Somatic symptom disorder (SSD) is defined as having one or more chronic somatic symptoms that are distressing or disruptive of daily life, as indicated by disproportionate and dysfunctional cognitive, emotional, and behavioral responses. For example, someone with SSD may present with chronic pain, bowel disruptions, or fatigue that is accompanied by anxiety, catastrophic thinking, and behavioral avoidance. The prevalence of SSD is 5 to 7% in the general population (1) and up to 17% in primary care (2). SSD tends to be chronic—up to 90% of patients have symptoms beyond 5 years (3)—and disruptive—the somatic symptoms in people with SSD are associated with psychiatric comorbidity and functional disability such as work impairment or early retirement (4).
One promising treatment for SSD is Emotional Awareness and Expression Therapy (EAET) (5). EAET includes psychoeducation about the central nervous system control of pain and other somatic symptoms, exploration of links between somatic symptoms and unresolved trauma, emotional processing of trauma and conflict, reattribution of somatic symptoms to emotional and brain-based processes, the development of a self-soothing capacity using self-compassion, and encouragement to improve adaptive interpersonal communication. EAET has been tested in randomized controlled trials (RCTs) for people with fibromyalgia (6), irritable bowel syndrome (7), urogenital pain (8), medically unexplained symptoms (9), and musculoskeletal pain (10, 11) and found to be superior to treatment as usual, education controls, and even cognitive behavioral therapy (CBT) (6, 10, 11). In our original RCT, which forms the basis for this follow-up study, an Internet-delivered version of EAET (I-EAET) was superior to a waitlist control in reducing somatic symptoms and pain intensity at post-treatment (small to medium effect size). It also showed superiority for somatic symptoms at the 4-month follow-up, response rates for somatic symptoms at follow-up, and also for depression and anxiety at post-treatment, but not at follow-up. (12). In other clinical trials of EAET, treatment effects have been maintained at short-term follow-up assessments, ranging from 2 to 6 months, but the effects of EAET in any modality after 6 months have not been studied. The purpose of the current secondary analyses was to examine whether the effects of I-EAET at post-treatment of our earlier trial were maintained at 12-month follow-up (12). Because the waitlist condition was offered I-EAET after the 4-month follow-up, the current analyses are within the I-EAET condition only and do not include a control condition.
2 Materials and methods2.1 Study designThe original RCT for people with persistent somatic symptoms (N = 74) compared I-EAET (n = 37) to a waitlist control (n = 37). All participants were diagnosed with SSD, with a physician ruling out diseases (e.g., cancer or rheumatoid arthritis) as the cause of the somatic symptoms. I-EAET lasted for 10 weeks and was provided through a secure web-platform (KI eHealth Core Facility) used by Karolinska Institute. The two study arms were compared at post-treatment and 4-month follow-up, at which point, the waitlist control participants were provided I-EAET. The recruitment, screening, randomization, measures, and intervention are fully described in (12). The trial was pre-registered at ClinicalTrials.gov (NCT04751825). Informed consent was given while registering for the study and included follow-up measurements. In this follow-up study, the 37 participants from the I-EAET condition were contacted 1 year after treatment termination to assess their primary and secondary outcomes.
2.2 Measures and statistical analysisTwo primary outcome measures were assessed at pre-treatment, post-treatment, as well as at 4-month and 12-month follow-ups. Somatic symptom severity during the last week was assessed with the Patient Health Questionnaire-15 (PHQ-15) (13, 14); items are rated 0 (“not bothered at all”), 1 (“bothered a little”), or 2 (“bothered a lot”) and summed. Pain intensity was assessed with four items from the Brief Pain Inventory (BPI-4): worst, least, and average pain over the past week, and current pain. Items are rated from 0 (“no pain”) to 10 (“worst imaginable pain”) and averaged (15).
Three secondary outcome measures were assessed at the same timepoints: the Patient Health Questionnaire-9 (PHQ-9) for depression (16), Generalized Anxiety Disorder-7 (GAD-7) for anxiety (17), and the Post Traumatic Stress Disorder Checklist (PCL-5) for trauma symptoms (18).
All 37 participants were included in the effect size analyses, applying intention to treat. The online software Psychometrica (19) was used for all effect size analyses. Welch tests, with SPSS 26 (20), were used for significance testing, comparing pre- and post-treatment to 12-month follow-up. Effect sizes in the range of 0.20–0.49 were considered small, 0.50–0.79 medium, and over 0.80 large (21).
Substantial response to treatment was defined as 50% or greater symptom reduction from pre- to post-treatment and to follow-up for each of the two primary outcomes (PHQ-15/somatic symptoms and BPI-4/pain intensity). Chi-square tests were conducted to examine condition differences in prevalence of substantial responders, using Psychometrica (19). Participants not providing follow-up data were excluded from these responder analyses.
The original trial registration noted that we would assess sleep-related outcomes (Epworth Sleepiness Scale, Insomnia Severity Index) and functional impairment (Sheehan Disability Scale); however, these measures were not included at the 12-month follow-up, due to research error. See Maroti et al. (12) for further elaboration.
3 Results3.1 Participants, adherence, attrition, and missing dataThe original sample of 37 participants was 81% female, age 23-64. Regarding psychiatric comorbidity, the most common diagnosis was depression—almost 70% had recurrent or ongoing depression. There was a range of somatic diagnoses, with IBS (25%) or migraine/severe headache (20%) as the most common. For details, see Maroti et al. (2022) (11).
All 10 I-EAET modules were completed by 83.8% of the 37 participants; the mean number of completed modules was 8.8. Attrition was very low; only 2 I-EAET participants (5.4%) terminated participation, and 34 of the 37 (92%) provided post-treatment data. At 12-month follow-up, 28 of 37 (76%) provided data for the two primary outcomes, and 26 of 37 (70%) provided data for three secondary outcomes.
3.2 Primary and secondary outcomesResults are displayed in Table 1. We compared within-condition effects from pre-treatment (baseline) to 12-month follow-up. Somatic symptoms were significantly reduced with a medium effect (d = 0.74, 95% CI 0.23–1.24, p = 0.005). Pain intensity had a small effect, but it was not significant. (d = 0.43, 95% CI: -0.06–0.93, p = 0.09). Comparing post-treatment assessment to 12-month follow-up, there was no significant change in somatic symptoms (d = -0.22, 95% CI: -0.72–0.28, p = 0.40), or pain intensity (d = -0.02, 95% CI: -0.52–0.48, p = 0.96).
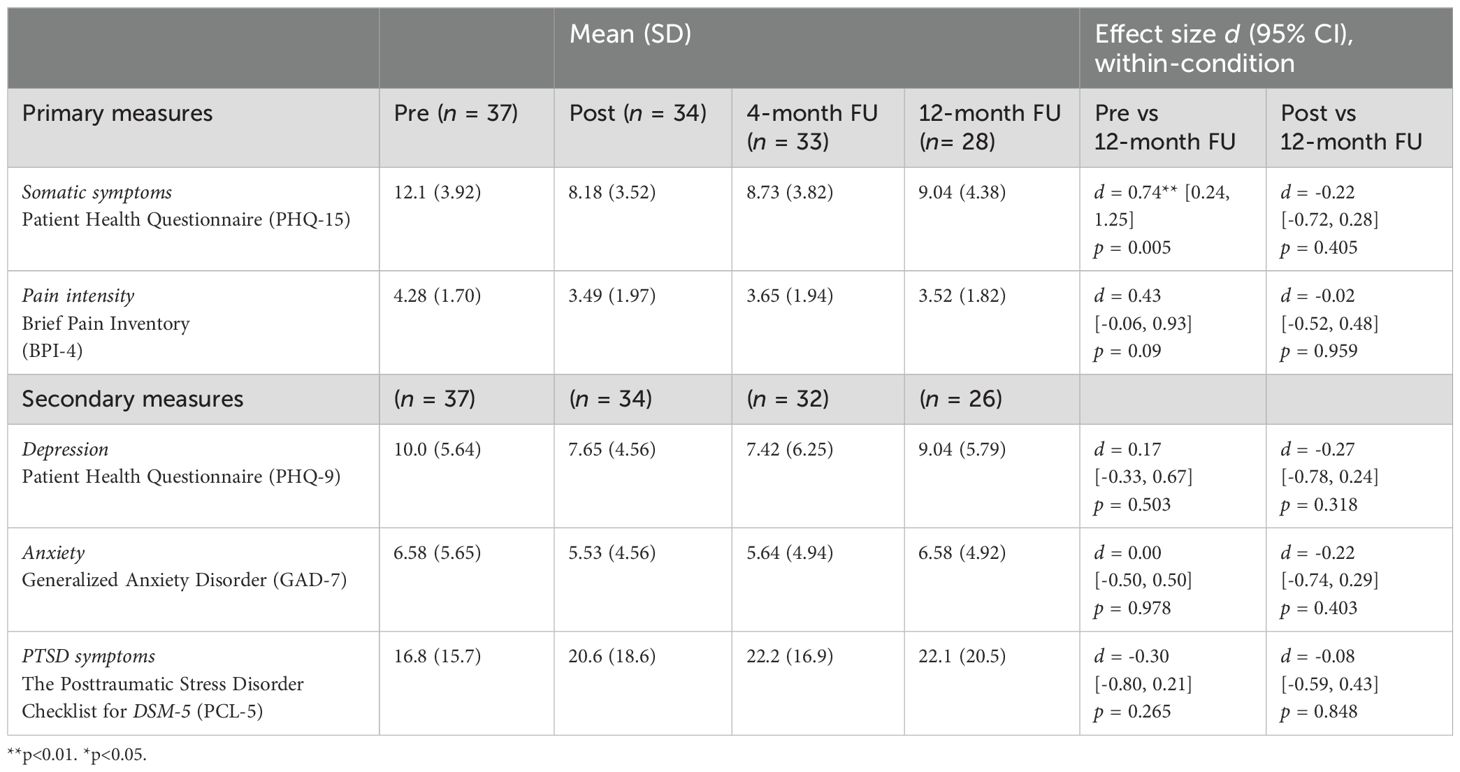
Table 1. Means, SDs, and effect sizes (Cohen’s d) for different outcome measures within the I-EAET condition only.
There were no significant changes in any of the secondary outcomes from pre-treatment to 12-month follow-up or from post-treatment to 12-month follow-up.
3.3 Responder analysesFor somatic symptoms (PHQ-15), 7 of 34 (21%) of I-EAET participants were classified as responders at post-treatment, and 7 of 28 (25%) were at follow-up; these percentages did not differ, X2 (1, N = 34) = 0.17, p = 0.68. For pain intensity, 8 of 34 (24%) participants were classified as responders at post-treatment, and 4 of 28 (12%) were responders at 12-month follow-up; these percentages did not differ, X2 (1, N = 34) = 0.84, p = 0.36. We did additional analyses to explore whether the 6 participants who provided post data but not 12-month follow-up data, differed from the 28 participants who did provide 12-month follow-up data. See Table 2. Statistical significance was tested with Welch tests at both primary measures, and Chi2-tests for response rates. All p-values were larger than 0.05, indicating no statistically significant differences at the main outcomes between the participants providing 12-month follow-up data and the ones who did not.
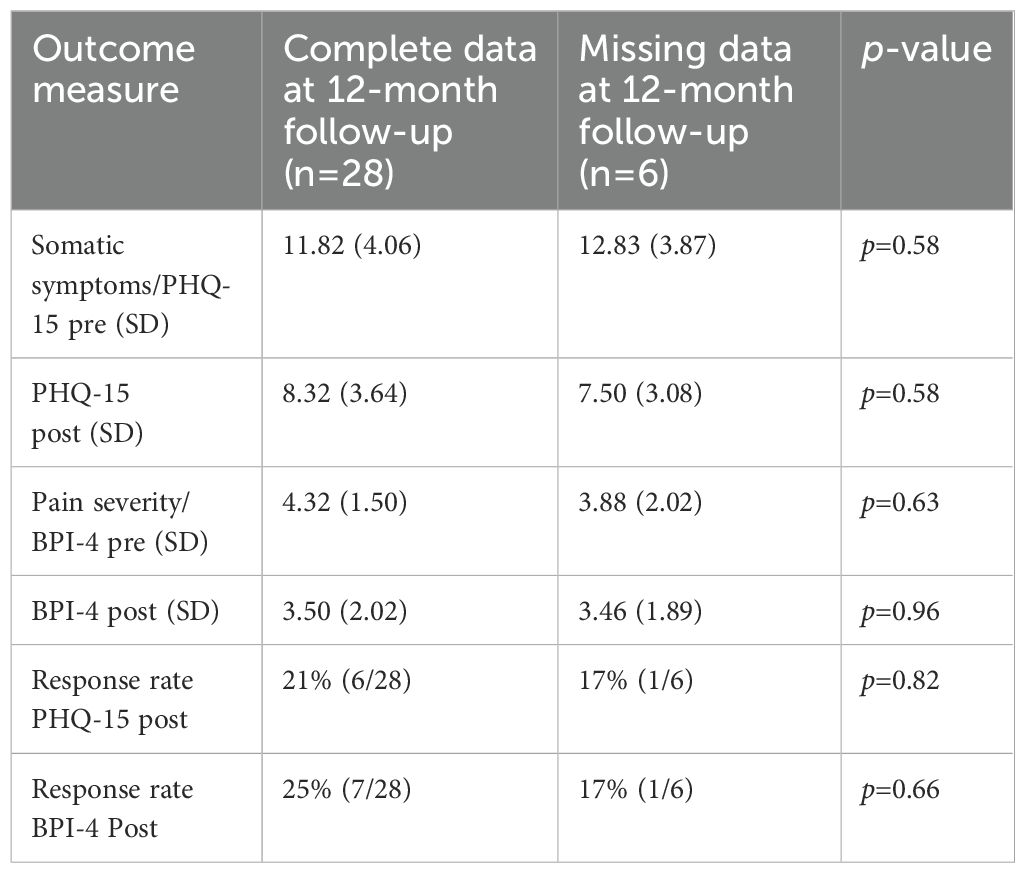
Table 2. Means, SDs, and significance tests comparing participants providing/not providing data at 12-month follow-up.
4 DiscussionThis is the first study to assess whether somatic symptom reduction at the end of EAET was maintained at 12-month follow-up. The significant post-treatment reductions in somatic symptoms for our participants who received I-EAET were largely maintained 12 months later, with a medium effect size, and the percentage of responders (50% or more reduction in somatic symptoms) was also maintained. The small trend toward increased somatic symptoms between post-treatment and 12-month follow-up was non-significant. The effect on pain severity at 12-month follow-up, however, was no longer significant. On secondary measures, treatment effects were originally not significant at post-treatment, and the 12-month follow-up reflected a return of anxiety and depression. The average levels of depression and anxiety at pre-treatment were, however, only in the mild range, and post-traumatic symptoms were in the subclinical range; these low levels on these measures likely limited improvement.
These results provide preliminary evidence that I-EAET has positive effects 12 months after treatment, at least on reducing somatic symptoms. One might compare these results with those of internet-cognitive behavioral therapy (I-CBT) for similar populations. The effects of I-CBT on pain, compared to controls at post-treatment, are small (22), and the few studies of I-CBT that have 12-month follow-ups have inconsistent results—two studies showed maintained benefits within-condition (23, 24), the third study had a small effect size within-group but not compared to the control condition (25). There is evidence for deterioration over time for people with chronic pain who are untreated (3), whereas the current study found that the positive effect of I-EAET on somatic symptoms was largely maintained. However, we still know too little about what works for people with somatic symptoms in the long-term, and I-EAET and I-CBT have never been directly compared in a trial.
This study has several limitations. Most importantly, these analyses at 12-month follow-up with only within-condition were not compared to a control condition because the original waitlist control participants were offered I-EAET 4 months post-treatment. This lack of a control group precludes concluding that the treatment itself led to the maintenance of improvements. We also do not know what other treatments participants might have obtained over the follow-up period, although the availability of effective treatments for SSD is limited. Moreover, it remains unclear whether the effects of I-EAET observed at the 12-month follow-up are generalizable to other forms of EAET, such as face-to-face individual therapy or group formats. Further, the small sample size limits the statistical power of the study to detect significant effects. Future studies should include control conditions and direct comparisons to other active treatments (e.g., internet-CBT) and have larger samples.
In conclusion, this study provides preliminary evidence that I-EAET has positive effects on somatic symptoms (although not other measures) for people with SSD at 12-month follow-up.
Data availability statementThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statementAs this study collected sensitive data about participants’ health, it was given an ethics review by the Swedish Ethical Review Authority (EPM, Etikprövningsmyndigheten). The application has been approved by EPM (06/11/2020). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsHH: Formal analysis, Methodology, Visualization, Writing – original draft. DM: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. ML: Methodology, Visualization, Writing – review & editing. RJ: Conceptualization, Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington: American Psychiatric Association (2013).
2. Haller H, Cramer H, Lauche R, Dobos G. Somatoform disorders and medically unexplained symptoms in primary care. Deutsches Aerzteblatt. (2015) 112(16):279–87. doi: 10.3238/arztebl.2015.0279
Crossref Full Text | Google Scholar
3. Rask MT, Rosendal M, Fenger-Grøn M, Bro F, Ørnbøl E, Fink P. Sick leave and work disability in primary care patients with recent-onset multiple medically unexplained symptoms and persistent somatoform disorders: a 10-year follow-up of the FIP study. Gen Hosp Psychiatry. (2015) 37:53–9. doi: 10.1016/j.genhosppsych.2014.10.007
Crossref Full Text | Google Scholar
4. Morales-Espinoza EM, Kostov B, Salami DC, Perez ZH, Rosalen AP, Molina JO, et al. Complexity, comorbidity, and health care costs associated with chronic widespread pain in primary care. Pain. (2016) 157:818–26. doi: 10.1097/j.pain.0000000000000440
Crossref Full Text | Google Scholar
5. Lumley MA, Schubiner H. Emotional awareness and expression therapy for chronic pain: rationale, principles and techniques, evidence, and critical review. Curr Rheumatol Rep. (2019) 21:30. doi: 10.1007/s11926-019-0829-6
Crossref Full Text | Google Scholar
6. Lumley MA, Schubiner H, Lockhart NA, Kidwell KM, Harte SE, Clauw DJ, et al. Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: a cluster-randomized controlled trial. Pain. (2017) 158:2354–63. doi: 10.1097/j.pain.0000000000001036
Crossref Full Text | Google Scholar
7. Thakur ER, Holmes HJ, Lockhart NA, Carty JN, Ziadni MS, Doherty HK, et al. Emotional awareness and expression training improves irritable bowel syndrome: A randomized controlled trial. Neurogastroenterol Motil. (2017) 29(12):10.1111/nmo.13143. doi: 10.1111/nmo.13143
Crossref Full Text | Google Scholar
8. Carty JN, Ziadni MS, Holmes HJ, Tomakowsky J, Peters K, Schubiner H, et al. The effects of a life stress emotional awareness and expression interview for women with chronic urogenital pain: A randomized controlled trial. Pain Med. (2019) 20:1321–9. doi: 10.1093/pm/pny182
Crossref Full Text | Google Scholar
9. Ziadni MS, Carty JN, Doherty HK, Porcerelli JH, Rapport LJ, Schubiner H, et al. A life-stress, emotional awareness, and expression interview for primary care patients with medically unexplained symptoms: A randomized controlled trial. Health Psychol. (2018) 37:282–90. doi: 10.1037/hea0000566
Crossref Full Text | Google Scholar
10. Yarns BC, Lumley MA, Cassidy JT, Steers WN, Osato S, Schubiner H, et al. Emotional awareness and expression therapy achieves greater pain reduction than cognitive behavioral therapy in older adults with chronic musculoskeletal pain: A preliminary randomized comparison. Trial Pain Med. (2020) 21:2811–22. doi: 10.1093/pm/pnaa145
Crossref Full Text | Google Scholar
11. Yarns BC, Jackson NJ, Alas A, Melrose RJ, Lumley MA, Sultzer DL. Emotional awareness and expression therapy vs cognitive behavioral therapy for chronic pain in older veterans: A randomized clinical trial. JAMA Netw Open. (2024) 7:e2415842. doi: 10.1001/jamanetworkopen.2024.15842
Crossref Full Text | Google Scholar
12. Maroti D, Lumley MA, Schubiner H, Lilliengren P, Bileviciute-Ljungar I, Ljótsson B, et al. Internet-based emotional awareness and expression therapy for somatic symptom disorder: A randomized controlled trial. J Psychosomatic Res. (2022) 163:111068. doi: 10.1016/j.jpsychores.2022.111068
Crossref Full Text | Google Scholar
13. Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. (2002) 64:258–66. doi: 10.1097/00006842-200203000-00008
Crossref Full Text | Google Scholar
14. Nordin S, Palmquist E, Nordin M. Psychometric evaluation and normative data for a Swedish version of the Patient Health Questionnaire 15-Item Somatic Symptom Severity Scale: Health and Disability. Scand J Psychol. (2013) 54:112–7. doi: 10.1111/sjop.12029
Crossref Full Text | Google Scholar
15. Atkinson TM, Mendoza TR, Sit L, Passik S, Scher HI, Cleeland C, et al. The brief pain inventory and its “Pain at its worst in the last 24 hours” Item: clinical trial endpoint considerations. Pain Med. (2010) 11:337–46. doi: 10.1111/j.1526-4637.2009.00774.x
Crossref Full Text | Google Scholar
16. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
Crossref Full Text | Google Scholar
17. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092. doi: 10.1001/archinte.166.10.1092
Crossref Full Text | Google Scholar
18. Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation: Posttraumatic Stress Disorder Checklist for DSM-5, Journal of traumtic stress. J Trauma Stress. (2015) 28:489–98. doi: 10.1002/jts.22059
Crossref Full Text | Google Scholar
20. IBM Corp. IBM SPSS Statistics, Version 29.0. (2022).
22. Terpstra J, van der Vaart R, van Beugen S, van Eersel R, Gkika I, Erdös D, et al. Guided internet-based cognitive-behavioral therapy for patients with chronic pain: A meta-analytic review. Internet Interventions. (2022) 30:100587. doi: 10.1016/j.invent.2022.100587
Crossref Full Text | Google Scholar
23. Dear BF, Gandy M, Karin E, Fogliati R, Fogliati VJ, Staples LG, et al. The pain course: 12- and 24-month outcomes from a randomized controlled trial of an internet-delivered pain management program provided with different levels of clinician support. J Pain. (2018) 19:1491–503. doi: 10.1016/j.jpain.2018.07.005
Crossref Full Text | Google Scholar
24. Ljótsson B, Hedman E, Lindfors P, Hursti T, Lindefors N, Andersson G, et al. Long-term follow-up of internet- delivered exposure and mindfulness based treatment for irritable bowel syndrome. Behav Res Ther. (2011) 49:58–61. doi: 10.1016/j.brat.2010.10.006
Crossref Full Text | Google Scholar
25. Kristjánsdóttir Ó. B., Fors EA, Eide E. A smartphone-based intervention with diaries and therapist feedback to reduce catastrophizing and increase functioning in women with chronic widespread pain: 11-month follow-up results of a randomized trial. J Med Internet Res. (2013) 15:e72. doi: 10.2196/jmir.2249
留言 (0)