According to previous studies, approximately 40% of patients with genital, urological and low gastrointestinal tumors have received pelvic radiotherapy (1). Although radiotherapy can destroy cancer cells and inhibit their spread (2, 3), it also triggers fibroblast senescence, affects mesenchymal cells differentiation, and even enhances colloid cells replication (4, 5), leading to changes in tissue stiffness and elasticity (6, 7). Consequently, radiotherapy for pelvic tumors can induce alterations in the pelvic floor structures, resulting in stress urinary incontinence (SUI). Due to the detrimental impact of radiotherapy on the management of SUI, numerous clinical trials of mid-urethral sling (MUS) have excluded individuals who have undergone pelvic radiotherapy from the study (8).
With the development of anti-incontinence surgery, MUS had gradually become the first choice for treatment of SUI (9). The treatment of gynaecological malignant tumors (e.g., hysterectomy and radiotherapy) can cause SUI. Of these, cervical and endometrial cancers require radical hysterectomy, and some patients choose radiotherapy as further treatment. The incidence of SUI is approximately 24%–29% in patients with cervical cancer (10), whereas it is 46.8% in patients with cervical cancer after hysterectomy (11). The incidence of urinary incontinence was higher in patients who received radiotherapy after hysterectomy compared to patients who only underwent hysterectomy (12).
The treatment of pelvic malignancies may result in dysfunction of urinary storage and urination. A study revealed that hysterectomy and radiotherapy affects the innervation of bladder, which leads to 9 bladder contractile dysfunction (13). Although there are few studies on urodynamic features after pelvic radiotherapy, we note that 15%–20% of patients in these studies developed detrusor instability and urinary frequency, accompanied by decreased bladder compliance and bladder overactivity after treatment (14–16). Meanwhile, compared to hysterectomy alone, radiotherapy can cause a decrease in pelvic floor muscles contractility (17). In a study of the effects of radiation and chemotherapy for cervical cancer on pelvic floor muscle function, it was found that radiation and chemotherapy resulted in pelvic floor dysfunction, especially at the end of treatment (18). Bladder dysfunction during storage and voiding after radical hysterectomy and radiation therapy often co-occurs with SUI, resulting in complicated conditions. Moreover, the fibrosis of pelvic floor tissue caused by radiotherapy can lead to more severe urinary incontinence. Currently, there is no consensus on the treatment of urinary incontinence after radiotherapy, thereby the treatments of physicians vary.
Despite the substantial burden of disease, only a limited number of researchers have focused on characterizing SUI following radiotherapy for female pelvic malignancies. MUS surgery has emerged as a widely favored and effective treatment for female SUI. In one research, MUS was administered to patients who had undergone radiotherapy and radical hysterectomy, which found that the recurrence rate of stress incontinence was 100% (19). Conventional sling procedures often struggle to control leakage effectively under tension-free conditions, primarily due to tissue stiffness following radiotherapy. Moreover, there is a notable absence of consensus regarding the management of female SUI after pelvic radiotherapy. Regarding this dilemma, we report our experience in managing patients who have undergone MUS operation for SUI post-radiotherapy, delineate the characteristics of SUI after pelvic radiotherapy, and present the results of this surgical intervention.
Materials and methods Study populationThis retrospective study focused on patients diagnosed with SUI subsequent to undergoing radiotherapy for pelvic tumors and who underwent MUS procedures at our institution between June 2015 and February 2022. The diagnosis was based on comprehensive history, clinical presentation, physical examination, uroflowmetry, PVR measurement and urodynamic testing. Data extracted included patient demographics, medical history, severity of SUI and any associated postoperative complications, and urogenital symptoms.
Patients were included in the study if they met the inclusion criteria: (1) Female patients >18 years of age. (2) Patients diagnosed with pelvic malignancy and treated with radiotherapy. (3) Patients treated with MUS. Patients with other urinary diseases (e.g., bladder neck obstruction, urethral stricture, bladder prolapse), who had undergone operation for urinary incontinence, or who were unable to complete follow-up were excluded from the study. The protocol was reviewed and approved by the institutional review committee of Beijing Chao-Yang Hospital (approval number: PX2020015) and informed consent was taken from all individual participants.
Procedure managementThe surgical procedures were generally carried out under general anesthesia. All procedures were performed by the same surgical team.
The patient was initially positioned in the gynecological posture. The operative area was prepped with a standard antiseptic solution and covered with multiple drapes. An 18 Fr Foley catheter was inserted to empty the bladder. Labia minor was suspended by fixation to the skin with nylon sutures a few centimeters above the vulvar ostium, inside the thigh folds, in order to expose the vulvar vestibulum. Inject 20 ml of saline into the vagina and urethral space. A median sagittal incision of the vaginal wall was started at this level and was continued proximally (towards the vaginal pouches) over a 1 cm distance, both vaginal mucosal and sub-mucosal tissues were incised. Minimal para-urethral sub-vaginal dissection was then carried out laterally with the scissors, over a few millimeters distance, on either side. After the dissection pathways were successfully established, the introducer was advanced towards the retropubic space. The bladder was filled with 300 ml of saline and then underwent an intraoperative cystoscopy to check for the presence of bladder injury. Subsequently, The abdominal compression test was performed with a bladder volume of 300 ml, aiming to adjust the tape to enable a drop of saline to escape from the outer meatus of the urethra upon strong abdominal compression. For procedure, the surgeons were instructed to place the sling with appropriate tension rather than “tension-free”. The tape ends were cut in the subcutaneous layer and the incisions were closed. Finally, the vaginal incision was closed with absorbable sutures.
Main study outcomes and follow-up evaluationThe primary endpoint was the ICI-Q-SF questionnaire for leakage symptom, and the secondary endpoints included uroflowmetry and postoperative complications such as dysuria and sling exposure. The postoperative objective success was defined as no urine leakage during a cough stress test or answering “no” to the ICIQ FLUTS question: “does urine leak when you are physically active, exert yourself, cough or sneeze?” (20, 21). Patients were discharged on criteria of improving urinary leakage symptom as well as Qmax > 15 ml/s or PVR < 50 ml. Patients who meet the criteria can be discharged. Patients underwent a review at 2 weeks post-surgery, where uroflowmetry and PVR were conducted at the outpatient clinic. Urinary symptoms were evaluated using the ICI-Q-SF questionnaire. Subsequently, all patients were followed up via telephone at 3, 6, and 12 months postoperatively. During follow-up period, ICI-Q-SF questionnaire, uroflowmetry, PVR, and any postoperative complications were recorded. Pre- and post-operative data of ICI-Q-SF scores, Qmax and PVR were compared for each patient.
Statistical analysisThe results were presented primarily using descriptive statistics due to the relatively small sample size. Where appropriate, continuous variables with normal distribution were presented as means ± standard deviation (SD) and compared by Paired t-test. While continuous variables with non-normal distribution were reported as median with interquartile range (P25, P75) and compared by Wilcoxon signed-rank test. All tests were two-sided with p value < 0.05 to be considered statistically significant. All statistical analyses were performed using SPSS statistical software version 26.0 (IBM, Chicago, IL, USA).
ResultsA total of 26 patients with objective evidence of SUI after radiotherapy for pelvic tumors were enrolled in our study. The mean age and body mass index were 59.35 ± 7.32 (range 43–70) years and 25.72 (22.79, 27.86) kg/m2, respectively. The baseline characteristics of patients, the history of pelvic tumors, and preoperative parameters of uroflowmetry were demonstrated in Table 1.
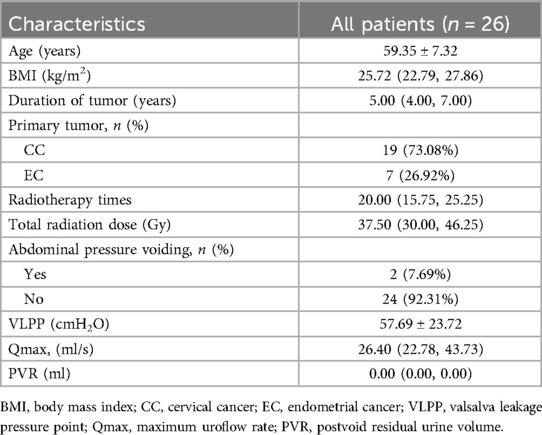
Table 1. Patient demographics and clinical features.
All 26 patients completed the surgery successfully. The comparison of Qmax and PVR at 2 weeks postoperative and preoperative was listed in Table 2. Postoperatively, the symptoms were improved and ICI-Q-SF was decreased significantly compared with the preoperative. As shown in Table 3, the ICIQ-SF scores were lower than the pre-operative at 2 weeks, 6 months and 1 year postoperatively, and the difference was statistically significant (p < 0.01).
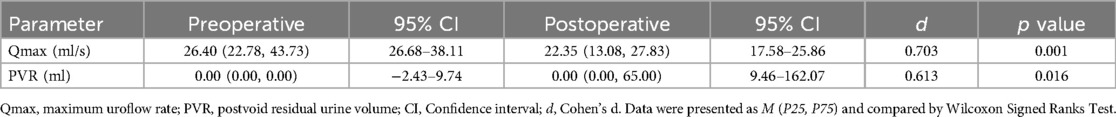
Table 2. Comparison of Qmax and PVR at 2 weeks postoperative and preoperative.
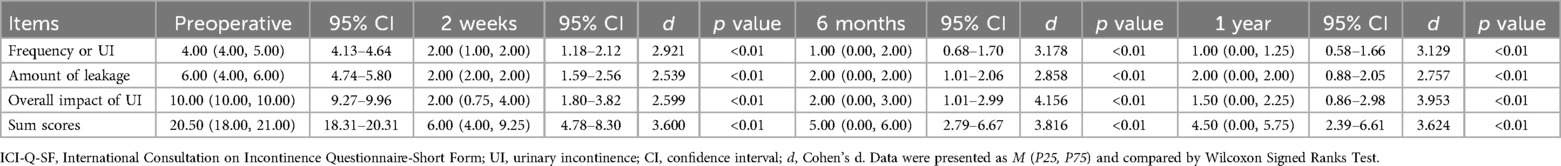
Table 3. Comparison of ICI-Q-SF scores before operation and 2 weeks, 6 months and 1 year after operation.
During our follow-up, 21 patients (80.77%, 95% CI: 0.621–0.915) were considered to have successfully improved after surgery. 5 patients (19.23%, 95% CI: 0.085–0.379) experienced dysuria after surgery, case 3 had no voiding trouble on postoperative day 1 and met the discharge criteria. At the first review 2 weeks after surgery, her Qmax was 3 ml/s and PVR was 700 ml, the patient was performed urethral dilatation at the outpatient clinic, and the patient had significant amelioration of symptoms after urethral dilatation with Qmax increasing from 3 ml/s to 15.7 ml/s and PVR decreasing from 700 ml to 0 ml. Case 5 experienced dysuria after 2 weeks postoperatively, the patient received multiple urethral dilatation with Qmax varying from 12 ml/s to 12.1 ml/s and PVR varying from 250 ml to 240 ml. There was no significant alleviation of dysuria symptoms at 3 months postoperatively, and the patient underwent sling release at 6 months post-surgery. After sling release, dysuria was relieved and the problem of urinary incontinence was well controlled. Moreover, the PVR of case 11, case 18, and case 24 at 2-week postoperative were 150 ml, 670 ml, and 200 ml, respectively. The patients were treated with urethral dilation, and their symptoms of dysuria were relieved with PVR of 0 ml. After 1-year follow-up, none of the patients had sling exposure.
DiscussionThe understanding of the presentation, diagnosis, and treatment of SUI has developed over the last 20 years. However, there is still no consensus on the treatment options and efficacy for urinary incontinence occurring in patients with cervical cancer, and the outcome may be affected by the primary disease. Pelvic floor muscle training (PFMT) is available as an initial therapeutic option; unfortunately, up to now, there is only limited clinical data on its effects (22). Periurethral or transurethral injection of bulking agents is currently the only treatment modality with research evidence to show its effectiveness for urinary incontinence after radiotherapy (23–25). Since the long-term effects of radiotherapy on the voiding function of the lower urinary tract are commonly irreversible and progressive, the manifestations of urinary incontinence post-radiotherapy exhibit variability. Opting for an incorrect therapeutic strategy may lead to symptom recurrence and exacerbation.
In our study, all patients had undergone radiotherapy for gynecological malignancies and subsequently developed urinary incontinence. All patients underwent trans-retropubic vaginal tape, with follow-up assessments conducted at 2 weeks, 6 months, and 1 year after surgery. Based on the ICI-Q-SF scores, symptom improvement was observed at the 2-week postoperative mark. Notably, beyond 6 months postoperatively, the sling fused with surrounding tissue and the subjective symptoms of the patients improved more significantly. The ICI-Q-SF score decreased from 20.50 (18.00, 21.00) to 4.50 (0.00, 5.75), (p<0.01). In contrast to previous extensive case analyses where MUS was ineffective after radiotherapy (26), 21 of 26 patients in the present study experienced improvement in symptoms. During the 1-year follow-up after the operation, there were no complications such as sling erosion, with only 1 case undergoing sling release surgery due to dysuria. Consequently, the success rate of the procedure was 80.77% at 1-year follow-up.
Among the 26 patients in our study, 2 patients exhibited abdominal pressure voiding during the preoperative urodynamic evaluation. Since both surgical treatment and radiotherapy of gynaecological tumors can affect bladder innervation and damage the detrusor function (13, 15). Currently, there is a lack of research addressing whether retropubic slings with appropriate tension might exacerbate dysuria in such patients. Case 5 had to undergo sling release surgery because of postoperative dysuria and urinary retention. However, it is worth noting that although the PVR in case 1 was 110 ml at 2 weeks after the procedure, the patient did not undergo further treatment and was followed up regularly and had a PVR of 0 ml after 1 month postoperatively. We consider the increased residual urine volume in this patient to be associated with postoperative periurethral edema, and the dysuria was relieved after the edema subsided 1 month after surgery. Therefore, for patients with urinary incontinence after radiotherapy, if the function of detrusor has been impaired before surgery, it is necessary to adequately communicate with the patient before the operation that there may be aggravation of dysuria or even urinary retention after treatment.
All patients with urinary incontinence after radiotherapy should undergo urodynamic examination before surgical intervention to exclude overflow urinary incontinence. While “tension-free” is a crucial aspect of retropubic tension-free vaginal tape (TVT) surgery, in the context of pelvic floor tissue stiffness after radiotherapy, appropriate sling tension on urethra becomes essential to relieve urinary leakage symptoms. The balance between the control of urinary incontinence symptoms and postoperative dysuria is difficult to meet. For example, the sling tension of case 5 that can control incontinence symptoms means severe dysuria or even urinary retention after surgery, but in 21 patients did not suffer from dysuria while controlling the symptoms of urinary leakage. This proved that proper sling tension is the key to successful surgical treatment of MUS for SUI after pelvic radiotherapy. Unfortunately, we failed to propose a method to quantify sling tension, which will be the priority of our future research endeavours.
The postoperative complications of urinary incontinence after radiotherapy are mainly sling erosion, which is related to the poor ability of tissue self-repair after radiotherapy and may also be associated with adhesions of the pelvic floor surrounding tissues after radiotherapy (27, 28). For the 26 patients in this study, we preserved the thickness of the vaginal wall and periurethral tissues as much as possible during the procedure, which we considered to avoid the occurrence of sling exposure. After 1-year follow-up, none of the patients had sling exposure.
This present study is innovative because few literatures focus on patients with SUI to research the specificity of urinary incontinence after radiotherapy. There is no definitive evidence that any treatment method has a better effect on this disease. In the present study, we involved the largest number of cases in such research works. Despite this, the results of our statistical analysis are still limited due to the small number of cases and the short follow-up period. We will obtain more cases and longer follow-up results in future works.
ConclusionMUS with appropriate sling tension emerged as a simple, safe, and effective treatment for SUI, with the improvement of incontinence in 80.77% of patients. It could be the first choice for patients who presented SUI after pelvic radiotherapy, and long-term follow-up is mandatory for patients presenting with SUI after treatment of pelvic tumors, as well as the treatment should be individualized.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Beijing Chao-Yang Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsXG: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. FW: Data curation, Formal Analysis, Writing – original draft. DZ: Data curation, Formal Analysis, Writing – original draft. PQ: Supervision, Visualization, Writing – original draft. YQ: Supervision, Writing – review & editing. BW: Supervision, Visualization, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Wedlake LJ, Andreyev HJN. Manipulating the consequential effect: an alternative approach to reducing pelvic radiation disease other than dose reduction. Curr Opin Support Palliat Care. (2011) 5(1):25–8. doi: 10.1097/SPC.0b013e328343ad2f
PubMed Abstract | Crossref Full Text | Google Scholar
2. Dong Y, Zheng Q, Wang Z, Lin X, You Y, Wu S, et al. Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J Hematol Oncol. (2019) 12(1):112. doi: 10.1186/s13045-019-0795-5
PubMed Abstract | Crossref Full Text | Google Scholar
3. Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. (2015) 17(5):678–88. doi: 10.1038/ncb3157
PubMed Abstract | Crossref Full Text | Google Scholar
4. Junker JPE, Kratz C, Tollbäck A, Kratz G. Mechanical tension stimulates the transdifferentiation of fibroblasts into myofibroblasts in human burn scars. Burns. (2008) 34(7):942–6. doi: 10.1016/j.burns.2008.01.010
PubMed Abstract | Crossref Full Text | Google Scholar
5. Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. (2011) 32(16):3921–30. doi: 10.1016/j.biomaterials.2011.02.019
PubMed Abstract | Crossref Full Text | Google Scholar
6. Streltsova O, Kiseleva E, Dudenkova V, Sergeeva E, Tararova E, Kochueva M, et al. Late changes in the extracellular matrix of the bladder after radiation therapy for pelvic tumors. Diagnostics (Basel). (2021) 11(9):1615. doi: 10.3390/diagnostics11091615
PubMed Abstract | Crossref Full Text | Google Scholar
7. Tuieng RJ, Cartmell SH, Kirwan CC, Sherratt MJ. The effects of ionising and non-ionising electromagnetic radiation on extracellular matrix proteins. Cells. (2021) 10(11):3041. doi: 10.3390/cells10113041
PubMed Abstract | Crossref Full Text | Google Scholar
8. Bezerra CA, Bruschini H, Cody DJ. Traditional suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. (2005) 3:CD001754. doi: 10.1002/14651858.CD001754.pub2
Crossref Full Text | Google Scholar
9. Viereck V, Bader W, Lobodasch K, Pauli F, Bentler R, Kölbl H. Guideline-Based strategies in the surgical treatment of female urinary incontinence: the new gold standard is almost the same as the old one. Geburtshilfe Frauenheilkd. (2016) 76(8):865–8. doi: 10.1055/s-0042-107079
PubMed Abstract | Crossref Full Text | Google Scholar
10. Ramaseshan AS, Felton J, Roque D, Rao G, Shipper AG, Sanses TVD. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. (2018) 29(4):459–76. doi: 10.1007/s00192-017-3467-4
PubMed Abstract | Crossref Full Text | Google Scholar
11. Cao TT, Wen HW, Gao YN, Lyu QB, Liu HX, Wang S, et al. Urodynamic assessment of bladder storage function after radical hysterectomy for cervical cancer. Chin Med J (Engl). (2020) 133(19):2274–80. doi: 10.1097/CM9.0000000000001014
PubMed Abstract | Crossref Full Text | Google Scholar
12. Shin JH, Gwak CH, Park MU, Choo MS. Effects of different types of hysterectomies on postoperative urodynamics and lower urinary tract symptoms. Investig Clin Urol. (2022) 63(2):207–13. doi: 10.4111/icu.20210393
PubMed Abstract | Crossref Full Text | Google Scholar
14. Lin HH, Sheu BC, Lo MC, Huang SC. Abnormal urodynamic findings after radical hysterectomy or pelvic irradiation for cervical cancer. Int J Gynaecol Obstet. (1998) 63(2):169–74. doi: 10.1016/S0020-7292(98)00158-1
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lajer H, Thranov IR, Bagi P, Aage Engelholm S. Evaluation of urologic morbidity after radiotherapy for cervical carcinoma by urodynamic examinations and patient voiding schemes: a prospective study. Int J Radiat Oncol Biol Phys. (2002) 54(5):1362–8. doi: 10.1016/S0360-3016(02)03033-X
PubMed Abstract | Crossref Full Text | Google Scholar
16. Katepratoom C, Manchana T, Amornwichet N. Lower urinary tract dysfunction and quality of life in cervical cancer survivors after concurrent chemoradiation versus radical hysterectomy. Int Urogynecol J. (2014) 25(1):91–6. doi: 10.1007/s00192-013-2151-6
PubMed Abstract | Crossref Full Text | Google Scholar
17. Bernard S, Moffet H, Plante M, Ouellet MP, Leblond J, Dumoulin C. Pelvic-floor properties in women reporting urinary incontinence after surgery and radiotherapy for endometrial cancer. Phys Ther. (2017) 97(4):438–48. doi: 10.1093/ptj/pzx012
PubMed Abstract | Crossref Full Text | Google Scholar
18. Miguel TP, Laurienzo CE, Faria EF, Sarri AJ, Castro IQ, Júnior RJA, et al. Chemoradiation for cervical cancer treatment portends high risk of pelvic floor dysfunction. PLoS One. (2020) 15(6):e0234389. doi: 10.1371/journal.pone.0234389
PubMed Abstract | Crossref Full Text | Google Scholar
20. Braga A, Castronovo F, Ottone A, Torella M, Salvatore S, Ruffolo AF, et al. Medium term outcomes of TVT-abbrevo for the treatment of stress urinary incontinence: efficacy and safety at 5-year follow-up. Medicina (Kaunas). (2022) 58(10):1412. doi: 10.3390/medicina58101412
PubMed Abstract | Crossref Full Text | Google Scholar
21. Offiah I, Freeman R. Long-term efficacy and complications of a multicentre randomised controlled trial comparing retropubic and transobturator mid-urethral slings: a prospective observational study. BJOG. (2021) 128(13):2191–9. doi: 10.1111/1471-0528.16899
PubMed Abstract | Crossref Full Text | Google Scholar
22. Bernard S, Ouellet MP, Moffet H, Roy JS, Dumoulin C. Effects of radiation therapy on the structure and function of the pelvic floor muscles of patients with cancer in the pelvic area: a systematic review. J Cancer Surviv. (2016) 10(2):351–62. doi: 10.1007/s11764-015-0481-8
PubMed Abstract | Crossref Full Text | Google Scholar
23. Kirchin V, Page T, Keegan PE, Atiemo KO, Cody JD, McClinton S, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. (2017) 7:CD003881. doi: 10.1002/14651858.CD003881.pub4
PubMed Abstract | Crossref Full Text | Google Scholar
24. Plotti F, Zullo MA, Sansone M, Calcagno M, Bellati F, Angioli R, et al. Post radical hysterectomy urinary incontinence: a prospective study of transurethral bulking agents injection. Gynecol Oncol. (2009) 112(1):90–4. doi: 10.1016/j.ygyno.2008.09.022
PubMed Abstract | Crossref Full Text | Google Scholar
25. Krhut J, Martan A, Jurakova M, Nemec D, Masata J, Zvara P. Treatment of stress urinary incontinence using polyacrylamide hydrogel in women after radiotherapy: 1-year follow-up. Int Urogynecol J. (2016) 27(2):301–5. doi: 10.1007/s00192-015-2834-2
PubMed Abstract | Crossref Full Text | Google Scholar
26. Chuang F-C, Kuo H-C. Management of lower urinary tract dysfunction after radical hysterectomy with or without radiotherapy for uterine cervical cancer. J Formos Med Assoc. (2009) 108(8):619–26. doi: 10.1016/S0929-6646(09)60382-X
PubMed Abstract | Crossref Full Text | Google Scholar
28. Guipaud O, Jaillet C, Clément-Colmou K, François A, Supiot S, Milliat F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br J Radiol. (2018) 91(1089):20170762. doi: 10.1259/bjr.20170762
留言 (0)