MDD.
This manuscript aims to: 1) investigate the underlying biomolecular mechanisms of MDD using a modeling approach, and 2) integrate this knowledge with a new comprehensive ‘spiraling risk factor model’ to inform integrated biopsychosocial treatment approaches for MDD. The research question guiding this work is: How can the biomolecular mechanisms underlying MDD be related to psychological and environmental risk factors, and how can they be integrated into a translational research framework?
Recent research suggests that MDD is more complex than a brain-only disease, involving a dysregulated hypothalamic-pituitary-adrenal (HPA) axis, a pro-inflammatory state, and dysbiosis of the microbiota-gut-brain axis (MGBA) (7). These complex mechanisms are mediated by key biomolecules such as cytokines enhancing the pro-inflammatory state like interleukin-6 (IL-6) and tumor necrosis factor (TNF) (14), short-chain fatty acids (SCFAs) (15, 16), cortisol (17), brain-derived neurotrophic factor (BDNF) (18), neurotransmitters (NTs) (19, 20), and lipopolysaccharide (LPS) (21). While fundamental knowledge about these underlying biomolecular mechanisms exists, a comprehensive picture of their interrelations is lacking. Therefore, we propose a schematic model incorporating the MGBA, HPA axis, and immune system in relation to MDD (Figure 2).
Moreover, MDD results from a combination of biomolecular, environmental, and psychological risk factors (7). Dysbiosis in the MGBA, imbalances in mental and physical conditions, lifestyle factors, and pre-existing treatments all contribute to the disorder. For example, van der Gronde et al. (5) describe how chronic stress and failure to cope can trigger a downward spiral of stress and anxiety, potentially leading to MDD. Thus, we aim to combine the available knowledge of the biomolecular mechanisms of MDD with psychological and environmental risk factors into a comprehensive spiraling risk factor model (Figure 3). This biopsychosocial model is intended to develop a translational research framework for the prevention and treatment of MDD.
2 MethodsFor our first aim, we carried out a systematic review that focused on research data concerning the role of the MGBA in the etiology of MDD, described in the sections 4 and 5 of this manuscript. The databases used were EMBASE, PubMed and Scopus, and the review has been pre-registered at PROSPERO under registration number CRD42020215412.
Studies were included that had a focus on the effects of stress, inflammation, microbiota, the gut-brain axis, external influences and depression.
Clinical trials and studies, as well as other research findings have been gathered through PubMed, Scopus and EMBASE using the following search strings:
- EMBASE: (‘depression’/exp OR ‘stress’/exp) AND (‘microflora’/exp OR ‘microbiome’/exp) AND ‘inflammation’/exp AND (2016:py OR 2017:py OR 2018:py OR 2019:py OR 2020:py) AND (‘article’/it OR ‘conference abstract’/it OR ‘review’/it).
- PubMed (limited from 2016-2020): (“depressive disorder”[All Fields] OR “stress”[All Fields]) AND (“microbiota”[All Fields] OR “gastrointestinal microbiome”[All Fields]) AND “inflammation”[All Fields] based on MeSH terms.
- Scopus: TITLE-ABS-KEY ((“depression” OR “stress”) AND (“microflora” OR “microbiome”) AND “inflammation”) AND (LIMIT-TO (PUBYEAR, 2020) OR LIMIT-TO (PUBYEAR, 2019) OR LIMIT-TO (PUBYEAR, 2018) OR LIMIT-TO (PUBYEAR, 2017) OR LIMIT-TO (PUBYEAR, 2016)).
The search strings were based on keywords relating to the research question, building on searching strategies of prior research. The keywords that had been found were (any derivations of) inflammation, stress, depressive disorder, gastrointestinal biome.
Only peer-reviewed studies written in English from 01 January 2016 until 14 August 2020 were included. The systematic review does not include translated studies, book chapters, conference abstracts, methodology reports and editorials.
Articles from the search were included by LV based on title and abstract and finally on full-text assessment. Judgements made regarding the inclusion of articles were carefully supervised by TP. Excluded articles and their specific exclusion rationality can be found in Supplementary Table 1. Risk of bias assessment was performed by LV and MB for the initial database search and reviewed by TP. Risk of bias assessment for externally included studies was performed by both MB and LV and reviewed by TP. Assessment was done manually and no automation tools were used.
A wide variety of studies regarding different aspects relating to our research question were included. This was done to maximize different perspectives regarding the research question. This includes, for example, the role of immune cells in MDD, as the microbiota can interact with these immune cells. But also some studies on for instance inflammatory bowel disease, as there are phenotypic similarities to MDD. Studies that offer minimal to no insight on MDD were excluded (TP, LV, MB).
To further maximize the identification of eligible articles related to the research question, external studies were included through websites and citation searching/snowballing, according to the PRISMA 2020 protocol (Figure 1, PRISMA flowchart).
For our second aim, we integrated the results of our systematic review for the first aim in a narrative review, described in section 6. Mainly PubMed was used to search for relevant publications, with the preference for recent systematic reviews and meta-analyses. To maximize different perspectives, externally found literature together with select additions of recent findings based on collective suggestions of the authors were added (applies to all sections of this article).
3 ResultsFor our systematic review (aim 1; section 4 and 5), from the 2262 articles originally retrieved via databases and registers, only 43 articles were included in the systematic review (Figure 1, PRISMA flowchart). Prior to screening, 667 articles were removed due to duplication. During screening, 1394 records were excluded on title, another 3 records were excluded due to inability to retrieve the record, yielding 198 articles assessed for eligibility. After abstract screening, full-text screening, and during writing, an additional 102, 8 and 45 records were excluded respectively. The details of the reason for exclusion can be found in Supplementary Table 1.
Figure 1. PRISMA flowchart: PRISMA 2020 flow diagram for new systematic review which included searches of databases, registers and other sources.
Through other methods including external sources and snowballing, an additional 34 articles were added. This yields a total of 77 articles the systematic review was based on.
For our narrative review (aim 2; section 6) we included an additional 89 articles found across mainly PubMed. As this second part of the article is a narrative review and not a systematic review, there is no PRISMA flow diagram shown for this part.
4 Major depressive disorder and possible biological etiological mechanismsTo develop a translational research framework for prevention and treatment of MDD, we start with what is known about the biological underlying mechanisms. As MDD is highly heterogeneous and associated with many comorbidities, the biology is intricate and not related to a specific factor. Partly, MDD has been associated with complex genetics and neurobiology (22). As most MDD patients experience a lot of stress because of a wide variety of stressors, we will also focus on the stress mechanisms (5, 15). Closely related to stress, the immune system is another universal finding in MDD, making the immune system an important mechanism as well (19). Furthermore, the disruption of the gut microbiome called ‘dysbiosis’ is underlying both the stress and the immune system, making it interesting for us to further elaborate on the topic of dysbiosis in this review. The MGBA plays a major role in the complex interplay of these mechanisms (21). The genetics, neurobiology, stress response, immune system, MGBA and the interplay of these processes related to MDD will be discussed in the upcoming sections (Table 1).
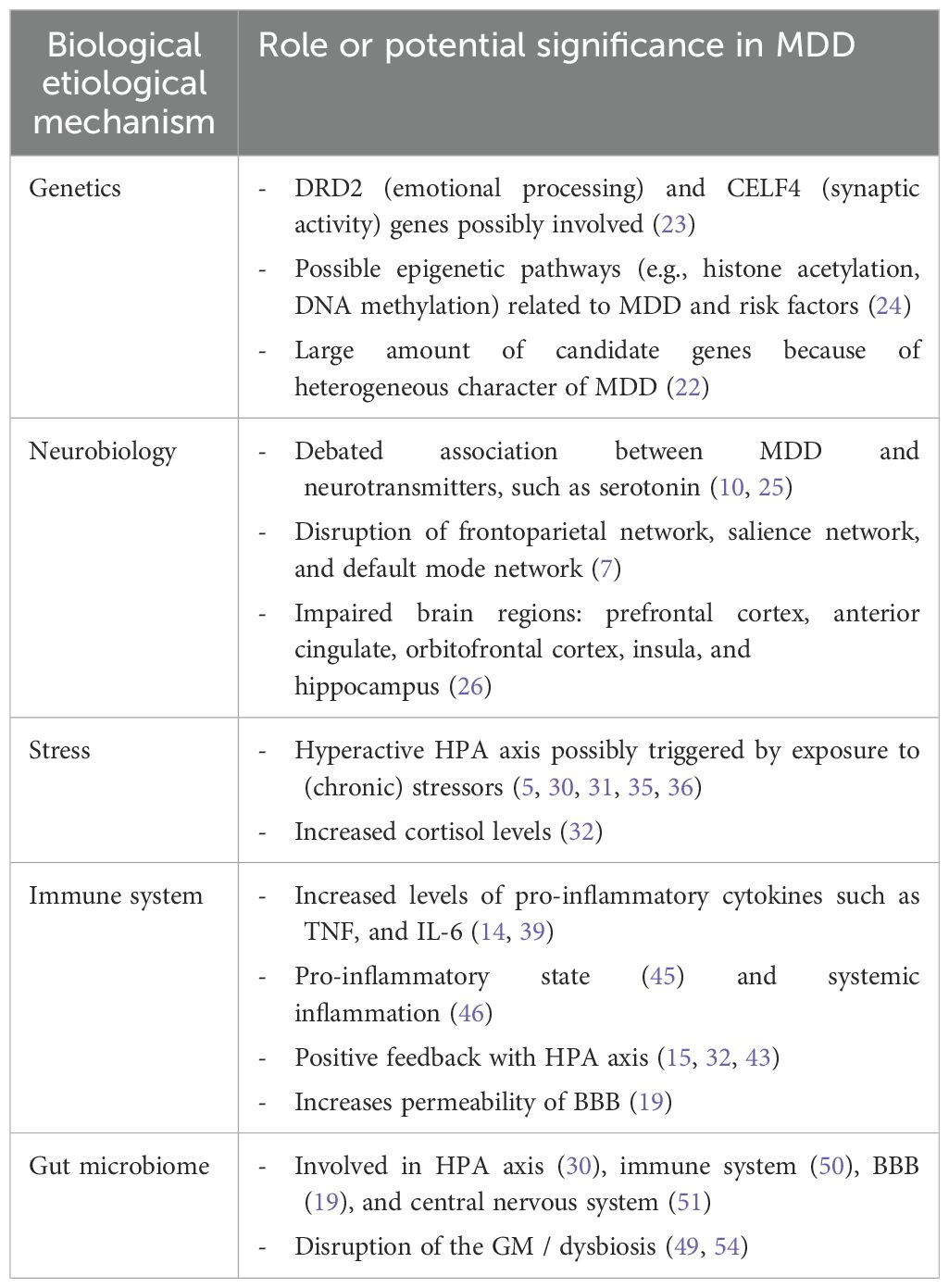
Table 1. Overview of (potential) biological etiological mechanisms in MDD and their impact on the disorder.
4.1 GeneticsAccording to recent research, children of individuals with MDD face a 35–40% likelihood of experiencing MDD in early adulthood, which is twice the risk observed in offspring of parents without MDD (5, 7). This includes both genetic and environmental factors within the family, in which both factors roughly contribute equally.
Examples of genes that implicate a neurobiological etiology of MDD are the dopamine receptor D2 (DRD2) gene, which is related to emotion processing, and the CUGBP Elav-Like Family Member 4 (CELF4) gene, which is associated with regulating synaptic activity for excitatory neurons (Table 1) (23). Compared to the majority of other mental disorders, the heritability of depression of approximately 37% is relatively low. Although large (up to one million participants) genome-wide association studies (GWAS) identified 178 genetic risk loci and 200 candidate genes, the specificity and robustness of these results are questionable (22). This might be due to the fact that researchers adopted minimal phenotyping methodology to identify cases to obtain robust statistical significance. This comes with a cost, resulting in signals that are insufficiently attributable to MDD. Moreover, effect sizes of GWAS results are rather small (7). Moreover, epigenetic processes could play a role in facilitating interactions between genes and the environment. Researchers found some markers related to MDD and risk factors (such as childhood trauma) and epigenetic pathways such as histone deacetylases and DNA methyltransferases (24). However, the sample sizes were small and for some studies only animal research was performed.
The considerably weak results of genetics involved in MDD may partly be explained by the fact that it is a complex and heterogeneous disease. Moreover, the high number of comorbidities many patients experience may result in a wide variety of contributing factors that are not reducible to single genes or single nucleotide polymorphisms. Additionally, in terms of treatment applications, it is hard to develop genetic treatments to help patients with MDD. However, the robustness of genetics underlying MDD may increase in the future.
4.2 NeurobiologyDespite the inconsistent findings between serotonin and MDD, depression is still thought to be a disease in which the brain plays a crucial role (Table 1) (10). Moreover, the study by Moncrieff et al. (10) has raised significant critiques regarding its reliability, as highlighted in multiple correspondences available on their webpage. An important example is that they misinterpret some of the reviewed data and suggested that serotonin reuptake inhibitor antidepressants, such as SSRIs, may decrease rather than enhance serotonin function (25). Furthermore, MDD exhibits high heterogeneity and is more complex than simply attributing MDD to serotonin or excluding the role of serotonin completely.
Besides serotonin, MDD is associated with the disruption of networks and different brain regions (7). Functional magnetic resonance imaging (fMRI) studies reported certain hypo- and/or hyperconnectivities in three neural networks, namely, the frontoparietal network (higher order cognitive processes), the salience network (emotional and motivational stimuli) and the default mode network (self-referential thinking). Brain regions in the central nervous system (CNS) associated with MDD are the prefrontal cortex, anterior cingulate, orbitofrontal cortex, and insula (7). These brain regions play a role in emotional processing and cognitive control. For instance, a study analyzing MRI data from 10.105 people (of which 2148 were MDD patients) showed that grey matter density of the orbitofrontal cortex, anterior cingulate cortex, and insula was reduced in MDD patients compared to healthy controls (26). Furthermore, other research demonstrated that decreased postmortem hippocampal volume is associated with MDD (27). This aligns with data from MRI studies showing subtle increment of hippocampal volume in remitting MDD patients (28). However, structural brain differences in individuals with MDD exhibit small effect sizes, and are not specific to MDD (7). These kinds of structural differences can be found in other mental disorders like anxiety disorder as well (29). Moreover, the mechanisms underlying these structural changes are possibly alterations in dysregulation of the HPA axis, the immune system and the gut-brain axis. Together with the fact that a large proportion of patients do not find adequate relief from antidepressants that directly target brain function (such as SSRIs), it is worthwhile to explore these alternative biological mechanisms (5).
4.3 StressHyperactivity of the HPA axis is found in many psychopathologies, including depression (30, 31). Symptoms of depression, such as disrupted sleep and hopelessness, have been associated with HPA axis impairments (Table 1). In humans, higher cortisol levels are found in more than 70% of MDD patients (32). Also in rats, research showed that an over-activated HPA axis increased anxiety and depressive-like behavior (15). Because of the significant relation between the HPA axis and depression, researchers believe that hyperactivity of the HPA axis is one of the most reliable biological markers of MDD (15, 33). High cortisol levels may therefore potentially function as a predictor for MDD onset (34). However, it is still not completely clear whether dysregulation of the HPA axis is a cause or consequence of depression.
The HPA axis starts its response by producing corticotropin-releasing hormone (CRH) in the hypothalamus, CRH then travels to the pituitary gland and stimulates the production of adrenocorticotropic hormone (ACTH) (17). ACTH subsequently travels to the adrenal gland via the blood and stimulates the production of glucocorticoids such as cortisol (Figure 2). Cortisol in turn inhibits its own production at both the pituitary gland and hypothalamus, creating a negative feedback loop.
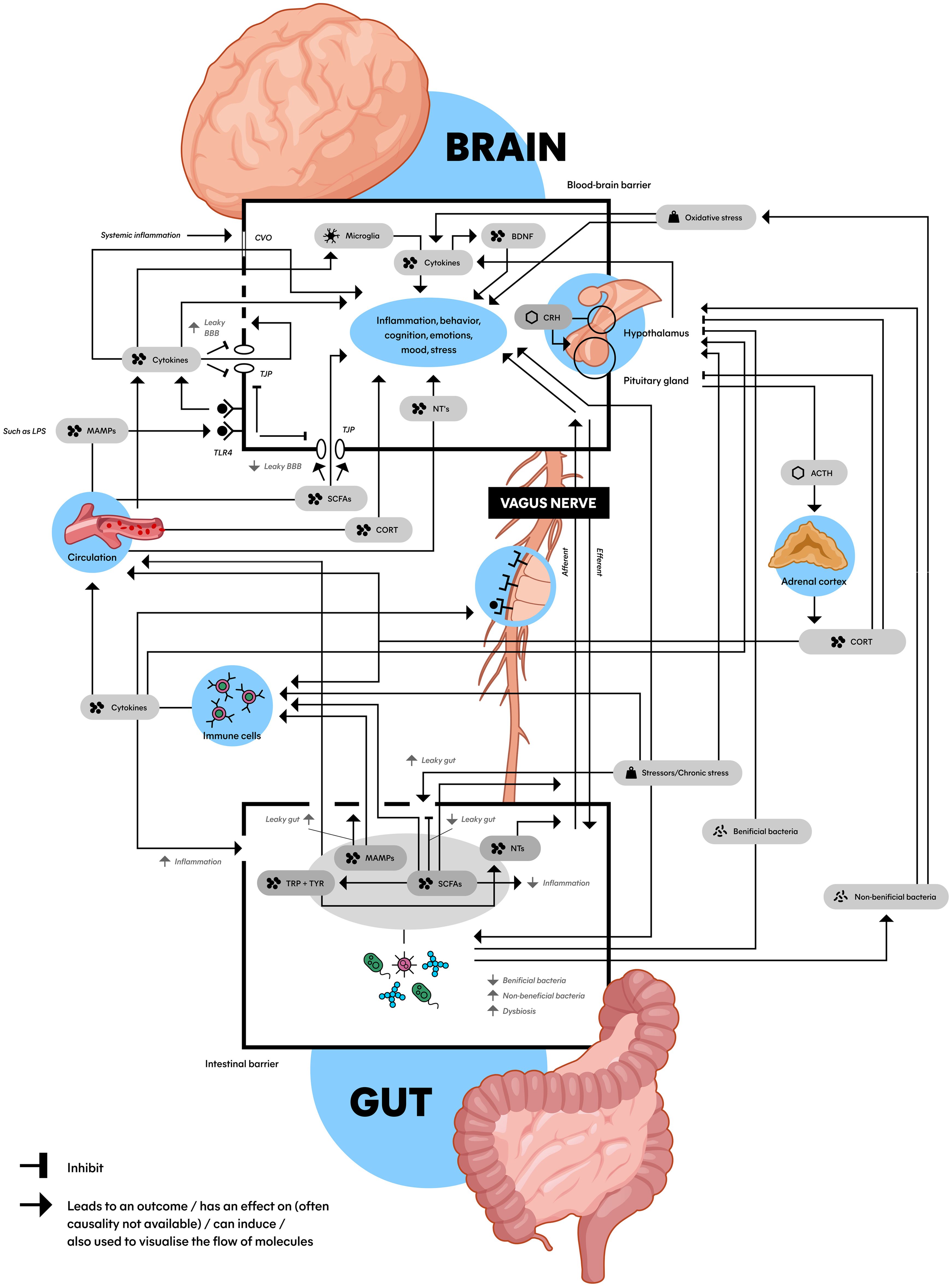
Figure 2. Schematic model of the microbiota gut-brain axis: An interplay between the HPA axis, the immune system, the gut microbiome and the brain connected by biomolecules. This figure shows the complex communication between the gut (bottom) and the brain (top) with a wide array of factors inducing, promoting, and inhibiting the stress (top right) and immune system (middle left) with an important role for the gut microbiota and microbial derivatives (bottom). Microbiota can be (indirectly) bidirectionally involved with a hyperactive HPA axis and pro-inflammatory state that play a role in the etiology of MDD. ACTH, adrenocorticotropic hormone; BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; CORT, cortisol; CRH, corticotropin-releasing hormone; CVO, circumventricular organs; LPS, lipopolysaccharide; MAMPs, microbe-associated molecular patterns; NTs, neurotransmitters (serotonin, dopamine and norepinephrine); SCFAs, short-chain fatty acids; TJP, tight junction protein; TLR4, toll-like receptor 4; TRP, tryptophan; TYR, tyrosine; Dashed line in BBB: impaired BBB integrity; Dashed in in gut epithelial barrier: leaky gut.
Possible reasons for the involvement of stress and the HPA axis in depression could be the fact that chronic stress and stressors can result in a psychological downward spiral in humans, which can cause difficulty adjusting to continuously stressful situations, which in turn is related to exhaustion and ultimately depression (5, 35). The continuous exposure to stressors can for instance disturb receptor signaling in the amygdala, ultimately leading to the HPA axis activation (36). Moreover, it is thought that overactive cortisol production can lead to damage of the hippocampus and increase vulnerability to MDD.
4.4 Immune system and the pro-inflammatory stateAnother consequence of stress and a dysregulated HPA axis can be suppression of immune function by affecting cytokines and cytokine production (30, 37). A recent comprehensive systematic review investigated the impact of MDD on 36 comorbid diseases found that the HPA axis was dysregulated, and the immune system was affected in these comorbidities (38). They also describe inflammation in general (whether induced by a disbalance in the HPA axis or not) as an underlying biological mechanism of MDD. This inflammation is related to elevated circulation of especially pro-inflammatory cytokines, for example TNF and IL-6, which are also associated with MDD (14, 39). Both hyperactivation of the HPA axis as well as immune activation during depressive episodes have been observed by other research (19). IL-6 (a marker of systemic inflammation) levels might even predict risk of the onset of MDD and poor antidepressant treatment response (40–42).
Pro-inflammatory cytokines can stimulate the HPA axis by binding to it (Figure 2) (15, 32, 43). This HPA axis hyperactivation can further increase the expression of cytokines, ultimately creating a positive feedback loop. Another important effect of pro-inflammatory cytokines and elevated stress is the increase in permeability of the blood-brain barrier (BBB) (Figure 2) (19). When BBB integrity is impaired (‘leaky BBB’), substances like pro-inflammatory cytokines can cross the BBB more easily, reaching the brain and affect mood and behavior (44). When pro-inflammatory cytokines disrupt the regulation of such mechanisms, the body can subsequently enter a state known as the ‘pro-inflammatory state’ (45, 46).
The pro-inflammatory state refers to a bodily condition characterized by malfunctioning of the immune system, indicated by elevated levels of proinflammatory cytokines (45). This systemic inflammation is seen as a physiological trigger of MDD (Table 1) (46). Research in humans showed that inflammatory factors are higher among depressed patients compared to controls. Studies found that inflammation can affect regional brain activity, neurogenesis and changes in microglia and astrocyte-specific markers in several brain regions (7). Moreover, pro-inflammatory drugs can induce depressive symptoms and increase the risk of MDD onset (47). Also in rodents, researchers found that systemic inflammation can cause long-term cognitive damage (48).
4.5 Gut microbiome in relation to MDDWhile the HPA axis and pro-inflammatory state are significant factors, we believe there is another crucial player in the etiology of MDD related to the stress- and immune system: the gut microbiome (GM) (39, 49). The importance of the GM therein stems from its ability to exert an effect on many of the previously discussed concepts: the HPA axis (30), the immune system (50), the CNS (51) and the BBB (Table 1, and Figure 2) (19). Additionally, over 90% of serotonin is produced in the gut (19). These findings indicate an intriguing relationship between the microbiota in the gut, the brain, and the development of MDD, warranting a more comprehensive analysis.
The GM encompasses all microorganisms including bacteria, viruses, fungi, and archaea (52). However, because the majority of information is derived from bacterial studies, we will primarily focus on bacteria in this manuscript. It’s important for human health, metabolism, protection against toxins, pathogens, and cytokine secretion (53, 54). Moreover, the GM can impact cognition and emotions, partly by producing or modifying NTs and neuropeptides (54). Altogether, the GM is influenced by numerous factors like genetics, environment, diet, antibiotics, probiotics, and prebiotics (21, 45).
When the gut is disrupted, leading to an imbalance known as ‘dysbiosis,’ it may contribute to mental health disorders like MDD (Table 1) (49, 54). Factors like pathogenic bacteria, stress, antibiotics, and diet are associated with dysbiosis. This imbalance is linked to a higher risk of comorbid conditions between gastrointestinal diseases such as irritable bowel syndrome (IBS) (55) and obesity (56) and stress-related disorders such as depression (49, 54). Additionally, the GM is associated with neurological and psychiatric disorders like schizophrenia, autism, Parkinson’s disease, and multiple sclerosis (49).
The link between the brain and GM is known as the MGBA, a bidirectional connection involving the CNS, enteric nervous system, and digestive system (21). It plays a role in gut movement, hormone and NT secretion, HPA axis, immune system. Given its central role, we will conduct a more comprehensive analysis of the MGBA in the upcoming section.
5 MGBA and MDDThe MGBA has been thought to play an important role in neurological and psychiatric disorders such as Parkinson’s disease, Alzheimer’s disease, autism spectrum disorder and MDD (30). Various studies show that the bidirectional interaction between the GM and the brain affects CNS development and cognitive functions such as stress regulation, behavior and mood (Figure 2) (21, 30). The fact that these CNS functions and pathways are impaired in MDD supports the idea that the MGBA is involved in depression. Moreover, the significant impact of microbiota in neural plasticity and circuitry wiring during neurodevelopment could heighten the vulnerability to stress-induced psychiatric disorders such as MDD (16).
Developmental research demonstrated that the GM is able to influence postnatal development of the HPA response in mice (57). Furthermore, the GM directly influences the development of the brain, observed in germ-free (GF) mice that show abnormal microglia morphology, modified gene expression, and an impaired functional response to stimulation (50). Besides directly influencing the developing brain, the GM also influences the mature brain and neurons (58).
Animal research supporting this idea involved fecal microbiota transplantation (FMT), a technique where researchers transplant fecal matter of human patients with MDD and healthy controls into rats with a depleted gut microbiome. In the rats that received fecal matter originating from MDD patients, the transplantation led to more behavioral and physiological characteristics typically seen in depression, compared to the rats that received fecal matter from healthy controls (59). Also, GF murine models compared to their non-GF counterparts showed remarkable alterations in the brain, immune system, HPA axis, microglia and BBB, which are implicated in anxiety and MDD behavior (58, 60). The relation between GF rodents and MDD may be attributed to the fact that GF mice show morphological alterations of neural dendrites in the amygdala and hippocampus (16). Interestingly, external stressors like maternal separation in rodents also led to behavioral despair, alterations in the HPA axis and changes in gut commensals (21). However, due to the fact that these studies are conducted in animals, it is crucial to exercise caution when interpreting the results, as they serve as mere “depression models”.
In humans, research showed similarity in fecal microbiota signatures of patients with irritable bowel syndrome (IBS) and patients with MDD (55, 61). IBS is characterized by gut dysbiosis, including abdominal pain and bloating. Research found that the prevalence of depression in groups of people with IBS (38.7%) was significantly higher than the prevalence of depression in the control group (6.5%) (62). Moreover, relative to the control groups, depression scores (assessed using the Hospital Anxiety and Depression Index and beck depression inventory) were higher in the IBS groups (63). Concerning microbiota, research showed that the concentration of Lactobacillus in feces was lower for healthy students in a period of intense stress compared to a period of mild stress (21). Furthermore, a systematic review found that depressed patients had a 58% higher risk of becoming obese, and obese patients faced a 55% higher likelihood of experiencing symptoms of depression throughout their lives, showing the bidirectionality of depression and the MGBA (56).
Though it is important to realize that these correlations are not necessarily causal relationships, they give a clear indication of a link between MDD and diseases related to the MGBA. In order to utilize this knowledge to develop treatment possibilities, it is crucial to understand the process through which the connection between MGBA and MDD is established.
5.1 The interplay between the dysbiosis, the pro-inflammatory state and a dysregulated HPA axis in MDDThe contribution of the MGBA in the etiology of MDD roughly consists of the complex network of interactions between dysbiosis, the pro-inflammatory state and a dysregulated HPA axis as major players (21, 50). For instance, chronic stressors influence the GM composition, resulting in activation of the HPA axis and elevation of the pro-inflammatory state (19). As mentioned earlier in this review, dysregulation of these mechanisms are known to worsen symptoms of MDD (30, 47, 49). To create an overview of current knowledge, we visualized these concepts into a model, which can be found in Figure 2. Though this is a simplified representation, it gives an impression of the processes at play. The connections in the Figure will be clarified in the next sections.
In section 4.3, we explained the interaction between the HPA axis and the brain, which can be found at the top right of Figure 2 (hypothalamus, pituitary gland, adrenal cortex, and biomolecules in between). As we discussed in section 4.4, the HPA axis is connected to the immune system, which is found at the middle left of Figure 2 (immune cells). The immune system can lead to systemic inflammation and impair BBB integrity, affecting mood and the brain visualized at the top of the image (44). The BBB is displayed as the top box with a black border in Figure 2. What becomes clear from section 4.5, is that these processes are connected to the gut, which is visualized at the bottom of the image. The bottom box with the black border represents the gut epithelial barrier, schematically displaying the biomolecules and processes related to the MGBA inside. Another crucial structure in connecting the brain to the GM is the vagus nerve, visualized in the middle of Figure 2 (64).
5.2 Vagus nerveThe vagus nerve (VN) is the tenth (X) cranial nerve which transmits afferent (sensory) and efferent (motor) sensory information towards and from the CNS to the periphery, forming a direct link between the brain and the gut (64). The VN communicates in a bi-directional relationship with the immune system and can be activated through short-chain fatty acids (SCFAs), and inflammatory processes in- and outside the periphery (65, 66). In other words, the VN forms a connection between the CNS and enteric nervous system mediated by immunoregulatory signals (19). The VN can affect appetite, mood, and sickness behavior, and possibly induce an immune response through efferent vagal signaling (65). Moreover, cytokine receptors that detect and react to inflammation are expressed on VN afferents. This in turn influences the activity of brain regions implicated in mood and motivation (60). Other research showed that vagotomy (removal of the VN) was associated with decreased neuronal activity and percentages of immune cells, and changes in gene expression and depression-related behavior in rodents (64, 67).
5.3 MicrogliaOther cells involved in the MGBA-related etiology of MDD are microglia (Figure 2). Microglia are macrophage-like cells in the brain, functioning as important immune cells that detect changes in the environment. Microglia are also involved with neuroinflammatory processes and are a part of MGBA communication, therefore possibly involved in the etiology of MDD (45). The GM plays a critical role in multiple aspects of microglia including maturation, morphology, and immunological function (68).
Microglia produce cytokines in the brain, and alterations in microglia and cytokines can result in neuroinflammation and is likely fundamental in MDD (60). Those cytokines may affect MDD through influencing growth factors (like BDNF) and the production of toxic metabolites. Additionally, neuronal destruction and the production of neurotoxic compounds may be related to symptoms of MDD. Moreover, there is a link between stress and regulation of immune responses that affect microglia in the brain, which can lead to neuroinflammation (69, 70).
5.4 BiomoleculesFor connecting the mechanisms (i.e., HPA axis, immune system, MGBA, VN) together, Figure 2 shows several biomolecules (such as SCFAs and lipopolysaccharides; LPS) that play their own part in the MGBA. These are interconnected, and play a role in the dysregulated HPA axis, pro-inflammatory state and dysbiosis. How these biomolecules exert their effect and connect the mechanisms described above will be explained in the next sections.
5.4.1 SCFAsLow levels of SCFAs have been associated with depressive-like behavior, compared to high levels of SCFAs (Figure 2) (15, 19, 71). SCFAs belong to the major gut bacteria metabolites and can offer relevant benefits in terms of depression relief, anti-inflammatory effects, neuroprotection, regulating T-cell induction, and a good BBB permeability balance (15, 16). This improvement of BBB integrity by butyrate (a SCFA) has been associated with the upregulation of tight junction protein (TJP) expression, which are proteins in the brain restricting substances to move freely between the brain and blood (71). Dysregulation of TJP is related to impaired BBB integrity, exposing the CNS to damaging substances. However, due to the MGBA being highly interconnected and SCFAs not being the only microbial metabolites, the causal link between SCFAs and the increase of the BBB remains uncertain. Moreover, reduction of SCFA-producing bacteria play an important role in dysbiosis, gut mucosal inflammation and loss of intestinal barrier integrity (leaky gut) (16).
5.4.2 LPSAnother important biomolecule involved in MGBA and MDD is LPS (21). Research suggests that LPS has the capability to trigger depressive-like behavior in animal models (72). LPS is a microbe-associated molecular pattern (MAMP) and a large constituent of gram-negative bacteria that binds to toll-like receptors (TLRs) located on immune cells (19, 73). MAMPs are microbial-derived products which can activate immune cells to promote the release of pro-inflammatory cytokines, which increases permeability of the intestinal barrier (‘leaky gut’) and the BBB, and influence CNS function and behavior (15). Additionally, when pro-inflammatory cytokines are able to cross the (damaged) BBB, they can interact with neurons which can lead to sickness behavior and MDD. The activation of TLRs can also activate the HPA axis, which may result in further increment of BBB permeability and gut-membrane-permeability, the latter associated with leaky gut (19, 45, 74).
5.4.3 Leaky gut and impaired BBB integrityA leaky gut can be the result of the gut epithelial barrier being damaged by dysbiosis and is displayed in Figure 2 as a dashed line (15, 47). The leaky gut has been associated with MDD through the immune system (60), and gut permeability markers are associated with patients with recent suicide attempts (42). Also stress in rodents might increase the leaky gut (74). However, direct mechanistic evidence between dysbiosis and a leaky gut is limited (75). What research does suggest, is that a leaky gut increases unregulated translocation of microbes over the lamina propria (thin layer of connective tissue, such as in the gastrointestinal tract). This can lead to, for instance, the infiltration of immune cells into the brain (71). It has been hypothesized that this infiltration can be pathogenic in the CNS because of the destructive properties of these cells.
Leaky regions in the BBB, called circumventricular organs, allow molecules and cytokines to travel to the brain, are related to systemic inflammation, and may cause altered brain function (76). Immune cells for instance produce cytokines like IL-17A, which further impair BBB integrity (displayed in Figure 2 as a dashed line) and contribute to neuroinflammation (77). This mechanism has been associated with CNS diseases such as multiple sclerosis and morbus Parkinson (78).
Moreover, a leaky gut allows LPS to activate even more TLRs inside and outside the gut. Because of increased BBB permeability and a leaky gut, LPS reaches systemic circulation and is therefore able to travel to the brain, where they can bind to TLR4 located on brain endothelial cells (cells which are part of the BBB), displayed at the top left of Figure 2 (79). Here, LPS can alter TJP expression, contributing to impaired BBB integrity, immune cell trafficking, and the release of more pro-inflammatory cytokines (71). Interestingly, TLR4 has been found to be upregulated in MDD patients (14). Moreover, when MDD patients were successfully treated, the TLR4 levels were found to be restored, suggesting their potential role in depression.
This example highlights how the immune system, the gut, BBB integrity and MDD are interrelated. However, there are many more complex interactions like these involved, but covering them each individually is outside the scope of this review (60, 64).
5.4.4 Neurotransmitters and other signaling moleculesOn top of the biomolecules described in the previous section, neurotransmitters (NTs) are thought to play an important role in the MGBA (Figure 2). Gut microbiota are able to secrete multiple NTs (and precursors), neuropeptides and metabolites. The NTs that are released by different bacteria species are GABA, acetylcholine, serotonin, dopamine, and histamine (19, 20). Another study even suggests that various Lactobacillus spp. can synthesize all the above-mentioned NTs (32). From the gut bacteria, NT (precursors) can travel through the blood or the VN to the brain (80).
Synthesis of NTs and neuropeptides that regulate cognition and behavior is in turn partly modulated by SCFA (mainly butyric and propionic acid) (81, 82). They enhance tyrosine and tryptophan hydroxylase expression, which are involved in dopamine, noradrenaline, and serotonin synthesis (81, 82) and have neuroprotective properties (Figure 2) (83).
5.4.5 MonoaminesSerotonin, dopamine, and norepinephrine are the monoamines that are mostly associated with MDD (7). All three are modulated by antidepressants such as SSRIs and noradrenaline and dopamine reuptake inhibitors. Serotonin is thought to be a crucial NT which is known as the primary regulator of mood and cognition (82, 84). Notably, 90%–95% of serotonin is compartmentalized in the gut, and serotonin production can be regulated by the GM (84). Another article mentions that patients with MDD generally have low circulating levels of tryptophan, possibly because low levels of plasma tryptophan are related to alterations in immune function (64, 76). As tryptophan goes predominantly through the kynurenine pathway, it is interesting that research showed that the kynurenine/tryptophan ratio was significantly higher in depressed individuals compared to healthy controls (59). Additionally, dopamine and noradrenaline are also NTs that have an influence on the CNS and are produced by microorganisms in the gut (Figure 2). For instance, stress in mice showed increased levels of dopamine and noradrenaline in the gut (85).
However, there is a major controversy about the association between MDD, serotonin and tryptophan. A recent paper involving 17 studies (systematic reviews, meta-analyses and more) concluded there is no consistent support for an association between serotonin and depression (10). Also, the relationship between tryptophan and serotonin remains weak. The weak relationship between MDD, dopamine and norepinephrine has not been investigated as comprehensively, but because of the controversy around serotonin, careful interpretation is required (10). This does not necessarily mean the monoamines are not involved in the MGBA, but the interaction is more complex and the relevance for MDD seems to be far less than previously assumed. However, it is important to reiterate that the study by Moncrieff et al. (10) has faced significant critiques regarding its reliability, emphasizing the complexity of serotonin’s role in MDD.
5.4.6 Nitric oxide & oxidative stressAnother NT associated with microbiota influencing MDD is nitric oxide (NO) (Figure 2). The gastrointestinal tract is rich in sources of NO and the GM is known to be involved in oxidative stress (81). Nanomolar concentrations of NO seem to have a neuroprotective effect, but excessive NO production can be neurotoxic – associated with neuroinflammation, cellular damage, axon degradation, and neurodegenerative disorders including MDD. Excess production of NO may lead to the generation of reactive oxygen species and reactive nitrogen species, both causing oxidative stress. This can lead to cellular and DNA damage. Depressed patients show a significant increase in oxidative stress (47, 81). This may have to do with the fact that overproduction of reactive oxygen species characterizes activation of the inflammatory pathway. Also, research shows that endogenous antioxidants can be decreased in MDD patients (81, 86). This is in line with research that showed that depletion of the GM might also affect the function of antioxidants (87).
5.4.7 Brain-derived neurotrophic factorAlterations in brain-derived neurotrophic factor (BDNF) modulation are also a risk factor of MDD in which microbiota can play a role (Figure 2) (18). BDNF is a neurotrophin and growth factor that has neuroprotective effects, and plays an essential role in the survival of neurons (64). It is widely expressed throughout the CNS and especially active in the hippocampus (30, 64). Decreased levels of BDNF in the hippocampus are associated with depression and are often seen as comorbidity in IBS and other inflammatory-bowel diseases (84). Furthermore, various treatments for depression, such as antidepressants, show an increase of BDNF expression in the brain (64). BDNF has also been used as a marker for antidepressant effects (70).
It is important to stress that bacteria can be considered beneficial or non-beneficial, as indicated in Figure 2. For example, some bacteria species can promote cytokine production (54) and increase anxiety (64), while other bacteria can reduce anxiety-like behavior (4, 17, 43). In line with these results, some studies found an effect of probiotics on the HPA axis (88), while others did not (89). Also, antibiotics can exert both positive effects and negative effects on the GM and MDD (69, 87).
Taking the information of section 4 and 5, we suggest that MDD is not a brain-only phenomenon, but a gut-brain interaction phenomenon. Genetics and neurobiology certainly play a role, but are not the sole cause. The HPA axis and immune system are part of the interconnected MGBA, and dysregulation of the HPA axis, a pro-inflammatory state and dysbiosis contribute to the development of MDD. Additionally, the bidirectional connections seen in Figure 2 regarding the HPA axis and GM, cytokines and the GM, cytokines and microglia and so forth highlight the overall bidirectional character of the MGBA. We believe that to treat depression more effectively, the MGBA is a crucial part to focus on and cannot be ignored.
6 Combining biomolecular mechanisms with a spiraling risk factor modelAlthough we believe the MGBA cannot be ignored in depression, MDD is a complex and heterogeneous disorder, in which psychological and environmental factors in addition to the biomolecular mechanisms contribute (5). This is why we propose a model that integrates the knowledge related to the biomolecular mechanisms underlying MDD with the psychological and environmental aspects (Figure 3). This spiraling model can be seen as the progression of an (im)balance in the condition of a person, divided in different groups of risk factors. Healthy individuals can obviously naturally encounter adverse life events and lead a less healthy lifestyle as well. This presents no problems while the balance is maintained. However, when a healthy individual slowly starts to experience a more depressed mood, all the factors in Figure 3 can have an impact on the condition of the patient and eventually move towards MDD in the worst case (5). Moreover, many risk factors interact in a stochastic manner, and can therefore contribute to the increase of other risk factors. For example, when an individual is victim of domestic violence, it may cause stress-related issues later in life which can, for example, lead to substance abuse. Subsequently, this substance abuse can worsen stress-related issues, forming a positive feedback loop, spiraling towards the development of MDD.
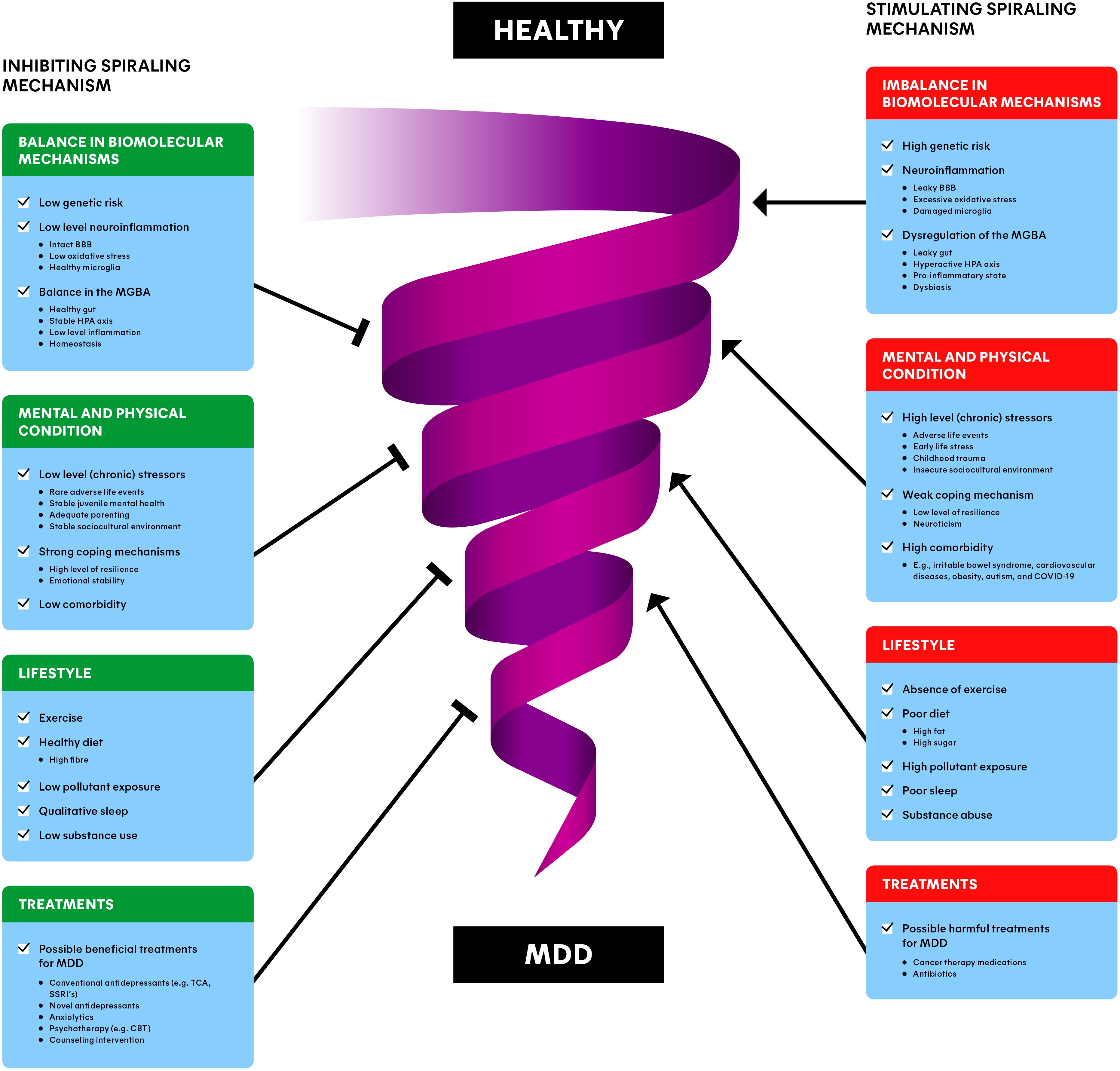
Figure 3. Spiraling risk factor model of major depressive disorder (MDD): inhibiting (left) and stimulating (right) risk factors interact dynamically, involved with the downward progression from a healthy individual to a depressed individual. The combination of balance in biomolecular mechanisms, the mental and physical condition, lifestyle and treatments of a patient/individual may provide the most promising and preventing interventions for MDD. BBB, blood-brain barrier; MGBA, microbiota-gut-brain axis; HPA axis, hypothalamic-pituitary-adrenal axis; MDD, major depressive disorder; TCA, tricyclic antidepressants; SSRI, selective serotonin reuptake inhibitor; CBT, cognitive behavioral therapy.
The spiral in Figure 3 represents this downward progression from a healthy individual to a MDD patient and vice versa. The involved factors often reside at opposing ends along a single dimension. For instance, an insecure sociocultural environment (e.g., unemployment or low income) is a risk factor, while a stable sociocultural environment (e.g., secure employment and high socioeconomic status) is a risk-reducing factor for depression (90). This is why each factor is depicted as a stimulating component (on the right) for the progression towards MDD, as well as an inhibiting component (on the left of the Figure), away from MDD. We differentiate between the balance in biomolecular mechanisms, the mental and physical condition, lifestyle, and (beneficial/harmful) treatments of individuals. Please note that the information described in section 4 and 5 is incorporated in the ‘Dysbiosis of the MGBA’ section. The upcoming sections are devoted to clarifying each of the groups of factors.
6.1 Balance in biomolecular mechanismsThe underlying biomolecular mechanisms are playing an important role in the etiology of MDD as described in section 4 and 5 (Figure 3). There are several genes (e.g., DRD2 and CLEF4) and epigenetics (e.g., histone deacetylases) associated with risk for developing MDD (7). Additionally, differences in brain regions like the hippocampus and the prefrontal cortex can play a role in depression. These risk factors can possibly disturb the balance of other biomolecular processes, or can be the result of other biomolecular imbalances, for instance related to the GM (Table 1) (7, 36).
A healthy individual with a low level of neuroinflammation naturally exhibits fluctuations in the levels of cytokines, stress-hormones and other biomolecules found in the gut (91, 92). This can become a problem when the homeostasis gets out of balance, for example because of MDD or comorbidities such as obesity and IBS (55, 61). Subsequently, the body can enter a state of dysbiosis (49, 54), pro-inflammatory state (45) or a hyperactive HPA axis (31), all signs of dysregulation of processes related to the MGBA (Figure 2). These states are associated with a leaky gut (60), impaired BBB integrity (77), disrupted levels of biomolecules (cytokines, cortisol, SCFA, LPS, monoamines, BDNF), oxidative stress (81), and impaired function of biological structures (VN, microglia) (18, 45, 64, 79) (Figure 2). Imbalances like these are in turn associated with neuroinflammation and MDD (Figure 3) (60, 69, 77). More detailed examples can be found in section 5.
6.2 Mental and physical conditionZooming out from the biomolecular mechanisms, the mental and physical condition of a depressed patient play a major role in the spiraling mechanism. Stress is a consistent finding in MDD, and there are numerous comorbid diseases related to depression (5, 7). To overcome adverse life events, individuals must find ways to cope with them (93). Another large factor that plays a role in the current mental and physical state of individuals is to what extent people experienced juvenile mental health problems and trauma in their childhood (94). The impact of (chronic) stressors (including early-life stress and sociocultural determinants; SDs), comorbidity, coping and childhood problems will be clarified in the upcoming sections (Figure 3).
6.2.1 (Chronic) stressorsApproximately 60–65% of MDD is explained by external factors such as adverse life events (5, 6). This is likely in large part the result of (chronic) stressors, as high levels of cortisol could potentially function as a predictive indicator of the risk of developing MDD (7). The source of stress could be in the past (e.g., early-life stress, childhood maltreatment or trauma) or it could be more recent (e.g., managing current life events and SDs) (Figure 3). On a psychological level, stressors can lead to avoiding, reducing, or predicting behavior towards a certain stressor (e.g., avoiding social interactions). From a biological perspective, stressors increase the level of cortisol of the individual, disrupting the HPA axis. Moreover, slightly higher cortisol levels in MDD patients have been found compared to controls (32, 34). Interestingly, MDD also increased cortisol levels in response to stressful stimuli. This fits the model as van der Gronde et al. (5) postulated: ‘depression is the result of a failure of coping mechanisms to control the stressors and a differential dysregulation in the stress system’.
6.2.2 Early-life stressAccording to a meta-analysis from 2019, people who went through early-life stress had higher odds of developing MDD prior to reaching the age of 18 years old compared to those who did not have a history of early-life stress (94) (Figure 3). They also found that the type of early-life stress plays a role in juvenile mental health. Poverty, illness/injury, and natural disasters were not associated with MDD, while emotional abuse and death of a family member were more strongly related to depression. Other than MDD, adverse childhood experiences are associated with significantly higher odds of anxiety, internalizing disorder, and suicidality in more extreme cases (95). Another large part of early-life stress in children and adolescents is negative behaviors of their parents (7). These negative behaviors could include hostile behavior and lower engagement. Additionally, depressed parents may also increase their children’s risk for developing depression.
Depression among children and adolescents is in turn associated with poor school attendance like absenteeism and truancy (96). These school performances may then again contribute to the downward depression spiral (Figure 3). Research trying to find potential underlying mechanisms demonstrate that resilience can partly protect against detrimental effects of child maltreatment (97). From a biomolecular perspective, research found altered HPA stress responses and lower levels of glucocorticoid receptor mRNA and other epigenetic differences in the hippocampus of humans who experienced childhood trauma (98).
6.2.3 Sociocultural determinantsSDs are also essentially external sources of (chronic) stressors and can be associated with MDD (90) (Figure 3). SDs include economic security, social protection, recent positive events (e.g., holidays), equality and neighborhood safety (7). Generally, because SDs differ to such a large extent between individuals, it is challenging to determine whether MDD is a cause or consequence of SDs and rule out confounding factors.
A report by the world health organization (WHO) described that in every age and phase of life, challenges such as poverty, violence, inequality, and environmental deprivation pose a threat to mental health (99). Additionally, a recent comprehensive review demonstrates that depression is associated with failure to complete secondary school, unemployment, work disability, lower income-earnings and household income (90). Vice versa, employment has been shown to lower the likelihood of depression, potentially possibly by enhanced autonomy, socioeconomic status, and personal growth opportunities. Interestingly, however, better SD conditions do not mean beneficial effects only by definition, as another paper found that higher parental education correlates with increased prevalence of alcohol and drug usage during early adulthood (100).
From a biomolecular viewpoint, earlier work reported that lower socioeconomic status during adolescence was linked to epigeneti
留言 (0)