Coronary revascularization, including percutaneous coronary intervention, coronary artery bypass grafting, and the combination of these two approaches (1), has brought about a revolutionary change in the treatment of coronary heart disease. However, the long-term outcomes of these therapeutic methods are not entirely favorable.
Research has demonstrated a considerable incidence of all-cause mortality and major adverse cardiovascular events (MACE) following coronary revascularization. Notably, over one-quarter of patients undergoing percutaneous coronary intervention (PCI) experience MACE, encompassing events such as death, non-fatal myocardial infarction (MI), or target vessel revascularization (TVR) (2). Head et al. (3) included 11 randomized trials to examine mortality between revascularization strategies and pooled data from 11,518 patients assigned to undergo PCI (n = 5,753) or CABG (n = 5,765). A meta-analysis revealed that the 5-year all-cause mortality rates were 11.2% following PCI and 9.2% after CABG. Consequently, it is imperative to implement preventive strategies aimed at identifying high-risk factors for adverse outcomes in patients undergoing coronary revascularization, with the ultimate goal of reducing mortality in this patient population.
Numerous factors influence the recovery and prognosis of patients following coronary revascularization, with malnutrition being a particularly significant concern. Malnutrition refers to a state of insufficient nutrient intake, absorption, or excessive depletion, or the consumption of a diet lacking in balance, leading to nutrient deficiencies. Recent evidence suggests that malnutrition is an important predictor of poor prognosis after cardiac surgery (4), and that pre-and post-operative nutritional supplementation can have a positive impact on myocardial glucose metabolism (5). Currently, a variety of scoring systems are used to assess the nutritional status of patients, with the GNRI (Global Nutritional Risk Assessment Index) being preferred for its accuracy and convenience (6). While studies have investigated the predictive utility of the GNRI for outcomes following PCI (7), research encompassing both PCI and CABG has yet to be reported.
MIMIC-IV database provides a large number of real clinical data of critically ill patients (8). An extensive body of research has meticulously explored databases, yielding numerous achievements with significant clinical application value. The objective of the present study was to examine the association between baseline nutritional status and clinical outcomes in patients undergoing coronary revascularization, utilizing the MIMIC-IV database. We posited that the GNRI could serve as a prognostic indicator for patients undergoing coronary revascularization, with the hypothesis that lower GNRI values would be correlated with poorer clinical outcomes.
2 Materials and methods 2.1 Data sourcesData were extracted from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database (https://mimic.mit.edu). This database is open source, records Beth Israel Deaconess Medical Center ICU adult case data from 2008 to 2019, and de-identifies all patient private information, thus exempting ethical approval and informed consent requirements. Author Shi Yue completed an online course at the National Institutes of Health and passed the Human Research Participant Protection exam to gain access to the MIMIC-IV database.
2.2 Study populationAdult patients admitted to ICU for the first time undergoing coronary revascularization surgery, including PCI and CABG, were included. AKI was defined as an increase in Scr levels ≥0.3 mg/dl within 48 h or an increase in Scr ≥1.5-fold from baseline within 7 days, passing the Kidney Disease: Improving Global Outcomes (KDIGO) guideline criteria. Patients who lacked serum albumin, height, and weight within 24 h of admission to ICU were excluded.
2.3 Data acquisitionDemographic information, Sequential Organ Failure Assessment (SOFA) scores, laboratory parameters, comorbidities, interventions, outcome events, etc. were extracted using Structured Query Language (SQL) running PostgreSQL (version 13.7.2). Demographic information includes age, gender, and BMI. Laboratory parameters were selected within 24 h after first admission to ICU, including red blood cell count, white blood cell count, hemoglobin, platelets, serum creatinine (Scr), urea nitrogen (BUN), albumin, blood glucose, potassium, and blood sodium. Concomitant diseases include heart failure, chronic kidney disease (CKD), hypertension, diabetes mellitus (DM), sepsis, and malignancy. Interventions included renal replacement therapy (RRT), diuretics, and vasoactive drugs, and outcome events included 28-day mortality, AKI incidence, and length of hospital stay.
2.4 Outliers and missing value managementVariables with outliers are processed by the winsorize method using the STATA winsor2 command, with cut-off points of 1% and 99%. Missing values were imputed using multiple imputation methods. Variables with missing values of more than 25% were excluded, such as lipids, transaminases, etc.
2.5 Study groups and endpointsGNRI is calculated as: 1.489× serum albumin (g/L) + 41.7 × body weight (kg)/ideal body weight (kg). Ideal weight was estimated according to Lorenz formula, i.e., ideal weight for men = height (cm) − 100 − [(height (cm) − 150)/4]; ideal weight for women = height (cm) − 100 − [(height (cm) − 150)/2.5]. When the actual weight/ideal weight ratio is greater than 1, set it to 1. All patients were divided into four groups according to GNRI quartiles, namely Q1 (86.37), Q2 (86.37–90.84), Q3 (90.85–96.78), and Q4 (≥96.79).Q4, Q3, Q2 and Q1 represent no malnutrition risk, mild, moderate and severe malnutrition risk, respectively.
The primary endpoint was mortality at 28 days, and secondary endpoints were the incidence of AKI and length of stay.
2.6 Statistical analysisContinuous variables for this study are expressed as medians (interquartile ranges) and comparisons are made by Kruskal-Wallis H due to their non-normal distribution. Categorical variables were expressed as n (%) and comparisons were made using chi-square or Fisher's exact test.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for GNRI and 28-day mortality, AKI were calculated using Cox proportional hazards models and adjusted for multiple confounding variables. Confounding variables were selected by clinical experience and stepwise regression (p < 0.05 for selection), and variables with variance inflation factors greater than 5 were excluded from the model. Kaplan-Meier survival analysis was used to assess 28-day mortality, and incidence of AKI based on GNRI strata, and differences between groups were assessed by Log-Rank test. The dose-response relationship between GNRI and 28-day mortality was investigated using restricted cubic splines (RCS). Subgroup analysis and interaction analysis were performed according to age, gender, BMI, heart failure, CKD, sepsis, and hypertension. In addition, multivariate linear regression was used to analyze the relationship between GNRI and length of hospital stay.
R software (Version 4.2.0) and STATA software (Version 16.0) were used for data analysis and visualization. A two-tailed test indicated that P < 0.05 was considered statistically significant.
3 Results 3.1 Baseline characteristicsA total of 1,168 patients with coronary revascularization were enrolled in this study. The screening flow chart is shown in Figure 1.
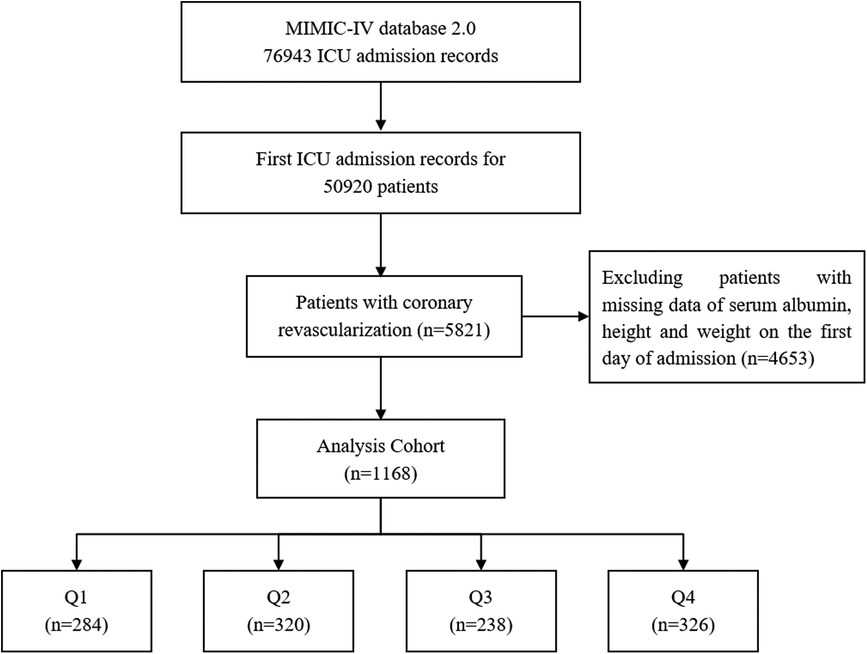
Figure 1. Screening flow chart.
The baseline characteristics of the four groups classified according to GNRI quartiles are shown in Table 1. Those with lower GNRI tended to be older, more female, had higher SOFA scores, higher prevalence of heart failure, CKD, sepsis, and use of RRT, diuretics, and vasoactive drugs. In laboratory indexes, RBC, hemoglobin, platelet, serum albumin, and sodium in patients with lower GNRI were lower than those in patients with higher GNRI, while WBC, BUN, and potassium were on the contrary. In addition, those with lower GNRI had higher 28-day mortality, greater risk of AKI, and longer hospital stays.
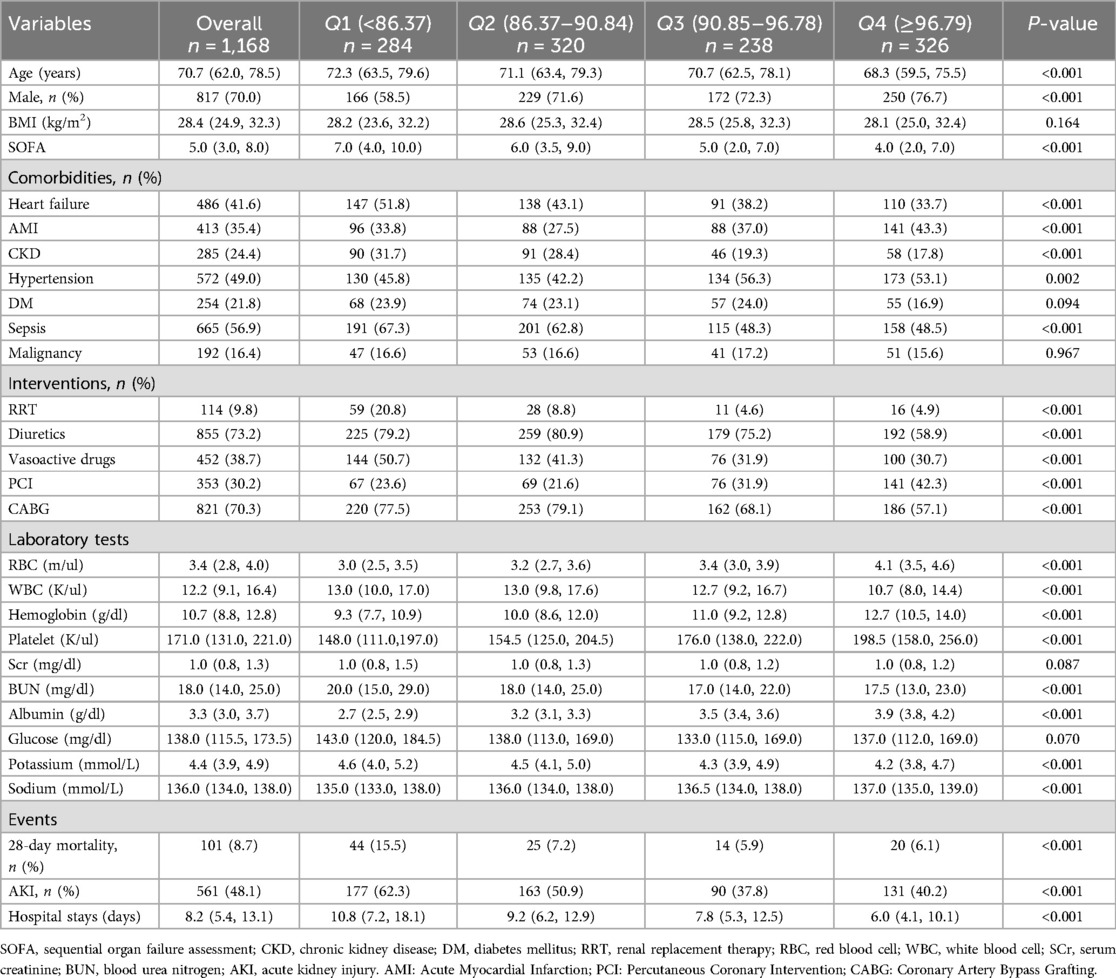
Table 1. Baseline characteristics according to GNRI quartiles.
3.2 Primary endpointOf the 1,168 patients, 101 died within 28 days, accounting for 8.7%. Kaplan–Meier curves (Figure 2) showed a significant increase in 28-day mortality for GNRI <86.37 (Log-rank P <0.0001).
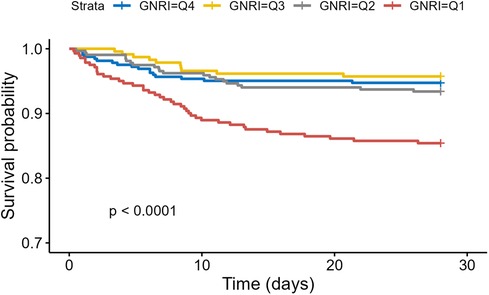
Figure 2. Kaplan–Meier survival analysis of 28-Day mortality based on GNRI stratification.
As shown in Table 2, both univariate and multivariate Cox regression models confirmed the independent effect of GNRI on 28-day mortality in patients with coronary revascularization. When GNRI is treated as a continuous variable, there is no significant difference between the unadjusted model and the unadjusted model. [HR, 0.93 (95% CI: 0.91, 0.96), P < 0.001], partially adjusted model GNRI was significantly negatively associated with 28-day mortality in both the fully adjusted model [HR, 0.94 (95% CI: 0.92, 0.96), P < 0.001] and the fully adjusted model [HR, 0.95 (95% CI: 0.93, 0.97), P < 0.001]. When GNRI was a categorical variable, the low GNRI group (Q1) was significantly associated with an increased risk of 28-day mortality: Unadjusted model [HR, 2.64 (95% CI: 1.56, 4.48), P < 0.001], partially adjusted model [HR, 2.30 (95% CI: 1.35, 3.92), P = 0.002], and fully adjusted model [HR, 1.97 (95% CI: 1.12, 3.46), P = 0.019].
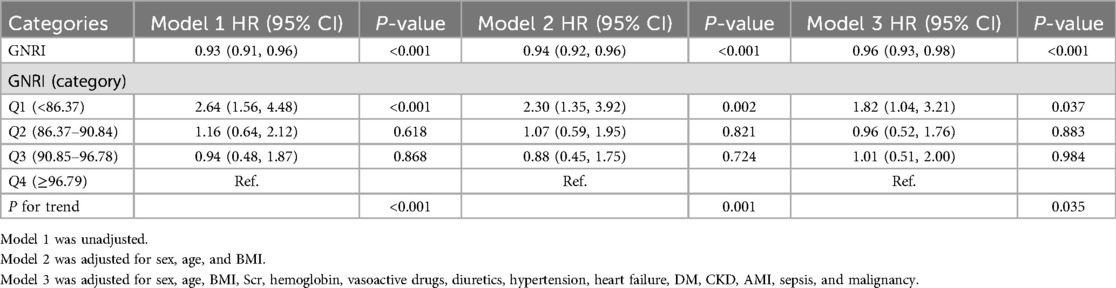
Table 2. Cox proportional hazard ratios for 28-day mortality.
RCS curves (Figure 3) showed a nonlinear association between GNRI and 28-day mortality after adjusting for some confounders (P for non-linearity <0.001).
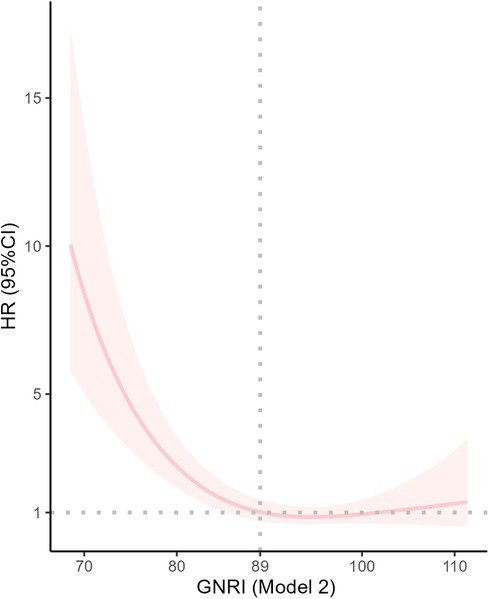
Figure 3. Dose response curves of GNRI and 28-Day mortality in patients undergoing coronary revascularization.
Subgroup analysis (Figure 4) showed a robust independent association between GNRI and 28-day mortality. Of note, GNRI had interaction effects with age (P for interaction = 0.016) and BMI (P for interaction = 0.024).
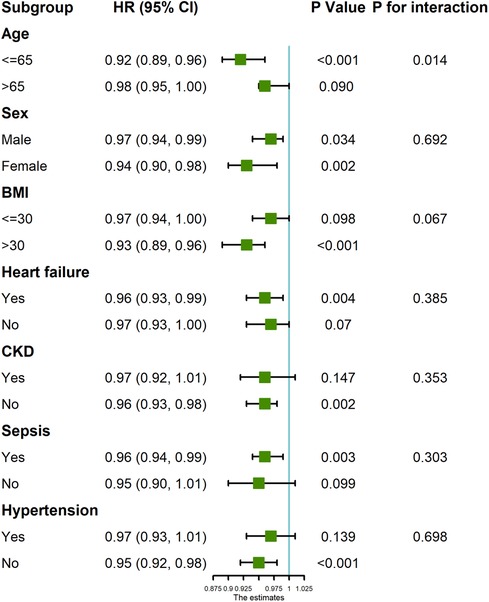
Figure 4. Forest plot of GNRI score and 28-day mortality in patients undergoing coronary revascularization.
3.3 Secondary endpointsWe further assessed the effect of GNRI on the incidence of AKI and length of stay in the study population. Figure 5 showed that cumulative AKI incidence was significantly higher for lower GNRI (Q1, Q2) (Log-rank P < 0.0001). Cox regression models (Table 3) also revealed a significant negative correlation between GNRI and AKI incidence when GNRI was a continuous variable (P < 0.001).
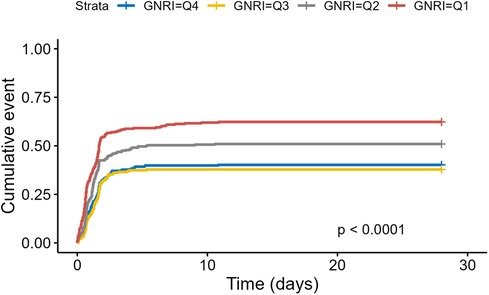
Figure 5. Kaplan–Meier survival analysis of incidence of AKI based on GNRI stratification.
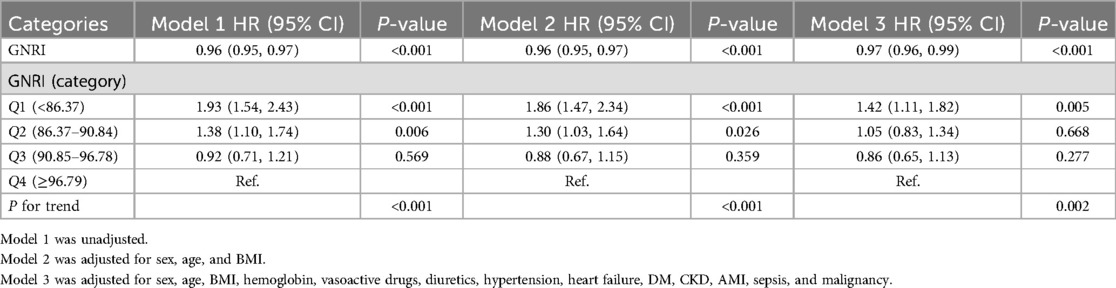
Table 3. Cox proportional hazard ratios for AKI incidence.
In addition, when GNRI was categorically stratified, the low GNRI group (Q1) was significantly associated with the risk of AKI: Unadjusted model [HR, 1.93 (95% CI: 1.54, 2.43), P < 0.001], partially adjusted model [HR, 1.86 (95% CI: 1.47, 2.34), P < 0.001], and fully adjusted model [HR, 1.40 (95% CI: 1.10, 1.79), P = 0.007].
Multiple linear regression models (Table 4) showed that the lower the GNRI, the longer the length of hospital stay, both as continuous and categorical variables.
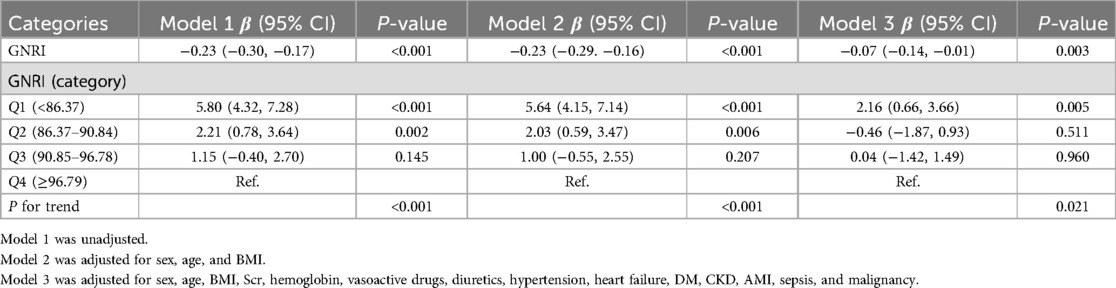
Table 4. Multiple linear regression of the association between GNRI and length of stay.
4 DiscussionCoronary revascularization is a cornerstone in the treatment of coronary heart disease; however, this procedure is not without its risks, as it is associated with a certain mortality rate and potential complications. Consequently, there is an imperative need to identify risk factors that influence postoperative prognosis. Malnutrition has been recognized as a significant predictor of adverse outcomes in various diseases, and GNRI is a widely utilized tool for assessing nutritional status. This study investigated the association between GNRI and key clinical outcomes, including mortality, the incidence of AKI, and the duration of hospitalization, in patients undergoing coronary revascularization. The findings revealed that patients with low GNRI scores exhibited higher mortality rates, an increased incidence of AKI, and prolonged hospital stays compared to those with high GNRI scores.
Malnutrition is a predictor of multiple diseases and surgical outcomes and is estimated to affect approximately 30% to 70% of hospitalized patients (9). The study found that approximately 20% of patients undergoing cardiac surgery experience weight loss during the postoperative period, and stress reactions to surgery and other comorbidities are potential factors that cause weight loss and lead to a malnourished state (10). Several nutritional indices have been developed to assess nutritional status, among which GNRI, which takes into account both serum albumin levels and body weight, is accurate comprehensive, and widely used. This study confirms the independent effect of GNRI on 28-day mortality in patients with coronary revascularization. This is consistent with the results of current studies. For example, Kanda et al. (11) evaluated the correlation between all-cause death and malnutrition in 268 AMI patients undergoing PCI and found that GNRI can predict the mortality rate within 1 month after PCI in AMI patients. Malnutrition may be associated with short-and long-term poor prognosis in AMI patients after PCI. Noike et al. (12) found that frailty and malnutrition were independent predictors of MACE in patients undergoing PCI for stable angina and were detrimental to prognosis. Similar results were found in CABG patients. Tasbulak et al. (13) found a strong correlation between nutritional status and long-term major adverse cardiovascular events (MACCE) and mortality in CABG patients, with CABG patients with low GNRI having higher mortality. In addition, an observational study showed that patients undergoing coronary artery bypass grafting with reduced GNRI had increased rates of MACE and decreased survival during long-term follow-up (14).
In addition to the association between GNRI and mortality, the study also found a significantly higher incidence of AKI in patients with lower GNRI and Cox regression models revealed a significant negative correlation between GNRI and AKI incidence. AKI is one of the possible complications of coronary revascularization surgery (15). The etiology is complex and unknown. Concomitant AKI increases the incidence of adverse medical events in patients undergoing surgery. Acute kidney injury (AKI) after PCI has been reported to be associated with significantly increased short-and long-term mortality and loss of renal function (16). One study showed that patients undergoing CABG had a higher risk of AKI than PCI intervention, but regardless of the revascularization strategy used, the impact of concomitant AKI on prognosis was significant (17). Aykut et al. (18) found that malnutrition and weakness were closely associated with AKI after CABG.
Finally, multiple linear regression models also showed that the lower the GNRI, the longer the length of hospital stay. Malnutrition affects the prognosis. Well-nourished patients recover from disease faster, resulting in faster discharge and reduced hospital costs.
5 LimitationsInevitably, this study has its limitations: Firstly, the study employed an observational design, which does not allow for complete control over potential confounding variables, and this may affect the interpretation of treatment effects. Secondly, the data source is from a single medical center, which may limit the generalizability and external applicability of the results. Additionally, the study only included critically ill patients undergoing revascularization in the ICU, failing to cover a broader patient population, which may affect the comprehensive understanding of treatment effects. Lastly, due to the lack of randomization, it is impossible to fully determine whether the observed treatment effects are indeed caused by the treatment itself or other unmeasured factors. To overcome these limitations, future studies can adopt a multi-center design, randomized controlled trials, and include a wider range of patient populations to more accurately assess treatment effects.
6 ConclusionNutritional level is associated with prognosis in patients undergoing coronary revascularization, and GNRI can be used as a predictor of postoperative mortality. This study shows that patients with low GNRI have higher mortality, AKI incidence, and longer hospital stays, suggesting that we can effectively predict prognosis after coronary revascularization by evaluating GNRI score, to implement relevant interventions to reduce postoperative mortality.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributionsBX: Writing – original draft, Writing – review & editing. YS: Writing – original draft, Writing – review & editing. ML: Writing – review & editing. ZJ: Writing – review & editing. WW: Methodology, Supervision, Writing – review & editing. YY: Methodology, Supervision, Writing – review & editing. MG: Methodology, Supervision, Writing – review & editing. LJ: Methodology, Supervision, Writing – review & editing. LY: Supervision, Writing – review & editing. JL: Supervision, Writing – review & editing. DS: Writing – review & editing. FZ: Funding acquisition, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Innovation Project of Chinese Academy of Traditional Chinese Medicine (CI2021A00910), and the Natural Science Foundation of China (Grant NO. 81973674).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
PubMed Abstract | Crossref Full Text | Google Scholar
2. Ding D, Huang J, Westra J, Cohen DJ, Chen Y, Andersen BK, et al. Immediate post-procedural functional assessment of percutaneous coronary intervention: current evidence and future directions. Eur Heart J. (2021) 42:2695–707. doi: 10.1093/eurheartj/ehab186
PubMed Abstract | Crossref Full Text | Google Scholar
3. Head SJ, Milojevic M, Daemen J, Ahn JM, Boersma E, Christiansen EH, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. (2018) 391:939–48. doi: 10.1016/S0140-6736(18)30423-9
PubMed Abstract | Crossref Full Text | Google Scholar
4. Rahman A, Agarwala R, Martin C, Nagpal D, Teitelbaum M, Heyland DK. Nutrition therapy in critically ill patients following cardiac surgery: defining and improving practice. JPEN J Parenter Enteral Nutr. (2017) 41:1188–94. doi: 10.1177/0148607116661839
PubMed Abstract | Crossref Full Text | Google Scholar
5. Visser M, Davids M, Verberne HJ, Kok WE, Tepaske R, Cocchieri R, et al. Nutrition before, during, and after surgery increases the arginine:asymmetric dimethylarginine ratio and relates to improved myocardial glucose metabolism: a randomized controlled trial. Am J Clin Nutr. (2014) 99:1440–9. doi: 10.3945/ajcn.113.075473
PubMed Abstract | Crossref Full Text | Google Scholar
6. Huang W, Xiao Y, Wang H, Li K. Association of geriatric nutritional risk index with the risk of osteoporosis in the elderly population in the NHANES. Front Endocrinol (Lausanne). (2022) 13:965487. doi: 10.3389/fendo.2022.965487
PubMed Abstract | Crossref Full Text | Google Scholar
7. Sun T, Ma M, Huang X, Zhang B, Chen Z, Zhao Z, et al. Prognostic impacts of geriatric nutritional risk index in patients with ischemic heart failure after percutaneous coronary intervention. Clin Nutr. (2023) 42:1260–7. doi: 10.1016/j.clnu.2023.05.023
PubMed Abstract | Crossref Full Text | Google Scholar
8. Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
PubMed Abstract | Crossref Full Text | Google Scholar
10. Brown JK, Singh K, Dumitru R, Chan E, Kim MP. The benefits of enhanced recovery after surgery programs and their application in cardiothoracic surgery. Methodist Debakey Cardiovasc J. (2018) 14:77–88. doi: 10.14797/mdcj-14-2-77
PubMed Abstract | Crossref Full Text | Google Scholar
11. Kanda D, Ikeda Y, Takumi T, Tokushige A, Sonoda T, Arikawa R, et al. Impact of nutritional status on prognosis in acute myocardial infarction patients undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. (2022) 22:3. doi: 10.1186/s12872-021-02448-x
PubMed Abstract | Crossref Full Text | Google Scholar
12. Noike R, Amano H, Hirano S, Tsubono M, Kojima Y, Oka Y, et al. Combined assessment of frailty and nutritional status can be a prognostic indicator after percutaneous coronary intervention. Heart Vessels. (2023) 38:332–9. doi: 10.1007/s00380-022-02176-y
PubMed Abstract | Crossref Full Text | Google Scholar
13. Tasbulak O, Guler A, Duran M, Sahin A, Bulut U, Avci Y, et al. Association between nutritional indices and long-term outcomes in patients undergoing isolated coronary artery bypass grafting. Cureus. (2021) 13:e16567. doi: 10.7759/cureus.16567
PubMed Abstract | Crossref Full Text | Google Scholar
14. Li Y, Wang Z, Sun T, Zhang B, Liang X. Geriatric nutritional risk index was associated with in-hospital mortality among cardiac intensive care unit patients. Front Nutr. (2023) 10:1218738. doi: 10.3389/fnut.2023.1218738
PubMed Abstract | Crossref Full Text | Google Scholar
15. Chang TI, Leong TK, Boothroyd DB, Hlatky MA, Go AS. Acute kidney injury after CABG versus PCI: an observational study using 2 cohorts. J Am Coll Cardiol. (2014) 64:985–94. doi: 10.1016/j.jacc.2014.04.077
PubMed Abstract | Crossref Full Text | Google Scholar
16. James MT, Har BJ, Tyrrell BD, Faris PD, Tan Z, Spertus JA, et al. Effect of clinical decision support with audit and feedback on prevention of acute kidney injury in patients undergoing coronary angiography: a randomized clinical trial. Jama. (2022) 328:839–49. doi: 10.1001/jama.2022.13382
PubMed Abstract | Crossref Full Text | Google Scholar
17. Shen W, Aguilar R, Montero AR, Fernandez SJ, Taylor AJ, Wilcox CS, et al. Acute kidney injury and in-hospital mortality after coronary artery bypass graft versus percutaneous coronary intervention: a nationwide study. Am J Nephrol. (2017) 45:217–25. doi: 10.1159/000455906
PubMed Abstract | Crossref Full Text | Google Scholar
18. Aykut A, Salman N. Poor nutritional status and frailty associated with acute kidney injury after cardiac surgery: a retrospective observational study. J Card Surg. (2022) 37:4755–61. doi: 10.1111/jocs.17134
留言 (0)