Obstructive sleep apnea (OSA), or obstructive sleep apnea hypopnea syndrome (OSAHS), is a condition in which patients suffer from repeated apnea and hypoventilation during sleep (1). Common clinical symptoms of OSA can often include loud and irregular snoring, choking or suffocating awakenings at night, sleep disturbances, daytime drowsiness, memory loss, and, in severe cases, cognitive decline and behavioral abnormalities (2). Researcher widely recognize OSA as a systemic disease that serves as an independent risk indicator for hypertension. It is closely linked to coronary atherosclerotic heart disease (CHD), heart failure, arrhythmias, and diabetes, and is a major cause of sudden death and road traffic accidents (3–6). Inflammation and oxidative stress are the biological mechanisms underlying the relationship between OSA and its various health complications (7). In the United States, about 26 percent of people aged between 30 and 70 receive an OSA diagnosis, and the prevalence of OSA increased as individual ages increased (8, 9). Moreover, eleven population-based epidemiological studies reported 22% OSA in males and 17% OSA in females (10). As a result, OSA is considered a threat to public health and a serious social problem.
Medical and epidemiological studies often employ cardiovascular health(CVH) to describe the level of cardiovascular disease risk in an individual or population (11, 12). The assessment often considers several risk factors, such as blood pressure, cholesterol levels, diabetes, tobacco use, dietary intake, physical activity, and body mass. As one of the most common respiratory system diseases, OSA, one of the most common respiratory system diseases, may induce hypertension and contribute to CHD, nocturnal angina, myocardial infarction, severe arrhythmias at night, psychiatric abnormalities, respiratory failure, secondary erythrocytosis, and increased blood viscosity (13). The high prevalence of shared risk factors between OSA and CVH implies a potential synergistic interaction between these diseases, potentially promoting either the initiation or progression of OSA; however, the intricate relationship remains unclear. Therefore, there is an urge to elucidate the intricate relationship of CVH with OSA symptoms.
Life's Essential 8 (LE 8) is the American Heart Association's(AHA) suggested algorithm to measure CVH, which serves as the vital evaluation for promoting and sustaining cardiovascular health (14). In 2022, the AHA updated LS7 to Life's Essential 8 to better address the complexities of modern health conditions and the living environment. Numerous studies have extensively studied LS7 metrics and consistently demonstrated an inverse correlation with coronary heart disease(CHD). Recent studies have established a clear inverse relationship between LE8 scores and the risks of cardiovascular disease (CVD) (15).
We performed a cross-sectional analysis utilizing data from the National Health and Nutrition Examination Survey (NHANES) to examine the correlation between CVH and OSA symptoms. Our findings suggested a potential association between LE 8 scores and OSA symptoms, even after adjustment for potential confounding factors.
Methods Data sourceNHANES, overseen by the Centers for Disease Control and Prevention (CDC), surveys the health and nutritional status of adults and children in the US with special emphasis on disease prevalence, adverse risk factors to health, and dietary intake (16). Public health research and policy is heavily dependent on NHANES data for understanding the overall population health, epidemiology of disease trends, and taking action (17). All NHANES procedures were conducted per the guidelines of The Ethical Review Committee at the National Centre for Health Statistics, and written informed consent from participants was obtained before initiation. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting cross-sectional studies (18). Thus, this study is not required for additional institutional review board approval.
Study design and populationIn our study, we employed data from NHANES spanning the years 2005–2008 and 2015–2018, comprising a total initial sample size of 39,722 participants. After excluding pregnant women, individuals under 20 years, and those with missing data on OSA symptoms, LE8 score-related indicators, marital status, income level, education level, BMI, alcohol consumption, smoking, and CVD, data from 12,540 participants was included in our analyses (Figure 1). We employ the List-wise Deletion method to remove entire rows that contain any missing values. This approach is advantageous due to its simplicity and ease of implementation, while also preserving the distribution of the data (Supplementary Material).
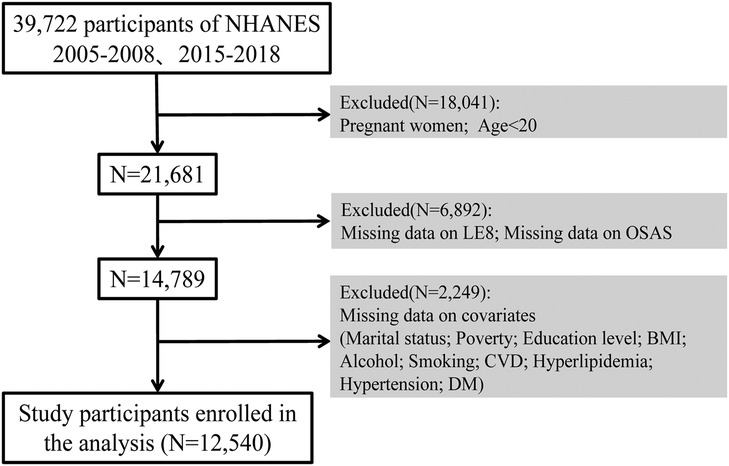
Figure 1. Selection of participants in the study. CVH, cardiovascular health; NHANES, national health and nutrition examination survey.
Definition of OSA symptomsThe assessment of OSA symptoms is based on the responses to three dichotomous questions (19). The two variables measured in the study were (1) frequency of snoring, (2) frequency of snoring/breathing stops, and (3) frequency of excessive daytime sleepiness. Individuals who exhibited the following characteristics were categorized as having symptoms of OSA: snoring at least 3 times per week, snoring or interrupted breathing at least 3 times per week, and feeling excessively sleepy during the day between 16 and 30 times per month.
Quantification of CVHThe LE8 metrics is determined by two primary criteria: health behaviors and health factors. It is specifically designed to measure an individual's overall level of CVH. The LE 8 metrics consist of the following components: dietary intake, physical activity, tobacco/nicotine exposure, sleep, BMI, HDL cholesterol, blood glucose, and blood pressure. The study employs an ordinal scoring system with a range of 0 to 100, where lower scores indicate poorer health, while higher scores indicate better health. The total LE8 score for each participant is calculated by summing the scores of all 8 components and dividing by 8. This framework includes comprehensive assessments of CVH at baseline, categorizing scores of <50 for low cardiovascular health; total scores of 50–79 for moderate cardiovascular health, and ≥80 for high cardiovascular health (20–22).
CovariatesThe covariates examined in this study encompass a range of factors, including age, gender (male and female), race and ethnicity (non-Hispanic black, non-Hispanic white, Mexican American, other Latino, and other races-including multiracial), marital status (married/living with a partner, never married, widowed/divorced/separated), personal income ratios [categorized as low income (1.3), middle income (1.3–3.49), and high income (≥3.5)], an education level (<high school, high school, college, or higher), BMI, alcohol, smoking, CVD, diabetes mellitus (DM), hypertension and hyperlipidemia (23–25).
We divided the smoking status into three categories: never smokers (less than 100 cigarettes in a lifetime), former smokers (quit after smoking more than 100 cigarettes), and current smokers. We categorized alcohol abuse status into three categories: never drinkers (less than 12 drinks in lifetime), former drinkers (less than 12 drinks in 1 year and no drinks in the last year, or no drinks in the last year but more than 12 drinks in lifetime), and currently drinking. CVD status refers to the existence or nonexistence of any of the subsequent diseases: CHD, congestive heart failure, heart attack, stroke, or angina. The American Diabetes Association criteria and self-report questionnaires define DM, and we consider a case of DM if any of the following conditions applies: (1) Doctor has informed you that you have diabetes; (2) Your glycated hemoglobin HbA1c level is equal to or greater than 6.5%; (3) The fasting blood glucose level should equal or exceed 7.0 mM/L.; (4) The random blood glucose level should equal or exceed 11.1 mM/L. (5) The two-hour OGTT blood glucose level should equal or exceed 11.1 mM/L. (6) Use of diabetes medicine or insulin.
Hypertension was assessed based on blood pressure measures taken during the physical examination, where systolic blood pressure was equal to or more than 140 mm Hg, or diastolic blood pressure was equal to or greater than 90 mm Hg. Moreover, It also needs to be determined by a physician's diagnosis of hypertension or receipt of hypertension. Definitions of hyperlipidemia include: (1) having a triglyceride level equal to or greater than 150 mg/dl.; (2) increased total cholesterol can be diagnosed if the triglyceride level is equal to or greater than 200 mg/dl, the LDL cholesterol level is equal to or greater than 130 mg/dl, or the HDL cholesterol level is below 40 mg/dl for males or 50 mg/dl for females.; and (3) The use of lipid-lowering medicines is also considered a factor in diagnosing these conditions.
Statistical analysesContinuous variables were presented as mean and standard deviation (SD), whereas categorical variables were displayed as frequency and weighted percentage. We used the Chi-square test to assess categorical data and the T-test to compare group differences in continuous variables.Our research aimed to determine the association between CVH and OSA symptoms. CVH is measured by LE 8 metrics (classified into high, medium, and low CVH groups). Participants in the low CVH group served as the reference category. We used a weighted logistic regression model to generate odds ratios (ORs) with 95% confidence intervals (CIs),taking into account into following adjustment variables: age, sex, race, marital status, poverty, education level, BMI, alcohol, smoking, CVD, DM, hyperlipidemia, and hypertension. In Crude model, we did not adjust for any confounders. In model 1, we adjusted for age, sex, and race. In model 3, we additionally adjusted for marital_status, poverty, education_level, BMI, alcohol, and smoking. In model 4, we additionally adjusted for CVD, DM, and hypertension, hyperlipidemia.
In addition, subgroup analyses were performed to investigate if the associations differed by sex (male and female) and age group (<60 and ≥60). Subgroup analyses were performed using stratified logistic regression models. The modifications and interactions of subgroups were inspected by likelihood ratio tests (26). Each stratification adjusted for the factors (race, marital_status, poverty, education_level, BMI, alcohol, smoking, CVD, DM, hypertension and hyperlipidemia). RCS was employed in weighted logistic regression models to examine the nonlinear relationships between the continuous LE8 and OSAS [Knots = 4, Reference: median (LE8) = 66.25].The selection of the number and location of knots in the RCS was guided by the Akaike information criterion (AIC) to strike a balance between optimal fit and overfitting (27).
The data were analyzed using R software version 4.2.2 provided by the R Statistical Computing Project and EmpowerStats (http://www.empowerstats.com). A two-sided test was applied, and statistical significance was defined as a P value < 0.05.the P value < 0.05 was considered statistically significant.
Results Population characteristicsThe baseline characteristics of the 12,540 participants in the study sample based on the weighted analyses are presented in Table 1. The sample comprised 6,360 (51.64%) females and 6,180 (48.36%) males. Among the participants, 3,785 participants (30.18%) had OSA symptoms, whereas 8,755 participants (69.82%) without OSA symptoms. Of the total participants, 8,222 (74.20%) were below 60 years old, and 4,318 (25.80%) were above 60 years old. Mexican Americans accounted for 7.40%, non-Hispanic black people accounted for 9.67%, non-Hispanic white people accounted for 71.77%, and the remaining accounted for other ethnicities, totaling 11.15%. The high CVH group consists of 2,272 participants, of whom 82.79% (1,881) did not exhibit OSA symptoms. In the moderate CVH group, there were 8,687 subjects, with 69.10% (6,003) not exhibiting OSA symptoms. The low CVH group had only 55.09% of participants without OSA symptoms. The mean LE8 score for subjects with OSA symptoms was 63.68, significantly lower than the 70.12 observed in the group without OSA symptoms (P < 0.001). As depicted in Table 1 participants with OSA symptoms were predominantly male, under 60 years old, married, highly educated, and former or current smokers. In addition, participants with OSA symptoms tend to have a BMI of 30 or higher, reported alcohol consumption, and did not have a history of CVD, DM, hyperlipidemia or hypertension.
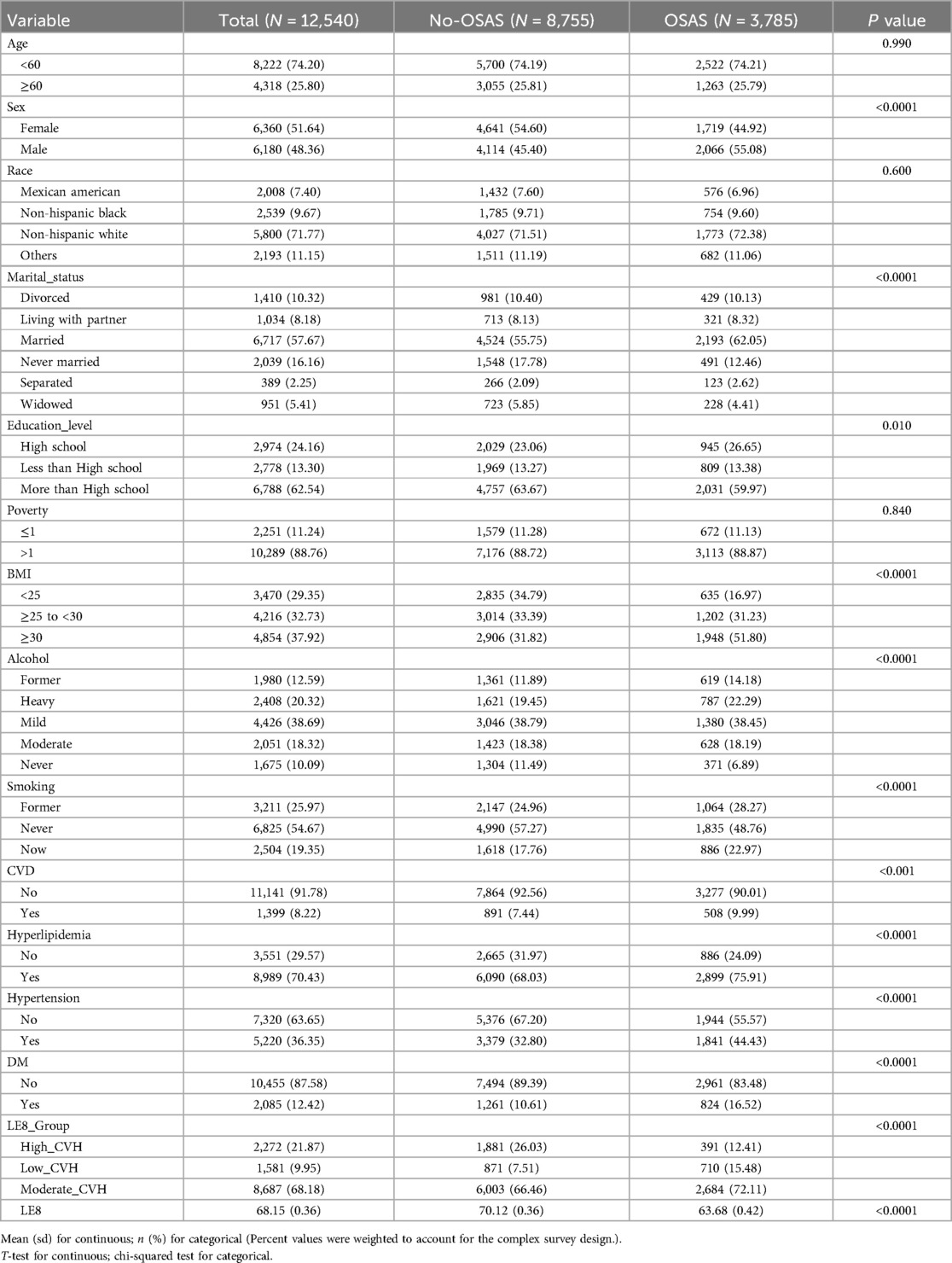
Table 1. Characteristics by cardiovascular health level.
Associations of Le8 with OSAThe results of the sample-weighted multivariate logistics regression analyses are presented in Table 2. Across all models, there was a significant inverse correlation between symptoms of OSA and LE8 metrics. The 95% CIs for the crude model, model 1, model 2, and model 3 were 0.968 (0.964,0.971), 0.967 (0.963, 0.971), 0.979 (0.974, 0.985), and 0.983 (0.978, 0.988), respectively, all with P < 0.0001. After adjustment for multiple covariates, the 95% CI in model 3 was 0.750 (0.630, 0.893) for the moderate CVH group and 0.573 (0.454,0.723) for the high CVH group. The trend P-value for all models was less than 0.0001, indicating a significant decrease in the risk of developing OSA symptoms with increasing LE8 scores.
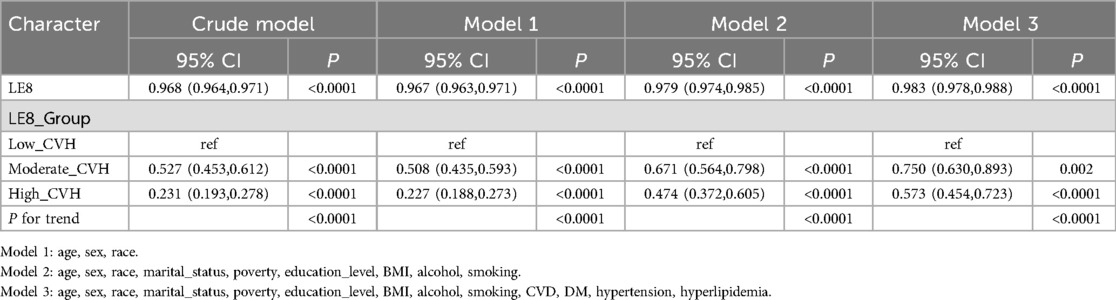
Table 2. Crude and adjusted association between life's essential 8 score and obstructive sleep apnea symptoms.
Subgroup analysesSubgroup analyses by age and gender in Table 3 specifically examine the relationship between LE8 scores and OSA. The results demonstrate a significant interaction between LE8 scores and OSA symptoms with age (P < 0.0001). The high cardiovascular health (CVH) group exhibited a lower risk of concurrent OSA symptoms (OR: 0.470; 95% CI: 0.345, 0.641) (Table 4). In the subgroup of male participants, lower LE8 scores were associated with OSA symptoms (OR: 0.982; 95% CI: 0.974, 0.989). In the subgroup of participants aged less than 60 years, lower LE8 scores were significantly associated with OSA symptoms (OR: 0.980; 95% CI: 0.973, 0.986) (Table 3).
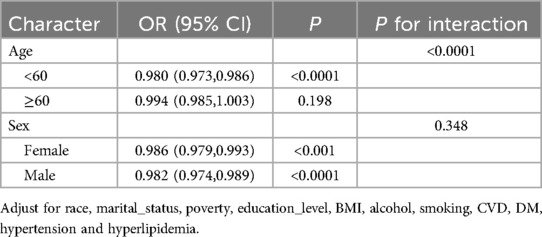
Table 3. Associations of life's essential 8 score for obstructive sleep apnea symptoms grouped by age, sex.
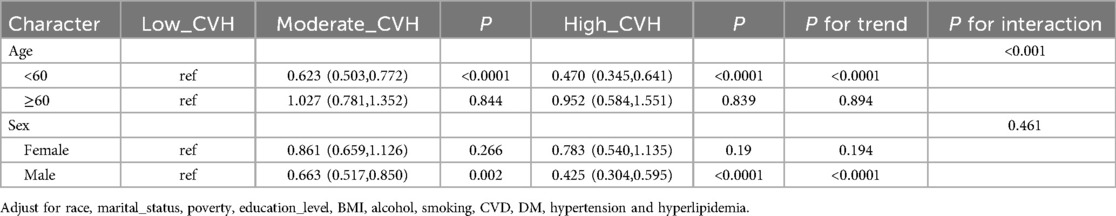
Table 4. The high cardiovascular health group showed a lower risk of developing OSA symptoms.
In summary, for men under 60 years of age, it was shown that those with high CVH grades had a notably reduced risk of experience concomitant OSA symptoms in comparison to those with low CVH grades. There was a notable correlation between the risk ratios and increasing CVH grade, suggesting that CVH grade is a significant risk factor in OSA. A substantial correlation was seen between age and CVH grade, suggesting that age may influence the relationship between CVH grade and OSA.
Dose-response analysis of Le8 score with OSAMultivariate corrected RCS analyses indicated a significant inversely nonlinear relationship between LE8 score and OSA (P for nonlinear = 0.0447; Figure 2). Our finding demonstrates a substantial decrease in OSA symptom prevalence with increased LE 8 scores.
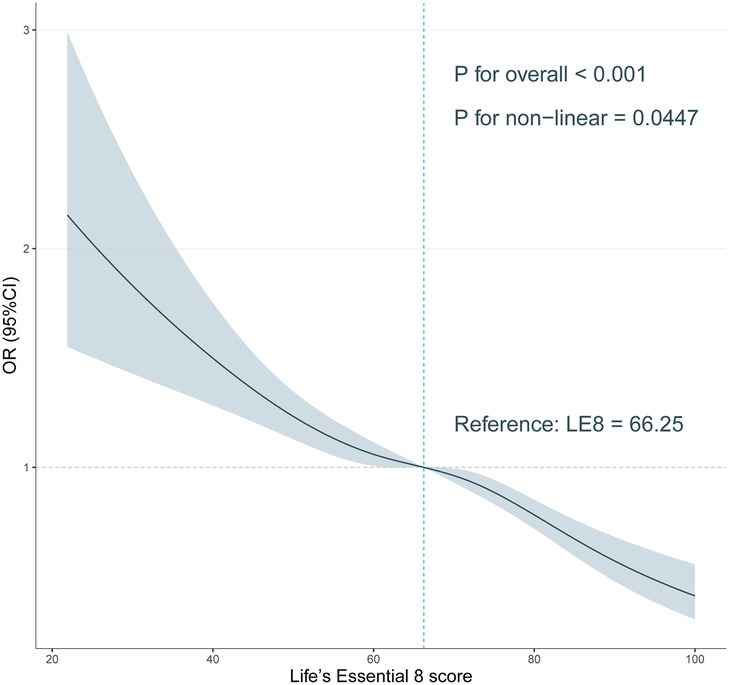
Figure 2. Association of Life’s Essential 8 score with obstructive sleep apnea symptoms in a restricted cubic spline model among all participants.
DiscussionOur study is one of the few to use the NHANES database to conduct a cross-sectional analysis to explore and understand the intrinsic relationship between CVH and OSA. We have observed that CVH subgroups may have a strong association with OSA symptoms, even after adjusting for confounders. The analyses conducted on 12,540 participants revealed that LE8 scores have a negative correlation with the proportion of OSA symptoms. This association was consistent across various demographic characteristics, including gender, race and ethnicity, income, and marital status. However, there was a significant interaction with age. Our findings highlight the importance of maintaining a high CVH to prevent OSA, echoing findings from Zhang et al. (28).
OSA is a common systemic disease that is closely related to diseases such as CHD and DM, as well as potentially causing sudden death and contributing to road traffic accidents, serving as a serious social problem along with a serious healthcare burden for the public (13, 29, 30). Individuals with OSA experience repeated upper airway obstruction and apnea during sleep, which may lead to a variety of cardiovascular problems, such as decreased oxygen saturation during sleep, a known risk factor for hypertension (31). Häusler et al. identified an association between sleep-related factors such as OSA and an elevated risk of CVD events, though the exact nature of this relationship remains incompletely understood (32).Recent research reveals a complex interplay among intermittent hypoxia, oxidative stress, inflammation, and autonomic dysregulation, which collectively contribute to increased cardiovascular risk in patients with OSA (33). Therefore, given the incomplete investigation of the association and the existing gap in evidence in the US, our cross-sectional study provides essential findings to contribute to the existing knowledge base.
From the analysis of the 12,540 participants included in the study, we found that the population with concomitant OSA symptoms was characterized by being male, having a BMI ≥30, having an alcohol consumption history, and being a former or current smoker (34). The prevalence of participants with concomitant OSA symptoms was notably higher among those diagnosed with CVD, hyperlipidemia, hypertension, or DM (P < 0.0001). This underscores the interconnectedness and shared risk factors among these conditions, such as unhealthy lifestyles, poor dietary habits, obesity, and smoking (35). We applied the LE8 scoring system to facilitate the quantitative assessment of participants' CVH status. The average LE 8 score was 63.68 in the group with OSA symptoms, which was significantly lower than the 70.12 in group without OSA symptoms (P < 0.0001). The proportion of participants with concomitant OSA symptoms was considerably higher in low CVH compared to the moderate CVH and high CVH groups. We analyzed the included participants in subgroups by age and gender and found a significant interaction between OSA symptoms with age and LE8 score (P < 0.0001). In subjects under 60 years of age, a reduced risk of concomitant OSA symptoms was observed in the high CVH group (OR: 0.470; 95% CI: 0.345, 0.641). The OR showed a significant increase with a higher CVH grade, indicating a significant interaction between age and CVH grading. For those under 60 years of age, maintaining a high LE 8 score significantly reduces the probability of developing OSA symptoms. However, this preventive effect appears less pronounced in individuals aged 60 and above. It is widely acknowledged that the risk of OSA may escalate with age, therefore, further research into preventive approaches aimed at mitigating OSA risk among older people becomes vital (36).
Our study is based on the NHANES database, selecting eligible samples for weighted analysis to explore the association between CVH and OSA. This finding can be extrapolated to the U.S. adult population-offering health policy insights and serving as a foundation for further research in public health interventions. Considering the interrelationship between CVH and OSA, enhancing public awareness and promoting CVH-related diagnosis and treatment may also be an effective means to improve OSA, which serves as an intervention that aims to address potential CVH issues and improve oxygen saturation (32, 37). At the same time, maintaining healthy lifestyle habits also serves as an effective method to improve OSA and CVH.
LimitationThis study also has some limitations. First, the identification of OSA symptoms relied primarily on the typical clinical presentations on the questionnaire, which may not capture all aspects of the condition. Some of the symptoms that are crucial for identifying OSA were not available, such as lifestyle behaviors, which may have resulted in some OSA participants not being identified (38). Moreover, using questionnaire-based assessments alone may overlook certain cases of OSA. Second, our study is limited to factors such as snoring, intermittent apnea, and drowsiness. This limitation could potentially result in some individuals with OSA not being identified (39). Third, the participants from the database lacked relevant information about professional laboratory sleep testing, potentially leaving out individuals with asymptomatic OSA from the OSA group. However, these missing individuals are likely to be undifferentiated and have no effect on the final analysis results (40). Fourth, our study is constrained by its retrospective and cross-sectional design, which introduces information bias stemming from missing data and complicates the establishment of a causal relationship between LE8 scores and OSA. Additionally, we did not employ Directed Acyclic Graphs (DAGs) to identify and elucidate potential confounding variables. Future research will concentrate on this aspect.
ConclusionThe findings of the research conducted on a large and diverse group of 12,540 individuals in the United States, using data from the NHANES, indicate a strong negative association between LE8 scores and OSA symptoms. Participants with higher LE8 scores were less likely to have concomitant OSA symptoms. These findings underscore the importance of adopting a healthy lifestyle and maintaining a high LE8 score to reduce the incidence of OSA symptoms and their potential role in preventing OSA-related complications.Future research should concentrate on investigating the causal relationships and clarifying the precise mechanisms underlying the relationship between CVH and OSA.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributionsQG: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. DD: Writing – original draft, Writing – review & editing. QZ: Visualization, Writing – original draft, Writing – review & editing. SH: Methodology, Software, Writing – original draft, Writing – review & editing. XQ: Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. ZD: Investigation, Writing – original draft, Writing – review & editing. XW: Writing – original draft, Writing – review & editing. YZ: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was partially funded by the National Natural Science Foundation of China (82071023), the International Science and Technology Cooperation Project of Henan Province (242102521064), and the Key Project of Medical Science and Technology of Henan Province (SBGJ202102160).
AcknowledgmentsWe sincerely appreciate the significant contributions made by all the authors towards this study, their invaluable efforts have been instrumental in its success.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1466752/full#supplementary-material
References6. Li N, Cai X, Zhu Q, Yao X, Lin M, Gan L, et al. Association between plasma homocysteine concentrations and the first ischemic stroke in hypertensive patients with obstructive sleep apnea: a 7-year retrospective cohort study from China. Dis Markers. (2021) 2021:9953858. doi: 10.1155/2021/9953858
PubMed Abstract | Crossref Full Text | Google Scholar
7. Lavalle S, Masiello E, Iannella G, Magliulo G, Pace A, Lechien JR, et al. Unraveling the complexities of oxidative stress and inflammation biomarkers in obstructive sleep apnea syndrome: a comprehensive review. Life. (2024) 14(4):425. doi: 10.3390/life14040425
PubMed Abstract | Crossref Full Text | Google Scholar
9. Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Rucker Coker T, et al. Screening for obstructive sleep apnea in adults: US preventive services task force recommendation statement. JAMA. (2022) 328(19):1945–50. doi: 10.1001/jama.2022.20304
PubMed Abstract | Crossref Full Text | Google Scholar
10. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. (2015) 7(8):1311–22. doi: 10.3978/j.issn.2072-1439.2015.06.11
PubMed Abstract | Crossref Full Text | Google Scholar
11. Ma H, Wang X, Xue Q, Li X, Liang Z, Heianza Y, et al. Cardiovascular health and life expectancy among adults in the United States. Circulation. (2023) 147(15):1137–46. doi: 10.1161/CIRCULATIONAHA.122.062457
PubMed Abstract | Crossref Full Text | Google Scholar
12. Sun Y, Yu Y, Zhang K, Yu B, Yu Y, Wang Y, et al. Association between Life’s essential 8 score and risk of premature mortality in people with and without type 2 diabetes: a prospective cohort study. Diabetes Metab Res Rev. (2023) 39(5):e3636. doi: 10.1002/dmrr.3636
PubMed Abstract | Crossref Full Text | Google Scholar
14. Sun J, Li Y, Zhao M, Yu X, Zhang C, Magnussen CG, et al. Association of the American heart Association’s new “Life's Essential 8” with all-cause and cardiovascular disease-specific mortality: prospective cohort study. BMC Med. (2023) 21(1):116. doi: 10.1186/s12916-023-02824-8
PubMed Abstract | Crossref Full Text | Google Scholar
15. Rempakos A, Prescott B, Mitchell GF, Vasan RS, Xanthakis V. Association of Life’s essential 8 with cardiovascular disease and mortality: the framingham heart study. J Am Heart Assoc. (2023) 12(23):e030764. doi: 10.1161/JAHA.123.030764
PubMed Abstract | Crossref Full Text | Google Scholar
16. Paulose-Ram R, Graber JE, Woodwell D, Ahluwalia N. The national health and nutrition examination survey (NHANES), 2021–2022: adapting data collection in a COVID-19 environment. Am J Public Health. (2021) 111(12):2149–56. doi: 10.2105/AJPH.2021.306517
PubMed Abstract | Crossref Full Text | Google Scholar
17. Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. (2016) 7(1):121–34. doi: 10.3945/an.115.009258
PubMed Abstract | Crossref Full Text | Google Scholar
19. Cavallino V, Rankin E, Popescu A, Gopang M, Hale L, Meliker JR. Antimony and sleep health outcomes: NHANES 2009–2016. Sleep Health. (2022) 8(4):373–9. doi: 10.1016/j.sleh.2022.05.005
PubMed Abstract | Crossref Full Text | Google Scholar
20. Shetty NS, Parcha V, Patel N, Yadav I, Basetty C, Li C, et al. AHA Life’s essential 8 and ideal cardiovascular health among young adults. Am J Prev Cardiol. (2023) 13:100452. doi: 10.1016/j.ajpc.2022.100452
PubMed Abstract | Crossref Full Text | Google Scholar
21. Herraiz-Adillo Á, Ahlqvist VH, Daka B, Wångdahl J, Wennberg P, Carlsson J, et al. Life’s essential 8 in relation to self-rated health and health-related quality of life in a large population-based sample: the SCAPIS project. Qual Life Res. (2024) 33(4):1003–14. doi: 10.1007/s11136-023-03580-1
PubMed Abstract | Crossref Full Text | Google Scholar
22. Qiu X, Wu Q, Zhang Y, Zhu Y, Yang M, Tao L. Association between life’s essential 8 and frailty status among cancer survivors in the United States: a cross-sectional analysis. BMC Public Health. (2024) 24(1):1287. doi: 10.1186/s12889-024-18741-1
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wang K, Zhao Y, Nie J, Xu H, Yu C, Wang S. Higher HEI-2015 score is associated with reduced risk of depression: result from NHANES 2005–2016. Nutrients. (2021) 13(2):348. doi: 10.3390/nu13020348
PubMed Abstract | Crossref Full Text | Google Scholar
24. Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. (2018) 15(12):e1002718. doi: 10.1371/journal.pmed.1002718
PubMed Abstract | Crossref Full Text | Google Scholar
25. ALHarthi SSY, Natto ZS, Midle JB, Gyurko R, O'Neill R, Steffensen B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J Periodontol. (2019) 90(1):16–25. doi: 10.1002/JPER.18-0183
PubMed Abstract | Crossref Full Text | Google Scholar
26. Shen R, Zou T. The association between cardiovascular health and depression: results from the 2007–2020 NHANES. Psychiatry Res. (2024) 331:115663. doi: 10.1016/j.psychres.2023.115663
PubMed Abstract | Crossref Full Text | Google Scholar
27. Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. (2020) 371:m4266. doi: 10.1136/bmj.m4266
PubMed Abstract | Crossref Full Text | Google Scholar
28. Zhang H, Zhang Z, Zhao Y, Song P, Chen X, Han P, et al. Association of the combination of obstructive sleep apnea risk and sleep duration with ideal cardiovascular health metrics in patients undergoing hemodialysis. BMC Nephrol. (2024) 25(1):77. doi: 10.1186/s12882-024-03517-x
PubMed Abstract | Crossref Full Text | Google Scholar
29. Haba-Rubio J, Marques-Vidal P, Andries D, Tobback N, Preisig M, Vollenweider P, et al. Objective sleep structure and cardiovascular risk factors in the general population: the HypnoLaus study. Sleep. (2015) 38(3):391–400. doi: 10.5665/sleep.4496
PubMed Abstract | Crossref Full Text | Google Scholar
30. Luo Q, Li N, Zhu Q, Yao X, Wang M, Heizhati M, et al. Non-dipping blood pressure pattern is associated with higher risk of new-onset diabetes in hypertensive patients with obstructive sleep apnea: UROSAH data. Front Endocrinol. (2023) 14:1083179. doi: 10.3389/fendo.2023.1083179
PubMed Abstract | Crossref Full Text | Google Scholar
31. Meszaros M, Bikov A. Obstructive sleep apnoea and lipid metabolism: the summary of evidence and future perspectives in the pathophysiology of OSA-associated dyslipidaemia. Biomedicines. (2022) 10(11):2754. doi: 10.3390/biomedicines10112754
PubMed Abstract | Crossref Full Text | Google Scholar
32. Häusler N, Marques-Vidal P, Heinzer R, Haba-Rubio J. How are sleep characteristics related to cardiovascular health? Results from the population-based HypnoLaus study. J Am Heart Assoc. (2019) 8(7):e011372. doi: 10.1161/JAHA.118.011372
PubMed Abstract | Crossref Full Text | Google Scholar
33. Maniaci A, Lavalle S, Parisi FM, Barbanti M, Cocuzza S, Iannella G, et al. Impact of obstructive sleep apnea and sympathetic nervous system on cardiac health: a comprehensive review. J Cardiovasc Dev Dis. (2024) 11(7):204. doi: 10.3390/jcdd11070204
PubMed Abstract | Crossref Full Text | Google Scholar
34. Pataka A, Kotoulas S, Kalamaras G, Tzinas A, Grigoriou I, Kasnaki N, et al. Does smoking affect OSA? What about smoking cessation? J Clin Med. (2022) 11(17):5164. doi: 10.3390/jcm11175164
PubMed Abstract | Crossref Full Text | Google Scholar
35. Zhao J, Cai X, Hu J, Song S, Zhu Q, Shen D, et al. J-Shaped relationship between weight-adjusted-waist index and cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Diabetes Metab Syndr Obes. (2024) 17:2671–81. doi: 10.2147/DMSO.S469376
PubMed Abstract | Crossref Full Text | Google Scholar
37. Makarem N, St-Onge MP, Liao M, Lloyd-Jones DM, Aggarwal B. Association of sleep characteristics with cardiovascular health among women and differences by race/ethnicity and menopausal status: findings from the American heart association go red for women strategically focused research network. Sleep Health. (2019) 5(5):501–8. doi: 10.1016/j.sleh.2019.05.005
PubMed Abstract | Crossref Full Text | Google Scholar
38. Duan X, Huang J, Zheng M, Zhao W, Lao L, Li H, et al. Association of healthy lifestyle with risk of obstructive sleep apnea: a cross-sectional study. BMC Pulm Med. (2022) 22(1):33. doi: 10.1186/s12890-021-01818-7
PubMed Abstract | Crossref Full Text | Google Scholar
40. Gu X, Tang D, Xuan Y, Shen Y, Lu LQ. Association between obstructive sleep apnea symptoms and gout in US population, a cross-sectional study.
留言 (0)