Felix Isso Bado1* , Nicolas Kagambega1,2
, Nicolas Kagambega1,2 and Adama Yameogo1,2
and Adama Yameogo1,2
1Geosciences and Environment Laboratory, Department of Earth Sciences, Joseph Ki-Zerbo University, Ouagadougou, Burkina Faso
2Mining Engineering Department, Higher School of Engineering, Yembila Abdoulaye Toguyeni University, Fada N’Gourma, Burkina Faso
Corresponding Author E-mail:isso_bado@ujkz.bf
Article Publishing History
Article Received on : 08 Oct 2024
Article Accepted on :
Article Published : 09 Dec 2024
Effective management and control of mining discharges is essential to minimise their negative impact on the environment and local communities. This study focuses on the assessment of the pollution risks that may be posed by Perkoa mining discharges. After characterizing the different mining discharges, the main physicochemical parameters were determined. The level and risk of pollution have also been assessed. The results show that these mining discharges are already oxidized, therefore acidic, with contents of potentially harmful elements well above the standards in force. They are therefore potentially a generator of acid mine drainage, which is harmful to the environment and communities.
KEYWORDS:Burkina Faso; Geochemistry; Mining discharges; Pollution; Zinc mine
Download this article as:Bado F. I, Kagambega N, Yameogo A. Geochemistry of Mining Discharges from the Ppolymetallic Deposit of the Perkoa Underground Zinc Mine in Central-western Burkina Faso – West Africa. Orient J Chem 2024;40(6).
Bado F. I, Kagambega N, Yameogo A. Geochemistry of Mining Discharges from the Ppolymetallic Deposit of the Perkoa Underground Zinc Mine in Central-western Burkina Faso – West Africa. Orient J Chem 2024;40(6). Available from: https://bit.ly/3OJs9Ku
Introduction
Over the last two decades, Burkina Faso has seen considerable growth in its mining sector, with around ten industrial mines being put into operation1. In 2023 in particular, production dominated by gold reached 57,338 tons.
Although the mining industry is a source of economic benefits and job creation, it also has harmful effects on the environment. These damages are felt on the human level, water resources, flora, fauna, landscape, soil and air in addition to the pressure on the territorial space 2-4.
In fact, the geochemistry of metal deposits, as well as the use of chemicals for the treatment of ores, increase the effects of pollution resulting from poor management of mining discharges5. Mining discharges containing sulphide minerals can lead to the generation of acid mine drainage (AMD), from the oxidation of sulphides6. In the absence of actions to control acid mine drainage, significant environmental damage can occur with the acidification of the environment and the release of pollutants. The phenomenon of acid mine drainage leads to the deterioration of water quality with the increase in acidity and the concentration of metals and oxyanions7. The Perkoa zinc deposit, in view of its polymetallic character, is dominated by sulphurous minerals such as iron-rich sphalerite, pyrite and pyrrhotite8.
The objective of this study is to assess the pollution risks that Perkoa mining discharges may represent, based on their geochemical characteristics.
Materials and Methods
Study Area
The study area is located approximately 135 kilometers from Ouagadougou, the capital of Burkina Faso, in the Center West region (Fig. 1), where the Perkoa zinc deposit was exploited from 2013 to 2022. Perkoa is located at a latitude of 12°19’ north and a longitude of 2°20’ west. The climate is of the northern Sudanese type with two seasons: a long dry season from October to May with temperatures between 15 and 41°C, and a rainy season from June to September with minimums and maximums varying between 22 and 35.3°C.
The Perkoa deposit is a stratiform hydrothermal mineralization of volcanogenic massive sulphide type containing zinc, lead and silver, located in the central part of the Boromo greenstone belt which are Paleoproterozoic formations locally composed of sedimentary and volcanic rocks, with magmatic intrusions9.
The geological units listed on the scale of the deposit are mainly massive andesites to the west, an intrusion of quartz diorite to the east, and felsic tuffs, observable between these two entities. Numerous dykes of dominant intermediate composition, acidic or basic, cut across these different geological units. The mineralization is mainly hosted in silicified felsic tuffs. It contains sphalerite (zinc sulfide), pyrite (iron sulfide), pyrrhotite (magnetic iron sulfide) and, incidentally, galena (lead sulfide) which is more or less argentiferous, contained in a matrix of quartz and barium sulfate9.
Sampling
Four samples were taken, representing the different types of discharges stored on the mine site, namely (Fig. 2):
“Oxidized” sterile rock (RS),
Grinder waste (RB),
Mining residues from the first cell of the tailings storage facility, called cell 1 (Rcel.1),
Mining residues from the third cell of the tailings storage facility, called cell 3 (Rcel.3).
Each sample (0.5-5 kg) is a composite of three to five sub-samples, located at a distance of 10-20 m from each other, collected from the four types of mining discharges. The samples taken were transported to the laboratory, where they were crushed, then pulverized to 75 micrometers, for the various analyzes.
Analysis
Particle size analysis provides information on the sizes and statistical distribution of particle sizes. It was determined by mechanical sieving using a column of sieves of decreasing diameters.
The pH and saturated paste conductivity (EC) were measured using a HACH multi-parameter device equipped with probes, in order to predict a possible generation of mine drainage (static test).
It is therefore a question of checking whether the mining discharges is already oxidized or not. This test consists of saturating each sample ground to a mesh of less than 1 mm in the presence of distilled water until a paste is obtained. After a 24-hour equilibrium period, pH and conductivity are measured. If the pH is less than 4, the sample is considered naturally acidogenic. This test is an initial indicator of pH and short-term reactivity of minerals. Also, a conductivity particularly higher than 2 mS/cm implies a significant quantity of soluble constituents10.
The determination of the metal content was carried out through laboratory analyses using ICP-OES. To do this, a one-gram mass of pulverized and homogenized solid sample is completely digested by an acid to obtain a solution.
Result and Discussion
Granulometry
The sizes corresponding to the passing percentages 10%, 30% and 60% were used to calculate the uniformity (UC) and curvature coefficients (Cc) (Table 1) according to the following formulas11 :

D10 corresponds to the diameter of the grains attributable to 10% passing and is commonly called the effective diameter. D60 is the particle diameter corresponding to 60% passing and D30 is the particle diameter corresponding to 30% passing.
UC and Cc correspond respectively to the uniformity coefficient and the curvature or concavity coefficient. The uniformity coefficient makes it possible to specify whether the particle size is spread out, discontinuous or uniform, i.e. the uniformity of the dimensions of the grains constituting the matrix. The lower the UC value, the more uniform the particle size. Values close to 1 reveal particles of uniform dimensions. For very narrowly spread grain sizes, such as beach sands, the values of UC = 2 to 3, whereas for very wide grain sizes UC can be greater than 1511.
The curvature coefficient is used to measure the degree of heterogeneity of the particle size, i.e. the relative importance of the different particle size classes. The particle size is said to be continuous if 1 < Cc < 6 and discontinuous if Cc < 1 or Cc > 6.
Table 1: Granulometric characteristics
Parameter
D10 (mm)
D30 (mm)
D60 (mm)
UC
Cc
Rcel.1
0.09
0.11
0.19
2.13
0.77
Rcel.3
0.09
0.11
0.20
2.30
0.75
RB
5.80
7.40
24
4.14
0.39
RS
6.80
18.70
27.50
4.04
1.87
The mining residues (Rcel.1 and Rcel.3) have very little spread, heterogeneous and fine granulometries (UC of 2.13 and 2.3 respectively, between 2 and 3), continuous and similar to those of sands. Their granulometry therefore covers the granulometric classes of fine sand (0.5mm to 80µm) and silt (80µm to 2µm).
The very coarse rejects (RB and RS) on the other hand present relatively spread out particle sizes (UC respectively of 4.14 and 4.04) and continuous.
Grain size is an important characteristic that influences the void ratio and porosity of mining residues. The particle size depends, among other things, on the mineralogy of the ore being mined, the degree of alteration of the ore, the grinding and separation or extraction process. Metals tend to concentrate in the fine fractions, while the coarse fractions act more as diluting agents5.
The particle size therefore influences the water content, the void ratio and the hydraulic conductivity of the mining residues and consequently the physical and chemical stability (oxidation and generation of acid mine drainage).
Mining residues (Rcel.1 and Rcel.3) is very problematic given its very fine particle size and the chemicals used during the treatment process.
pH and conductivity in saturated paste
Four classes can be distinguished based on pH values and electrical conductivity (EC) (Table 2)12.
Table 2: Potential risk of acid mine drainage (AMD) generation from pH and electrical conductivity (EC)12
pH
EC (µS/cm)
Class
Diagnostic
pH ≤ 4
> 1000
I
AMD proven
4 < pH < 5
> 750
II
AMD in development confirmed
5 < pH < 6
> 500
III
AMD in potential development
pH > 6
≤ 500
IV
No risk of AMD
The pH values (Table 3) indicate that all mining discharges are acidic, therefore oxidized and do not comply with the standards in force, with the exception of the crusher discharge (RB) whose pH is at the limit of the acceptable threshold value and therefore also problematic.
As for electrical conductivity, the values are well above the standards required in Burkina Faso. They are 198 to 550 times the threshold value for soil quality.
In accordance with the classification of (Table 2) 12, the pH and electrical conductivity values of the Perkoa mine discharges (Table 3) show that Rcel.1 and Rcel.3 are in class II (4 < pH < 5 and EC > 750 µS/cm), that is, where the development of mine drainage is confirmed. These two materials have pH values of 4.1 and 4.66 with EC values of 1101 and 745 µS/cm respectively.
The grinder discharge (RB) belongs to class III (5 < pH < 6 and EC > 500 µS/cm), therefore with a potential development of acid mine drainage.
The sterile discharge (RS) has a pH of 4.81 but with an electrical conductivity of 396 µS/cm. The pH value indicates that the sterile waste is already oxidized (therefore acidic) even though the electrical conductivity is less than 500 µS/cm. Which shows that these materials are also problematic.
Table 3: Main physicochemical parameters
As
Cd
Cr
Co
Cu
Fe
Pb
Ni
Zn
pH
EC
Unity
mg/kg
mg/kg
mg/kg
mg/kg
mg/kg
mg/kg
mg/kg
mg/kg
mg/kg
µs/Cm
DL
1
0.1
0.2
0.5
2
5
1
0.5
1
—
—
Rcel.1
491
150
270
< DL
74
210169
1335
50
50664
4.1
1101
Rcel.3
537
< DL
120
27
174
196623
583
51
14689
4.66
745
RB
504
35
310
19
69
55351
2322
59
6071
5.88
682
RS
915
14
69
< DL
35
56421
4240
20
1306
4.81
396
Quality standard
20
1
75
25
50
5
100
50
200
5.5-8
2
DL : Detection Limit
Contents of potentially harmful elements (PHEs)
The contents of potentially harmful elements are recorded in Table 3. For the mining residue of cell 1 (Rcel.1), the levels are significantly higher than the standards in force with the exception of cobalt and nickel, for which the value is at the limit of the threshold value (50 mg/kg).
As regards cell 3 (Rcel.3), apart from cadmium (content below the detection limit), all other metals show values which are well above the standards in force.
Regarding the grinder discharge (RB), only the cobalt content meets the acceptable values with regard to soil quality.
For sterile discharge (RS), cobalt, copper and nickel have contents lower than the standards in force.
The very high iron content of all mining discharges could be explained by the mineralogy of the deposit which is rich in pyrite (iron sulfide) and pyrrhotite (magnetic iron sulfide). The relatively high levels of potentially harmful elements in mining discharges may result in potential pollution of the soil, which will therefore become unsuitable for agriculture and toxic for plants13,14,15.
Geoaccumulation index (Igeo)
The geo-accumulation index was used to assess the level of contamination, in other words, the level of accumulation of potentially harmful elements in the various mining discharges (Table 4). It is calculated according to the formula below16.
Igeo = log2 (Cx /1.5 × Bgx)
Cx = the measured content of a given element (metal) x,
Bgx = geochemical background for element x.
1.5 = constant that takes into account fluctuations in the content of a given substance in the environment17.
Table 4: Geoaccumulation Index Classification
Igeo Value
Igeo Class
Quality designation
> 5
6
Extremely contaminated
4-5
5
Heavily to extremely contaminated
3-4
4
Heavily contaminated
2-3
3
Moderately to heavily contaminated
1-2
2
Moderately contaminated
0-1
1
Uncontaminated to moderately contaminated
0
0
Not contaminated
The Igeo values are recorded in Table 5 and allow the following classes to be distinguished :
Table 5: Geoaccumulation index values
Mining discharges
As
Cd
Cr
Co
Cu
Fe
Pb
Ni
Zn
Rcel.1
0.33
1.00
0.07
0.00
0.02
140.60
0.11
0.01
3.40
Rcel.3
0.36
0.00
0.03
0.02
0.04
131.53
0.05
0.01
0.98
RB
0.34
0.23
0.08
0.01
0.01
37.03
0.19
0.02
0.41
RS
0.61
0.09
0.02
0.00
0.01
37.74
0.34
0.01
0.09
Not contaminated (Class 0) with all potentially harmful elements except iron, for mining discharges Rcel.3, RB and RS and with regard to mining residue Rcel.1, As, Cr, Co, Cu, Pb and Ni.
Uncontaminated to moderately contaminated (Class 1) with cadmium for mining residue Rcel.1 only.
Heavily contaminated (Class 4) with zinc for mining residue Rcel.1 only.
Extremely contaminated (Class 6) with iron for all mining discharges.
Contamination factor (CF)
The contamination factor (CF) is used to determine the level of metallic contamination in mining discharges. The FC corresponds to the ratio of the measured content of an element x in the tested material and the background content of the same element.
CF = Cx / Bgx
Cx = the measured content of a given element (metal) x,
Bgx = geochemical background for element x.
According to the classification of18, the following different levels of contamination can be distinguished (Table 6).
Table 6: Different classes of contamination factor18
CF Value
CF Class
Contamination level
> 6
IV
Very high contamination
3 – 4
III
Considerable contamination
1 – 3
II
Moderate contamination
< 1
I
No or low contamination
The values of the contamination factor (Table 7), show that the following classes can be highlighted:
All mining discharges are non- to low-contamination (Class I) with chromium, cobalt, copper and nickel. The same applies to Rcel.3 and RS in relation to cadmium. The same observation can be noted for Rcel.1, Rcel.3 and RB releases with regard to lead.
Rcel.1, Rcel.3 and RB are moderately contaminated (Class II) with arsenic. The same applies to RB in relation to cadmium and zinc and RS in relation to lead.
Rcel.1 presents considerable contamination (Class III) in cadmium, as do Rcel.3 and RS, respectively in arsenic and zinc.
All mining discharges are very heavily contaminated (Class IV) with iron as in the case of the geo-accumulation index.
Table 7: Contamination Factor Values
Mining discharges
As
Cd
Cr
Co
Cu
Fe
Pb
Ni
Zn
Rcel.1
1.64
5.00
0.34
0.00
0.07
700.56
0.53
0.07
16.89
Rcel.3
1.79
0.00
0.15
0.09
0.17
655.41
0.23
0.07
4.89
RB
1.68
1.17
0.39
0.06
0.07
184.50
0.93
0.08
2.02
RS
3.05
0.47
0.09
0.00
0.04
188.07
1.70
0.03
0.44
Pollution Risk Index (PI)
The pollution risk index (PI) is used to assess the pollution of mining discharges by PHEs19-21. This index corresponds to multi-element pollution by its formula. Thus, the formula used is that proposed by22, modified by23.
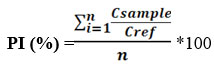
Where Csample is the sample concentration and Cref: reference concentration and n: the number of PHEs considered.
Depending on the IP value, the pollution risk can be divided into four classes23 :
For IP = 0, this indicates that there is no risk of pollution ;
If IP < 100%, the risk of pollution is low ;
IP = 100%, corresponds to the threshold of the risk of pollution or moderate pollution ;
For IP > 100%, the risk of pollution is high or high pollution.
The values of the pollution risk index (Table 8) are all less than 100%, which means that the risk of pollution is low23.
Table 8: Values of pollution risk indices (PI)
Mining discharges
Sum of CF
PI
Rcel.1
725.11
80.57
Rcel.3
662.82
73.65
RB
190.91
21.21
RS
193.87
21.54
The risk of pollution is low but not zero or non-existent. The exposure of mining discharges to meteoric agents could accelerate the oxidation process already very significant with acidic pH (4.1 to 5.8) which promote the release and solubilization of metals and consequently would lead to environmental pollution24-26.
Source and origin of pollution
The Pearson correlation matrix (Table 9) indicates a strong positive correlation between pH and PHEs such as lead and nickel, meaning that increasing lead and nickel contents lead to decreasing pH. In fact, all mining discharges have relatively high lead and nickel contents (with the exception of RS discharges where the nickel content is below the standard) and acidic pH values. A negative correlation is however observed between pH and cadmium, cobalt and zinc.
A strong correlation is also observed between electrical conductivity (EC) and cadmium and zinc. These would therefore be the cause of the increase in electrical conductivity observed in mining discharges.
This strong positive correlation also exists between arsenic and PHEs such as cobalt and copper. However, the correlation between arsenic and cadmium, chromium, lead and zinc is significantly negative.
Cadmium is very strongly and positively correlated with zinc, but significantly negatively with nickel.
Chromium, on the other hand, has a strong positive correlation with lead and nickel. The correlation between chromium and cobalt, and copper is significantly negative.
As for cobalt, it is strongly correlated with copper. Its correlation with lead and nickel is significantly negative.
Copper is negatively correlated with lead. The same observation can be made regarding nickel and zinc. Lead, on the other hand, is strongly correlated with nickel.
Table 9: Pearson Correlation Matrix
Variables
pH
EC
As
Cd
Cr
Co
Cu
Pb
Ni
Zn
pH
1
EC
-0,83
1
As
0,07
-0,61
1
Cd
-0,89
0,99
-0,51
1
Cr
0,40
0,18
-0,89
0,06
1
Co
-0,67
0,14
0,67
0,26
-0,95
1
Cu
-0,25
-0,34
0,95
-0,21
-0,98
0,89
1
Pb
0,73
-0,22
-0,64
-0,34
0,92
-0,99
-0,85
1
Ni
0,98
-0,69
-0,14
-0,78
0,58
-0,81
-0,45
0,85
1
Zn
-0,85
0,99
-0,58
0,99
0,14
0,18
-0,29
-0,26
-0,72
1
Values in bold are different from 0 at a significance level of r=0.05
These observations show that the positive correlations could correspond to a relationship on their mineralogical or substitution origin27,28. In fact, in most deposits containing cobalt we find arsenic, as well as sulfides and arsenides of copper, nickel and cobalt. In addition, cobalt can be obtained by partial calcination of sulfide ores in the presence of flux, which can be the case for mining residues that have undergone treatment processes to extract marketable zinc. Arsenic is a common element in Birimian greenstone belts in Burkina Faso3.
If we refer to the polymetallic deposit of Perkoa, it contains sphalerite (zinc sulfide), pyrite (iron sulfide), pyrrhotite (magnetic iron sulfide) and, incidentally, galena (lead sulfide) more or less argentiferous, contained in a matrix of quartz and barium sulfate9. This mineralogical composition also confirms certain correlations observed at the level of the Pearson correlation matrix. The same applies to the indices and factors used to assess the level of pollution, which show that the most problematic elements (with the exception of iron) are arsenic, cadmium, lead and zinc.
Principal component analysis (PCA) of physicochemical parameters of mining discharges indicate three distinct groups (Fig. 3). The contribution of F1 in ACP corresponds to 55.67% variability, while that of F2 is 44.33% variability.
The first group is represented by the pH parameter with chromium, nickel and lead. The second is composed of the EC parameter with cadmium and zinc and the last group by arsenic, cobalt and copper. The F1 axis would represent the parameters causing pollution from the oxidation and solubilization of metals such as chromium, nickel and lead causing the drop in pH and cadmium and zinc which would cause the increase in electrical conductivity.
Arsenic, copper, cadmium and zinc are variables influenced by factor F2, the source of pollution of which would be linked to the destabilization of materials by oxidation in contact with oxygen and/or water. It is also noted that if the content of arsenic and copper increases, that of cadmium and zinc decreases and vice versa. The CE value appears to be related to the Cu and Zn contents (Fig. 2).
Conclusion
The physicochemical parameters of the mining discharges from the Perkoa zinc mine show that these materials are oxidized and acidic (pH ranging from 4.1 to 5.8) with potential or confirmed development of acid mine drainage. The levels of the various potentially harmful elements are well above the standards in force. They relate to the polymetallic character of the deposit which is very rich in sulfides. The assessment of the level of pollution reveals that the most problematic elements are arsenic, cadmium, iron, lead and zinc. Their oxidation and solubilization would therefore be at the origin of the very considerable drop in pH and the relatively very high values of electrical conductivity.
Acknowledgement
The authors thank Dr Moussa PALENFO who made the field work possible and the Higher Education Support Program (PAES) for the financial contribution as well as the laboratory of the Bureau of Mines and Geology of Burkina (BUMIGEB).
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
References
MEMC., Statistical yearbook 2023 of the Ministry of Energy, Mine and Quarries (MEMC)., Report, 2024, French.Loska K, Wiechula D, Barska B, Cebula E, Chojnecka A. Assessment of Arsenic enrichment of cultivated soils in Southern Poland. Polish., Journal of Environmental studies. 2003 2, 87-192.Kagambega, N., Sawadogo, S. and Gordio, A., High arsenic enrichment in water and soils from Sambayourou watershed, Burkina Faso (West Africa)., International Journal of Environmental Monitoring and Analysis, 2014, 2(3): 6-12.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Related Posts
留言 (0)