Coarctation of the aortic arch (CoA) usually occurs at the descending part of the aortic arch, the distal end of the left subclavian artery, and the junction of the ductus arteriosus. It has become a common congenital heart anomaly in clinical practice, accounting for 5%–8% of congenital heart disease (CHD) (1, 2). Commonly seen in males, with a male to female ratio of 3.5:1, it can exist alone, and cardiac surgery can also cause aortic constriction. More than 50% of patients often have other congenital cardiovascular malformations, mainly including patent ductus arteriosus, ventricular septal defect, and bivalve aortic valve. In clinical practice, for systolic arterial pressure gradient greater than 20 mmHg (1 mmHg = 0.133 kPa). Congenital coarctation of the aorta can be diagnosed by combining with corresponding imaging changes (1, 2). However, the surgery for CoA was complicated and the mortality and complication rate were high. In recent years, percutaneous balloon dilatation covered stent implantation is a new technology for the treatment of CoA (3, 4). With the continuous improvement of surgical techniques, the mortality rate has decreased (5). In recent years, with the continuous development of intervention technology, intervention therapy has made rapid progress in the treatment of congenital heart disease. Especially in the past decade, NuMED stents and balloon in balloon (BIB, NuMed Corporation, USA) catheters specifically designed for the treatment of vascular obstructive diseases related to congenital heart disease have increasingly attracted the attention of clinical doctors (6, 7).
Structural heart disease (SHD) mediates Treatment requires a deep understanding of perioperative cardiac pathophysiology and interventional instruments, and traditional imaging examinations are no longer sufficient to meet these requirements. There are still shortcomings in the development of specialized instruments, medical education, medical imaging, and nursing cooperation in transcatheter intervention surgery, and there are also differences in understanding the 3D, 4D and artificial intelligence (8). Futhermore, studys have indicated that the application of CFD simulations may be useful for blood flow reconstruction for clinical conditions in circulatory system (9, 10). In order to bridge the gap between virtual reality and integrate the advantages of computer science and biomedical engineering, 3D printing, computer modeling, and artificial intelligence (AI) may play their advantageous roles. Our research team has applied 3D printing technology for preoperative multimodal imaging evaluation and in vitro simulation in cardiovascular intervention surgeries such as TAVR, TMVR, PVL, etc., and has achieved good results in assisting in the formulation of intervention surgical plans and physician training. Similarly, we would like to conduct an application study using 3D printing technology for preoperative evaluation and simulation of aortic arch constriction covered stent implantation, in order to further improve the role of 3D printing technology in preoperative imaging evaluation of structural heart disease.
Materials and methods General information of patientsA total of 10 patients with CoA underwent percutaneous balloon expandable covered stent implantation in Cardiovascular Surgery, Xijing Hospital from December 2018 to December 2023 were enrolled. Among them, there were 6 males and 4 females whose average age was (27.68 ± 13.45) years. Routine arterial blood pressure measurements showed that patients had different degrees of hypertension (right upper limb), and lower limb blood pressure was generally lower than upper limb blood pressure by 20 mmHg or more. There were 2 cases with ventricular septal defect and 2 cases with patent ductus arteriosus. All patients underwent percutaneous human-to-human occlusion at the same time. One case had mild aortic insufficiency. Preoperative ECG, echocardiography and aortic CTA were performed to confirm the clinical diagnosis. Routine preoperative biochemical examinations were performed to assess the patient's condition. All patients were diagnosed with CoA for the first time and none undergo plain angioplasty before stent placement. All selected patients have obtained the informed consent of their patients and their families, and signed the informed consent form for surgery (Figure 1).
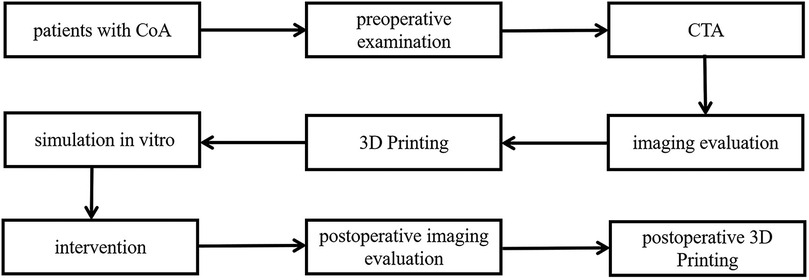
Figure 1. Protocal of the experiment.
3D Printing and preoperative simulation in vitroAll patients underwent chest area scanning using Siemens Flash dual source CT machine (Siemens AG, Germany), with a scanning range from the aortic arch to the pubic symphysis, with a scanning layer thickness of 7.0 mm and a reconstruction layer thickness of 0.75 mm. A total of 100 ml of iopromide (300 mgI/ml, Bayer Schering Pharma AG, Germany) was injected through the elbow vein at a rate of 3.0 ml/s. Import CT scan data in DICOM (digital Imaging and Communications in Medicine) format into Mimics 21.0 (Materialise, Leuven, Belgium) software for whole heart and aortic reconstruction, with a focus on reconstructing the site of aortic constriction and adjacent vascular structures. The extraction range of printed model image data in the reconstructed data includes the whole heart and aorta, which are segmented and surface modified with surrounding tissues to form an STL (STereoLithography) format file and imported into Magic software for model surface modification. After that, the data is imported into a Form2 printer (Stratasys Corporation, USA), and the printing materials are mainly silicone and relatively hard transparent resin materials (Clear V4, Formlabs Corporation, USA), in order to fully simulate the structure of the patient's aorta and create a model of the patient's heart and aorta. Before intervention, a 3D printed model and a covered stent delivery system were used in vitro to conduct personalized analysis of the anatomical structure of the patient's heart and aortic constriction site. The procedure of the guide wire, catheter, and delivery system through the aortic constriction site was simulated, and the surgical process was simulated in vitro. The optimal position and angle for releasing the covered stent were determined, and a personalized surgical plan was developed (Figures 2–4).
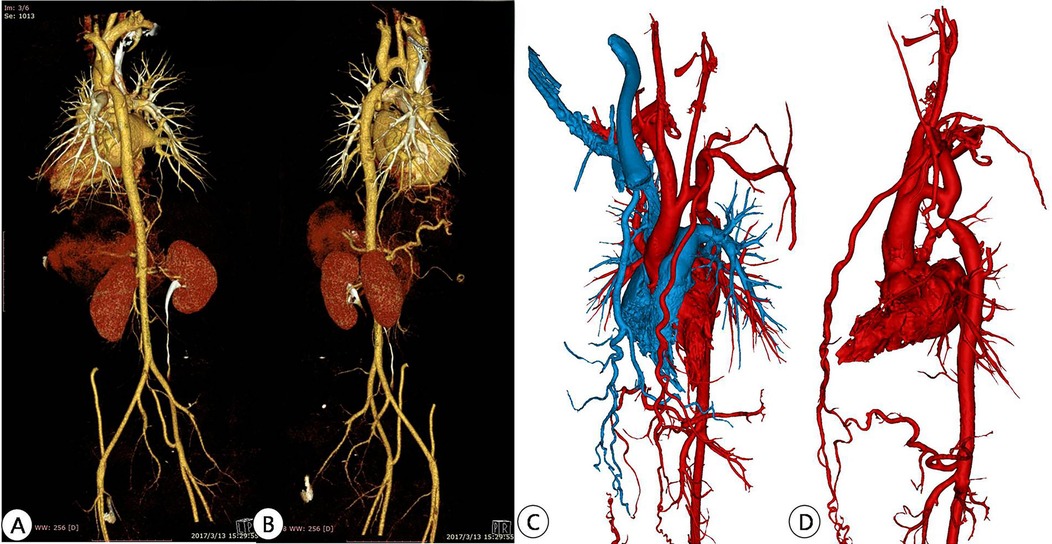
Figure 2. Preoperative CTA reconstruction image of the patient. (A,B) CTA reconstruction imaging shows the location of aortic arch constriction; (C,D) Using computer reconstruction technology, segment the anatomical structure around aortic constriction and highlight the location of aortic constriction.
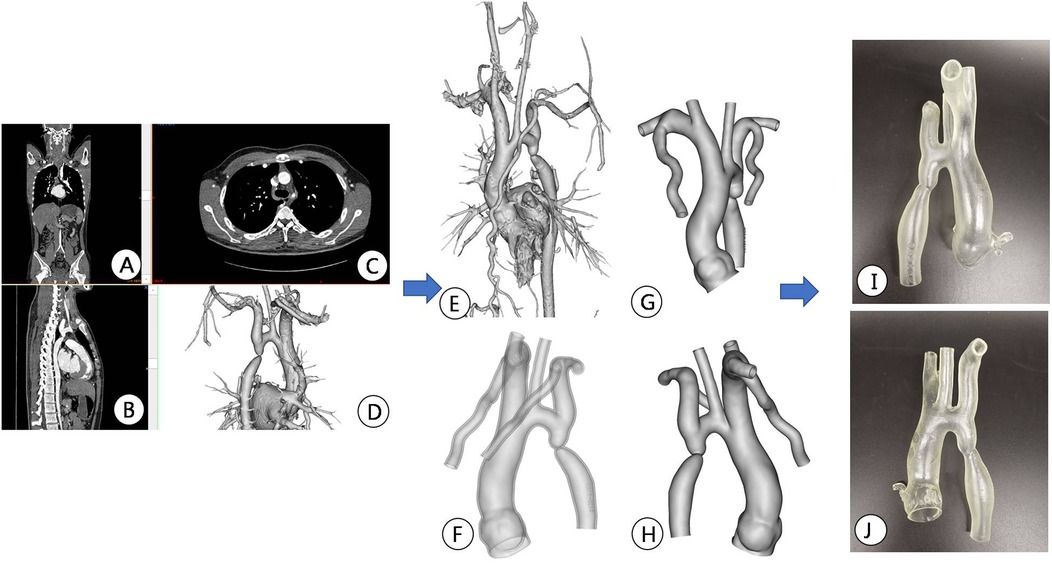
Figure 3. Preoperative CT image data reconstruction and 3D printing model of patients. (A–C) Display CTA images of the aorta in different sections; (D,E) Mimics software displays the location of active artery constriction at different angles after reconstruction; (F–H) segmentation, decoration, and reconstruction of images for subsequent 3D printing; (I,J) 3D printed aortic constriction model displayed on different sides.
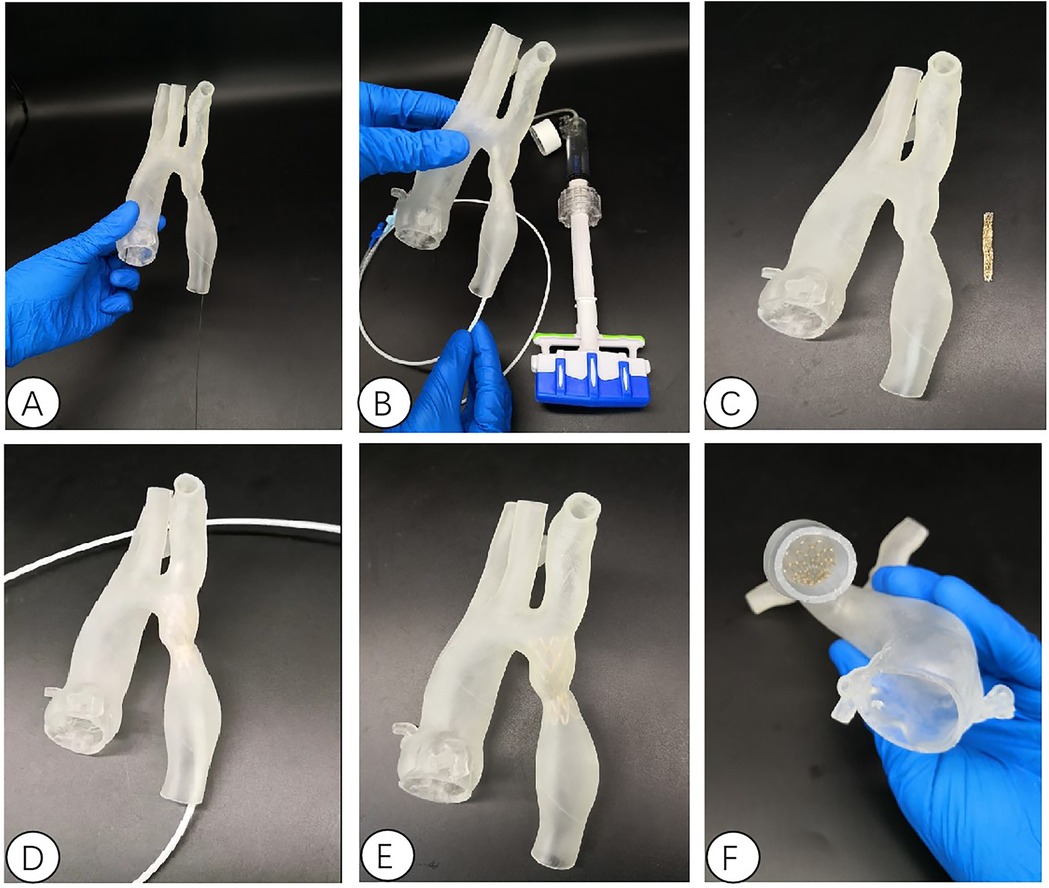
Figure 4. Interventional simulation using 3D printed models. (A) 3D printed aortic constriction model; (B) Using a 3D printing model of aortic constriction to simulate balloon dilation in vitro; (C) 3D printed aortic constriction model and stent with covered membrane; (D) in vitro simulated stent release at aortic constriction; The position relationship and fit degree between the (E,F) bracket and the 3D printed model after its release.
Procedure of interventionIn the hybridization room, all patients were in supine position and operated under local anesthesia. Seldinger technique was used to puncture the left radial artery and the right femoral artery. The sheaths of 5F and 6F arteries were placed respectively. After intravenous administration of heparin (1 mg/kg), the pigtail catheter was placed into the ascending aorta via the left radial artery. The narrowing position, length, degree, proximal and distal aorta diameter, the relationship with the surrounding vessels and the presence of other vascular lesions were defined by multi-angle projection angiography. The right coronary artery catheter and the super slippery loach guide wire were placed through the right femoral artery. The guide wire catheter passed through the narrowing lesion to the ascending aorta. The aorta was measured before and after stenosis and the Lunderquist guide wire (Cook Medical Trading Co., Ltd., China) was exchanged. Cheatham-Platinum covered stent (CP stent, Shenzhen Lifetech Scientific Medical Equipment Co., Ltd., China) and balloon in balloon (BIB, NuMed Corporation, USA) catheter of suitable length were selected. Balloon and covered stent were assembled in vitro. Steel protective inner core was built into balloon, and outer sheath tube protective stent was pre-placed on BIB balloon respectively. The 12F transporting sheath tube was inserted along the Lunderquist guide wire, the inner core was removed, the guide wire was retained, and the selected BIB balloon catheter and CP stent system for aortic coarctation were fed into the 12F transporting sheath tube. After satisfactory positioning, the stent was released, and the contrast agent was pre-dilated by injecting a high-pressure surgical syringe through a red interface. The contrast agent was injected by a high-pressure hand syringe through a blue interface rapidly and fully. Extracorporeal balloon dilatation showed lumbar sign of stenosis and rapid withdrawal of syringe. Angiography and manometry were performed again to observe the position and shape of stent, and aortic pressure was measured before and after stenosis. After satisfactory pressure difference, the catheter was withdrawn. The femoral artery puncture point was sutured with Perclose ProGlide vascular suture (Abbott Company, USA) and compressed and bandaged (Figure 5).
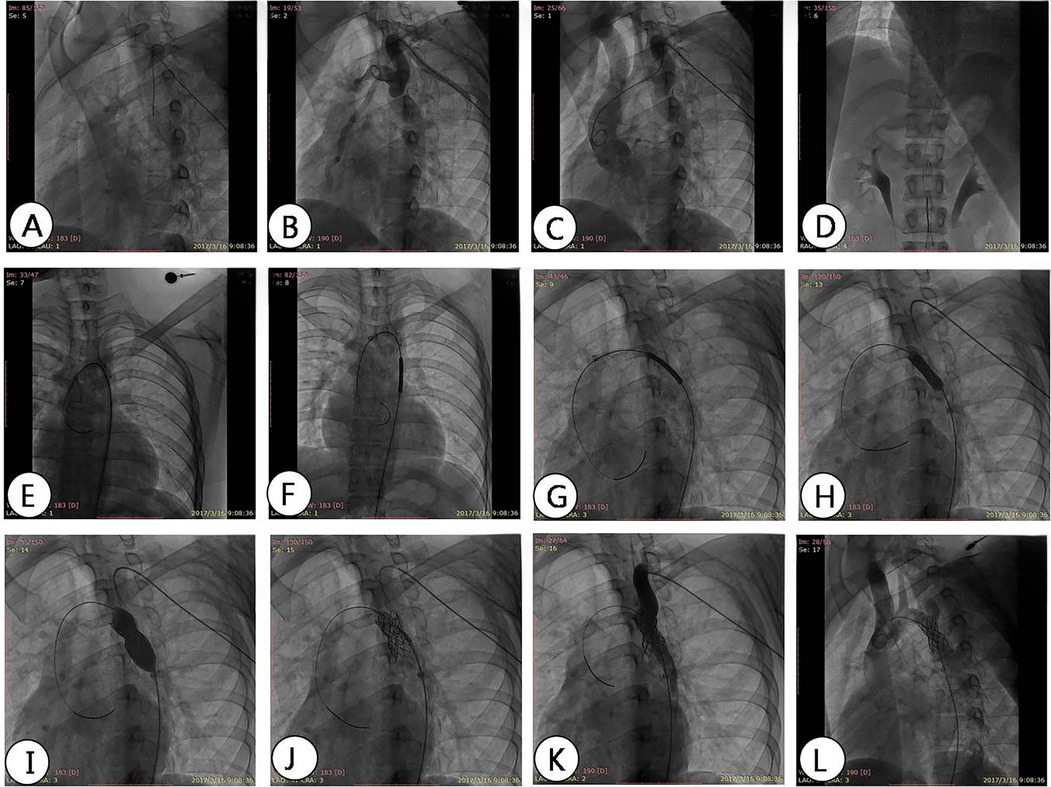
Figure 5. DSA angiography shows the process of stent implantation. (A) The contrast catheter passes through the constriction of the aorta; (B) angiography shows the location and degree of aortic arch constriction; (C) changing the angle of imaging to show narrowing of the aortic arch; (D) feeding into the conveying system from the femoral artery; (E) the conveying system; (F–H) transports the bracket to the predetermined position, narrows the plane, and performs imaging at different angles to determine the release position; (I) balloon dilation and release of stent; (J) evacuate the conveying system; (K,L) angiography shows the position of the covered stent and evaluates the pressure difference after expansion.
Postoperative treatment and follow-upAt least 3 months after the operation, the patient persisted in taking dual antiplatelet aggregation drugs orally. After 3, 6, 12 months to the hospital for review, 1 year after the annual outpatient follow-up. Ask patients about the improvement of general symptoms, measure the blood pressure of limbs, and review the color echocardiography, aortic CTA and 3D printing if necessary.
Statistical analysisAll demographic, valve-related procedural and outcome data, and clinical and anatomic data were obtained from a retrospective review of patient charts and procedural records. Statistical analyses were conducted using SPSS 22.0 software (IBM SPSS Statistics for Macintosh, Version 22.0, IBM Corp, Armonk, NY). Continuous variables are presented as means ± SD and categorical variables are expressed as percentages. Univariable comparisons were performed with the Student unpaired t-test for continuous normally distributed data and the chi-square test was used for categorical data. Values of P < 0.05 were considered statistically significant.
ResultsAll the patients with CoA were successfully implanted with covered stents. The narrowest mean diameter of CoA increased from (4.35 ± 2.61) mm before operation to (16.84 ± 1.99) mm immediately after operation (P < 0.05), and the mean transconstrictive systolic pressure difference decreased from (81.29 ± 18.72) mmHg before operation to (15.52 ± 7.47) mmHg after operation (P < 0.05). The mean systolic blood pressure of the right upper limb decreased from (182.05 ± 38.99) mmHg preoperatively to (141.95 ± 32.11) mmHg postoperatively (P < 0.05); the mean systolic blood pressure of the lower limb increased from (121.52 ± 27.84) mmHg preoperatively to (131.81 ± 32.39) mmHg postoperatively (P < 0.05) (Figure 6, Table 1). A total of two patients with VSD and PDA underwent interventional occlusion at the same time, and the shunt disappeared completely after operation. All patients had good healing of puncture points, no subcutaneous hematoma and vascular injury, no aortic dissection, aneurysm, pseudoaneurysm and other manifestations. After 12 months of follow-up, the clinical symptoms of the patients were relieved obviously, and the difference of systolic pressure between upper and lower limbs was less than 20 mmHg. Postoperative re-examination of aortic CTA showed that the stent was in good position and shape, and no dissection or aneurysm occurred (Figures 6, 7).
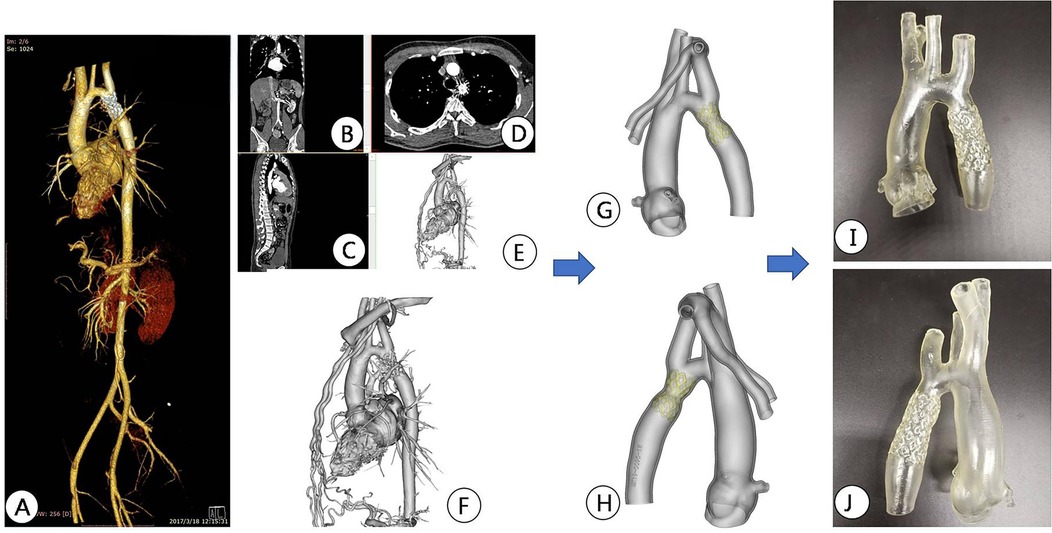
Figure 6. CT image reconstruction and 3D printing model after aortic coarctation surgery. (A) Postoperative aortic CTA reconstruction imaging showed stent position; (B–D) CTA images were imported into Mimics software and displayed different sections; (E,F) reconstruction images after stent implantation; (G,H) reconstructed images were segmented and modified for subsequent 3D printing; (I,J) 3D printed models after stent implantation.
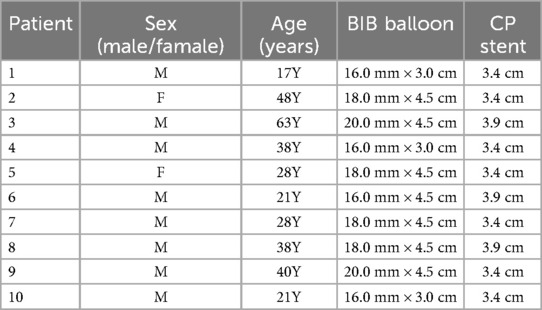
Table 1. Information about the balloon and stent used by the patient.
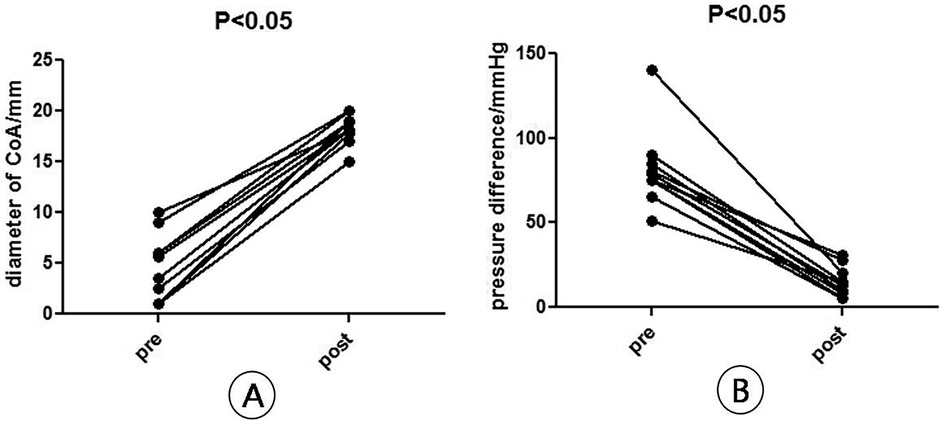
Figure 7. Diameter and pressure difference (B) at the narrowest point of arch constriction before and after surgery. (A) Diameter of CoA, n = 10, means ± SD, pre vs. post, P < 0.05; (B) pressure difference of CoA, n = 10, means ± SD, pre vs. post, P < 0.05.
DiscussionCoA refers to congenital coarctation of the aorta near the ductus arteriosus or ductal ligament, accounting for 5%–8% of congenital heart disease. Since 1991, O'Laughlin et al. (7) successfully treated one case of aortic constriction with balloon dilation stent for the first time, endovascular therapy has received widespread praise with the continuous development of interventional techniques. Interventional treatment for CoA includes balloon angioplasty (BA) and stent implantation (SI). In 2008, the American College of Cardiology and the College of Cardiology (ACC/AHA) guidelines (5) recommended interventional treatment for adult CoA, including: ① narrowing of the proximal and distal arterial peak pressure difference ≥20 mmHg, or ≤20 mmHg with significant narrowing or collateral blood flow imaging evidence; ② Recurrent or discontinuous narrowing with arterial pressure difference ≥20 mmHg. BA uses balloon dilation to expand the vascular lumen, which is simple to operate and effective, but can damage the intima and some media of the narrowed segment of the aortic wall. The use of a single BA to treat fibrous scar tissue in CoA cases can cause the narrowing segment to expand and retract, resulting in a higher incidence of restenosis (10%–15%) and the possibility of aneurysm formation. The incidence is higher in children and infants with CoA cases (11), and its application is limited. Compared with BA, SI uses intravascular stents to dilate narrowed arteries, and the continuous support of the stent on the vascular wall can effectively prevent the occurrence of restenosis. However, the problem of complications such as dissection and aneurysm caused by the injury of the aortic wall during bare stent placement still exists. For SI complications, Golden and Hellenbran (12) reviewed 565 cases of CoA stent placement from 17 institutions between 2002 and 2007, according to the Congenital Cardiovascular Interventional Study Consortium (CCISC). Among them, the pressure gradient after stent placement was less than 20 mmHg, accounting for 97% of all cases 9%. The incidence of acute complications was 81/565 (14.3%), including aneurysm formation (6/565, 1.1%), intimal tear (8/565, 1.4%), interlayer (9/565, 1.6%), bracket displacement (28/565, 5%), airbag rupture (13/565, 2.3%) and peripheral vascular complications including cerebrovascular accidents (17/565, 3.1%). Therefore, postoperative restenosis is a problem that needs to be considered. After the successful treatment of aortic aneurysm with CP stent, it was introduced into the interventional treatment of aortic constriction, effectively solving the problem of complications such as dissection and aneurysm caused by damage to the aortic wall during percutaneous balloon dilation and bare stent placement, significantly improving the safety of stent placement, and expanding the treatment indications for CoA (13). The NuMED CP stent is a novel stent system specifically designed for the treatment of aortic constriction in adolescents and adults. It belongs to the balloon expansion stent, mainly composed of NuMed BIB balloon and NuMed CP stent. BIB balloon is a dual balloon catheter (BIB catheter), and the CP stent is made of platinum material and covered with polytetrafluoroethylene membrane. The CP stent is not only beneficial for preventing restenosis, but its characteristics also include: high strength of the stent after expansion, strong plasticity of the alloy material, and small pressure on the aortic wall due to blunt edges; Large expandable range and low shortening rate; Previous balloon dilation of the stent can lead to a trumpet shaped opening at the proximal and distal ends, which can easily damage the aortic wall. Additionally, during the dilation process, the stent may move, resulting in inaccurate or displaced positioning of the stent. The BIB balloon is used to expand both the inner and outer balloons. Even with strong and high-pressure expansion, the stent will not have a small shape in the middle and a large shape at both ends, which helps with stent positioning. When the stent position is found to be unsatisfactory during internal balloon expansion, the stent position can be further adjusted to reduce the occurrence of complications. This reduces the probability of damage to blood vessels and balloons. Due to the PTFE membrane covering the stent, complications such as local aortic aneurysm or aortic dissection can be effectively avoided. At present, the CP balloon expandable CP stent has been widely used in clinical practice (6).
The results of this group of cases show that all 10 patients have successfully undergone CP stent placement surgery, and CP stents have good short-term and medium-term effects. Postoperative follow-up CTA showed good stent shape and position, and no serious complications such as stent displacement, rupture, restenosis, aortic dissection, or aneurysm were observed. CP stents pose a risk of blocking arterial blood flow perfusion in the aortic branch. Blocking the spinal artery can lead to serious neurological complications, such as paraplegia. However, the spinal artery usually originates below the level of the descending aorta diaphragm (beyond the 9th thoracic vertebra, >90% or more), so spinal artery occlusion cannot occur. In addition, when the hypoplasia of the aortic arch affects the left subclavian artery, CP stents can be used in combination with bare stents. The limitation of using CP stents for the treatment of aortic constriction is that the existing stents and pushers are 8–12 F, and the requirement for femoral artery diameter is not suitable for children. Even in older children, sheaths within this size range can cause damage to the femoral artery. Therefore, technological improvements in the delivery system will expand the applicability of stent therapy for aortic constriction. For young patients, due to the continued growth and development of the aorta, stent placement can cause relative restenosis of the stent segment. Therefore, research on biodegradable scaffolds and growable scaffolds will solve this problem. The former can gradually degrade automatically, eliminating the shortcomings of metal scaffolds that do not grow with growth and development. It can also protect damaged intima and reduce complications. The latter is composed of two symmetrical half stainless steel stents connected by absorbable wires to form a complete stent, which can be repeatedly expanded, while often requires multiple percutaneous balloon dilation or re stent placement after surgery (14). CP stent replacement is a safe and effective method for treating aortic stenosis in adolescents and adults. The short-term results are satisfactory. However, the long-term effects require long-term follow-up observation and more case studies.
The use of 3D models for individual patient model reconstruction in preoperative planning and surgical planning has been applied in structural heart disease surgery (15–18). 3D printing technology can facilitate the smooth completion of complex surgeries, especially in dealing with challenging interventional treatment cases. There have been many successful cases of using 3D printing technology to assist surgical simulation and preoperative evaluation in the cardiovascular field both domestically and internationally (19, 20). This study utilized 3D printing technology to create models of the entire heart and aorta. Prior to surgery, the patient's anatomical structure of the heart was fully understood. A 3D printing model that accurately replicated the anatomical structure of the patient's lesion site can provide a visual reference for the surgeon before surgery, and together with the results of ultrasound and CT examinations, it can be used as preoperative multimodal imaging evaluation data. At the same time, the 3D printing model is also a tool for surgical simulation, including the location and angle of the stent release, in vitro measurement and simulation of corresponding indicators, and combined with preoperative ultrasound and other imaging measurement tools to achieve the role of preoperative multimodal imaging evaluation.
This study validated the effectiveness and safety of interventional treatment for CoA, while also reflecting the role of 3D printing technology in assisting preoperative and postoperative imaging evaluation. With the rapid development of imaging and computer technology, the accuracy and simulation level of cardiovascular 3D printing are constantly improving. The 3D printing technology compensates for the shortcomings of traditional imaging technology in displaying complex structures, especially 3D reconstruction, which enables cardiovascular physicians to have a more intuitive understanding of the morphology and structure of disease changes, bringing new ideas for the precise treatment of heart disease patients (21–23).
Study limitationsTo gain a better insight into the auxiliary role of 3D printing technology in CoA disease, population-based multicenter case-control studies with a prospective design are needed.
ConclusionsTo sum up, the mastery of indications and the clinical experience of the surgeon are very important for the success of the operation as well as the role of 3D printing technology in assisting preoperative and postoperative imaging evaluation. Percutaneous balloon dilatation covered stent implantation for CoA in adolescents and adults has a definite short-term and medium-term effect, high success rate and few related complications. The long-term effect needs further study.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by The study protocol was approved by the ethics committee of Xijing Hospital (KY20150205). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsQH: Conceptualization, Data curation, Formal Analysis, Validation, Investigation, Methodology, Supervision, Project administration, Software, Visualization, Writing – review & editing. CX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. YL: Funding acquisition, Supervision, Validation, Writing – review & editing. PJ: Supervision, Validation, Writing – review & editing. MZ: Validation, Writing – review & editing. ZT: Visualization, Writing – review & editing. RQ: Writing – review & editing, Visualization. BC: Visualization, Writing – review & editing. JY: Writing – review & editing, Funding acquisition, Project administration, Supervision.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Key Research and Development Programs (2022YFC2503402), National Natural Science Foundation of China (82370375), Shaanxi Provincial Key Research and Development Plan (2023-YBSF-105), the 74th General Project of China Postdoctoral Science Foundation (2023M744296), Youth Project of Shaanxi Provincial Natural Science Basic Research Program (2024JC-YBQN-0793) and Start-up funding for postdoctoral research of Air Force Medical University (qd20230216).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Wu MH, Chen HC, Lu CW, Wang JK, Huang SC, Huang SK. Prevalence of congenital heart disease at live birth in Taiwan. J Pediatr. (2010) 156(5):782–5. doi: 10.1016/j.jpeds.2009.11.062
PubMed Abstract | Crossref Full Text | Google Scholar
3. Gray WH, Wells WJ, Starnes VA, Kumar SR. Arch augmentation via median sternotomy for coarctation of aorta with proximal arch hypoplasia. Ann Thorac Surg. (2018) 106(4):1214–9. doi: 10.1016/j.athoracsur.2018.04.025
PubMed Abstract | Crossref Full Text | Google Scholar
4. Suzuki R, Mikamo A, Matsuno Y, Fujita A, Kurazumi H, Okada M, et al. Endovascular treatment of intercostal artery aneurysm associated with aortic coarctation. Ann Thorac Surg. (2019) 108(1):e51–2. doi: 10.1016/j.athoracsur.2019.01.052
PubMed Abstract | Crossref Full Text | Google Scholar
7. Hamdan A, Nassar M, Monakier D, Assali A, Shafir G, Vaknin-Assa H, et al. Differentiating primary from secondary hypertrophy based on the coronary lumen volume to myocardial mass relationship. JACC Cardiovasc Imaging. (2018) 11(12):1926–8. doi: 10.1016/j.jcmg.2018.06.012
PubMed Abstract | Crossref Full Text | Google Scholar
8. Polanczyk A, Podgorski M, Wozniak T, Stefanczyk L, Strzelecki M. Computational fluid dynamics as an engineering tool for the reconstruction of hemodynamics after carotid artery stenosis operation: a case study. Medicina. (2018) 54(3):42. doi: 10.3390/medicina54030042
PubMed Abstract | Crossref Full Text | Google Scholar
9. Hangge P, Pershad Y, Witting AA, Albadawi H, Oklu R. Three-dimensional (3D) printing and its applications for aortic diseases. Cardiovasc Diagn Ther. (2018) 8(Suppl 1):S19–25. doi: 10.21037/cdt.2017.10.02
PubMed Abstract | Crossref Full Text | Google Scholar
10. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol. (2008) 52(23):e143–263. doi: 10.1016/j.jacc.2008.10.001
PubMed Abstract | Crossref Full Text | Google Scholar
11. Parra-Bravo JR, Reséndiz-Balderas M, Francisco-Candelario R, García H, Chávez-Fernández MA, Beirana-Palencia LG, et al. Angioplastía con balón de la coartación aórtica nativa en niños menores de 12 meses: resultado inicial y a mediano plazo [Balloon angioplasty for native aortic coarctation in children younger than 12 months: immediate and medium-term results]. Arch Cardiol Mex. (2007) 77(3):217–25. Spanish.18050934
PubMed Abstract | Google Scholar
13. Bruckheimer E, Dagan T, Amir G, Birk E. Covered cheatham-platinum stents for serial dilation of severe native aortic coarctation. Catheter Cardiovasc Interv. (2009) 74(1):117–23. doi: 10.1002/ccd.21923
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ewert P, Peters B, Nagdyman N, Miera O, Kühne T, Berger F. Early and mid-term results with the growth stent–a possible concept for transcatheter treatment of aortic coarctation from infancy to adulthood by stent implantation? Catheter Cardiovasc Interv. (2008) 71(1):120–6. doi: 10.1002/ccd.21397
PubMed Abstract | Crossref Full Text | Google Scholar
15. Moore RA, Riggs KW, Kourtidou S, Schneider K, Szugye N, Troja W, et al. Three-dimensional printing and virtual surgery for congenital heart procedural planning. Birth Defects Res. (2018) 110(13):1082–90. doi: 10.1002/bdr2.1370
PubMed Abstract | Crossref Full Text | Google Scholar
16. Olivieri LJ, Krieger A, Loke YH, Nath DS, Kim PC, Sable CA. Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: feasibility and relative accuracy. J Am Soc Echocardiogr. (2015) 28(4):392–7. doi: 10.1016/j.echo.2014.12.016
PubMed Abstract | Crossref Full Text | Google Scholar
17. Sun Z, Lee SY. A systematic review of 3-D printing in cardiovascular and cerebrovascular diseases. Anatol J Cardiol. (2017) 17(6):423–35. doi: 10.14744/AnatolJCardiol.2017.7464
PubMed Abstract | Crossref Full Text | Google Scholar
18. Luo H, Meyer-Szary J, Wang Z, Sabiniewicz R, Liu Y. Three-dimensional printing in cardiology: current applications and future challenges. Cardiol J. (2017) 24(4):436–44. doi: 10.5603/CJ.a2017.0056
PubMed Abstract | Crossref Full Text | Google Scholar
19. Olivieri L, Krieger A, Chen MY, Kim P, Kanter JP. 3D Heart model guides complex stent angioplasty of pulmonary venous baffle obstruction in a mustard repair of D-TGA. Int J Cardiol. (2014) 172(2):e297–8. doi: 10.1016/j.ijcard.2013.12.192
PubMed Abstract | Crossref Full Text | Google Scholar
20. Ripley B, Levin D, Kelil T, Hermsen JL, Kim S, Maki JH, et al. 3D printing from MRI data: harnessing strengths and minimizing weaknesses. J Magn Reson Imaging. (2017) 45(3):635–45. doi: 10.1002/jmri.25526
PubMed Abstract | Crossref Full Text | Google Scholar
21. Hadeed K, Acar P, Dulac Y, Cuttone F, Alacoque X, Karsenty C. Cardiac 3D printing for better understanding of congenital heart disease. Arch Cardiovasc Dis. (2018) 111(1):1–4. doi: 10.1016/j.acvd.2017.10.001
PubMed Abstract | Crossref Full Text | Google Scholar
22. Iriart X, Ciobotaru V, Martin C, Cochet H, Jalal Z, Thambo JB, et al. Role of cardiac imaging and three-dimensional printing in percutaneous appendage closure. Arch Cardiovasc Dis. (2018) 111(6–7):411–20. doi: 10.1016/j.acvd.2018.04.005
PubMed Abstract | Crossref Full Text | Google Scholar
23. Yoo SJ, Spray T, Austin EH 3rd, Yun TJ, van Arsdell GS. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg. (2017) 153(6):1530–40. doi: 10.1016/j.jtcvs.2016.12.054
留言 (0)