Many in situ cells, such as epithelial cells and neurons, show polarity and have more than two types of well-differentiated plasma membrane domains. Epithelial cells develop apical membranes for absorption or secretion and basolateral membranes for cell viability. Neurons contain dendrites for receiving signals and axons for transmitting signals to subsequent cells. These specific plasma membrane domains are formed and maintained by the accurate supply of specific membrane components from the trans-Golgi network (TGN) (Levic and Bagnat, 2022; Rodriguez-Boulan et al., 2005; Rodriguez-Boulan and Macara, 2014). These membrane flows are known as polarized transport and essential for human health because their impairment results in genetic disorders, including microvillus inclusion disease, polycystic kidney disease, and retinal degeneration (Charron et al., 2000; Cotton et al., 2013; El Ghouzzi and Boncompain, 2022; Hollingsworth and Gross, 2012; Müller et al., 2008; Ryan et al., 2010; Schneeberger et al., 2015; Vogel et al., 2017; Xiong and Bellen, 2013). Thus, it is important to understand the mechanisms of polarized transport.
Highly polarized Drosophila photoreceptors serve as excellent genetic model systems for studying the mechanisms of polarized transport (Hebbar et al., 2020; Schopf and Huber, 2017). By observing whole-mounted retinas using confocal microscopy, three distinct plasma membrane domains encompassing hundreds of photoreceptors were visualized simultaneously (Karagiosis and Ready, 2004). The first domain is the photoreceptive membrane domain, known as the rhabdomere, which forms at the center of the apical plasma membrane during pupal development. Proteins involved in phototransduction, such as the photosensitive molecule rhodopsin1 (Rh1), the Ca2+-permeable channel transient receptor potential, and chaoptin, a protein essential for rhabdomere architecture, localize specifically in the rhabdomeres (Hardie and Juusola, 2015). The second domain is the peripheral apical domain surrounding the rhabdomere, referred to as the stalk membrane, where the apical determinant Crumbs (Crb) is localized (Pellikka et al., 2002; Pocha et al., 2011). The third domain is the basolateral membrane, which is separated from the apical membrane by an adherens junction; Na+/K+-ATPase localizes to the basolateral membrane, similar to typical polarized epithelial cells (Yasuhara et al., 2000).
Several screening studies to identify proteins involved in polarized transport or recycling in fly retinas have been performed in the last decade (Laffafian and Tepass, 2019; Pocha et al., 2011; Satoh et al., 2013; Satoh et al., 2016a; Xiong et al., 2012; Yano et al., 2012; Feizy et al., 2024; Wang et al., 2014; Xiong et al., 2012; Zeger et al., 2024). These screenings, along with other studies, have revealed a number of proteins involved in transport to either the rhabdomere or basolateral membrane, as well as in recycling processes. Two sets of Rab proteins and their guanine nucleotide exchange factors, Rab6/Rich and Rab11/Pcs, regulate Rh1 transport to the rhabdomeres, and Rab10/Crag is required for basolateral transport, although Crag also works as Rab11GEF in adult photoreceptors under light conditions (Iwanami et al., 2016; Nakamura et al., 2020; Otsuka et al., 2019; Satoh et al., 2005; Tong et al., 2011; Xiong et al., 2012). The lack of motor proteins such as kinesin (Khc and Klc), dynein (Dhc64c), and MyoV (didum), or a subunit of tethering complex exocysts, causes Rh1-containing vesicles to accumulate in the cytoplasm (Beronja et al., 2005; Laffafian and Tepass, 2019; Li et al., 2007; Pocha et al., 2011; Wu et al., 2005; Yano et al., 2012). Conversely, the absence of adapter protein complex-1, clathrin, or glycosylphosphatidylinositol synthesis induces the mislocalization of the basolateral membrane protein Na+/K+-ATPase to the apical domains (Nakamura et al., 2020; Satoh et al., 2013). It is not known whether there is a specific transport pathway to the stalk membrane. To date, no factor involved in such a pathway has been identified. In addition, the stalk/rhabdomere boundary lacks an obvious physical structure separating these subdomains, such as an adherens junction separating the stalk and basolateral subdomains (Schopf and Huber, 2017). These results suggest that proteins destined for the apical plasma membrane are transported by the same pathway and then segregate into the stalk and rhabdomere subdomains by soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-independent mechanisms.
SNARE proteins are indispensable components for membrane fusion (Grissom et al., 2020; Hong and Lev, 2014; Jahn et al., 2024; Stanton and Hughson, 2023). They are classified as R-SNAREs and Q-SNAREs based on the presence of conserved arginine or glutamine in the SNARE motif. Q-SNAREs are further subdivided into Qa-, Qb-, and Qc-SNAREs based on their homology to syntaxin and SNAP25. In Drosophila photoreceptors, phenotypes of loss-of-function of six SNAREs have been reported: Sec22, an R-SNARE that is essential for endoplasmic reticulum (ER) morphology and photoreceptor morphogenesis; Gos28, a Qb-SNARE that mediates intra-Golgi transport of Rh1 and is required for photoreceptor survival; Syx5, a Qa-SNARE that regulates ER-to-Golgi transport; Syx7, a Qa-SNARE that is required for the degradation of large amounts of endocytosed apical components; and R-SNAREs nSyb and Syb that are required for the post-Golgi transport of Rh1, which was recently reported (Laffafian and Tepass, 2019; Rosenbaum et al., 2014; Satoh et al., 2016b; Yamashita et al., 2022; Zhao et al., 2015). These studies highlight the functions of SNAREs in photoreceptor morphogenesis and survival in flies; however, SNAREs involved in polarized transport have not been identified.
In this study, we used RNAi knockdown and a somatic knockout (KO) approach to search for SNARE proteins involved in post-Golgi transport to the rhabdomere, stalk, or basolateral membrane. Surprisingly, in the post-Golgi transport, none of the SNAREs are exclusively involved in the transport to a specific destination on the plasma membrane domains, although each SNARE shows some preference for certain membrane domains. In particular, Syx1A, Snap24, and Snap25 appear to be required for all plasma membrane domains.
2 Materials and methods2.1 Construction of the plasmids and transgenic fliesDetails of the DNA constructs used in this paper are given in Supplemental Materials and Methods. Molecular cloning was performed according to standard protocols. In brief, PCRs were performed using a KOD One PCR Master Mix (TOYOBO Osaka, Japan), ligations were performed using Ligation high Ver.2 (TOYOBO), and Gibson assemblies were performed using a NEBuilder HiFi DNA Assembly Master Mix (New England Biolabs, Ipswich, MA, United States). The ligated or assembled DNAs were chemically transformed into E. coli OmniMAX 2T1R (Thermo Fisher Scientific, Waltham, MA, United States). Sequence files of plasmids constructed for this paper are available in Dryad (https://doi.org/10.5061/dryad.wwpzgmssx) and given in Supplementary Table S3. Primers used for PCR and adapters for fill-in are given in Supplementary Table S4.
2.2 Drosophila stocks and geneticsFlies were grown at 20°C–25°C on standard cornmeal–glucose–agar–yeast food. The following fly stocks were used: Rh1-Gal4 (from Dr. Hama, Kyoto Sangyo University) and vas-Cas9 (stock number 51325, Bloomington Drosophila Stock Center, Bloomington, IN, United States: BL51325). The SNARE RNAi lines used in this study are given in Supplementary Table S1. X chromosomes of SNARE RNAi lines obtained from the stock centers were replaced with those carrying eyeless-Flippase (eyFLP) and UAS-Dicer. These lines were crossed with CoinFLP-ActGal4 (BL58751) (Bosch et al., 2015) or CoinFLP-longGMR-Gal4, which were prepared in this work (see below). The SNARE gRNA lines used in this study are given in Supplementary Table S2. These lines were crossed with CoinFLP-longGMR-FLAG::Cas9-H2B::NG or CoinFLP-Act5C-FLAG::Cas9-H2B::GFP, which were prepared in this work (see below). To obtain Syx7 null mosaic eyes, w*;; FRT80B, Syx71/TM6B (BL52000) and w*;; FRT80B, Syx74/T (2;3) TSTL, CyO; TM6B, Tb1 (BL51644) were crossed to y w ey-FLP;; P3RFP FRT80B (Satoh et al., 2013). To obtain Syx6 null mosaic eyes, y w ey-FLP; FRT42D Syx6Δ5/CyO GFP prepared in this work (see below) were crossed to y w ey-FLP; FRT42D P3RFP (Satoh et al., 2013). To obtain Syx13 null mosaic eyes, y w ey-FLP; Syx13Δ11 FRT80B/TM6B prepared in this work (see below) were crossed to y w ey-FLP;; P3RFP FRT80B (Satoh et al., 2013).
2.3 Generation of transgenic fliesA Phi31-based vector, pCFD4w-Syx6, was injected into the embryo of a phi31 host line (y[1] MZH-2A w[*]; MZH-68E, BL24485) to obtain gRNA-Syx6. P-element-based vectors were co-injected with a helper plasmid pUC hsPI (DGRC Stock 1001; https://dgrc.bio.indiana.edu//stock/1001; RRID:DGRC_1001) into the embryo of a host line, w1118. The plasmid of pP-3xlongGMR-coinFLP-HFCas9-H2B-2xNG was injected into the w1118 embryo by BestGene (CA, United States).
2.4 Generation of null alleles for Syx13 and Syx6 genes by the CRISPR/Cas9 systemThe male flies carrying Syx13-gRNA on second (BL84021), y sc v sev/Y; PattP40, were crossed to females carrying vas-Cas9 and FRT80B, y w MZH-2A;; Syx13wt FRT80B. Each male offspring, y w MZH-2A/ Y; PattP40/+; Syx13KO FRT80B, was individually crossed with females, y w eyFLP;; P3RFP FRT80B. Each male offspring with RFP-mosaic eyes, y w eyFLP/Y;; Syx13KO FRT80B/P3RFP FRT80B, was crossed with females, y w eyFLP;; Dr P/TM6B GFP, to establish stock lines, and the males were lysed for genotyping. The genomic region, including the target site of CRISPR-Cas9, was amplified with Syx13-GF1 and Syx13-GR1 and sequenced (Supplementary Tables S3, S4). Syx13Δ11 carrying 11 base deletions (GCTTCAGTCCG) was identified as the null allele.
The males flies carrying Syx6-gRNA on third, w/Y;; Syx6-gRNA/TM6B, were crossed to females carrying vas-Cas9 and FRT42D, y w MZH-2A; Syx6wt FRT42D. Each male offspring, y w MZH-2A/Y; Syx6KO FRT42D/+; Syx6-gRNA/+, was individually crossed with females, y w eyFLP; P3RFP FRT42D. Each male offspring with RFP-mosaic eyes, y w eyFLP/Y; Syx6KO FRT42D/P3RFP FRT42D, was crossed with females, y w eyFLP; Sp P/CyO GFP, to establish stock lines, and the males were lysed for genotyping. The genomic region, including the target site of CRISPR-Cas9, was amplified with Syx6-GF3 and Syx6-GR3 and then sequenced (Supplementary Tables S3, S4). Syx6Δ5 carrying five base deletions (TGTCG) was identified as the null allele.
2.5 ImmunohistochemistryFixation and staining methods were performed as described previously (Otsuka et al., 2019; Satoh and Ready, 2005). The primary antisera were as follows: rabbit anti-Rh1 (1:1,000) (Satoh et al., 2005), mouse monoclonal anti-Na+/K+-ATPase alpha subunit (α5: 1:500 ascite; Developmental Studies Hybridoma Bank (DSHB) Iowa City, IA, United States), mouse anti-Na+/K+-ATPase beta subunit (Nrv) (1:10) (DSHB, IA, United States), mouse anti-Eys (1:10) (DSHB, IA, United States), rabbit anti-Myc (1:300) (Medical and Biological Laboratories, Nagoya, Japan; No. 562), rabbit anti-HA (1:300; cat. no. 561, Medical and Biological Laboratories, Nagoya, Japan), mouse anti-Syx1A (1:15) (DSHB, IA, United States), rabbit anti-TRP (1:1000) (Dr. Montell, University of California, Santa Barbara, United States), rat anti-Crb (1:300) (Dr. Tepass, University of Toronto, Canada), rabbit anti-Snap29 (1:300; provided by Dr. Paden, University of Sheffield, United Kingdom), rabbit anti-Sec22 (1:300) (Dr. Paden, University of Sheffield, United Kingdom), guinea pig anti-Rab6 (1:300) (Iwanami et al., 2016), and rat anti-Rab11 (1:300) (Otsuka et al., 2019). The secondary antibodies were anti-mouse, anti-rabbit, and/or anti-rat antibodies labeled with Alexa Fluor 488, 568, and 647, respectively (1:300; Life Technologies, Carlsbad, CA, United States). Phalloidin-conjugated Alexa Fluor 568 (1:100; Life Technologies, Carlsbad, CA, United States) was used for F-actin staining. Images of samples were recorded using a FV3000 confocal microscope (UPLXAPO60XO 1.30 NA and UPlanSApo 60 × S2 1.42 NA objective lens; Olympus, Tokyo, Japan). To minimize bleed-through, each signal in double- or triple-stained samples was imaged sequentially. The images were processed in accordance with the Guidelines for Proper Digital Image Handling using ImageJ and/or Affinity Photo (Serif Europe Ltd., West Bridgford, Nottinghamshire, United Kingdom) (Schindelin et al., 2012). For the quantification of the intensity of Rh1 and Na+/K+-ATPase staining in the photoreceptor cytoplasm, we used more than three mosaic retinas with more than eight wild-type and more than six mutant photoreceptors in each retina. For the quantification of Rh1, the area of cytoplasm or whole cells and their staining intensities were measured, and for the quantification of Na+/K+-ATPase-α and Nrv, the staining intensities of the cytoplasm were measured using Fiji (Schindelin et al., 2012).
2.6 Electron microscopyElectron microscopy was performed as described previously (Satoh et al., 1997). The samples were observed under a JEM-1400 and JEM-1400Plus electron microscope (JEOL, Tokyo, Japan), and montages were prepared using a CCD camera system (JEOL). The phenotypes were investigated using the section with the depth where a couple of photoreceptor nuclei within the ommatidia were observed.
3 Results3.1 Three-step RNAi mosaic eye screeningMosaic retinas, consisting of both wild-type and mutant cells, provide a powerful tool for assessing the phenotype of mutant photoreceptors because they can be compared side-by-side with wild-type cells. The previously reported CoinFLP system (Bosch et al., 2015) enables mosaic RNAi knockdown screening by automatically generating a reliable ratio of mutant-to-wild-type tissues in a developmentally controlled manner. In brief, in the CoinFLP system, strong and constitutive expression of the Gal4 trans-activator from the Act5C promoter is blocked by the “transcriptional stop cassette” until it is unlocked by recombination between two canonical flippase recognition target (FRT) sites mediated by FLP recombinase. Instead, in approximately half of the cells, the recombination between two FRT3 sites anchors a “transcriptional stop cassette” upstream of Gal4, resulting in the permanent inactivation of Gal4 expression. Combining CoinFLP with any FLP lines that are expressed in the tissues and the timing of interest, in our case, retinas, allows the generation of mosaic tissues consisting of mutant and wild-type cells. In this study, we used the promoter of the eyeless gene, the master regulator of the eye, to express the FLP recombinase at an early stage of eye imaginal disk development. We named this configuration eyeless–CoinFLP–Act5C–Gal4 (Bosch et al., 2015) and used it as the first step on the screen.
The expression of 59 types of RNAi constructs driven by eyeless–CoinFLP–Act5C–Gal4 resulted in 7 lines with well-grown Gal4-positive clones showing obvious phenotypes, 35 lines without any phenotype, and 17 lines with no Gal4-positive clones or cells with phenotypes too severe for analysis (Figure 1A). We hypothesized that the loss of Gal4-positive cells was the result of a lack of clonal growth or cell survival caused by the constant expression of the RNAi construct.
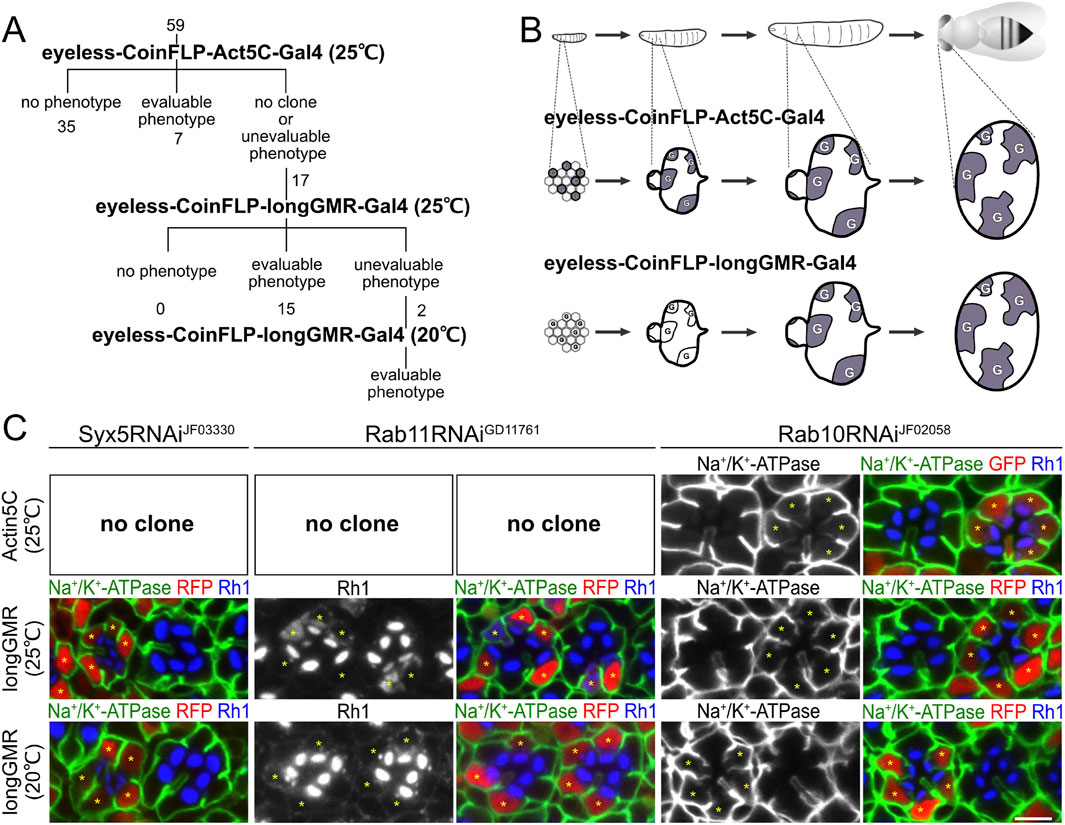
Figure 1. Three-step RNAi screening of SNAREs involved in polarized transport in Drosophila photoreceptors. (A) Schematic diagram of RNAi screening of SNAREs involved in polarized transport. (B) Schematic diagram of the Gal4 expression pattern in developing eyes by eyeless–CoinFLP–Act5C–Gal4 and eyeless–CoinFLP–longGMR–Gal4. Alteration of the original schematic diagram in the study by Bosch et al. (2015). (C) Immunostaining of Syx5RNAiJF03330-, Rab11RNAiGD11761-, or Rab10RNAiJF02058-expressing retina by eyeless–CoinFLP–Act5C–Gal4 and eyeless–CoinFLP–longGMR–Gal4 using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. GFP or RFP (red) and asterisks represent the cells expressing RNAi constructs. Scale bar: 5 μm (C).
To evaluate the effects of the 17 RNAi lines, we attempted to delay the onset of Gal4 expression in the second step of screening. Both the GMR and longGMR promoters have been used to induce expression in all developing retinal cells, starting in the third-instar larvae, i.e., posterior to the morphogenetic furrow (Wernet et al., 2003). The longGMR promoter provides milder but more photoreceptor-specific expression than the GMR promoter (Wernet et al., 2003). Thus, we generated a transgenic fly with the CoinFLP–Gal4 construct under the control of the longGMR promoter by replacing the Act5C promoter. On combining it with an eyeless-FLP, we referred to this configuration as eyeless–CoinFLP–longGMR–Gal4 (Figure 1B). We crossed them with 17 lines of RNAi constructs, which failed to obtain evaluable clones when crossed with the eyeless–CoinFLP–Act5C–Gal4 system. Gal4-positive clones with milder and more evaluable phenotypes were obtained from 15 of the 17 RNAi lines. However, two of these lines exhibited phenotypes that were too severe for evaluation. As the third step in the screen, we reared the flies at lower temperatures to reduce the effect of RNAi because Gal4 activity is temperature-dependent. Flies expressing these two RNAi constructs in eyeless–CoinFLP–longGMR–Gal4 were maintained at low temperatures, resulting in the eventual acquisition of evaluable clones.
3.2 Validation of the three-step RNAi-mosaic eye screeningTo validate the results of the three-step RNAi screening, we first examined the phenotypes induced by Syx5, Rab10, or Rab11 RNAi constructs under the three conditions and compared them with the phenotypes previously reported for the loss of the Syx5, Rab10, and Rab11 genes (Figure 1C). Syx5 is involved in early Golgi trafficking in fly photoreceptor cells; in homozygous clones of hypomorphic allele Syx5EP2313, ommatidia are smaller than those in wild-type clones, and the photoreceptors have less Rh1 localizing only in the rhabdomeres (Satoh et al., 2016b). The RNAi construct targeting Syx5, Syx5RNAiJF03330, failed to produce any clones when driven by eyeless–CoinFLP–Act5C–Gal4 but produced reasonably grown clones when driven by eyeless–CoinFLP–longGMR–Gal4. In such clones, the ommatidia are small, and the photoreceptors have less Rh1, which is limited only to the rhabdomeres and replicates the phenotypes of the homozygous Syx5EP2313 clones. We previously reported that Rab11 is involved in post-Golgi transport to the rhabdomeres; in the photoreceptor homozygous null mutant Rab11EP3017 and photoreceptors expressing the dominant-negative mutant Rab11N124I, rhabdomeres are small, and Rh1 is not concentrated in the rhabdomeres but accumulates in the cytoplasm (Satoh et al., 2005). In this study, Rab11RNAiGD11761 did not produce any clones by eyeless–CoinFLP–Act5C–Gal4 but produced them by eyeless–CoinFLP–longGMR–Gal4. Rhabdomeres were small, and Rh1 accumulated in the cytoplasm of clones expressing Rab11RNAiGD11761, which perfectly phenocopied Rab11EP3017 homozygous cells and photoreceptors expressing dominant-negative Rab11N124I. In Rab11RNAiGD11761 clones generated by eyeless–CoinFLP–longGMR–Gal4 at lower temperatures, the rhabdomeres grew to a normal size, and only low Rh1 accumulation was detected. Thus, the strength of the phenotypes can be manipulated by the type of CoinFLP–Gal4 driver and temperature. In contrast, the phenotype of the Rab10RNAiJF02058 clone was not significantly attenuated by the type of CoinFLP–Gal4 driver or ambient temperature. Rab10 is required for the basolateral transport of Na+/K+-ATPase in fly photoreceptors, and dominant-negative Rab10S23N expression or Rab10 deficiency induces the mislocalization of Na+/K+-ATPase to the stalk membrane (Nakamura et al., 2020; Ochi et al., 2022). In Rab10RNAiJF02058 clones generated with either eyeless–CoinFLP–Act5C–Gal4 or eyeless–CoinFLP–longGMR–Gal4, Na+/K+-ATPase was mislocalized to the stalk membrane, even when grown at a lower temperature (20°C). These results indicate that the three-step RNAi mosaic screening scheme can mitigate the difficulties arising from the unpredictable efficacy of RNAi, thus providing a powerful method for screening genes involved in biosynthetic transport pathways in Drosophila photoreceptors.
3.3 Classification of SNARE proteins by knockdown phenotypesThrough the three-step mosaic screening of 59 SNARE RNAi lines, we identified 24 lines with meaningful phenotypes (Supplementary Table S1). The lines were classified into three categories (Table 1). Category I represents RNAi lines that induce a strong reduction in Rh1 in the rhabdomeres and accumulate Rh1 in the cytoplasm. This phenotype is typically observed in photoreceptors lacking genes involved in post-Golgi transport to the rhabdomeres, as shown by Rab11RNAiGD11761 (Figure 1C) (Li et al., 2007; Otsuka et al., 2019; Satoh et al., 2005; Yamashita et al., 2022). A limited Na+/K+-ATPase signal of both alpha (α) is also detected in the cytoplasm. Category I included three RNAi lines against nSyb (Figures 2A, D, E) and three against Ykt6 (Figures 2B, F, G). Category II represents RNAi lines that induce not only weak Rh1 and Na+/K+-ATPase-α cytoplasmic accumulation but also strong Na+/K+-ATPase mislocalization to the stalk membrane and shortened basolateral membranes. The latter is a characteristic phenotype caused by the loss of genes involved in post-Golgi trafficking to the basolateral membrane, as observed in Rab10RNAiJF02058 (Figure 1C). Four RNAi lines targeting Snap29 (Figure 4) and two lines targeting Syx1A (Figure 3) were classified as category II. Category III represents RNAi lines that induce a large reduction in Rh1 in the rhabdomeres without the cytoplasmic accumulation of Rh1. This phenotype is typical of photoreceptors deficient in ER-to-Golgi or intra-Golgi trafficking, as observed in Syx5RNAiJF03330 (Figure 1C), but it can also be observed in the defects of genes that regulate other processes. At least one RNAi line for the genes Syx6, Syx7, Syx8, Syx13, Syx18, Bet1, Use1, Sec20, Sec22, and Syb was classified as category III (Figure 5).

Table 1. List of RNAi and gRNA constructs giving the eye phenotypes.
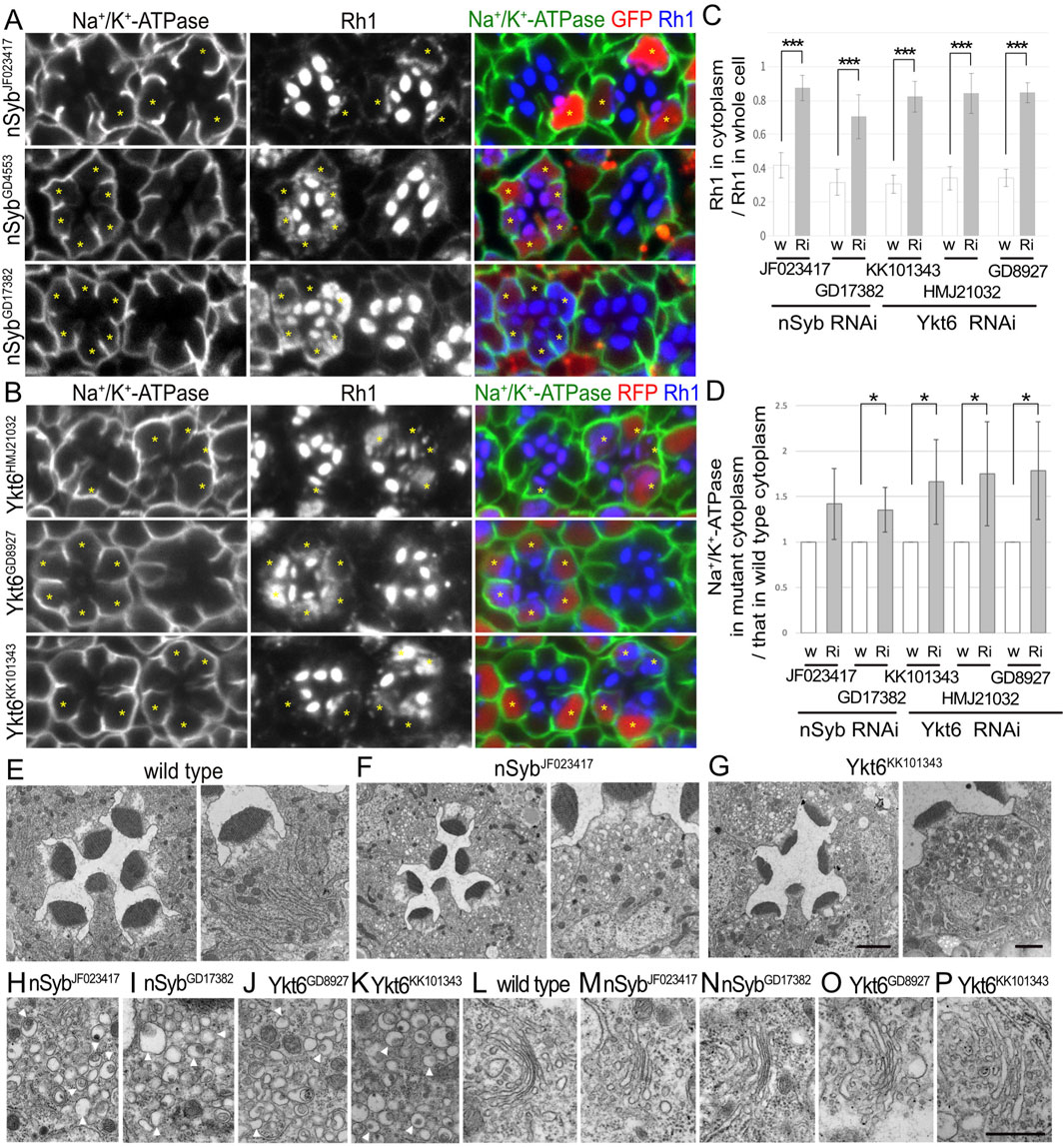
Figure 2. nSyb and Ykt6 are required for the post-Golgi transport toward the rhabdomeres. (A) Immunostaining of nSybRNAiJF023417-, nSybRNAiGD4553-, or nSybRNAiGD17382-expressing retina by eyeless–CoinFLP–Act5C–Gal4 using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. GFP (red) and asterisks represent the cells expressing RNAi constructs. (B) Immunostaining of Ykt6RNAiHMJ21032-, Ykt6RNAiGD8927-, or Ykt6RNAiKK101343-expressing retina by eyeless–CoinFLP–longGMR–Gal4 using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. RFP (red) and asterisks represent the cells expressing RNAi constructs. (C) The ratio of integrated fluorescence density for Rh1 staining of the cytoplasm compared with that of whole cells was plotted. White bars indicate wild-type cells (w), and gray bars indicate the cells expressing RNAi constructs (Ri). Error bars indicate the SD with five retinas. Significance according to two-tailed unpaired Student’s t-test: ***p < 0.001. (D) The ratio of integrated fluorescence density for Na+/K+-ATPase-α staining of the cytoplasm in the cells expressing RNAi constructs compared with that in wild-type cells was plotted. White bars indicate wild-type cells (w), and gray bars indicate the cells expressing RNAi constructs (Ri). Error bars indicate the SD with four retinas. Significance according to two-tailed unpaired Student’s t-test: *p < 0.05. (E–G) Electron micrographs of the wild-type (D), nSybRNAiJF023417(E), Ykt6RNAiGD8927(F), or Ykt6RNAiKK101343(G) ommatidium (left) and photoreceptor (right). (H–K) Electron micrographs of the vesicles accumulated in the cytoplasm of the photoreceptors expressing nSybRNAiJF023417(H), nSybRNAiGD17382(I), Ykt6RNAiGD8927(J), or Ykt6RNAiKK101343(K). Arrowheads indicate unusual irregular-shaped vesicles. (L) Electron micrographs of the Golgi stacks of wild-type photoreceptors. (M–P) Electron micrographs of the Golgi stacks of the photoreceptors expressing nSybRNAiJF023417(M), SybRNAiGD17382(L, N), Ykt6RNAiGD8927(O), or Ykt6RNAiKK101343(P). Scale bar: 5 μm (A, B), 2 μm [(E–G) left], 1 μm [(E–G) right], and 500 nm (H–P).
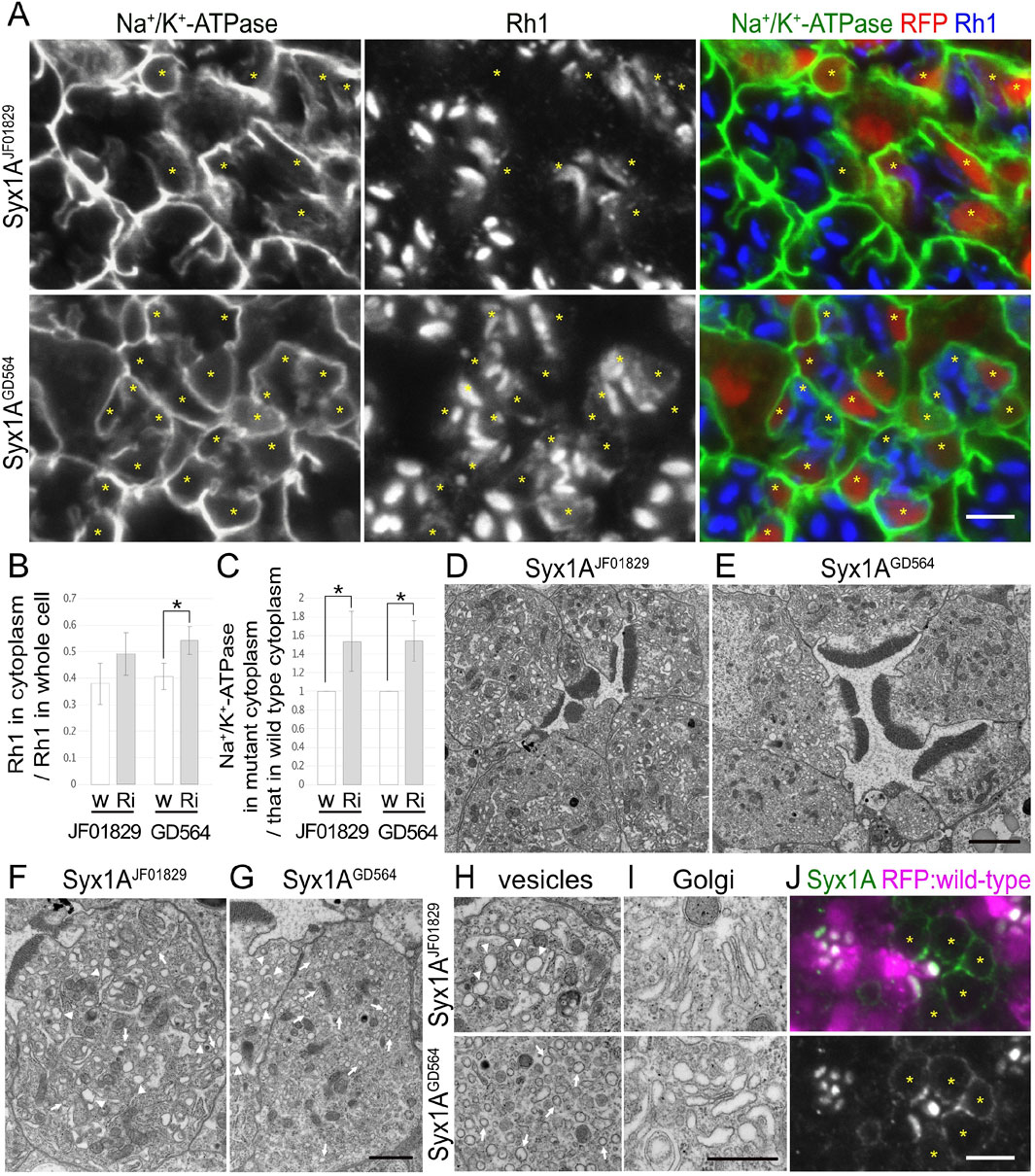
Figure 3. Syx1A is required for the polarized transports toward both the rhabdomeres and basolateral membrane. (A) Immunostaining of Syx1ARNAiJF01829- or Syx1ARNAiGD564-expressing retina by eyeless–CoinFLP–longGMR–Gal4 using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. RFP (red) and asterisks represent the cells expressing RNAi constructs. (B) The ratio of integrated fluorescence density for Rh1 staining of the cytoplasm compared with that of whole cells was plotted. White bars indicate wild-type cells (w), and gray bars indicate the cells expressing RNAi constructs (Ri). Error bars indicate the SD with four retinas. Significance according to two-tailed unpaired Student’s t-test: *p < 0.05. (C) The ratio of integrated fluorescence density for Na+/K+-ATPase-α staining of the cytoplasm in the cells expressing RNAi constructs compared with that in wild-type cells was plotted. White bars indicate wild-type cells (w), and gray bars indicate the cells expressing RNAi constructs (Ri). Error bars indicate the SD with four retinas. Significance according to two-tailed unpaired Student’s t-test: *p < 0.05. (D, E) Electron micrographs of the ommatidium expressing Syx1ARNAiJF01829(D) or Syx1ARNAiGD564(E). (F, G) Electron micrographs of the photoreceptor expressing Syx1ARNAiJF01829(F) or Syx1ARNAiGD564(G). Arrowheads and arrows indicate unusual irregular-shaped vesicles and normal vesicles, respectively. (H) Electron micrographs of the vesicles accumulating in the cytoplasm in the photoreceptors expressing Syx1ARNAiJF01829 (upper) and Syx1ARNAiGD564 (lower). Arrowheads and arrows indicate unusual irregular-shaped vesicles and normal vesicles, respectively. (I) Electron micrographs of Golgi stacks in the photoreceptors expressing Syx1ARNAiJF01829 (upper) and Syx1ARNAiGD564 (lower). (J) Immunostaining of EMC3Δ4 mosaic retina using anti-Syx1A antibody (green). RFP (magenta) and asterisks represent the wild-type cells and mutant cells, respectively. Scale bar: 5 μm (A), 2 μm (D, E), 1 μm (F, G), 500 nm (H, I), and 5 μm (J).
3.4 Two R-SNAREs, nSyb and Ykt6, are strongly involved in post-Golgi transport of Rh1We recently reported that photoreceptor cells homozygous for null or hypomorphic alleles of nSyb show a defect in Rh1 transport to the rhabdomeres, accompanied by massive cytoplasmic accumulation of Rh1 (Yamashita et al., 2022). As expected, the expression of nSybRNAiJF023417, nSybRNAiGD4553, or nSybRNAiGD17382 reduced Rh1 in the rhabdomeres and strongly induced Rh1 accumulation in the cytoplasm (Figure 2A). In nSyb-knockdown photoreceptors in nSybRNAiJF023417 or nSybRNAiGD17382 mosaic retinas, 87% ± 7% or 70% ± 13% of Rh1 was localized to the cytoplasm in nSyb-knockdown photoreceptors, whereas only 42% ± 7% or 32% ± 8% of Rh1 was localized in the cytoplasm in control cells in the same retinas (Figure 2C). In contrast to the strong inhibitory effect on Rh1 transport, the basolateral transport of Na+/K+-ATPase was not severely affected by the expression of nSybRNAiJF023417, nSybRNAiGD4553, or nSybRNAiGD17382. Mislocalization of Na+/K+-ATPase was not observed, but some Na+/K+-ATPase α-signals were found in the cytoplasm (Figure 2A). In nSyb-reduced photoreceptors in nSybRNAiJF023417 and nSybRNAiGD17382 mosaic retinas, cytoplasmic Na+/K+-ATPase-α was increased to 1.42 ± 0.39-fold and 1.35 ± 0.20-fold, respectively, of that in wild-type cells (Figure 2D), although the significance was achieved only in photoreceptors expressing nSybRNAiJF023417. We next investigated the localization of a phototransduction channel in the rhabdomeres, transient receptor potential (TRP), Na+/K+-ATPase beta subunit (Nrv), inter-rhabdomeric space (IRS)-making protein, Eys, and a stalk membrane protein, Crb. Some TRP and Nrv are accumulated in the cytoplasm, similar to Rh1 and Na+/K+-ATPase-α in nSyb-reduced photoreceptors (Supplementary Figure S1A left). In nSyb-reduced photoreceptors in nSybRNAiGD17382 mosaic retinas, cytoplasmic Nrv was increased to 2.00 ± 1.12-fold of that in wild-type cells (Supplementary Figure S1C), although there was no significance. However, Eys and Crb were normally localized in the IRS and stalk membrane (Supplementary Figure S1A, right). These results indicated that nSyb mainly regulates the transport to the rhabdomeres. Na+/K+-ATPase accumulation in cytoplasm is surprising, but a normal level of Na+/K+-ATPase in the basolateral membrane indicates that the role of nSyb in basolateral transport is rather limited compared to the rhabdomere transport.
In addition, the expression of Ykt6RNAiHMJ21032, Ykt6RNAiGD8927, and Ykt6RNAiKK101343 strongly induced Rh1 accumulation in the cytoplasm (Figure 2B). In Ykt6-reduced photoreceptors in Ykt6RNAiHMJ21032, Ykt6RNAiGD8927, and Ykt6RNAiKK101343 mosaic retinas, 84% ± 12%, 85% ± 6%, and 82% ± 9% of Rh1 were localized in the cytoplasm, respectively, whereas only 34% ± 7%, 34% ± 5%, and 30% ± 5% of Rh1 were localized to the cytoplasm in control cells of the same retina, respectively (Figure 2C). These three RNAi lines also induced some cytoplasmic accumulation and minor mislocalization of Na+/K+-ATPaseα (Figure 2B), although shrinkage of the basolateral membrane was not prominent. In Ykt6-reduced photoreceptors in Ykt6RNAiHMJ21032, Ykt6RNAiGD8927, and Ykt6RNAiKK101343 mosaic retinas, cytoplasmic Na+/K+-ATPase-α was increased to 1.75 ± 0.57-fold, 1.79 ± 0.54-fold, and 1.73 ± 0.47-fold, respectively, of that in wild-type cells (Figure 4C). Some Nrv, TRP, Eys, and Crb were also accumulated in the cytoplasm (Supplementary Figures S1B, C). In Ykt6-reduced photoreceptors in Ykt6RNAiKK101343 and Ykt6RNAiGD8927 mosaic retinas, cytoplasmic Nrv was significantly increased to 2.22 ± 0.70-fold and 2.14 ± 0.74-fold, respectively, of that in wild-type cells (Supplementary Figure S1C). Ykt6 might be involved in the post-Golgi transport toward the rhabdomeres, stalk, and basolateral membrane, although the effects on rhabdomere transport were quite severe compared to the other domains. To confirm the involvement of Ykt6 in post-Golgi transport, we attempted to observe the phenotypes of Ykt6 homozygous null clones in the mosaic retina; however, neither Ykt6C nor Ykt6G0155 homozygous clones were obtained, indicating the strong cell lethality of Ykt6 null alleles.
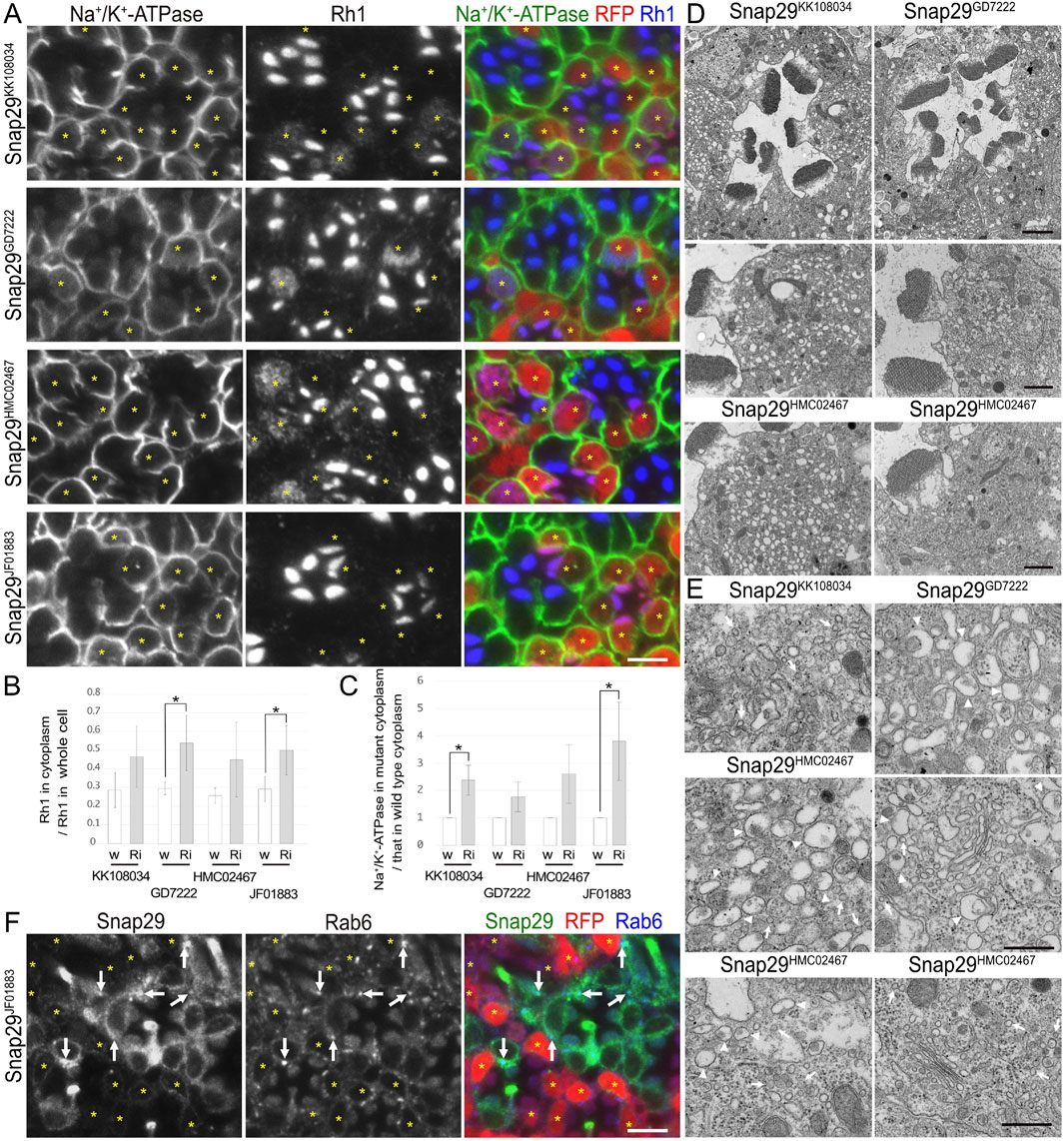
Figure 4. Snap29 is required for the polarized transports toward both the rhabdomeres and basolateral membrane. (A) Immunostaining of Snap29RNAiKK108034-, Snap29RNAiGD7222-, Snap29RNAiHMC02467-, or Snap29RNAiJF01883-expressing retina by eyeless–CoinFLP–longGMR–Gal4 using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. RFP (red) and asterisks represent the cells expressing RNAi constructs. (B) The ratio of integrated fluorescence density for Rh1 staining of the cytoplasm compared with that of whole cells was plotted. White bars indicate wild-type cells (w), and gray bars indicate the cells expressing RNAi constructs (Ri). Error bars indicate the SD with four retinas. Significance according to two-tailed unpaired Student’s t-test: *p < 0.05. (C) The ratio of integrated fluorescence density for Na+/K+-ATPase-α staining of the cytoplasm in the cells expressing RNAi constructs compared with that in wild-type cells was plotted. White bars indicate wild-type cells (w), and gray bars indicate the cells expressing RNAi constructs (Ri). Error bars indicate the SD with four retinas. Significance according to two-tailed unpaired Student’s t-test: *p < 0.05. (D) Electron micrographs of the ommatidia or photoreceptors expressing Snap29RNAi. Used RNAi lines are indicated. (E) Electron micrographs of the vesicles accumulated in the cytoplasm or Golgi stacks in the cells expressing Snap29RNAi. Used RNAi lines are indicated. Arrowheads and arrows indicate unusual irregular-shaped vesicles and normal vesicles, respectively. (F) Immunostaining of the retina expressing Snap29RNAiJF01883 using anti-Snap29 (green) and Rab6 (blue) antibodies. RFP (red) and asterisks represent the cells expressing Snap29RNAiJF01883. Arrows indicate Snap29 localization on Golgi stacks. Scale bar: 5 μm (A), 2 μm [(D) in ommatidia], 1 μm [(D) in photoreceptors], 500 nm (E), and 5 μm (F).
To understand the detailed phenotype of nSyb or Ykt6 RNAi knockdown, thin sections of pupal photoreceptors were examined using electron microscopy (Figures 2E–P). Photoreceptor cells expressing nSybRNAiJF023417, nSybRNAiGD17382, Ykt6RNAiGD8927, and Ykt6RNAiKK101343 exhibited smaller rhabdomeres and accumulated cytoplasmic vesicles. These vesicles were irregularly shaped and typically 200 nm in size, which was much larger than normal secretory vesicles (Figures 2H–K, arrowheads). The appearance of these vesicles was similar to that of pleomorphic vesicles that accumulated in the cytoplasm of photoreceptors lacking factors for post-Golgi transport to rhabdomeres, Rab11, Rip11, didum, or exocyst complexes (Beronja et al., 2005; Li et al., 2007; Satoh et al., 2005). Golgi stacks showed normal morphologies in nSyb and Ykt6 knockdown photoreceptors (Figures 2L–P). nSyb localized particularly at the base of the rhabdomeres with Rab11 (Supplementary Figure S1D, arrows) and also Golgi stacks (Yamashita et al., 2022). These results indicate that both nSyb and Ykt6 are required for post-Golgi transport.
3.5 Syx1A and Snap29 are involved in post-Golgi transport for both rhabdomeres and basolateral membranesIn addition to the nSyb and Ykt6 RNAi constructs, Syx1A and Snap29RNAi constructs also induced the cytoplasmic accumulation of Rh1, but their effects were rather limited (Figures 3A, 4A). In Syx1A-knockdown photoreceptors in Syx1ARNAiJF01829 and Syx1ARNAiGD564 mosaic retinas, 49% ± 8% and 54% ± 5% of Rh1 were localized in the cytoplasm, respectively, whereas only 38% ± 8% and 40% ± 10% of Rh1 were localized in the cytoplasm of control cells in the same retinas (Figure 3B). In Snap29-reduced photoreceptors in Snap29RNAiKK108034, Snap29RNAiGD7222, Snap29RNAiHMC02467, and Snap29RNAiJF01883 mosaic retinas, 46% ± 16%, 54% ± 15%, 45% ± 20%, and 50% ± 13% of Rh1 were localized in the cytoplasm, respectively, whereas only 29% ± 9%, 29% ± 3%, 26% ± 4%, and 29% ± 7% of Rh1 were localized in the cytoplasm of the control cells in the same retina (Figure 4B).
In addition to Rh1, Na+/K+-ATPase-α was accumulated in the cytoplasm in Syx1A or Snap29-knockdown photoreceptor cells in similar or severer levels than those in nSyb- or Ykt6-deficient photoreceptors (Figures 3A, 4A). In Syx1A-reduced photoreceptors in Syx1ARNAiJF01829 and Syx1ARNAiGD564 mosaic retinas, cytoplasmic Na+/K+-ATPase-α was significantly increased to 1.54 ± 0.32-fold and 1.54 ± 0.22-fold in wild-type cells (Figure 3C). In Snap29-reduced photoreceptors in Snap29RNAiKK108034, Snap29RNAiGD7222, Snap29RNAiHMC02467, and Snap29RNAiJF01883 mosaic retinas, cytoplasmic Na+/K+-ATPase-α was increased to 2.37 ± 0.55-fold, 1.76 ± 0.55-fold, 2.59 ± 1.07-fold, and 3.79 ± 1.43-fold, respectively, of that in wild-type cells (Figure 4C), although the significance was achieved only in photoreceptors expressing Snap29RNAiKK108034 and Snap29RNAiJF01883. Moreover, the shrinkage of the basolateral membrane was prominent in both Syx1A and Snap29-deficient photoreceptors (Figures 3A, 4A), which is in contrast to nSyb or Ykt6-deficient photoreceptors. Syx1A reduction by Syx1AJF01829 expression and Snap29 reduction by Snap29RNAiJF01883 or Snap29RNAiHMC02467 expression were confirmed by anti-Syx1A or anti-Snap29 antibody staining (Figure 4F; Supplementary Figures S2A, B). Nrv, TRP, Eys, and Crb localizations were also investigated in Snap29RNAiKK108034 and Snap29RNAiGD7222 mosaic retinas. Only Nrv was accumulated in the cytoplasm, and the localization of TRP, Eys, and Crb was not affected (Supplementary Figures S2C, D). In Snap29-reduced photoreceptors in Snap29RNAiKK108034 and Snap29RNAiGD7222 mosaic retinas, cytoplasmic Nrv was significantly increased to 1.39 ± 0.27-fold and 1.40 ± 0.25-fold, respectively, of that in wild-type cells (Supplementary Figure S1C).
Similar to nSyb or Ykt6-knockdown photoreceptors, electron microscopy results showed that in photoreceptors expressing either Syx1ARNAiJF01829, Syx1ARNAiGD564, Snap29RNAiKK108034, Snap29RNAiGD7222, Snap29RNAiHMC02467, or Snap29RNAiJF01883, the rhabdomeres were thinner and vesicles accumulated in the cytoplasm (Figures 3D–H, 4D). However, these vesicles were of two types: large pleomorphic vesicles, as observed in nSyb or Ykt6 mutants (Figures 3F–H, 4E, arrowheads), and regularly shaped vesicles with diameters of approximately 100 nm, which are typical secretory vesicles (Figures 3F–H, 4E, arrows). These regular small vesicles were likely post-Golgi vesicles destined for the basolateral membranes. In contrast, the Golgi stacks were unaffected by the reduction in Syx1A or Snap29 in photoreceptors (Figures 3I, 4E; Supplementary Figure S2E).
Next, we examined the localization of endogenous Syx1A and Snap29 using specific antibodies (Figures 3J, 4F). Syx1A appeared to localize exclusively to the rhabdomeres in wild-type cells (Figure 3J), but its basolateral localization was pronounced in EMC3-deficient cells, which have unpacked small rhabdomeres (Satoh et al., 2015). Even if Syx1A is located on both the basolateral and rhabdomere membranes, it may only stain the rhabdomeres because the rhabdomere membranes are too condensed in wild-type cells. Immunostaining of Snap29RNAiJF01883 mosaic retina showed an anti-Snap29 antibody in the cytoplasm (Figure 4F) and Golgi stacks (Figure 4F arrows) in the wild-type cells. These results are consistent with the hypothesis that Syx1A and Snap29 are involved in post-Golgi trafficking.
3.6 Candidate genes involved in the transport between the ER and Golgi stacksCategory III represents RNAi lines that induce a large reduction in Rh1 in rhabdomeres without ectopic accumulation anywhere in the cell (Figure 5). Two Syx7RNAi constructs, Syx7RNAiKK101990 and Syx7RNAiGD2767, showed a mild reduction in Rh1 expression and disorganization of rhabdomeres (Figures 5A, B). It is reported that Syx7 is involved in protein degradation in the stalk, rhabdomeres, and basolateral membrane, as well as in the secretion of proteins (Laffafian and Tepass, 2019). As Syx7 null alleles, Syx71 and Syx74 are available from stock centers but are lethal; we examined the phenotypes of FRT mosaic retinas with wild-type and homozygous photoreceptors of these null mutants. Clones homozygous for Syx71 or Syx74 were small, and the ommatidia often contained fewer photoreceptors, indicating that Syx71 or Syx74 alleles were cell-lethal (Figures 5C, D). However, photoreceptors homozygous for either Syx71 or Syx74 were not severely malformed, although the rhabdomeres of Syx71 but not Syx74 homozygous photoreceptors were thinner or more disorganized (Figures 5C, D). In contrast, Rh1-containing large vesicles (Figure 5C arrows), supposed to be multivesicular bodies (Satoh et al., 2005; Satoh and Ready, 2005), were not found in Syx71 homozygous photoreceptors (Figure 5C), suggesting impaired endocytosis in Syx7 null cells, which is consistent with the results of previous studies (Laffafian and Tepass, 2019). Thus, we excluded the possibility that Syx7 is involved in ER–Golgi trafficking or polarized transport.
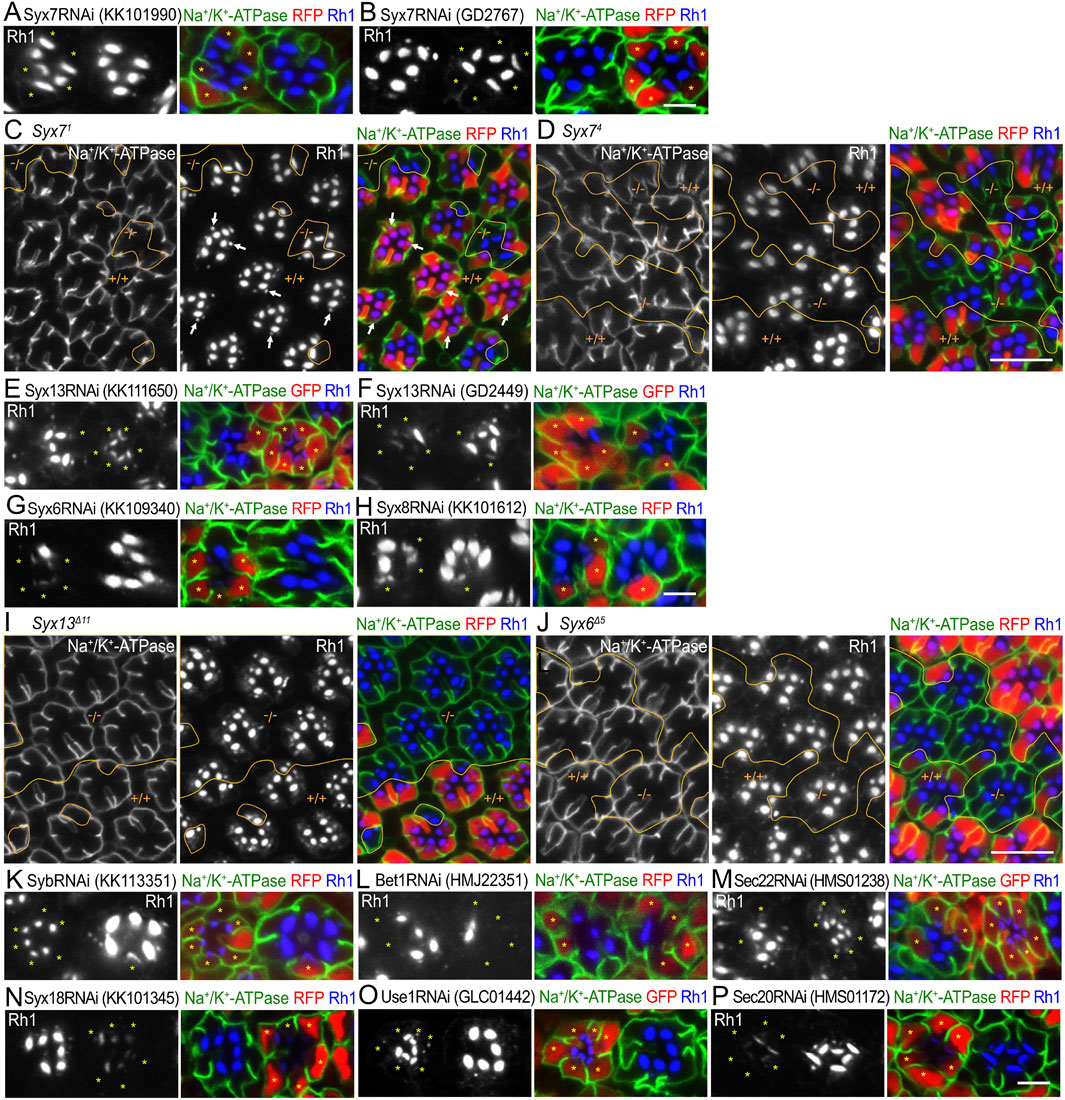
Figure 5. SNARE RNAi mosaic retinas showing category-III phenotypes (A, B). Immunostaining of Syx7RNAiKK101990(A) or Syx7RNAiGD2767(B)-expressing retina by eyeless–CoinFLP–longGMR–Gal4 using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. RFP (red) and asterisks represent the cells expressing RNAi constructs. (C, D) Immunostaining of Syx71(C) or Syx74(D) mosaic retina using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. RFP (red) represents wild-type cells. Arrows indicate Rh1-containing large vesicles. (E–H) Immunostaining of SNARE RNAi construct-expressing retina by eyeless–CoinFLP–Act5C–Gal4 (E, F) or eyeless–CoinFLP–longGMR–Gal4 (G, H) using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. GFP or RFP (red) and asterisks represent the cells expressing RNAi constructs. (I, J) Immunostaining of Syx13Δ11(I) or Syx6Δ5(J) mosaic retina using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. RFP (red) represents the wild-type cells. (K–P) Immunostaining of SNARE RNAi construct-expressing retina by eyeless-CoinFLP–Act5C–Gal4 (M, O) or eyeless–CoinFLP–longGMR–Gal4 (K, L, N, P) using anti-Na+/K+-ATPase-α (green) and anti-Rh1 (blue) antibodies. GFP or RFP (red) and asterisks represent the cells expressing RNAi constructs. (K) SybRNAiKK113351, (L) Bet1RNAiHMJ22351, (M) Sec22RNAiHMS01238, (N) Syx18RNAiKK101345, (O) Use1RNAiGLC01442, and (P) Sec20RNAiHMS01172. Scale bar: 5 μm (A, B), 10 μm (C, D), 5 μm (E–H), 10 μm (I, J), and 5 μm (K–P).
Two Syx13RNAi constructs, Syx13RNAiKK111650 and Syx13RNAiGD2449 (Figures 5E, F); one Syx6RNAi construct, Syx6RNAiKK109340 (Figure 5G); and one construct Syx8RNAiKK101612 (Figure 5H) showed a strong reduction in Rh1. Since null alleles for Syx13, Syx6, and Syx8 were not available, we attempted to generate them using the CRISPR/Cas9 system. Null alleles of Syx13 and Syx6 were successfully generated; however, we failed to obtain a null allele of Syx8. We examined the phenotypes of photoreceptors homozygous for these null alleles in FRT mosaic retinas. The clones homozygous for either Syx13Δ11 or Syx6Δ5 were large but did not show an obvious phenotype (Figures 5I, J). Similarly, one SybRNAi construct, SybRNAiKK113351, showed a severe reduction in Rh1 (Figure 5K); however, we previously showed that there is no phenotype for Syb21-15 or Syb25-77 (Yamashita et al., 2022). Thus, the RNAi constructs for Syx13, Syx6, and Syb were mistargeted to other genes, and Syx13, Syx6, and Syb were not required for transport between the ER and Golgi stacks or for polarized transport.
Bet1, Syx5, and Sec22 were involved in anterograde trafficking between the ER and Golgi stacks via COPII vesicles. As expected, a strong reduction in Rh1 was observed upon expressing the RNAi construct Bet1RNAiHMJ22351 or Sec22RNAiHMS01238 (Figures 5L, M). Sec22 reduction by Sec22RNAiHMS01238 expression was confirmed by anti-Sec22 antibody staining (Supplementary Figure S2F). Syx18, Use1, Sec20, and Sec22 are SNAREs of Golgi-derived COPI vesicles that fuse with the ER membrane. The expression of RNAi constructs of Syx18KK101345, Use1GLC01442, or Sec20HMS01172 showed strong effects on Rh1 levels (Figures 5N–P). The detailed phenotypes of these SNAREs, which are involved in anterograde and retrograde transport between the ER and Golgi, are described elsewhere (Tago et al., 2024).
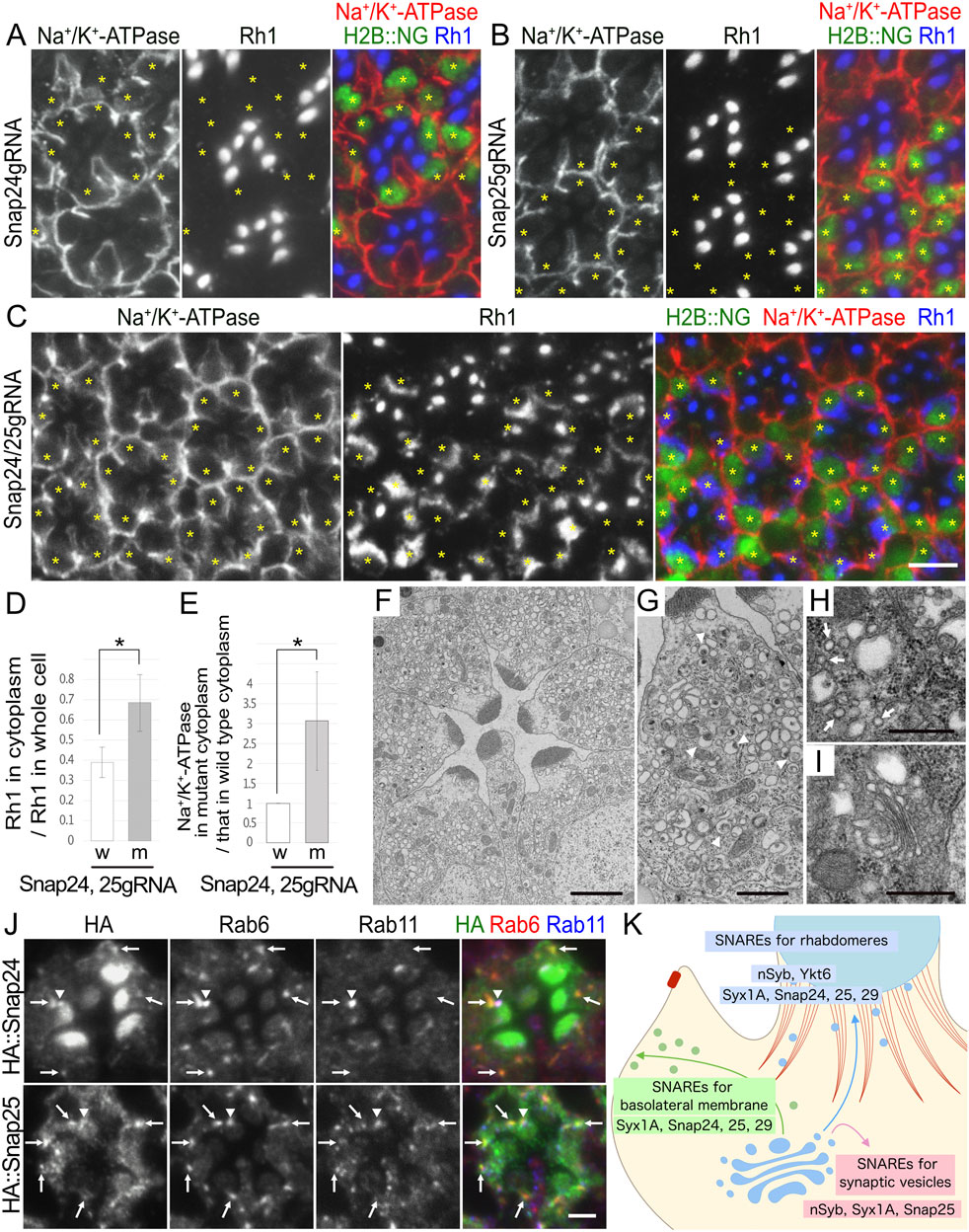
3.7 CRISPR/Cas9–mosaic eye screeningTo expand and complement the mosaic RNAi screening described above, we attempted to utilize the growing resources of transgenic gRNA lines in a scheme involving the instant somatic KO of target genes in the mosaic retina. A project to generate gRNA lines covering every gene in the fly genome is underway using DRSC/TRiP functional genomics resources. In their in vivo CRISPR project, fly lines with gRNA designed to knockout genes were called TKO lines. We obtained TKO lines for 15 SNARE genes. Other gRNA lines for Snap24/Snap25/Snap29 were also used in a previous study (Poe et al., 2019). The gRNA lines we used in this study are given in Supplementary Table S2. To generate a mosaic of Cas9 expression in the retina, we generated fly lines carrying constructs of CoinFLP–longGMR–FLAG::Cas9–T2A–Histone2B::2x NeonGreen (H2B::NG) and CoinFLP–Act5C–FLAG::Cas9–T2A–Histone2B::GFP (H2B::GFP). These lines were combined with eyeless-FLP to induce the mosaic expression of FLAG::Cas9 and H2B::NG/GFP. We named them eyeless–CoinFLP–longGMR–FLAG::Cas9-H2B::NG or eyeless–CoinFLP–Act5C-FLAG::Cas9–H2B::GFP, which can generate the somatic mosaic of CRISPR knockout by a single cross with gRNA lines.
Somatic mosaic retinas were generated to test this scheme. We used eyeless-CoinFLP–longGMR–FLAG::Cas9–H2B::NG, which was designed to perform somatic CRISPR KOs in third-instar larvae. Unfortunately, none of the SNARE TKO lines showed any phenotype; however, a previously reported gRNA line expressing both Snap24gRNA and Snap25gRNA (Poe et al., 2019) showed a category-II phenotype: Rh1 accumulation in the cytoplasm and Na+/K+-ATPase mislocalization to the stalk membrane when these two gRNAs were co-activated by eyeless–CoinFLP–longGMR–FLAG::Cas9-H2B::NG. In contrast, when Snap24gRNA and Snap25gRNA were activated independently, no phenotype was observed in the Cas9-positive clones (Figures 6A–C). In triple-KO photoreceptors of Snap24, Snap25, and Snap29, mosaic retinas contained many Cas9-positive photoreceptors, but none of them showed a category-II phenotype. Instead, the mosaic retinas often contained patches lacking photoreceptors, indicating cell death before the late pupal stage (Supplementary Figure S3A). These results collectively indicate that when CRISPR/Cas9 generates alleles with sufficiently reduced function in all targeted loci, Snap24/Snap25 double-KO photoreceptors are viable, and Snap24/Snap25/Snap29 triple-KO photoreceptors are lethal; however, when one or more of the targeted genes received no or insufficient change by CRISPR/Cas9, the cells could generate photoreceptors in good shape.
留言 (0)