Antibiotics were used to cure bacterial infections in the past (Muñoz et al., 2024). However, with the decline of antibiotic discovery and the evolution of drug resistance, current antibiotic resistance has become a public health crisis (Hutchings et al., 2019). Therefore, it is urgent to explore the mechanism of antibiotic resistance and find a way to eliminate drug resistance in bacteria (Aleksandrowicz et al., 2023).
It is found that certain combinations of Chinese herbal extracts and antibiotics exhibit synergistic effects against E. coli through distinct mechanisms (Porras et al., 2021). For instance, Quercetin could let E. coli regain susceptibility to colistin by enhancing its destructive effects by destroying the cell membrane of E. coli (Lin et al., 2021). Artesunate enhanced the inhibitory effect of various β-lactam antibiotics against MDR E. coli by inhibiting the expression of efflux pump genes (Wei et al., 2020). Magnolol enhanced the sensitivity of MDR E. coli to cefquinome and reversed the resistance of MDR E. coli (Tong et al., 2023b).
Kaempferol belongs to flavonoids, which can be found in a variety of herbs. Besides anticarcinogenic and anti-inflammatory effects, kaempferol and its extensions also show antibacterial, antifungal, and antiprotozoal effects (Periferakis et al., 2022). In addition, kaempferol has excellent anti-diabetic effects (Yang et al., 2022) and neuroprotective effects (Chang et al., 2022). The previous research showed that some kaempferol derivatives had inhibitory effects on E. coli biofilm (Periferakis et al., 2022), and they could also destroy the integrity of bacterial cell membranes (Lin et al., 2020).
This study aimed to explore the mechanism of kaempferol restoring the sensitivity of ESBLs E. coli to ceftiofur, focusing on the biofilm formation and β-lactamase, and the therapeutic effect in vivo was also studied.
2 Results 2.1 Ceftiofur and kaempferol susceptibility testingTo explore the antimicrobial activity of Ceftiofur and Kaempferol, the Minimal Inhibitory Concentration (MIC) of 6 ESBLs E. coli isolates and ATCC®25922™ were detected. The result showed that all 6 ESBLs E. coli isolates were resistant to ceftiofur (Table 1).
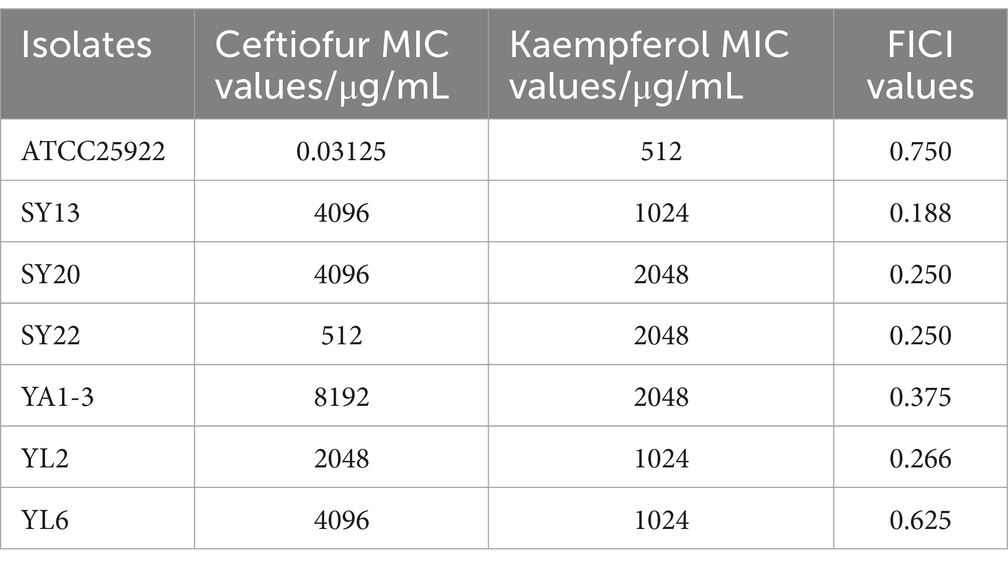
Table 1. MIC values and FICI values of Ceftiofur and kaempferol to ATCC® 25922TM and 6 ESBLs E. coli isolates.
2.2 Synergistic of Kaempferol with ceftiofur against ESBLs Escherichia coliTo evaluate the potential synergistic effect of kaempferol with ceftiofur against ESBLs E. coli, checkerboard assays were performed. As shown in Table 1 and Figure 1, the rate of synergistic effects was 83.33%. The rate of additive effects was 16.67%. Notably, the FICI value of ATCC®25922™ ≤ 0.75, indicating that kaempferol combined with ceftiofur inhibited ESBLs E coli partially synergistically. Compared with monotherapy, the dose of ceftiofur in combination treatment was 4 to 64 lower, which suggested that kaempferol eliminated the resistance of ESBLs E. coli to ceftiofur.
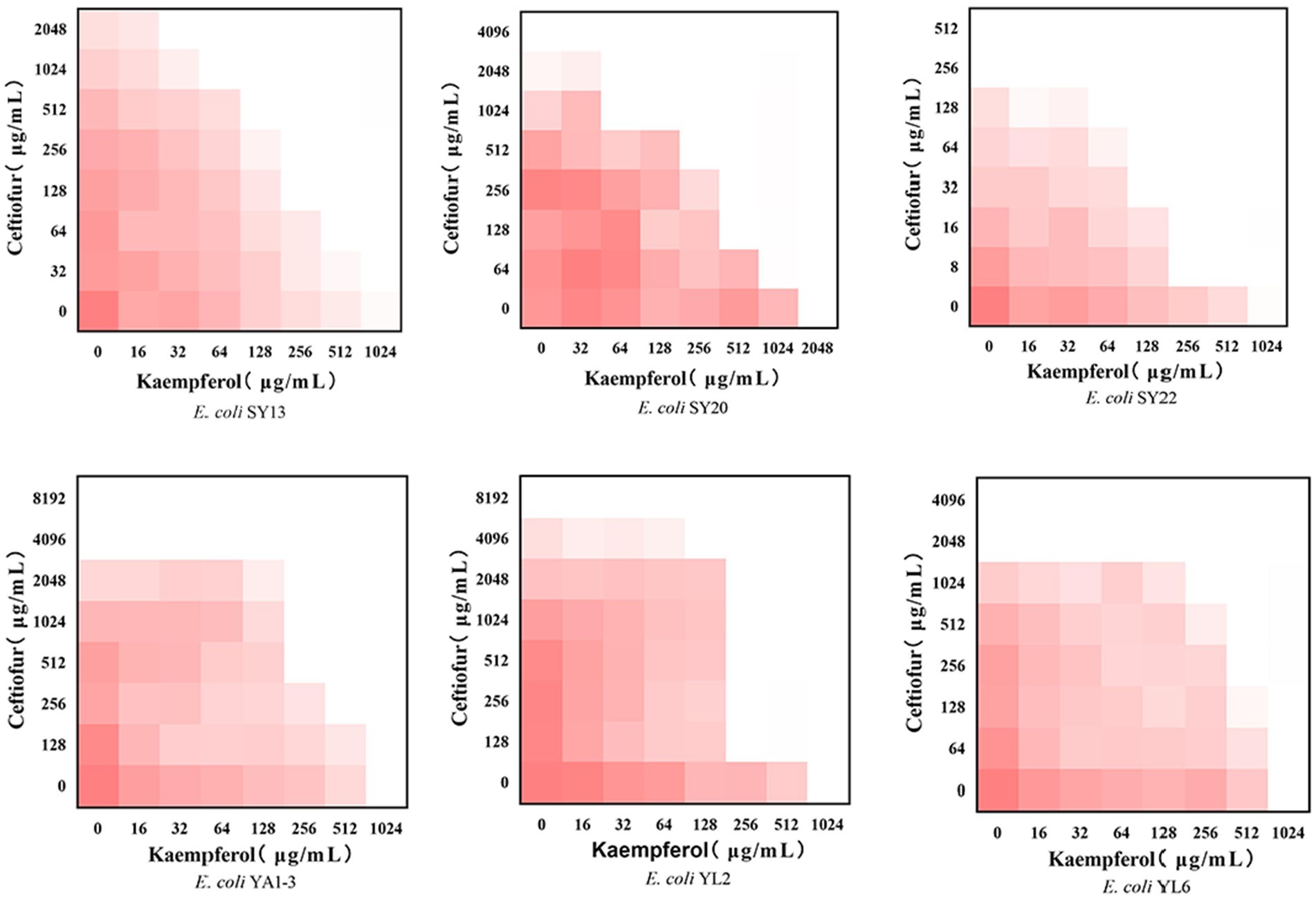
Figure 1. Checkerboard broth assays for kaempferol and ceftiofur against 6 ESBLs Escherichia coli strains. Dark red regions represent higher bacterial cell density.
2.3 Kaempferol enhances ceftiofur efficacy and minimize the emergence of resistanceAlthough the checkerboard test showed kaempferol to enhance ceftiofur, direct tests of synergistic inhibitory effects and synergistic bactericidal activity may reinforce these findings. Based on the results of MIC and FIC assays, three strains of ESBLs E. coli (SY13, SY20, SY22) were choosed for the next test.
The growth curves were performed to analyze the inhibitory effects of the combination of kaempferol and ceftiofur against ESBLs E. coli. As shown in Figure 2A, compared with the CEF group and the KAE group, the CEF + KAE group showed better effects on inhibiting the growth of ESBLs E. coli within 2 to 24 h. The results suggested that the combination of kaempferol and ceftiofur may be an ideal bacterial-inhibiting combination.
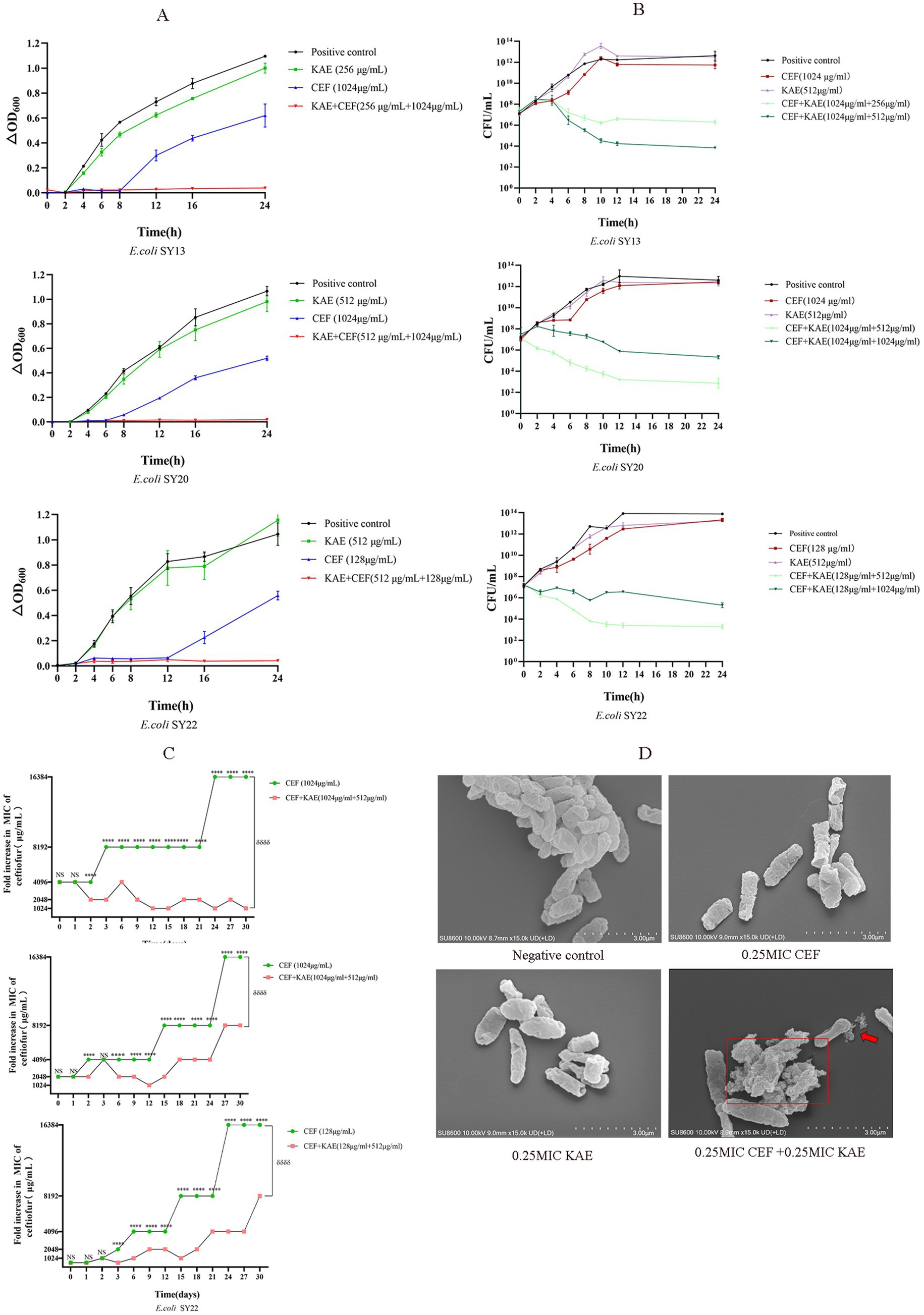
Figure 2. Kaempferol Enhances Ceftiofur Efficacy and Minimize the Emergence of Resistanc. (A). The growth curves. (B). Time-dependent killing. (C). The addition of kaempferol (0.25MIC) prevents the evolution of ceftiofur resistance to E. coli. (D). Morphological changes of ESBLs E. coli (SY20) with different treatments under SEM.
The time-kill curves were performed to evaluate the bactericidal effect of the combination of kaempferol and ceftiofur against ESBLs E. coli. As shown in Figure 2B, compared with the KAE group and the CEF group, at all concentrations tested, the combination of kaempferol and ceftiofur exhibited an enhanced bactericidal effect against the three tested ESBLs E. coli strains within 24 h. After 12 h treatment, the differences in the total bacteria counts between the KAE + CEF group (the low concentration group) and the CEF group reached 105- to 107-fold, and this gap lasted for 12 h. Notably, the differences in the population between the low-concentration KAE + CEF group and the high-concentration KAE + CEF group reached 102- to 103-fold from 8 to 24 h, which indicated that the bactericidal effect appeared to be kaempferol dose-dependent.
Scanning Electron Microscopy (SEM) was used to observe the morphological changes of ESBLs E. coli (SY20) directly. As shown in Figure 2D, the surface of cells in the KAE + CEF group showed depression, shrinkage, and even rupture, and lysis, indicating that the ability of ceftiofur to disrupt cell surface structures could be enhanced by kaempferol.
Finally, to understand the role of kaempferol in the development of ceftiofur resistance, we performed serial passages of ESBLs E. coli with ceftiofur (0.25 MIC) in the presence and absence of kaempferol (0.25 MIC) during 30 d. As shown in Figure 2C, the growth of the ESBLs E. coli resistance in the KAE + CEF group was significantly lower than that in the CEF group (p < 0.001), this indicates that kaempferol can slow down the development of ESBLs E. coli resistance to ceftiofur.
In conclusion, kaempferol can enhance the antibacterial and bactericidal effects of ceftiofur and minimize the emergence of ESBLs E. coli Resistance to ceftiofur.
2.4 Kaempferol enhances the biofilm-damaging ability of ceftiofurConsidering the anti-biofilm activity of kaempferol and ESBLs E. coli developed ceftiofur-resistance by producing β-lactamase. We speculated that kaempferol restored bacterial sensitivity to ceftiofur by affecting biofilm formation, quorum sensing, and β-lactamase activity.
Biofilm formation of ESBLs E. coli was measured by crystal violet staining. As shown in Figure 3A, the absorbance of OD570 in the CEF + KAE group was significantly lower than in the monotherapy groups (p < 0.0001). The high-level kaempferol (0.5 MIC) combined with the same level of ceftiofur exhibited the same destructive effect on biofilm formation as the low-level group. The above results suggested that the combination of kaempferol and ceftiofur affects the biofilm formation in ESBLs E. coli.
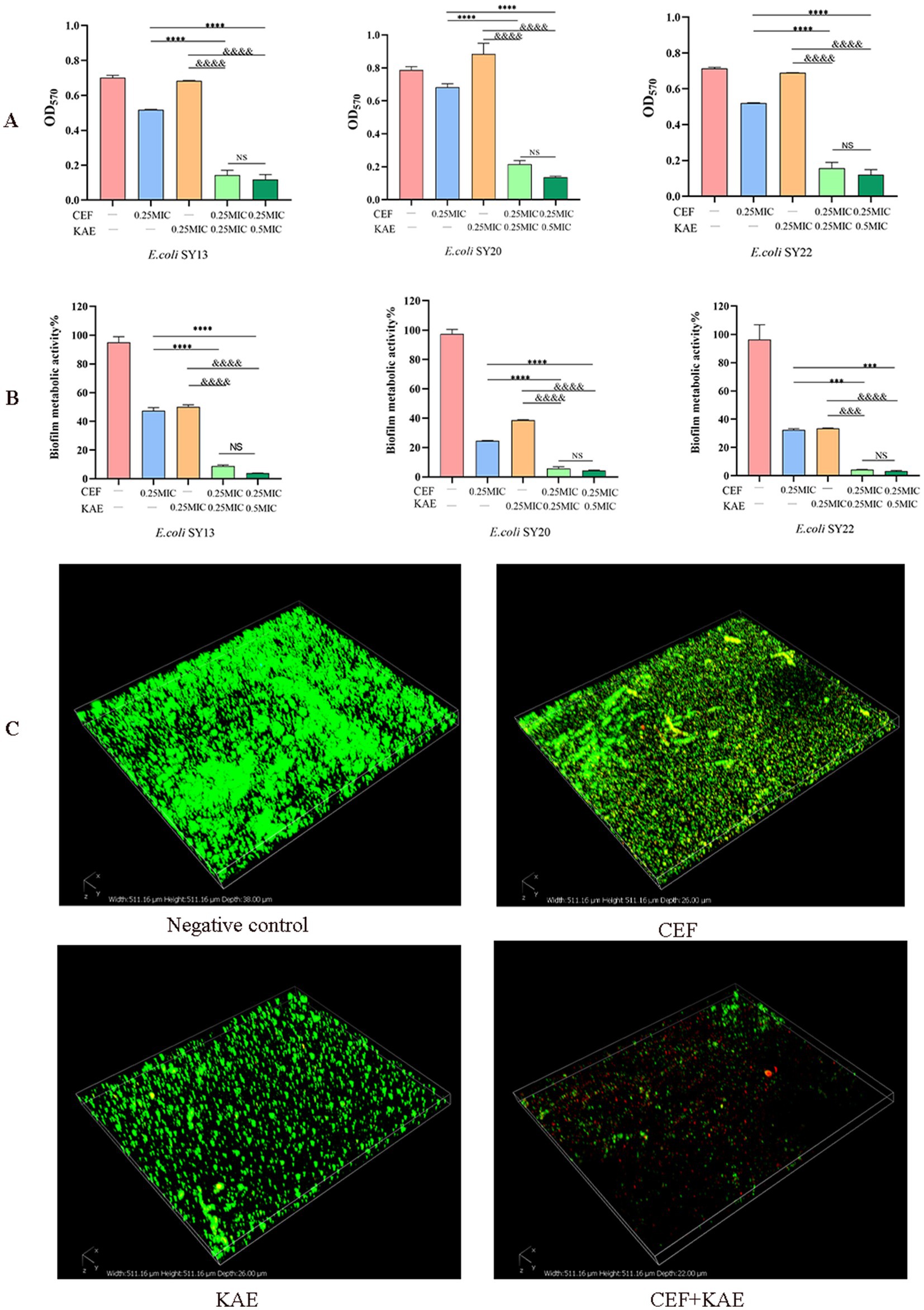
Figure 3. Kaempferol enhances the biofilm-damaging ability of Ceftiofur. (A) The absorbance at OD570 of crystal violet staining of ESBLs E. coli treated with Kaempferol (KAE) and Ceftiofur (CEF) in different combinations or levels. (B) The absorbance at OD570 of MTT staining of ESBLs E. coli treated with kaempferol (KAE) and ceftiofur (CEF) in different combinations or levels. (C) Biofilm of ESBLs E. coli (SY20) after different treatments, dead ESBLs E. coli in the biofilm were stained red by PI, and all the ESBLs E. coli were stained green by SYTO9. CEF: ceftiofur; KAE: kaempferol.
To further investigate the effect of different treatments on the metabolic activity of biofilm cells, the Methyl thiazolyl tetrazolium (MTT) colorimetric method was performed. As shown in Figure 3B, the absorbance of OD570 in the CEF + KAE group was significantly (p < 0.0001) lower than in the monotherapy groups. The above results suggested that the combination of kaempferol and ceftiofur could affect the metabolic activity of biofilm.
To observe the destruction of different treatments on biofilm, Confocal Laser Scanning Microscopy (CLSM) was performed to evaluate the changes in biofilm activity (Figure 3C). Compared with the monotherapy group and negative control, ceftiofur combined with kaempferol killed a large population of ESBLs E. coli (SY20) and showed severe shedding of biofilm. Considering the phenomenon of SY20 shedding in the CEF + KAE group, we speculated that the combination of kaempferol and ceftiofur might affect E. coli adhesion and aggregation.
To further explore whether kaempferol affected the ESBLs E. coli (SY20) aggregation, we studied from three aspects: motility, adhesion, and surface characteristics.
The motility of SY20 was assessed in the presence and absence of kaempferol by measuring the diameters of the swarming and swimming zones. Compared with the CEF group, the CEF + KAE group significantly prevented the swarming motility (p < 0.05; Figure 4A) and swimming motility (p < 0.001; Figure 4B) of ESBLs E. coli.
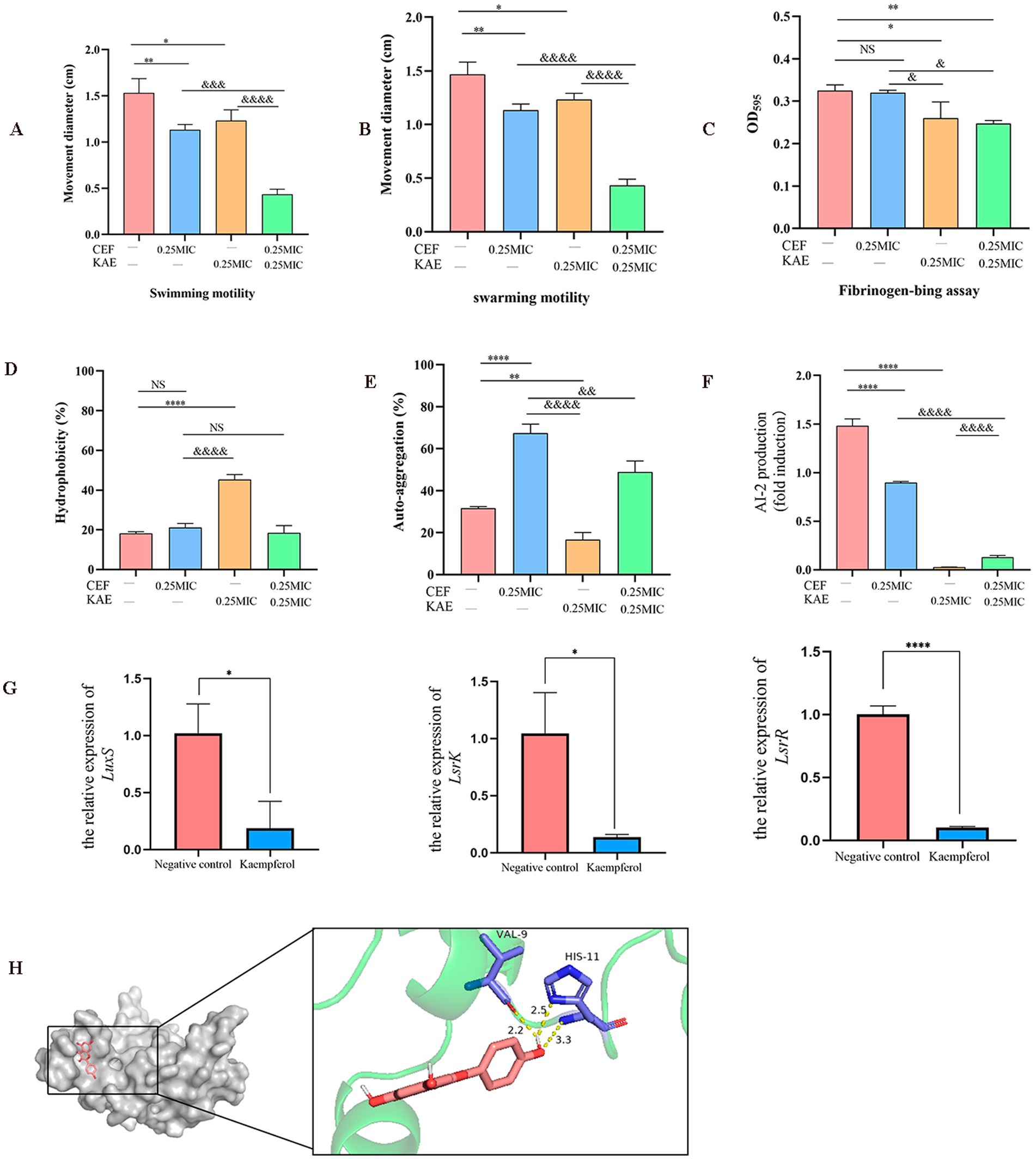
Figure 4. Using ESBLs E. coli (SY20) as an example, the effects of kaempferol on the adhesion, aggregation, and LuxS/AI-2 during the formation of Escherichia coli biofilm were measured. (A). Swimming motility. (B). Swarming.motility. (C). Fibrinogen-bing assay. (D). Hydrophobicity. (E). Self-aggregation. (F). Measurement of AI-2 activity using bioluminescence assay. (G). The relative expression of LuxS, LsrR, and LsrK by RT-qPCR. (H). Molecular docking of kaempferol and LuxS protein.
Then we test the effect of kaempferol on SY20 adhesion by fibrinogen-bing assay. Compared with the CEF group, the CEF + KAE group significantly reduced the adhesion ability of SY20 to the fibrinogen (p < 0.05; Figure 4C), the results showed that kaempferol may affect the attachment phase of biofilm formation by reducing the SY20 adhesion.
The surface characteristics (hydrophobicity and self-aggregation) of SY20 were tested to explore their relationship with aggregation in ESBLs E. coli. The hydrophobicity of E. coli can regulate their adhesion on diverse surfaces, compared with the CEF control, the KAE group significantly enhanced the hydrophobicity of SY20 (p < 0.0001), but there was no significant difference between this group and CEF + KAE group (p > 0.05; Figure 4D). The self-aggregation of E. coli contributes to the improvement of bacterial biofilm morphology. In the present study, we found that under the stress of ceftiofur, the self-aggregation ability of SY20 was significantly enhanced, while kaempferol could reduce this stress (Figure 4E).
On the other hand, LuxS/AI-2 QS as the key to regulating the biofilm formation and motility of E. coli were also considered. Compared with the group without kaempferol treatment, the AI-2 activities within the supernatant of biofilms of ESBLs E. coli with kaempferol treatment were significantly decreased (p < 0.001; Figure 4F). Meanwhile, the relative expression levels of the group than in the Negative control group. Finally, We found that kaempferol can bind tightly to the two residues of VAL-9 and HIS-11 in the active center of LuxS protein by forming three hydrogen bonds (Binding energy as −8.422 kcal/mol) (Figure 4H).
2.5 Kaempferol inhibits the blaCTX-M-27 protein activityConsidering ESBLs E. coli developed ceftiofur resistance by producing β-lactamase, we speculated that kaempferol restored bacterial sensitivity to ceftiofur by inhibiting the β-lactamase activity.
RT-qPCR was used to simulate the effect of kaempferol on the expression level of the blaCTX-M-27 gene and investigated the interaction between kaempferol and beta-lactamase active centers through molecular docking (Figure 5B). The result showed that kaempferol can reduce the relative expression of blaCTX-M-27 and bind tightly to the active center of blaCTX-M-27 (Binding energy as −8.425 kcal/mol). Therefore, nitrocefin tests were used to detect the effect of kaempferol on blaCTX-M-27 protein activity. The result showed that kaempferol significantly (p < 0.05) inhibited the hydrolytic activity of blaCTX-M-27 protein (Figure 5C). These data suggest that kaempferol has the potential to enhance cefotifo against ESBLs by inhibiting β-lactamase activity.
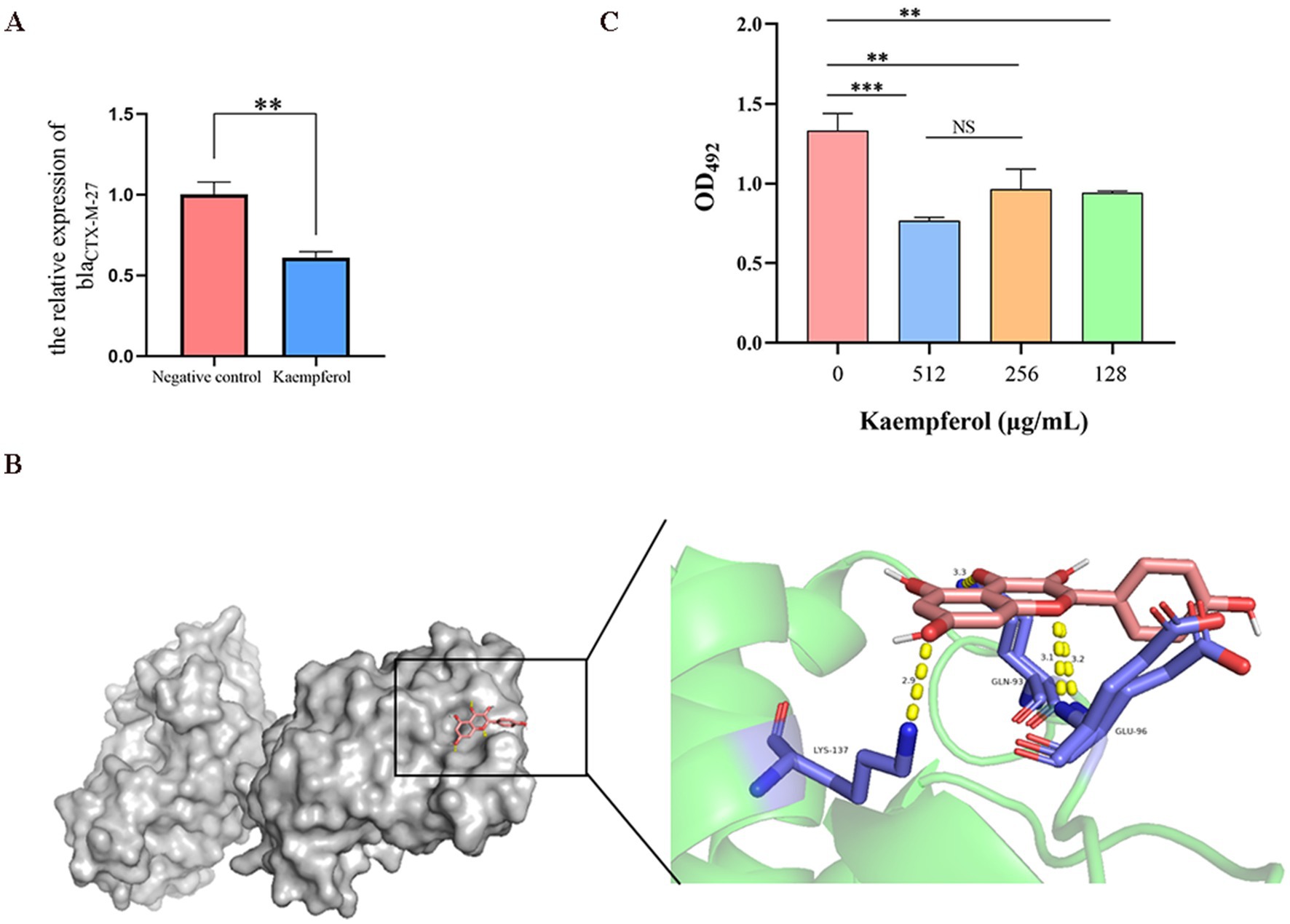
Figure 5. Kaempferol inhibits the blaCTX-M-27 proteion activity of ESBLs E. coli. (A). The relative expression of blaCTX-M-27 by RT-qPCR. (B). Molecular docking of kaempferol and blaCTX-M-27 protein. (C). The effect of kaempferol on protein activity was determined by nitrocefin test.
2.6 Transcriptome analysis was further verifiedThe comparison of treatment with a combination of ceftiofur alone revealed an up-regulation of 331 genes and a down-regulation of 442 genes (Figure 6A). KEGG (Figure 6B) and Go (Figure 6C) enrichment analysis showed that these differentially expressed genes (DEGs) were involved in pathways related to E. coli resistance, such as β-lactam resistance, biofilm formation, and quorum sensing, this is consistent with our previous speculation that kaempferol with ceftiofur plays a synergistic role by affecting the biofilm formation and β-lactamase activity (Figure 6D). KEGG enrichment (Figure 6B) analysis showed that KAE + CEF affected multiple metabolic pathways, including Glyoxylate and dicarboxylate metabolism, Alanine, aspartate and glutamate metabolism, Glycine, serine and threonine metabolism, Arginine and proline metabolism etc. Go enrichment analysis oxidation–reduction process, cellular protein metabolic process etc., suggests that kaempferol may restore E. coli sensitivity to ceftiofur by influencing E. coli metabolic indications, and it provides a new direction for our future research.
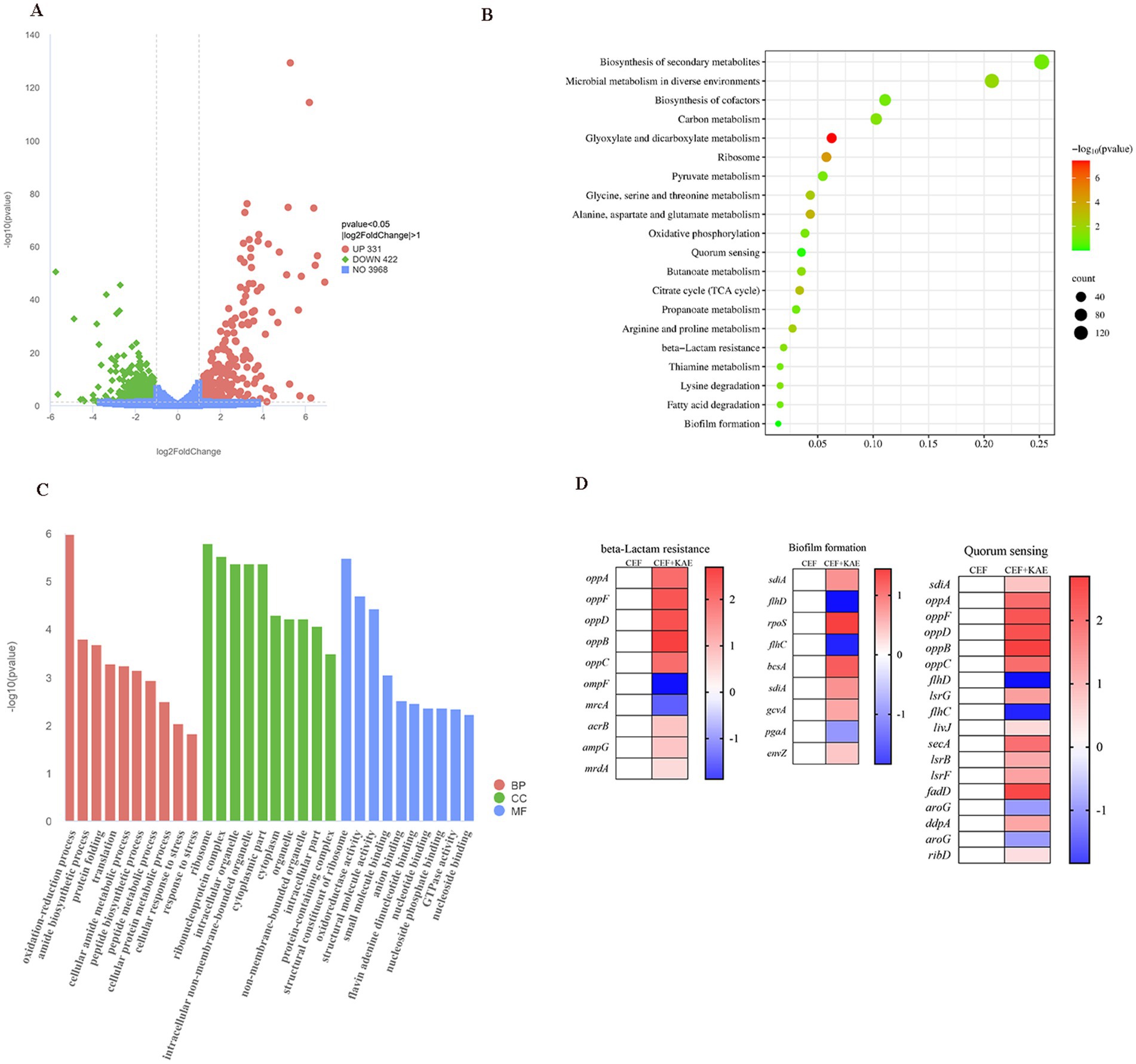
Figure 6. Transcriptome analysis of E. coli SY20 after exposure to ceftiofur alone or the combination of ceftiofur and kaempferol. (A). Volcano plot. (B). KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis of the DEGs in E. coli SY20. (C). GO (Gene Ontology) annotation analysis of the DEGs in E. coli SY20. (D). Selected differential expression genes related to E. coli drug resistance involved in Biofilm formation, Quorum sensing system, and β-lactamase activity.
Collectively, transcription analyses of E. coli (SY20) further indicated our previous study that kaempferol enhanced the inhibitory and killing effect of ceftiofur on ESBLs E. coli by influencing AI-2 Quorum sensing system, biofilm formation, and β-lactamase activity.
2.7 Kaempferol restores ceftiofur activity in vivoConsidering the excellent synergistic bactericidal activity of the combination of Kaempferol and Ceftiofur against E. coli in vitro, we reasoned that kaempferol would reverse ceftiofur resistance in vivo and thus recover its clinical efficacy. To confirm this, a mouse intestinal inflammation model infected with E. coli (SY20) was constructed and used for this speculation. There was no significant difference between the COM group and the CEF group (p > 0.05), but the COM group obtained a survival benefit trend than the CEF group (Figure 7A). The COM group significantly reduced intestinal bacterial load than the CEF group (Figure 7B). Histopathology damage in mice with SY20 challenge was alleviated, as manifested by the higher villus height (Figure 7C) and crypt depth (Figure 7D) in the COM group and KAE group than in the CEF group, indicating that the integrity of intestinal villi was protected by kaempferol, but there was no significant difference in codling ratio between CEF group and KAE group (Figure 7E).
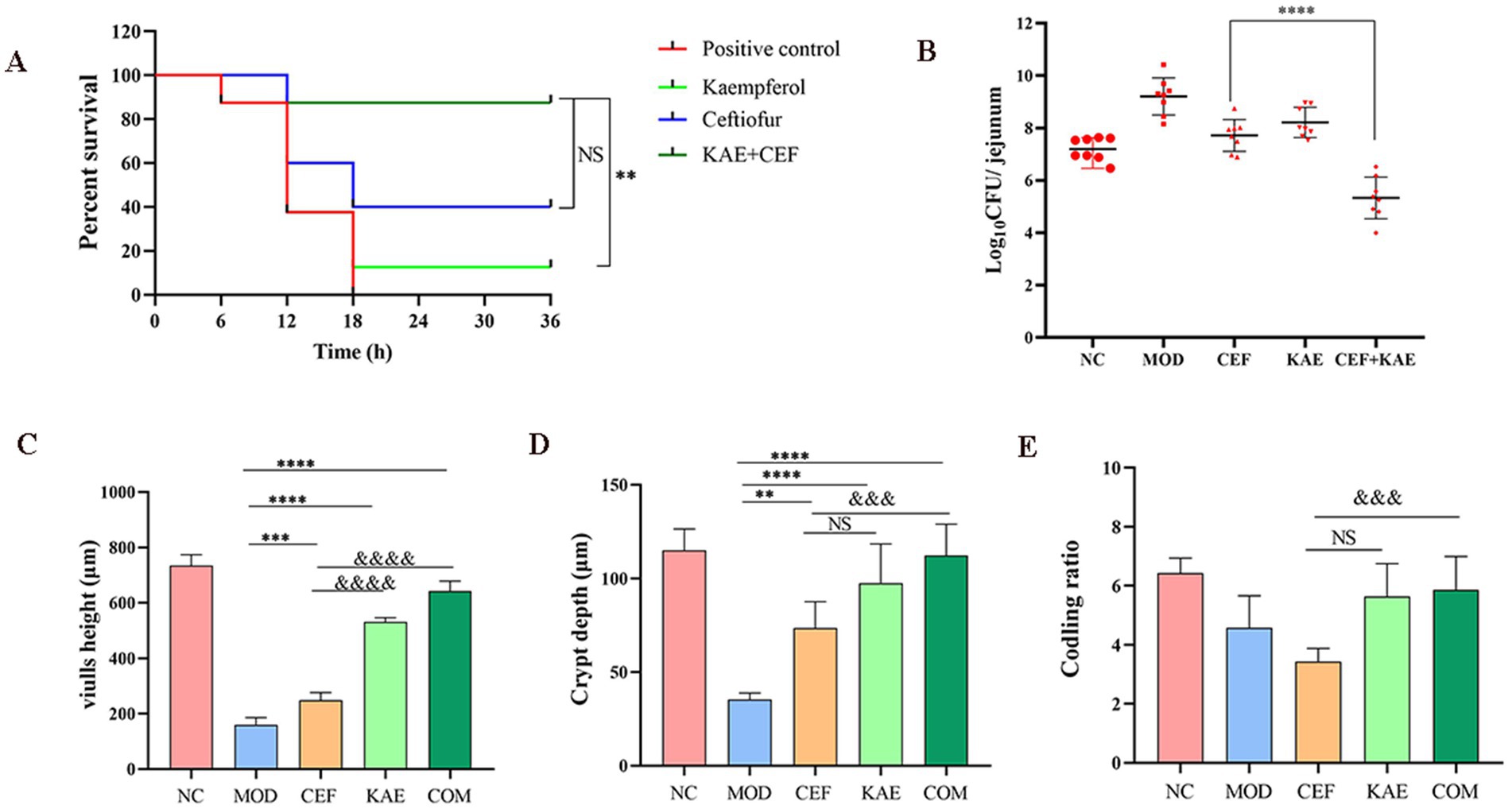
Figure 7. Kaempferol rescues ceftiofur activity in vivo. (A). Survival rates. (B). Intestinal bacterial load. (C). Length of villi in the small intestine. (D). Crypt depth. (E). Coding ratio. NC: Negative control; MOD: model group; CEF: ceftiofur group; KAE: kaempferol group; COM: combination group.
As shown in Figure 8A, the jejunum villus was broken, and fragmentation occurred in the CEF group and MOD group. Unexpectedly, compared with the CEF group, the expression of NF-κB p65 proteins (Figure 8B), TLR proteins (Figure 8C), and MYD88 proteins (Figure 8D) were significantly reduced in the COM group. It is showed that kaempferol combined with ceftiofur exerted anti-inflammatory activity by affecting the NF-κB/TLR pathway (Figure 8A).
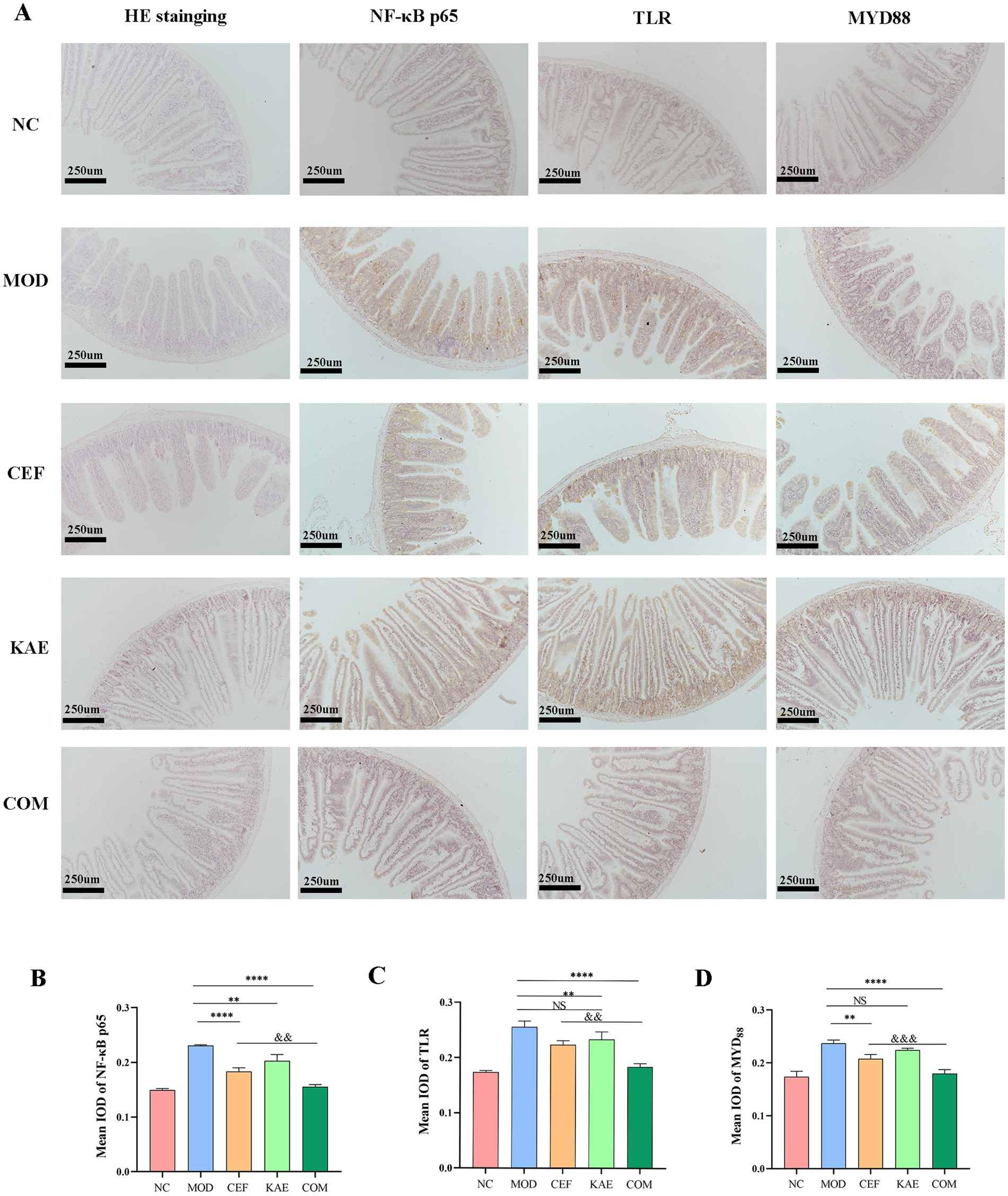
Figure 8. The distribution and expression analysis of inflammatory proteins and morphological changes in mice jejunum under different treatments. (A). HE staining and Immunohistochemistry. (B). The mean IOD of NF-κB. (C). The mean IOD of TLR. (D). The mean IOD of MYD88. NC:Negative control; MOD: model group; CEF: ceftiofur group; KAE: kaempferol group; COM: combination group.
In conclusion, kaempferol fully restores the activity of ceftiofur in mous infection models by relieving TLR4/NF- κB pathway.
3 DiscussionIn the prevailing epoch, antibiotic resistance is one of the critical threats (Hutchings et al., 2019). Despite the notion that ceftiofur has been widely recognized as one of the critical clinical antibiotics against E. coli infection, its clinical efficacy has greatly decreased due to the increasing resistance of E. coli. Therefore, the identification of potent adjuvants is of great importance (Wright, 2016).
As a previous study showed, kaempferol is a potential anti-inflammatory, antioxidant, and antibacterial compound (Chagas et al., 2022). Additionally, kaempferol can also inhibit the primary attachment phase of Staphylococcus aureus biofilm formation (Ming et al., 2017). In this study, kaempferol showed synergistic inhibitory against ESBLs E. coli combined with ceftiofur, which is the first report about the antibacterial effect of this combination against ESBLs E. coli.
The biofilm plays an important role in the formation of drug resistance in bacteria (Ciofu et al., 2022). Some previous studies found that mature biofilms can reduce the susceptibility of bacteria to ceftiofur (Ster et al., 2017). For example, the non-Neisseria gonorrhoeae suffered a lower survival rate than the aggregated Neisseria gonorrhoeae (Wang L C, et al., 2018), as the biofilm plays a key role in bacterial aggregation (Arnaouteli et al., 2021). In another previous study, kaempferol showed the ability to inhibit the initial attachment stage of Staphylococcus aureus biofilm formation by reducing the expression of adhesion-related genes (Chagas et al., 2022). As a result of that, we hypothesized that kaempferol could enhance the bacteriostatic effect of ceftiofur on E. coli by inhibiting the formation of biofilm. To study the possible mechanism of the antibacterial effect of kaempferol combined with ceftiofur, we evaluated the biofilm destruction activity of the combination. After being treated with kaempferol and ceftiofur together, the biofilm was significantly damaged, and its activity was inhibited. Furthermore, the effects of kaempferol on the adhesion and aggregation of E. coli in the initial stage were evaluated, the results showed found that the motility, adhesion, and self-aggregation of ESBLs E. coli were inhibited by the combination of ceftiofur and kaempferol, while kaempferol can increase the surface hydrophobicity of ESBLs E. coli when used alone, but the combination has no effect, this confirms our previous speculation. The effect of biofilm formation may be one of the reasons for the antibacterial effect of kaempferol combined with ceftiofur.
Quorum sensing (QS) system plays an important role in various bacterial processes, including drug resistance and biofilm formation, and it can help bacteria adapt to the external environment (Wang et al., 2023). AI-2 is a QS signal that mediates communication within and between many bacterial species (Wang et al., 2022), and the LuxS is a synthase involved in the synthesis of AI-2 (Wang Y, et al., 2018), inhibited the expression of the LuxS gene can affect the formation of biofilm in E. coli (Zong et al., 2024; Yu et al., 2021), the LuxS /AI-2 QS system of E. coli has been shown to regulate the formation of biofilms (Bai et al., 2022), it provides a new direction for inhibiting biofilms. Considering the anti-biofilm function of kaempferol, we speculate that kaempferol exerts its anti-biofilm effect by regulating the AI-2 QS system. So we measured the relative expression of LuxS /AI-2 system-related genes by RT-qPCR, including luxS, LsrR, and LsrK, LsrR, and LsrK regulate AI-2 uptake, playing a key role in the processing of AI-2, reducing the expression of LsrK and LsrR can inhibit the formation of AI-2, and then affect biofilm formation (Zuberi et al., 2017). Used Molecular Docking to confirm the interaction relationship between kaempferol and LuxS protein active center, and then we tested the effect of kaempferol on the molecular weight of the AI-2 signal. As expected, it is found that the kaempferol inhibited the AI-2 QS system and reduced the molecular weight of the AI-2 signal in ESBLs E. coli. Considering the anti-biofilm activity of kaempferol and its inhibition of LuxS/AI-2 QS, kaempferol might exert a synergistic effect on other antibiotics, more specific research needs to be conducted in the future.
Furthermore, ESBLs E. coli produce β-lactamases that hydrolyze β-lactam rings, thereby inactivating the drug, which is one of the main causes of resistance to β-lactams (Zhu et al., 2013; Nasrollahian et al., 2024). This study also pays attention to the effect of kaempferol on β-lactamases, and the isolates SY20 were blaCTX-M-27 of β-lactamases used. The result shows that kaempferol can reduce the relative expression of the blaCTX-M-27 gene, tightly bound to the active center of the blaCTX-M-27 protein, inhibit the hydrolytic activity of blaCTX-M-27 protein, this provides a new direction for our future research.
Previous studies (Qu et al., 2021) have shown that kaempferol can restore intestinal microbiota, and this study found that the bacterial load of the kaempferol group was lower than that of the ceftiofur group and the model group was also preliminarily verified. Besides, we found that kaempferol has a strong protective effect on intestinal villi, considering the role of villi in protecting the small intestine from bacterial invasion (Rostami Nejad et al., 2015), which may be one of the reasons for kaempferol affecting bacterial load. The anti-inflammatory of kaempferol has been partially reported. For example, kaempferol inhibits the activation of inflammatory NF-κB transcription factors through NIK/IKK and MAPKs in aged rat kidneys (Park et al., 2009), meanwhile, kaempferol alleviates enteritis in mice by inhibiting the kaempferol alleviates enteritis in mice by inhibiting the TLR4/NF-κB (Qu et al., 2021). Based on the aforesaid research, it was discovered that apart from the direct potentiation of ceftiofur, kaempferol assisted ceftiofur could alleviate inflammatory responses by modulating the NF-κB/TLR pathway through reducing the expression of NF-κB p65 proteins, TLR proteins, and MYD88 proteins. However, more works is still required to explain the underlying mechanisms of the anti-inflammatory and intestinal protective properties of kaempferol against ESBLs E. coli infections.
One potential limitation of the study is that the authors had to employ ESBLs E. coli. Although the study used ATCC®25922™ as the control to observe the combined antibacterial activity of kaempferol with ceftiofur against non-ESBLS E.coli, its inhibitory mechanism and in vivo therapeutic effect against non-ESBLS E. coli cannot be verified, this provides a new direction for our future work.
In conclusion, our data have shown that kaempferol exhibits potent synergistic activity with ceftiofur both in vitro and in vivo. The discovery of kaempferol as a novel ceftiofur adjuvant highlights the huge potential of compounds extracted from herbs against bacterial infections diseases. Nevertheless, the mechanism of this synergistic activity remains to be elucidated in the future.
4 Materials and methods 4.1 StrainsStrains proved to be ESBLs E. coli (Table 2) and were kept in the laboratory of the College of Veterinary Medicine, Northwest A&F University (Tong et al., 2023a).
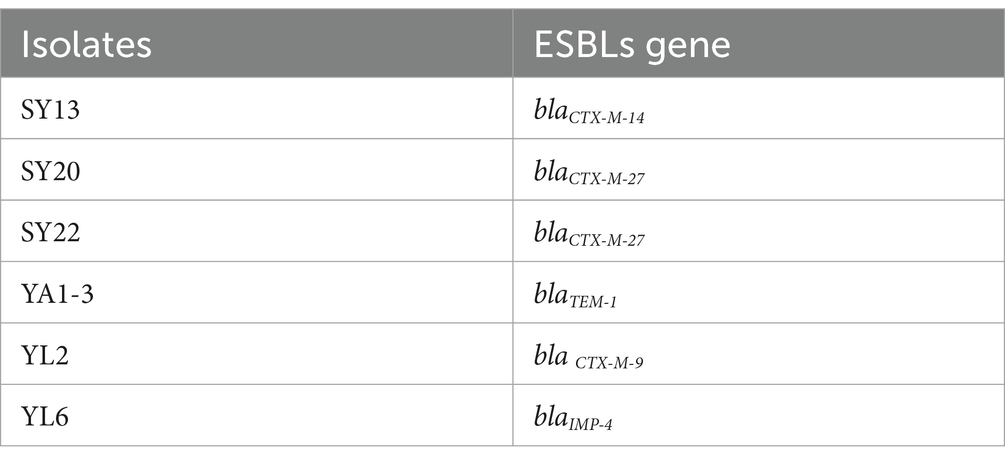
Table 2. THE ESBLs gene information of isolates.
4.2 Checkerboard assayThe combined antibacterial effect of kaempferol and ceftiofur was assessed by checkerboard assays (Tong et al., 2023a). In brief, both kaempferol and cefiofur were diluted to prepare seven gradient concentrations ranging from 1/16 MIC to 2 MIC. Each vertical column of tubes contained an identical concentration of drug A, while each horizontal row of tubes contained the same concentration of drug B. Bacterial suspension was inoculated into each tube to achieve a final density of approximately 1 × 106 CFU/mL. Single-drug control tubes and blank control tubes were also prepared, with E. coli ATCC® 25,922™ used as a sensitivity control strain. Six ESBLs isolates were employed as experimental bacteria. All tubes were incubated at 37°C for 16 h under aerobic conditions. The experiment was conducted in triplicate. The fractional inhibitory concentration index (FICI) was calculated according to the following formula (Table 3).
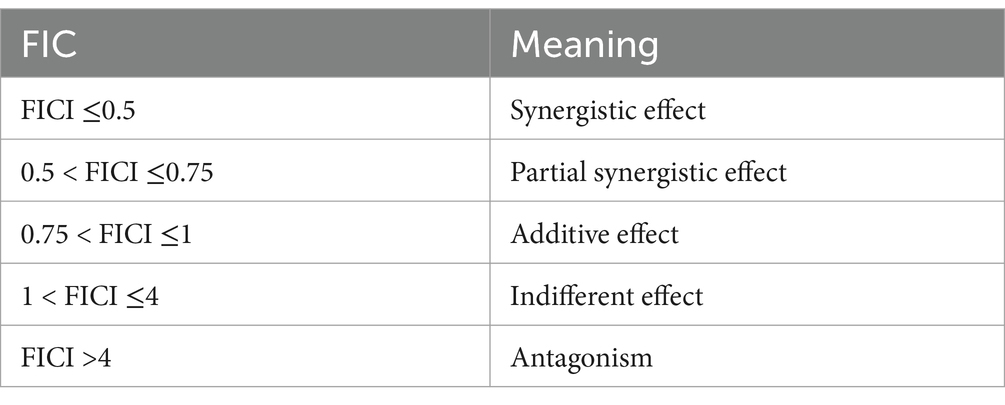
Table 3. FICI values and criteria definitions.
FICI = MIC of kaempferol in combination/MIC of kaempferol alone + MIC of ceftiofur in combination/MIC of ceftiofur alone.
4.3 Time-kill curvesTime-kill assays (Liu et al., 2020) were employed to assess the synergistic antibacterial effects of kaempferol and ceftiofur against ESBL-producing E. coli by quantifying the reduction in CFU/mL over a 24-h period. Different concentrations of kaempferol and ceftiofur were co-incubated with an equal volume of ESBLS E. coli culture, while Mueller-Hinton broth (MHB) was used as a control in place of kaempferol or ceftiofur. All samples were incubated at 37°C, and aliquots (100 μL) were collected at 0, 2, 4, 6, 8, 10,12, and 24 h for colony counting after three rounds of centrifugation and resuspension to remove residual antimicrobial agents. Each assay was repeated in triplicate.
4.4 Growth curvesThe growth curve was utilized to assess the inhibitory effect of the combination on ESBLs E. coli from the 24-h time point until the logarithmic phase. Kaempferol and ceftiofur were co-incubated with an equal volume of E. coli culture at 0.25 MIC of kaempferol and ceftiofur. MHB was added instead of kaempferol or ceftiofur as a control. The initial concentration of bacterial culture was 1 × 106 CFU/mL. All samples were incubated at 37°C, and after 0, 2, 4, 6, 8, 12, and 24 h of incubation, 100 μL samples were extracted for measuring absorbance at OD600. Curves depicting absorbance changes at OD600 over time were plotted, with each assay being repeated in triplicate.
4.5 Resistance development studiesESBLs E. coli in the exponential phase were diluted 1:1000 into fresh TSB media supplemented with 0.25 × MIC of ceftiofur or ceftiofur plus 0.25 × MIC of kaempferol. After incubation at 37°C for 24 h, the MIC of the culture was determined by two-fold serial dilutions in 96-well microtiter plates. Simultaneously, this culture was diluted to an adjusted 0.25 × MIC of drugs for subsequent passages. This process was repeated for a duration of 30 days, and the MIC values were measured at intervals of 0, 1, 2, 3, 6, 9, 12, 15, 18, 21, 24, 27, and 30 days. With each assay being repeated in triplicate.
4.6 Scanning electron microscopeThe isolates were incubated in MHB with 0.25 MIC of kaempferol monotherapy, 0.25 MIC of ceftiofur monotherapy, or a combination of 0.25 MIC of kaempferol and 0.25 MIC of ceftiofur for 10 h. As a control, MHB was added instead of kaempferol or ceftiofur. After the incubation period, the bacteria were collected and washed with PBS, followed by fixation with 4% glutaraldehyde for 3 h. Subsequently, the bacteria underwent dehydration using graded ethanol before undergoing carbon dioxide critical point drying and gold spraying prior to scanning electron microscopy.
4.7 Crystal violet stainingThe overnight bacterial cultures were adjusted to 0.5 McFarland in each well of 96-well microtiter plates using MHB broth. Subsequently, 10 μL of the diluted cultures were dispensed into each well and treated with kaempferol monotherapy, ceftiofur monotherapy, or a combination of kaempferol and ceftiofur. MHB was added to achieve a final volume of 200 μL/well. The microplates were completely covered with parafilm to prevent sample evaporation and incubated for 48 h. Planktonic cells were removed, and the attached cells were gently washed twice with a sterile physiological saline solution. Then, 200 μL of methanol/well was added and left for 20 min to fix the sessile cells. After discarding the methanol, the plates were left under a laminar flow cap until complete dryness (at least 30 min). Adhered cell staining was achieved by adding 200 μL of a 2% w/v crystal violet solution to each well for 20 min followed by gentle washing and drying. A volume of 200 μL of glacial acetic acid at a concentration of 20% w/v was added to release the bound dye (Caputo et al., 2022). The absorbance was measured at OD570. The concentration of all drugs mentioned above was set at 0.25 MIC level. Each assay was repeated in triplicate.
4.8 MTT stainingMTT staining was employed to evaluate the metabolic activity of biofilm cells. Overnight bacterial cultures, grown at the appropriate temperatures, were adjusted to 0.5 McFarland and then exposed to kaempferol monotherapy, ceftiofur monotherapy, or a combination of kaempferol and ceftiofur. After 48 h of incubation, the bacterial suspension was removed, and 150 μL of PBS and 30 μL of 0.3% MTT were added to microplates which were maintained at 37°C. Following a 2-h incubation period, the MTT solution was discarded; subsequently, after two washing steps with 200 μL of sterile physiological solution, 200 μL of DMSO was added for dissolution of formazan crystals before measuring absorbance at OD595. The concentration for all aforementioned drugs was set at 0.25 MIC. Triplicate tests were conducted, and average results were recorded for reproducibility.
4.9 Confocal laser scanning microscopyAs outlined in a previous investigation (Sun et al., 2021), biofilm formation in E. coli was evaluated using CLSM with slight adjustments. E. coli SY20 suspension supplemented with MHB was inoculated into a 24-well plate with a cover and incubated at 37°C for 24 h. Following the removal of the suspension, the wells were washed with PBS (pH = 7.2). After 4 h of treatment with kaempferol monotherapy, cefotifo monotherapy, or combined kaempferol and cefotifo, the solution was aspirated, and the wells were rinsed again with PBS (pH = 7.2). The biofilm was stained with PI and SYTO9 (PI stains dead bacteria, while SYTO9 stains all bacteria) for CLSM observation.
4.10 Swarming and swimming motility assaysAs described in a previous study (De La Fuente-Núñez et al., 2012), swarming experiments were conducted on 1% LB agar plates for swarming and 0.3% LB agar plates for swimming, with the addition of four different treatments (negative control, 0.25MIC kaempferol, 0.25MIC ceftiofur, and 0.25MIC kaempferol +0.25MIC ceftiofur). E. coli drops were placed in the center of the agar plate, and the diameter was measured after a 24-h incubation period. Triplicate tests were conducted, and average results were recorded for reproducibility.
4.11 Fibrinogen-binding assayIn a previous study (Ming et al., 2017), the SY20 isolate was cultured overnight and then diluted 1:100 in sterile TSB. The culture was divided into four treatment groups, as detailed in section 4.10. Upon reaching an OD600 of 0.5, all cells were collected by centrifugation (5,000 × g for 5 min) and suspended in PBS to achieve an OD600 of 1.0. Subsequently, the resuspended cells were seeded onto polystyrene Costar 96-well plates coated with fibrinogen (pre-incubated overnight with 20 μg/mL bovine fibrinogen at 4°C) and incubated for 1 h at 37°C. After removing the supernatant, the cells were washed with PBS and fixed with a solution of 25% (v/v) formaldehyde for fixation. Following a duration of 30 min, the adherent bacteria underwent another round of washing with PBS before being stained with CV solution at a concentration of 12.5 g/L for a period of 10 min. The wells were subsequently washed again with PBS and allowed to dry before measuring different samples at OD595.Triplicate tests were conducted, and average results were recorded for reproducibility.
4.12 Hydrophobicity and self-aggregationThe surface hydrophobicity of the SY20 isolate was investigated in four groups, as outlined in section 4.10. The bacterial culture was adjusted to a concentration of 1.0 × 105 CFU/mL and subjected to four different treatments in TSB at 37°C for 4 h.
Cultured cells were centrifuged at 12,000× g at 4°C for 5 min. The resulting precipitates were then rinsed twice. The collected cells were re-dissolved in PBS to adjust the OD600 to 0.5 ± 0.05 (ODinitial). Two milliliters of each suspension were mixed with chloroform (0.5 mL) and vortexed for 2 min. After incubating at room temperature for 15 min, the upper aqueous layer was collected, and its absorbance was measured at OD600 (ODtreatment). The hydrophobicity (%) was calculated using the following equation (Ming et al., 2017):
Hydrophobicity (%) = (1 − ODtreatment / ODinitial) × 100.
Auto-aggregation was performed using a modified version of the previously described method (Lee et al., 2021). The cell culture was adjusted to a final concentration of 1.0 × 105 CFU/mL, and an ε-PL solution at 1/2 × MIC was mixed in a 1:1 ratio and then incubated at 37°C for 24 h. Non-treated cells were used as the control. Five milliliters of the mixture were collected and statically incubated at 4°C for 24 h. After incubation, the upper aqueous layer was measured at 600 nm (ODtreatment). The sample was then vortexed and measured again at OD600 (ODinitial). Auto-aggregation (%) was calculated using the following equation:
Auto-aggregation (%) = (1 − ODtreatment/ODinitial) × 100.
4.13 AI-2 assaysTo investigate the impact of kaempferol on AI-2 activity, SY20 isolates were cultured overnight at 37°C. The bacterial cultures were then diluted to a concentration of 105 CFU/mL and divided into four test groups as outlined in section 4.10. Subsequently, the diluted bacterial cultures were incubated at 37°C for 12 h and centrifuged at 10,000 g for 10 min at 4°C. The negative control consisted of the supernatant obtained by centrifuging E. coli DH5α under the same culture conditions. The supernatants were filtered through a 0.22 μm filter and stored at −80°C. To assess AI-2 activity in each test group, V. harveyi BB170 cultured overnight at 28°C was diluted 5,000-fold with AB medium. Ninety microliters of the BB170 diluted culture, along with 10 μL of AI-2 supernatants (prepared from the above test group), were incubated at 28°C in the dark for 6 h, and the bioluminescence value was measured. The test was repeated 3 times independently. The test results are displayed in the form of ratio: luminescence value of each test group/luminescence value of E. coli DH5α (Li et al., 2021).
4.14 RT-qPCRRT-qPCR was employed to assess the combined impact of kaempferol and ceftiofur on the LusX/AI-2 QS system-regulated genes in the SY20 The SY20 isolate was separately incubated in TSB containing kaempferol and ceftiofur, as well as only TSB, for 24 h. RNA extraction was performed using the triazole method, with RNA concentration determined by spectrophotometry and integrity assessed via agarose gel electrophoresis. Subsequently, cDNA synthesis was carried out using an Integrated First-strand cDNA Synthesis kit, followed by qRT-PCR analysis using 2 × Fast HS SYBR QPCR Mixture. The resulting qPCR data were analyzed for relative changes in gene expression levels based on the 2−∆∆Ct method (Zuo et al., 2023). Primers listed in Table 4 were utilized for this study.
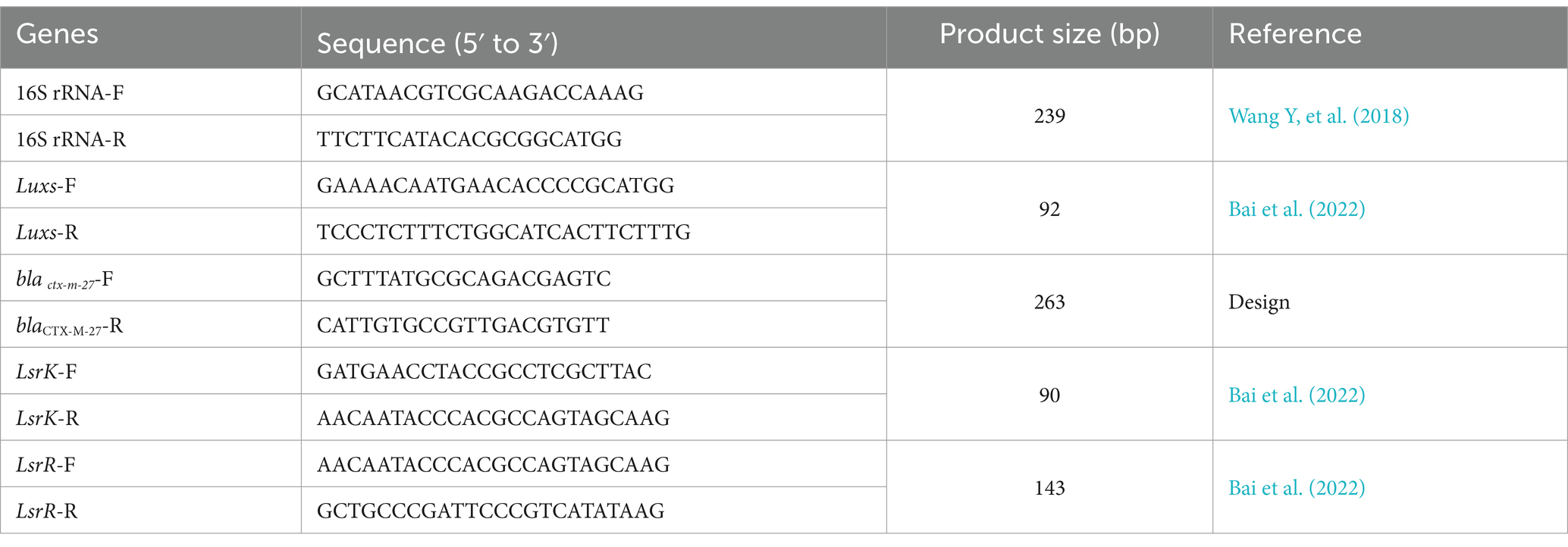
Table 4. Primer sequences used for qRT-PCR amplification.
4.15 Molecular docking assayTo assess the binding affinity of kaempferol with LuxS protein and blaCTX-M-27 protein, we conducted a molecular docking assay using Autodock4 software. The chemical structure of kaempferol was retrieved from the PubChem database, while the structures of E. coli LuxS protein and blaCTX-M-27 protein were obtained from UniProtKB. We assessed the binding capability between kaempferol and LuxS protein using Autodock Vina, Pymol was used to visualize the results (Huang, 2023). Furthermore, binding scores lower than −5 Kcal/mol were indicative of binding activity.
4.16 Nitrocefin assayNitrocefin assay (Teng et al., 2019) was used to assess the effect of kaempferol on blaCTX-M-27 protein activity. The SY20 isolate was cultured to OD600 nm = 0.6 at 37°C, after centrifugation, the bacteria were resuspended in sterile phosphate buffer (pH = 7.2) and broken by ultrasound in an ice bath. After the completion of the ultrasound, blaCTX-M-27 protein crude extract was obtained from the supernatant after centrifugation at 12000 rpm for 10 min at 4°C. BlaCTX-M-27 protein rude extract was incubated with various concentrations of kaempferol (128, 256, 512 μg/mL) in phosphate buffer (pH = 7.2) at 37°C for 5 min, and then, 50 μg/mL of nitrocefin was added to the mixture. After 10 min of incubation, the samples were read at OD492 nm to determine the level of nitrocefin hydrolysis.
4.17 Transcriptome analysisTranscriptome sequencing services were conducted by Novogene-Beijing. SY20 of ESBLs E.coli were treated 0.25MIC kaempferol and 0.25MIC kaempferol +0.25MIC ceftiofur procedure involved reactivating the test bacteria in 400 mL of MH broth and incubating them at 37°C for 4 h until reaching the early log phase. Subsequently, drug solutions were added to achieve the desired final concentration and then incubated at 37°C for an additional 4 h. The cultures were then centrifuged at 4°C, the supernatant was discarded, and the samples were flash-frozen in liquid nitrogen. Utilizing established RNA extraction protocols. RNA integrity and total quantity were evaluated using an Agilent 2,100 bioanalyzer. Ribosomal RNA (rRNA) was depleted from the total RNA to enrich for mRNA using probes, and libraries were constructed in a strand-specific manner. Subsequently, different libraries were pooled based on effective concentration and desired sequencing output for Illumina sequencing, which involved quality analysis of the sequencing, quantitative gene expression analysis, GO enrichment analysis, KEGG enrichment analysis, GSEA enrichment analysis, etc.
4.18 Mouse jejunum inflammation modelAll mice were divided into five groups, with eight female SPF Kunming mice in each group receiving intraperitoneal injections of the lowest lethal dose of E. coli SY20 suspension at a concentration of 1 × 108 CFU/mL. Following a 2-h infection period, the mice were administered a single intraperitoneal dose of kaempferol (50 mg/kg, KAE group), ceftiofur (50 mg/kg, CEF group), or kaempferol and ceftiofur (50 + 50 mg/kg, COM group). The negative control group received 0.9% Nacl injections (NC group). The survival rate of the treated mice was monitored for up to 36 h.
4.19 HE staining and immunohistochemistryThe jejunum were collected, washed with PBS, fixed with a 4% paraformaldehyde solution, dehydrated with ethanol, embedded in paraffin, sliced, and stained with Hematoxylin & Eosin (HE) staining. The samples were examined with a microscope, and the villi length and crypt depth were recorded (Tang et al., 2021).
After fixation in 4% paraformaldehyde solution, the jejunum sections were embedded and subjected to immunohistochemical staining for detection of TLR4, NF-κB p65, and MYD88 antibodies. The samples were observed by staining with hematoxylin. Finally, the samples were examined with a microscope, and the results were measured. Three fields were observed in each sample,and the integrated optical density (IOD) was calculated (Tang et al., 2021).
4.20 Statistical analysisStatistical analysis was conducted using GraphPad Prism 8 and SPSS software. The data are presented as the mean ± SD. The statistical significance of differences was assessed using a t-test for two groups or a one-way ANOVA test for multiple groups, the p-value of survival rates were determined by log-rank test. p value <0.05 was considered statistically significant for all comparisons. All figures were generated using GraphPad Prism 8.0.1 and edited with Photoshop 2021.
Data availability statementThe original contributions presented in the study are publicly available. This data can be found here: [https://doi.org/10.6084/m9.figshare.27686070.v1].
Ethics statementThe animal study was approved by Animal Ethical and Welfare Committee of Northwest A&F University (Approval: NWLA-2021-063). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributionsP-CL: Writing – original draft, Writing – review & editing. Y-CT: Writing – review & editing. X-LX: Writing – original draft. Y-PF: Writing – review & editing. W-RM: Writing – review & editing. Y-QL: Writing – review & editing. SZ: Writing – review & editing. S-ZQ: Writing – review & editing. W-MZ: Funding acquisition, Writing – review & editing.
留言 (0)