Osteoporosis is a metabolic bone disease strongly associated with age. As global populations age, the incidence of osteoporosis continues to rise annually, imposing significant burdens on society (1). Osteoporotic fractures represent its most severe complication and are closely linked to patient mortality (2). Effective prevention of these complications is crucial for enhancing osteoporosis management.
Handgrip strength, quantified using a dynamometer, is a crucial indicator of muscle strength (3) and demonstrates a close relationship with osteoporosis. Research has shown that handgrip strength is associated with lumbar (4) and distal radius bone mineral density (BMD) (5). Additionally, low handgrip strength in postmenopausal Japanese women correlates with an increased risk of site-specific fractures over 10–15 years (6). Furthermore, diminished grip strength has been linked to higher mortality rates following hip fractures (7).
Handgrip strength measurement offers objectivity and high repeatability, making it a valuable tool for health and disease prediction research. As a simple, reliable, and inexpensive evaluation tool, handgrip strength assessment has shown significant predictive and diagnostic value in conditions such as sarcopenia (8), osteoporosis, and osteoporotic fractures (9). Previous studies have demonstrated that grip strength can serve as a predictor of total and cardiovascular mortality (10), and may also have a potential predictive role in the risk of all-cause death in elderly women with reduced bone mass (11). Grip strength also plays a pivotal role in osteosarcopenia (12).
While grip strength shows promise as a prognostic indicator for several diseases and correlates closely with BMD, research exploring its impact on long-term outcomes in individuals with decreased bone mass remains limited. Hence, this study investigates the relationship between grip strength and long-term mortality risk among people with osteopenia, aiming to provide evidence-based insights for their health management.
2 Method 2.1 Data sourceData concerning individuals with osteopenia were sourced from the publicly available National Health and Nutrition Examination Survey (NHANES) database. Mortality data were obtained from publicly released datasets by the United States Centers for Disease Control and Prevention (CDC). The NHANES database, managed by the National Center for Health Statistics (NCHS), aims to gather comprehensive health data across all age demographics in the United States. The NHANES protocol underwent evaluation by the NCHS Research Ethics Review Board, ensuring that all participants provided informed consent. We extracted data from the survey year 2013–2014, including BMD measurements, demographic details (age, gender, race, marital status, family income), anthropometric measures (height, weight, BMI, waist circumference), and questionnaire responses covering smoking habits, diabetes history, cardiovascular disease history, stroke history, and blood pressure levels. Detailed descriptions and measurement methodologies for each variable are available on the NHANES website.
2.2 BMD and low bone massWe collected BMD data from the total femur, femoral neck, trochanter, and intertrochanter regions for the period spanning 2013 to 2014. BMD measurements were conducted using dual-energy X-ray absorptiometry (DEXA). The data were acquired with the Hologic QDR-4500A fan-beam densitometer (Hologic, Inc., Bedford, Massachusetts) equipped with Apex 3.2 software. Prior to each measurement, the densitometer was calibrated to ensure accuracy.
Osteoporosis and osteopenia were characterized by low bone mass. The criteria for these conditions were established according to the World Health Organization (WHO) standards (13). Osteopenia was defined as BMD values ranging from 1 to 2.5 standard deviations (SD) below the mean of young adult male and female reference populations. Osteoporosis was defined as BMD values more than 2.5 SD below the mean of the young reference population. The threshold of BMD for osteopenia and osteoporosis was based on the study of Looker et al. (14). The specific BMD thresholds for males and females can be found in the original study.
2.3 Hand grip strengthHand grip strength was assessed using a hand grip meter. Each hand was tested three times, and the maximum sum of these readings across both hands was recorded as the comprehensive grip strength. We used half of the comprehensive grip strength as our metric for grip strength data. Given the known disparity in grip strength between males and females, we analyzed and categorized grip strength separately for each gender. We adopt the definition of sarcopenia for the European population as defined by the European Working Group on Sarcopenia in Older Adults (EWGSOP) (15). According to this standard, low grip strength is considered when male grip strength is below 27 kg and female grip strength is below 16 kg.
2.4 CovariatesThrough an analysis of previously published studies, we identified factors associated with grip strength, osteoporosis, or mortality risk. These include general demographic characteristics such as age, gender, race, education level, household income poverty ratio (16, 17), marital status (18), body measurements including height (cm), weight (kg), and BMI (<25, 25–30, ≥30) (19). Additionally, we considered blood pressure (mmHg), past medical history including diabetes (yes/no), cardiovascular diseases: congestive heart failure (yes/no), coronary heart disease (yes/no), angina pectoris (yes/no), heart attack (yes/no), stroke (yes/no) and fracture (yes/no) (20, 21).
2.5 Sources of death dataThe death data originates from the CDC and is linked to the NHANES database using a specific Inclusion Number. Further details regarding data conversions and links are available on the corresponding website.
2.6 Statistical analysisCount data were presented as mean ± standard deviation, while measurement data were expressed as percentages. The analysis utilized a Cox regression model to examine the relationship between grip strength and mortality risk through both univariate and multivariate regression analyses. To assess result robustness, various models were constructed by adjusting covariates. Model 0 remained unadjusted. Model 1 incorporates general demographic characteristics including gender, age, race, marital status, family income, and education level. Model 2 builds upon Model 1 by adjusting for BMI and blood pressure. Model 3 further adjusts for smoking based on Model 2. Model 4 incorporates factors potentially increasing mortality risk, such as congestive heart failure, coronary heart disease, angina pectoris, myocardial infarction, and stroke. To handle missing data, we applied multiple imputation (MI) with 5 replications using the chained equation approach via the R mice package, enhancing statistical power and reducing bias. Five sets of complete data were generated, and their effect values were integrated. Model 5 represents the post-MI integrated effect values based on the fully adjusted model. Interaction and stratified analyses were conducted using subgroup variables. Schoenfeld’s global and individual tests were applied to assess the time-varying covariances in the Cox proportional hazards regression analysis.
All analyses were performed using R version 4.22 and FreeStatisticsV1.9.2 software. A significance level of p < 0.05 was applied for determining statistical significance.
3 Results 3.1 Baseline characteristicsThe demographic profile of the study population is depicted in Table 1. A total of 1,343 individuals with decreased bone mass were enrolled, comprising 54.1% women. The mean age of the cohort was 62.0 ± 11.8 years. Among them, 137 participants exhibited low grip strength, while 1,206 had normal grip strength. By the end of 2015, 148 patients had deceased, with an average follow-up duration of 69.5 ± 13.9 months.
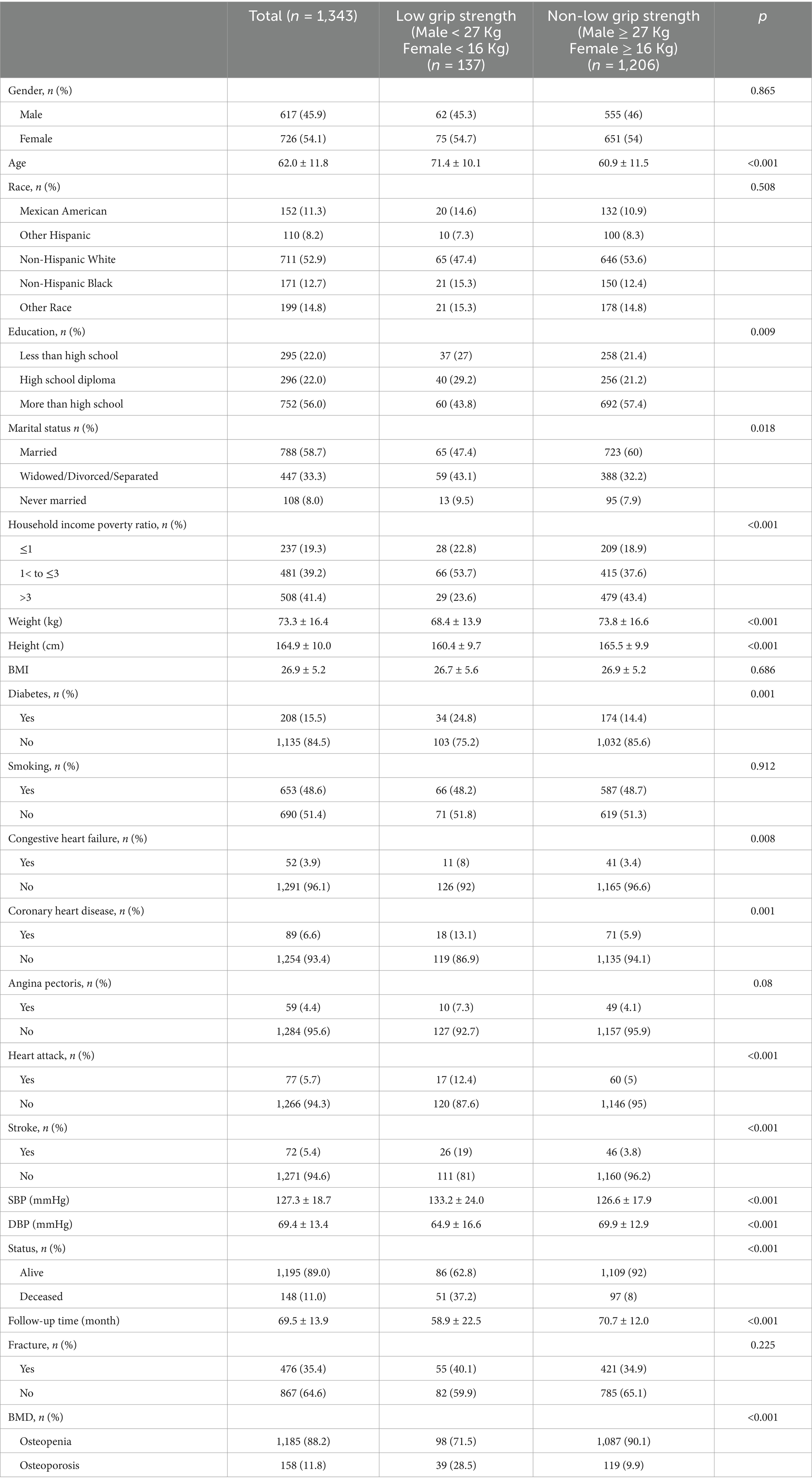
Table 1. Basic characteristics of included data.
Analysis of baseline data revealed that individuals with higher grip strength were predominantly younger, more educated, and had a higher weight and height. Additionally, this subgroup exhibited lower prevalence of cardiovascular and cerebrovascular diseases.
3.2 Association between grip strength and mortalityTable 2 presents the results of multivariate regression analysis investigating the association between grip strength and the risk of all-cause mortality among individuals with decreased bone mass. The analysis revealed a gradual reduction in the risk of mortality with increasing grip strength as a continuous variable (HR = 0.90, 95% CI: 0.87–0.93, p < 0.001). When comparing individuals with low grip strength to those with non-low grip strength, the latter exhibited a 56% lower risk of all-cause mortality (HR = 0.44, 95% CI: 0.29–0.67, p < 0.001). This trend remained consistent across various adjustment models, as depicted in Figure 1.
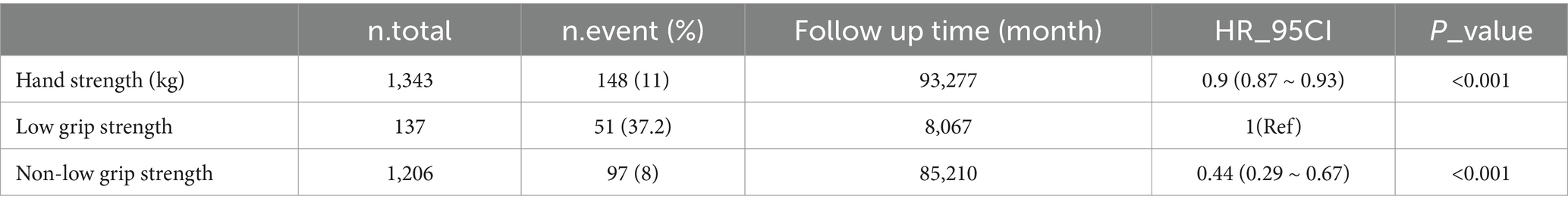
Table 2. Cox regression analysis results between grip strength and risk of all-cause mortality after adjusting for covariates.
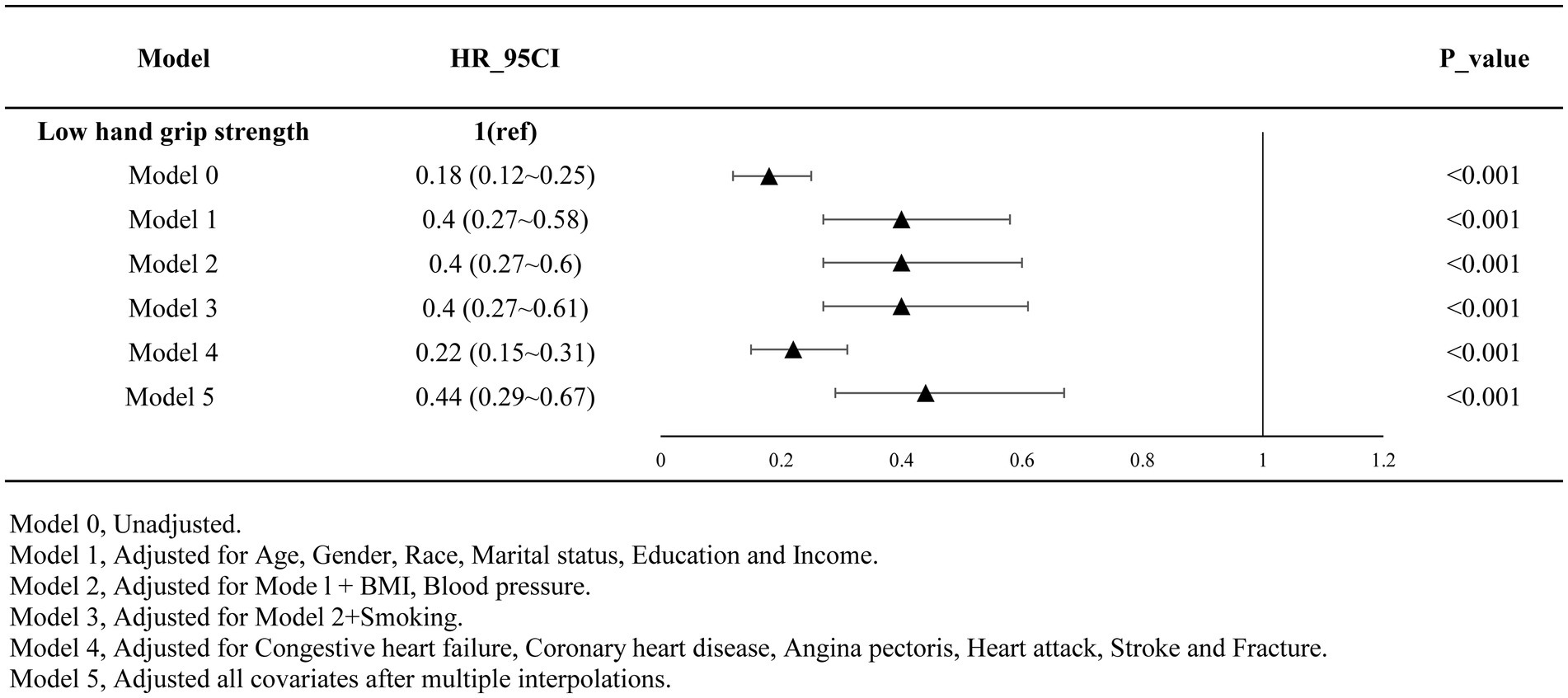
Figure 1. Results after multi-model adjustment (adjusted).
3.3 Subgroup analysisFigure 2 displays the results of our subgroup analysis. Subgroup analysis results indicated that, although no significant difference was observed, the negative correlation trend of high grip strength on the risk of all-cause mortality among each subgroup remained consistent with the main analysis. Notably, a history of coronary heart disease exhibited a significant interaction with grip strength and the risk of all-cause mortality (p < 0.05).
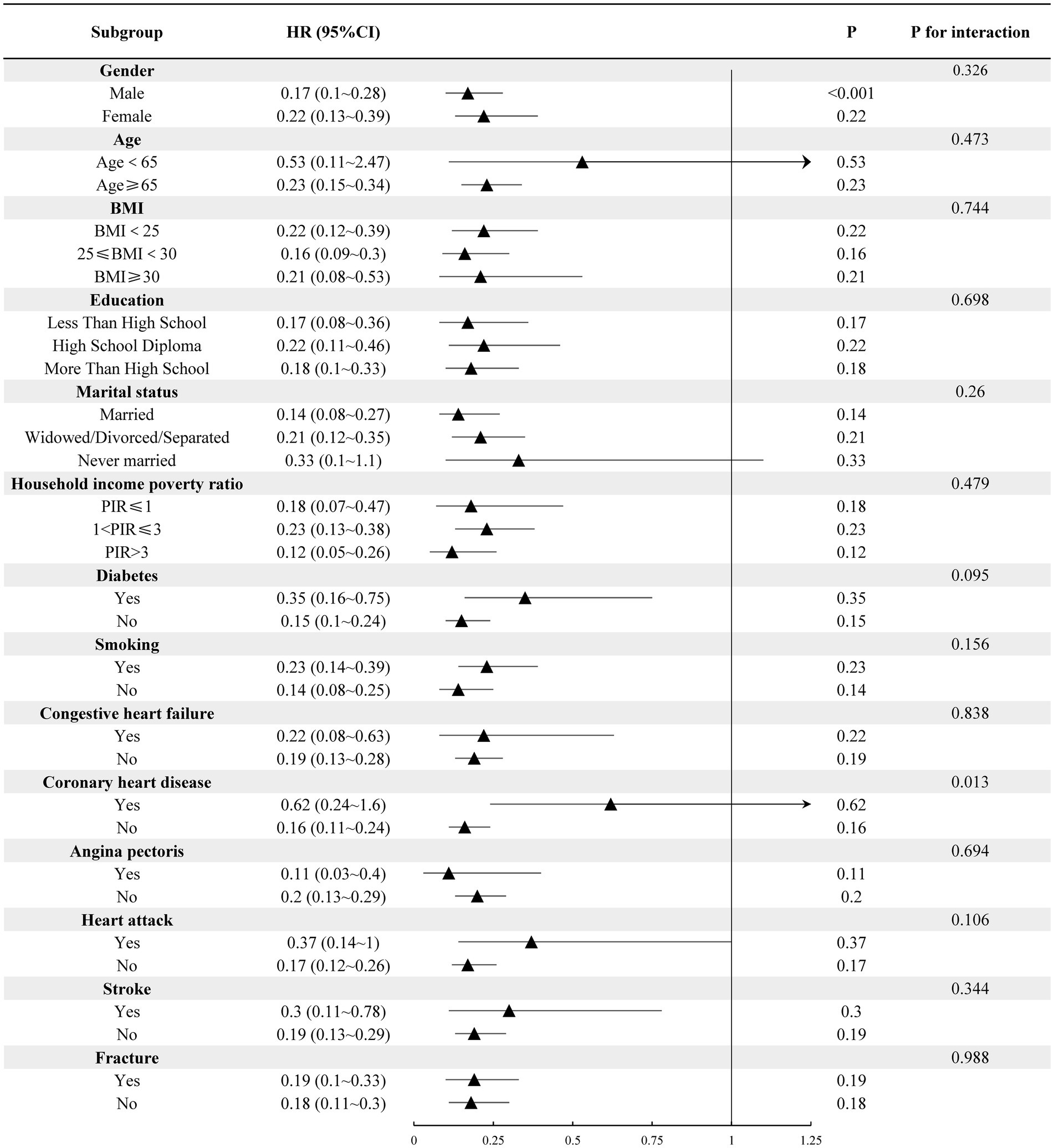
Figure 2. Subgroup analysis of hand grip strength and all-cause mortality risk in individuals with decreased bone mass.
3.4 Cox proportional hazard assumption testAccording to the Cox proportional hazards regression model, the hazard ratio of individual variables, such as hand grip strength, remains constant over time. We conducted a Cox proportional hazards model test using Schoenfeld residuals to evaluate the continuous and categorical variables of grip strength, respectively. The results, depicted in Figure 3, demonstrate that Schoenfeld’s individual and global tests do not suggest a violation of the proportional hazards assumption (p > 0.05).
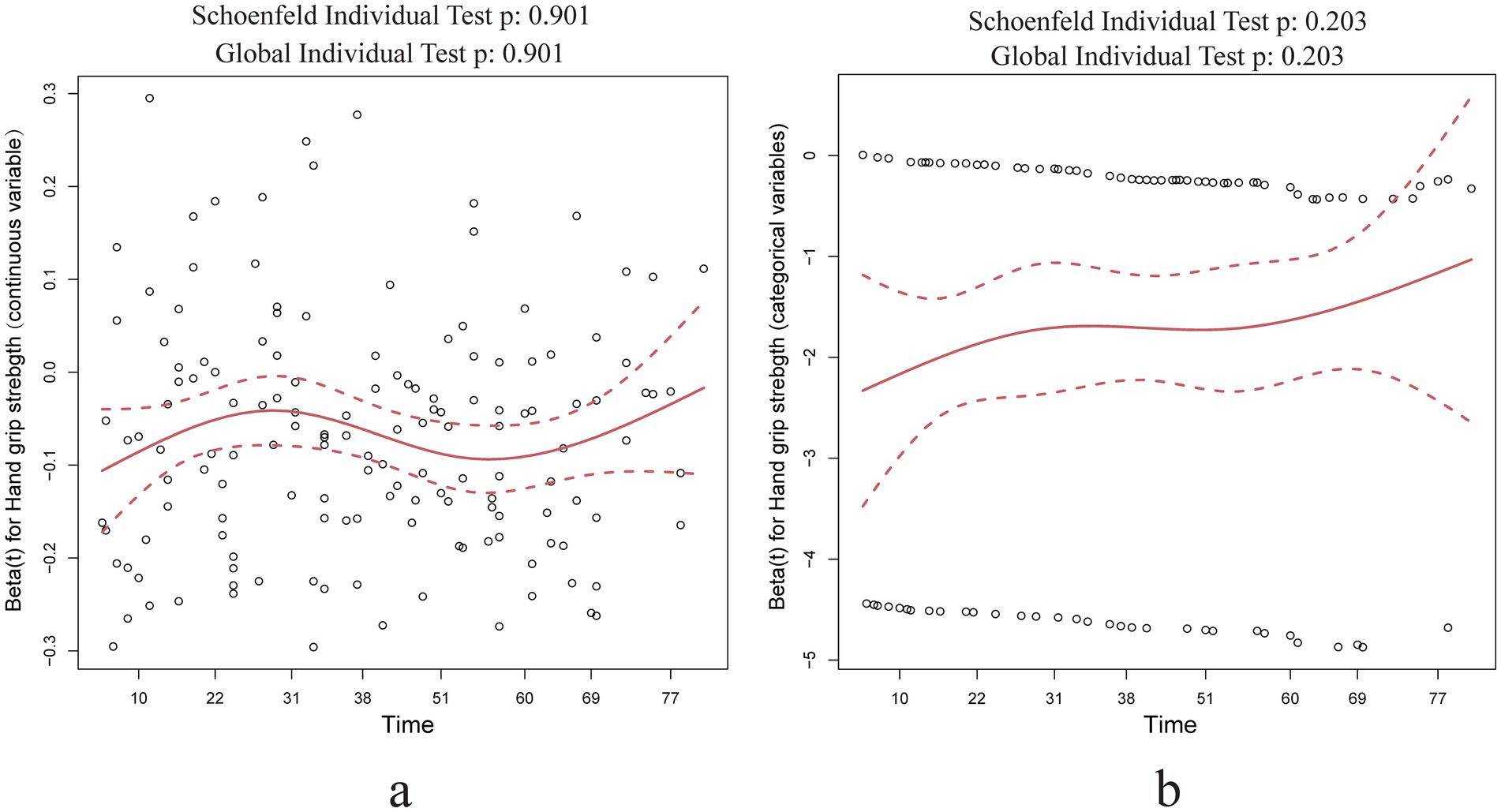
Figure 3. Schoenfeld residual test results of hand grip strength in Cox regression analysis. (a) Hand grip strength as a continuous variable Schoenfeld residual test results; (b) hand grip strength as a categorical variable Schoenfeld residual test results.
4 DiscussionOur findings indicate that low grip strength may elevate the risk of all-cause mortality in individuals with decreased bone mass. Specifically, compared to those with higher grip strength, individuals with low grip strength experience a 56% higher risk of all-cause mortality. This trend persists across adjustments in our model and in stratified analyses. Our sensitivity analysis also verifies the reliability of the model.
The association between grip strength and health outcomes has been extensively investigated. Previous studies have consistently linked lower grip strength to increased risks of all-cause mortality, cardiovascular mortality, myocardial infarction, and stroke (22). Additionally, grip strength has been associated with heightened risks of all-cause dementia and mortality (23). Studies have also demonstrated poorer outcomes in patients with type 2 diabetes who exhibit lower grip strength, suggesting the inclusion of grip strength monitoring in their health management (24).
Varying perspectives exist on the influence of grip strength on the risk of all-cause mortality among individuals with decreased bone mass. A study reported that decreased bone mass in the distal forearm is associated with an elevated risk of all-cause death, and this association remains unaffected by high grip strength (25). Conversely, a cohort study involving 909 participants in the UK (26) revealed that low grip strength significantly elevates the risk of cardiovascular and all-cause mortality, whereas femoral neck BMD does not correlate with any risk of death. Another investigation, involving 1,032 subjects, indicated that those with muscle weakness or osteopenia face considerably higher mortality risks than those without these conditions (27). Additionally, individuals with reduced muscle mass exhibited notably higher mortality risks. However, the coexistence of osteopenia and osteodystrophy did not significantly augment fracture or mortality risks beyond those linked to each condition independently, emphasizing the distinctions between osteopenia, sarcopenia, and hypodynamia. In contrast, a prospective cohort study of elderly women (11) demonstrated that the potential osteosarcopenia group exhibited a heightened risk of 10-year hip fracture and mortality compared to the normal group or those with isolated low bone mass, aligning with our findings. Nonetheless, our study presents divergent conclusions from previous research, potentially attributed to variations in definitions of decreased bone mass, sample sizes, and adjustments for confounding variables. The discordant conclusions on this subject may be influenced by several factors, including the lack of unified criteria for defining osteomyopenia, differences in BMD measurement sites, sample size, follow-up duration, data censoring, and other variables. Given the pivotal role of grip strength in predicting adverse risk outcomes and the absence of a consensus on this matter, continued investigation into this topic is necessary.
The impact of grip strength on the risk of all-cause mortality in individuals with decreased bone mass primarily arises from reduced muscle strength, increasing the risk of fractures—a relationship well-documented in previous studies (28–31). Fractures, particularly hip fractures, are significantly linked to elevated mortality risk (32). Additionally, grip strength is viewed as an indicator of nutritional status, with higher grip strength correlating with better physical function (33). Lower grip strength often indicates a higher prevalence of chronic diseases, thereby diminishing the body’s resilience to adverse outcomes.
Our study observed the interaction of coronary heart disease in the effect of grip strength on the risk of all-cause death in people with low bone mass. The relationship between CHD and grip strength is bidirectional. Grip strength has demonstrated significant predictive value for the risk of CHD and associated mortality (34–36). Conversely, patients with CHD often exhibit a tendency to reduce grip strength. A prospective Finnish study (37) with a 22-year follow-up period reported a marked decrease in hand grip strength among CHD patients, corroborated by another study (38), indicating that the risk escalates over the course of the disease. CHD is a pivotal determinant of all-cause mortality in patients, a topic extensively covered in previous research. Furthermore, decreased grip strength in CHD patients may exacerbate the risk of all-cause death. However, the specific impact of CHD on grip strength in osteopenia populations remains underexplored and warrants further investigation. Several limitations exist within this study. First, to maintain data integrity, cases with missing information were excluded, thereby reducing the number of included cases to some extent. Second, as the study primarily involves the American population, the generalization of results to other populations remains uncertain. Third, due to its observational design, causal associations cannot be established, and residual confounding factors may not be entirely excluded. Fourth, without weight analysis, the findings may not fully represent the characteristics of the entire NHANES survey population. Finally, the number of deaths involved is relatively small compared to the sample size, which may limit the statistical power of the study. Further verification through studies with larger sample sizes is necessary.
5 ConclusionLow grip strength is associated with an increased risk of all-course mortality among individuals with decreased bone mass. Integrating routine monitoring of grip strength in patients with osteopenia and promoting the maintenance or enhancement of grip strength in this population may introduce a novel strategy for health management among these individuals.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe NHANES protocol underwent evaluation by the NCHS Research Ethics Review Board, ensuring that all participants provided informed consent.
Author contributionsHS: Writing – original draft, Writing – review & editing. JL: Conceptualization, Writing – review & editing. RT: Formal analysis, Visualization, Writing – review & editing. XZ: Data curation, Investigation, Writing – review & editing. XQ: Conceptualization, Supervision, Writing – review & editing. CQ: Data curation, Visualization, Writing – review & editing. WQ: Conceptualization, Funding acquisition, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 82074570).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Qu, L, Zuo, X, Yu, J, Duan, R, and Zhao, B. Association of Inflammatory Markers with all-cause mortality and cardiovascular mortality in postmenopausal women with osteoporosis or osteopenia. BMC Womens Health. (2023) 23:487. doi: 10.1186/s12905-023-02631-6
PubMed Abstract | Crossref Full Text | Google Scholar
2. Shangguan, X, Xiong, J, Shi, S, Liao, Y, Chen, L, Deng, J, et al. Impact of the malnutrition on mortality in patients with osteoporosis: a cohort study from NHANES 2005-2010. Front Nutr. (2022) 9:868166. doi: 10.3389/fnut.2022.868166
PubMed Abstract | Crossref Full Text | Google Scholar
3. Alshahrani, A, Samy Abdrabo, M, Aly, SM, Alshahrani, MS, Alqhtani, RS, Asiri, F, et al. Effect of smartphone usage on neck muscle endurance, hand grip and pinch strength among healthy college students: a cross-sectional study. Int J Environ Res Public Health. (2021) 18:6290. doi: 10.3390/ijerph18126290
PubMed Abstract | Crossref Full Text | Google Scholar
4. Song, J, Liu, T, Zhao, J, Wang, S, Dang, X, and Wang, W. Causal associations of hand grip strength with bone mineral density and fracture risk: a Mendelian randomization study. Front Endocrinol. (2022) 13:1020750. doi: 10.3389/fendo.2022.1020750
PubMed Abstract | Crossref Full Text | Google Scholar
5. Di Monaco, M, Di Monaco, R, Manca, M, and Cavanna, A. Handgrip strength is an independent predictor of distal radius bone mineral density in postmenopausal women. Clin Rheumatol. (2000) 19:473–6. doi: 10.1007/s100670070009
Crossref Full Text | Google Scholar
6. Kamiya, K, Kajita, E, Tachiki, T, Ikehara, S, Kouda, K, Sato, Y, et al. Association between hand-grip strength and site-specific risks of major osteoporotic fracture: results from the Japanese population-based osteoporosis cohort study. Maturitas. (2019) 130:13–20. doi: 10.1016/j.maturitas.2019.09.008
PubMed Abstract | Crossref Full Text | Google Scholar
7. Gutierrez-Hermosillo, H, de Leon-Gonzalez, ED, Medina-Chavez, JH, Torres-Naranjo, F, Martinez-Cordero, C, and Ferrari, S. Hand grip strength and early mortality after hip fracture. Arch Osteoporos. (2020) 15:185. doi: 10.1007/s11657-020-00750-3
Crossref Full Text | Google Scholar
8. Cruz-Jentoft, AJ, Baeyens, JP, Bauer, JM, Boirie, Y, Cederholm, T, Landi, F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
PubMed Abstract | Crossref Full Text | Google Scholar
9. Cheung, AM, and Papaioannou, A. Osteoporosis Canada scientific advisory council guidelines C. Bone-density testing interval and transition to osteoporosis. N Engl J Med. (2012) 366:1546. doi: 10.1056/NEJMc1201933
Crossref Full Text | Google Scholar
10. Soysal, P, Hurst, C, Demurtas, J, Firth, J, Howden, R, Yang, L, et al. Handgrip strength and health outcomes: umbrella review of systematic reviews with Meta-analyses of observational studies. J Sport Health Sci. (2021) 10:290–5. doi: 10.1016/j.jshs.2020.06.009
PubMed Abstract | Crossref Full Text | Google Scholar
11. Paulin, TK, Malmgren, L, McGuigan, FE, and Akesson, KE. Osteosarcopenia: prevalence and 10-year fracture and mortality risk – a longitudinal, population-based study of 75-year-old women. Calcif Tissue Int. (2024) 114:315–25. doi: 10.1007/s00223-023-01181-1
PubMed Abstract | Crossref Full Text | Google Scholar
12. Hamad, B, Basaran, S, and Coskun, BI. Osteosarcopenia among postmenopausal women and handgrip strength as a practical method for predicting the risk. Aging Clin Exp Res. (2020) 32:1923–30. doi: 10.1007/s40520-019-01399-w
PubMed Abstract | Crossref Full Text | Google Scholar
13. Kanis, JA, Melton, LJ 3rd, Christiansen, C, Johnston, CC, and Khaltaev, N. The diagnosis of osteoporosis. J Bone Miner Res. (1994) 9:1137–41. doi: 10.1002/jbmr.5650090802
Crossref Full Text | Google Scholar
14. Looker, AC, Orwoll, ES, Johnston, CC Jr, Lindsay, RL, Wahner, HW, Dunn, WL, et al. Prevalence of low femoral bone density in older U.S. adults from Nhanes iii. J Bone Miner Res. (1997) 12:1761–8. doi: 10.1359/jbmr.1997.12.11.1761
Crossref Full Text | Google Scholar
15. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyere, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
PubMed Abstract | Crossref Full Text | Google Scholar
16. Lane, NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. (2006) 194:S3–S11. doi: 10.1016/j.ajog.2005.08.047
Crossref Full Text | Google Scholar
17. Younesi Asl, L, Kashanian, M, Najmi, Z, Mahdavi, A, and SafarpourLima, Z. Risk factors of osteoporosis and osteopenia in postmenopausal women based on the L2-L4 Bmd T score of the lumbar spine: a study in Iran. Gynecol Endocrinol. (2023) 39:2205959. doi: 10.1080/09513590.2023.2205959
PubMed Abstract | Crossref Full Text | Google Scholar
19. Tang, G, Feng, L, Pei, Y, Gu, Z, Chen, T, and Feng, Z. Low BMI, blood calcium and vitamin D, kyphosis time, and outdoor activity time are independent risk factors for osteoporosis in postmenopausal women. Front Endocrinol. (2023) 14:1154927. doi: 10.3389/fendo.2023.1154927
Crossref Full Text | Google Scholar
20. Long, G, Liu, C, Liang, T, Zhang, Z, Qin, Z, and Zhan, X. Predictors of osteoporotic fracture in postmenopausal women: a meta-analysis. J Orthop Surg Res. (2023) 18:574. doi: 10.1186/s13018-023-04051-6
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ma, X, Xin, D, She, R, Liu, D, Ge, J, and Mei, Z. Novel insight into Cgas-sting pathway in ischemic stroke: from pre- to post-disease. Front Immunol. (2023) 14:1275408. doi: 10.3389/fimmu.2023.1275408
PubMed Abstract | Crossref Full Text | Google Scholar
22. Strand, BH, Cooper, R, Bergland, A, Jorgensen, L, Schirmer, H, Skirbekk, V, et al. The Association of Grip Strength from midlife onwards with all-cause and cause-specific mortality over 17 years of follow-up in the Tromso study. J Epidemiol Community Health. (2016) 70:1214–21. doi: 10.1136/jech-2015-206776
PubMed Abstract | Crossref Full Text | Google Scholar
23. Esteban-Cornejo, I, Ho, FK, Petermann-Rocha, F, Lyall, DM, Martinez-Gomez, D, Cabanas-Sanchez, V, et al. Handgrip strength and all-cause dementia incidence and mortality: findings from the UK biobank prospective cohort study. J Cachexia Sarcopenia Muscle. (2022) 13:1514–25. doi: 10.1002/jcsm.12857
PubMed Abstract | Crossref Full Text | Google Scholar
24. Hamasaki, H. What can hand grip strength tell us about type 2 diabetes?: mortality, morbidities and risk of diabetes. Expert Rev Endocrinol Metab. (2021) 16:237–50. doi: 10.1080/17446651.2021.1967743
PubMed Abstract | Crossref Full Text | Google Scholar
25. Hauger, AV, Bergland, A, Holvik, K, Stahle, A, Emaus, N, and Strand, BH. Osteoporosis and osteopenia in the distal forearm predict all-cause mortality independent of grip strength: 22-year follow-up in the population-based Tromso study. Osteoporos Int. (2018) 29:2447–56. doi: 10.1007/s00198-018-4653-z
PubMed Abstract | Crossref Full Text | Google Scholar
26. Westbury, LD, Laskou, F, Patel, HP, Cooper, C, and Dennison, EM. Mortality, bone density and grip strength: lessons from the past and Hope for the future? Rheumatol Adv Pract. (2024) 8:46. doi: 10.1093/rap/rkae046
PubMed Abstract | Crossref Full Text | Google Scholar
27. Balogun, S, Winzenberg, T, Wills, K, Scott, D, Callisaya, M, Cicuttini, F, et al. Prospective associations of Osteosarcopenia and Osteodynapenia with incident fracture and mortality over 10 years in community-dwelling older adults. Arch Gerontol Geriatr. (2019) 82:67–73. doi: 10.1016/j.archger.2019.01.015
PubMed Abstract | Crossref Full Text | Google Scholar
28. Sogaard, AJ, Magnus, JH, Bjornerem, A, Holvik, K, Ranhoff, AH, Emaus, N, et al. Grip strength in men and women aged 50-79 years is associated with non-vertebral osteoporotic fracture during 15 years follow-up: the Tromso study 1994-1995. Osteoporos Int. (2020) 31:131–40. doi: 10.1007/s00198-019-05191-4
PubMed Abstract | Crossref Full Text | Google Scholar
29. Dixon, WG, Lunt, M, Pye, SR, Reeve, J, Felsenberg, D, Silman, AJ, et al. Low grip strength is associated with bone mineral density and vertebral fracture in women. Rheumatology. (2005) 44:642–6. doi: 10.1093/rheumatology/keh569
PubMed Abstract | Crossref Full Text | Google Scholar
30. Albrand, G, Munoz, F, Sornay-Rendu, E, DuBoeuf, F, and Delmas, PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the Ofely study. Bone. (2003) 32:78–85. doi: 10.1016/s8756-3282(02)00919-5
PubMed Abstract | Crossref Full Text | Google Scholar
31. Kim, SW, Lee, HA, and Cho, EH. Low handgrip strength is associated with low bone mineral density and fragility fractures in postmenopausal healthy Korean women. J Korean Med Sci. (2012) 27:744–7. doi: 10.3346/jkms.2012.27.7.744
PubMed Abstract | Crossref Full Text | Google Scholar
32. Barrett, JA, Baron, JA, and Beach, ML. Mortality and pulmonary embolism after fracture in the elderly. Osteoporos Int. (2003) 14:889–94. doi: 10.1007/s00198-003-1494-0
Crossref Full Text | Google Scholar
33. Norman, K, Stobaus, N, Gonzalez, MC, Schulzke, JD, and Pirlich, M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. (2011) 30:135–42. doi: 10.1016/j.clnu.2010.09.010
PubMed Abstract | Crossref Full Text | Google Scholar
34. He, J, Huang, M, Li, N, Zha, L, and Yuan, J. Genetic association and potential mediators between sarcopenia and coronary heart disease: a bidirectional two-sample, two-step Mendelian randomization study. Nutrients. (2023) 15:3013. doi: 10.3390/nu15133013
PubMed Abstract | Crossref Full Text | Google Scholar
35. Liu, X, Wang, Y, Wang, Z, Li, L, Yang, H, Liu, J, et al. Association between sarcopenia-related traits and cardiovascular diseases: a bi-directional Mendelian randomization study. Front Endocrinol. (2023) 14:1237971. doi: 10.3389/fendo.2023.1237971
PubMed Abstract | Crossref Full Text | Google Scholar
36. Xiao, M, Lu, Y, Li, H, and Zhao, Z. Association between handgrip strength and mortality of patients with coronary artery disease: a meta-analysis. Clin Cardiol. (2024) 47:e24322. doi: 10.1002/clc.24322
PubMed Abstract | Crossref Full Text | Google Scholar
37. Stenholm, S, Tiainen, K, Rantanen, T, Sainio, P, Heliovaara, M, Impivaara, O, et al. Long-term determinants of muscle strength decline: prospective evidence from the 22-year Mini-Finland follow-up survey. J Am Geriatr Soc. (2012) 60:77–85. doi: 10.1111/j.1532-5415.2011.03779.x
PubMed Abstract | Crossref Full Text | Google Scholar
38. Wen, QR, Wu, M, Liu, Q, Lyu, J, Guo, Y, Bian, Z, et al. Correlation between chronic diseases and low muscle mass, strength and quality in adults in China. Zhonghua Liu Xing Bing Xue Za Zhi. (2021) 42:1948–54. doi: 10.3760/cma.j.cn112338-20200910-01146
留言 (0)