Spinal muscular atrophy (SMA) is an autosomal-recessive degenerative neuromuscular disease affecting lower motor neurons due to the absence of the survival of motor neurons (SMN1) gene (1). This results in progressive denervation and muscle weakness, predominately affecting the proximal muscles (2). SMA occurs in 1 out of 10,000 to 20,000 live births (3, 4), with age of onset and clinical severity often correlated with the number of survival motor neurons 2 (SMN2) copies (5).
Those individuals with one or two SMN2 copies are typically non-ambulatory, whereas individuals with three to four SMN2 copies can walk either household or community distances (6). Although this trend is noted, recent systematic review suggests that SMN2 copy number alone does not predict walking ability (7). Studies have also found that sex, along with SMN2 copy number, is a predictor of disease severity related to ambulatory function, with males more severely affected than females (5, 8). Although it is not clear how sex-based differences are affected by ambulatory function, specifically.
As the disease progresses, individuals with SMA may lose their ability to ambulate due to weakness, fatigue, or injuries resulting from falls. So far, clinicians have not been able to accurately predict loss of ambulation in SMA. A history of falls is often reported prior to complete loss of ambulation (9, 10). Limited research assessed the association between ambulation and falls in SMA, including a multiple-case study design of five adults and two children showing that strength, gait velocity, and variability in gait pattern were not predictors of falls but stride-length variability was a significant risk factor (9). In contrast, gait speed is a validated predictor of fall risk in other neurological diseases such as Huntington’s disease (11, 12), Parkinson’s disease (13, 14), and stroke (15).
Within neuromuscular disease, there is limited data on gait speed in adults. One study assessed children with Charcot–Marie Tooth Disease finding a gait speed of 0.94 m/s, significantly slower when compared to healthy controls (16). Another study found that gait speed was slower and stride length was shorter in children with neuromuscular disease (Duchenne Muscular Dystrophy, Becker Muscular Dystrophy, and Charcot–Marie-Tooth) compared to typical developing peers (17). In healthy adults between 30 and 39 years old, gait speeds tend to range from 1.32 to 1.55 m/s in men and 1.19 to 1.48 m/s in women (18). Clinicians use the 10 Meter Walk Test or 6 Minute Walk Test (6MWT) to assess gait speed and stratify an individual as a community or household ambulatory. In healthy adults, those with a gait speed of <0.8 m/s were found to be at an increased risk of falling and were classified as household ambulators (19). In an upper and lower motor neuron disease such as amyotrophic lateral sclerosis (ALS), changes in gait including shorter stride length, increased stride duration, slower walking speed, and increased gait variability were noted in comparison to healthy adults (20–23). These spatial–temporal changes in gait were associated with lack of independence, lower quality of life, and increased risk for falls (24). A study investigating the changes in lower extremity function across clinical phenotypes in ALS also found that a history of falls serves as an early indicator of disease progression and severity (25). Assessing fall risk in the neuromuscular population is important as it can signal a progressive loss in ambulation (25).
Fatigue, defined as an overwhelming sense of tiredness, lack of energy, feeling of physical or mental exhaustion or both (26–28), is reported in 81–100% of patients with SMA and found to be a major barrier for participation in physical activities, recreational activities, and overall mobility (29–31). The term fatigue can be broken down into two domains: physical and mental fatigue (26). Physical fatigue can be captured as fatigability which can be measured by the performance on an outcome measure (26). Studies have used the 6MWT to capture rate of speed decline across the minutes of the test as a means of capturing fatigability in ambulant patients. One study found a 17% decline in speed between the first minute and the last minute of the 6MWT in individuals with SMA, capturing fatigability during ambulation (32). In a healthy cohort, a study found a typical percent decline in speed from the first minute to the last minute during the 6MWT was 1.4% (33).
However, to date, no study has been able to associate fall risk with a quantifiable value in SMA. The purpose of this study was to investigate falls in adults with genetically confirmed 5q-SMA and the predicting factors associated with falls. We hypothesized that individuals with reported falls would express greater fatigability and slower gait speed when compared to individuals who are not falling.
2 Materials and methods 2.1 Study designA retrospective chart review of 53 charts was conducted at the Ohio State University Wexner Medical Center. This study was approved by the institutional review board. The study period occurred between 2017 and 2023.
2.2 Study populationInclusion criteria included: age > 18 years old, genetic confirmation of 5q-SMA, and ability to ambulate at least 10 meters with or without an assistive device. Exclusion criteria included: lack of follow-up post baseline assessment.
2.3 Study overviewCollected data included age, sex, initial age at symptom onset, SMN2 copy number, body mass index (BMI), and 6MWT (34, 35) distance in individuals that report falls or no falls with an average follow up of 36 months (range of 12–66 months). Clinic visits occurred on average every 6 months post baseline visit. During the clinic visits, the functional assessments were performed according to standardized practice by neurologic physical therapists who specialize in neuromuscular conditions (35). A fall history was obtained by the physical therapist or physician asking the patient if they had experienced any falls since their last visit and if any of those falls led to injuries or hospitalizations. For the 6MWT, individuals were permitted to use an assistive device if the individual used one daily. To assess gait speed and fatigability, the 6MWT speeds at 1, 2, and 6 min were used for analysis during the visit preceding the reported fall to best predict the speed at which the individual was ambulating with prior to their fall. Multiple time points with the 6MWT were analyzed to attempt to capture the possible fatigability by assessing the change in gait speed over time. Fatigability was represented as the percent difference in speeds between minutes 1 and 2, minutes 2 and 6, and minutes 1 and 6. We recorded the pre-fall gait speed as it best reflects their gait characteristics preceding the fall. We recorded the slowest speed to investigate a minimum gait speed that does not correspond with fall. We also recorded the fastest speed to document a range in ambulation. Additionally, we calculated the median gait speed for each individual as a robust measure of central tendency. As a comparison, everyone’s slowest, fastest and median 6MWT speeds at 1, 2, and 6 min were analyzed in both cohorts. Standardized motivation was provided at each minute of the test.
2.4 Statistical analysisA chi–squared test was used to compare categorical variables. For continuous variables, 2-sampled t-tests (for normally distributed data) and the Mann – Whitney U test (for non-normally distributed data) were used to compare the faller and non-faller groups.
3 ResultsFourteen ambulatory patients (9 men, 5 women; median age 33.50; range 17–48) with SMA met enrollment criteria and were classified as fallers (Fall, n = 10) and non-fallers (NFall, n = 4) based on subjective report during clinic visits. Patients had clinic visits every 6 months for a minimum of 2 years. The average follow-up time was 36 months (range 12–66 months). All participants were receiving Nusinersen except for one in the fall cohort who was receiving Risdiplam consistently throughout the entire study period. None of the participants used assistive devices during the 6MWT. However, in the community for longer distances, 60% of Fall cohort used wheelchairs compared to 25% in the NFall cohort. Age, sex, initial age at symptom onset, and BMI were similar between the falling and non-falling cohorts as shown in Table 1. All patients with 3 SMN2 copies were in the fall cohort, whereas people with 4 or more copies were distributed between the two cohorts.
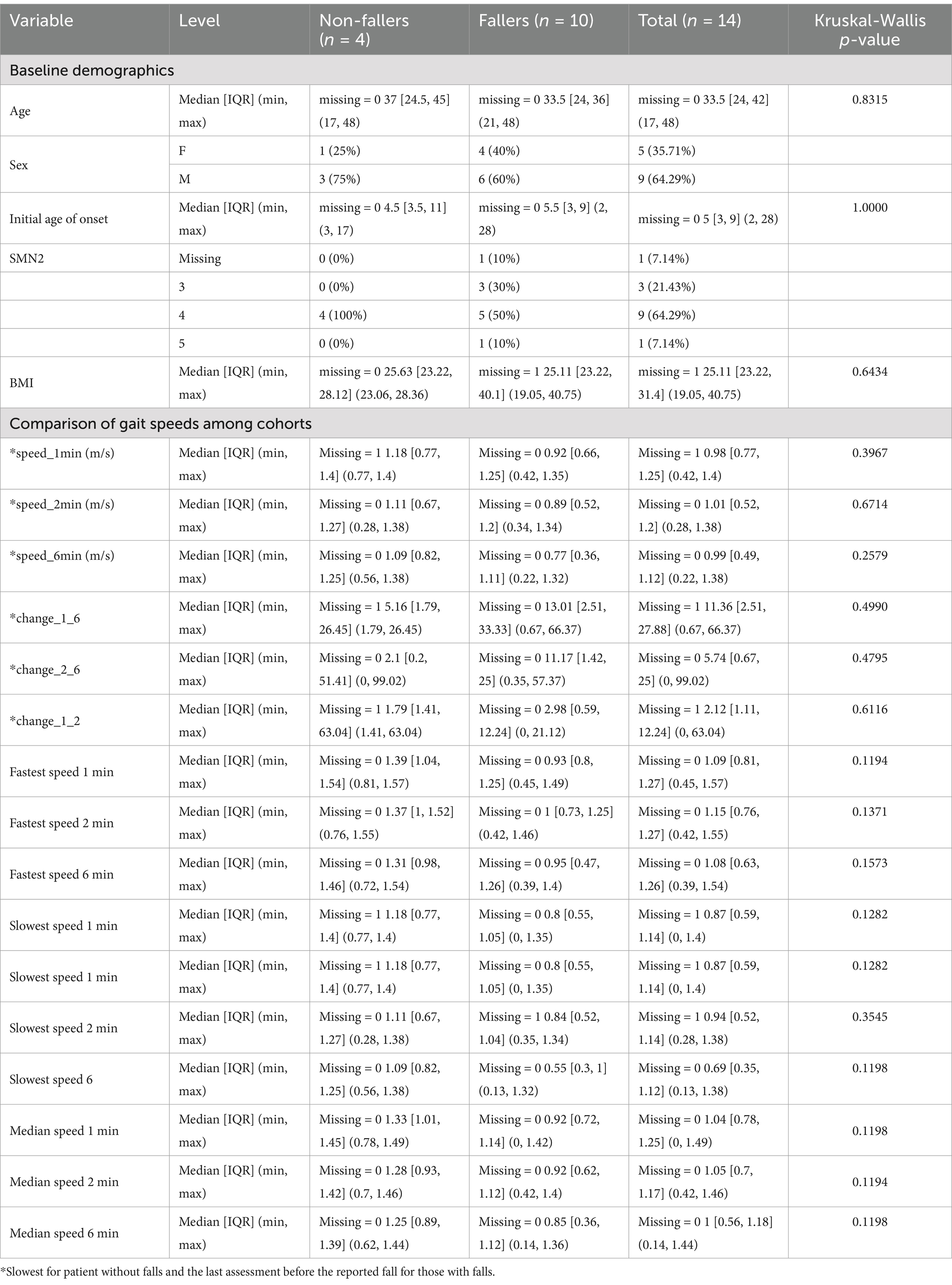
Table 1. Comparison of baseline demographics and gait speeds between the cohorts.
No falls or injuries occurred during the testing. The distances walked and corresponding gait speeds at 6 min between the falling and non-falling groups were not associated with predicting fall risk as shown in Table 1.
In the Fall cohort, 7 of the 10 patients (70%) had multiple falls over the course of the study period, with two (20%) of those individuals having falls sustaining serious lower extremity fractures resulting in hospitalizations and loss of ambulation. Per physical therapy and physician documentation, no other serious injuries or hospitalizations occurred in the remaining patients.
The gait speeds across minutes 1, 2, and 6 in the 6MWT are illustrated in Figures 1, 2 for both cohorts. We compared the median pre-fall gait speed in the Fall cohort to the slow and fast median gait speeds of the NFall cohort to decern if there was a range of speeds where patients are at a higher risk for falls. Despite considerable overlap between pre-fall gait speeds for fallers and median speeds for non-fallers, a distinct trend emerged. In the Fall cohort, the median speeds at 1, 2, and 6 min, measured at the visit preceding the fall, were 0.92 m/s, 0.89 m/s, and 0.77 m/s, respectively, with one individual reaching 1.32 m/s at 6 min. For non-fallers, the median slowest speeds were 1.18 m/s, 1.11 m/s, and 1.09 m/s, and the median fastest speeds were 1.39 m/s, 1.37 m/s, and 1.31 m/s at minutes 1, 2, and 6, respectively. Therefore, we found that the slowest median speeds at 1, 2, and 6 min for non-fallers were nominally faster than the speed recorded prior to a fall. To further explore the distribution of gait speeds at 1, 2, and 6 min between fallers and non-fallers, the median gait speeds for each participant were calculated across study period and compared between the two groups. In the Fall cohort, the median speeds at 1, 2, and 6 min were 0.92 m/s, 0.92 m/s, and 0.85 m/s, respectively. For the NFall cohort, the median gait speeds across the study period were 1.33 m/s, 1.28 m/s, and 1.25 m/s at minutes 1, 2, and 6, respectively. We therefore noted a trend where individuals who reported falls tended to have gait speeds below 1.0 m/s while those without reported falls tended to have gait speeds above 1.0 m/s shown in Table 1 and Figure 2C.
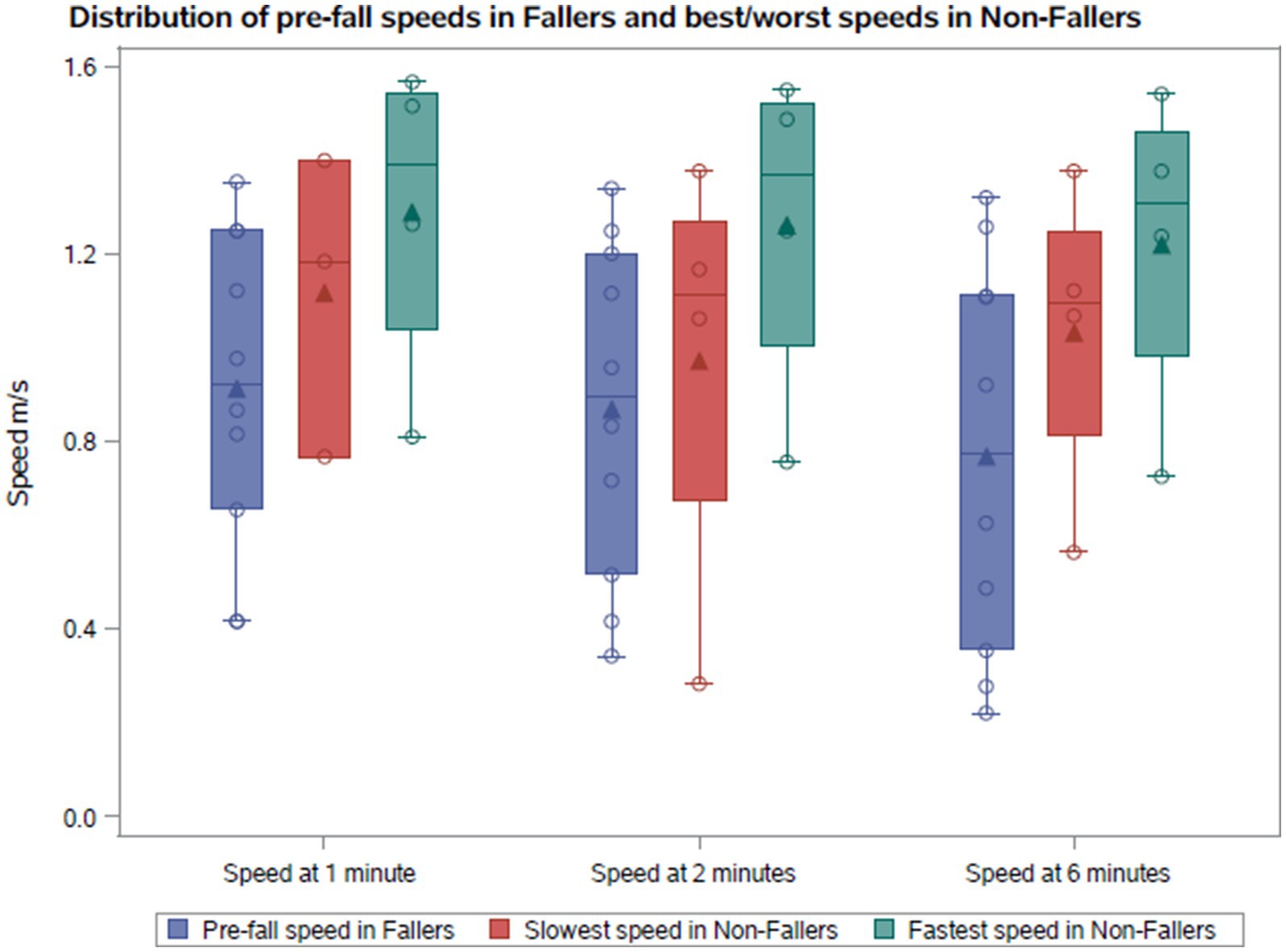
Figure 1. Comparison of the pre-fall gait speeds with the fastest and slowest gait speeds in non-fallers.

Figure 2. (A) Comparison of the slowest recorded gait speeds between the NFall and Fall cohorts. (B) Comparison of the fastest recorded gait speeds between the NFall and Fall cohorts. (C) Comparison of the median gait speeds between the NFall and Fall cohorts.
Similarly, a trend was observed where fallers walk with a slower gait speed than non-fallers. The slowest median speeds at 1, 2, and 6 min were 0.80 m/s, 0.84 m/s, and 0.55 m/s for fallers, compared to 1.18 m/s, 1.11 m/s, and 1.09 m/s for non-fallers as shown in Figure 2A. Whereas the fastest median gait speeds at 1, 2, and 6 min were 0.93 m/s, 1.0 m/s, and 0.95 m/s for fallers, compared to 1.39 m/s, 1.37 m/s, and 1.31 m/s for non-fallers shown in Figure 2B.
Additionally, we captured the percent difference in gait speeds across minutes 1, 2, and 6 during the 6MWT in both groups. Patients who fell showed a nominally greater decline in gait speeds between minutes 1 and 6, as well as 2 and 6, compared to non-fallers during the same intervals, though this difference was not statistically significant. One patient who reported falls demonstrated a 66.37% decline in gait speed from minute 1 to minute 6 in the 6MWT, which is substantially greater than the greatest percent decline in the NFall cohort of 26.45%.
4 DiscussionOur retrospective study assessed the association between reported falls and both gait speed and fatigability in adults with SMA. We cannot draw firm conclusions due to the small sample size of 14 individuals. However, our findings highlight important trends found in the SMA population in regard to describing falls. There was not a statistically significant association between falls and gait speed in this small pilot study; however, we observed a tendency for NFall cohort to have faster gait speeds compared to Fall cohort. In addition, we found that the Fall cohort had a greater relative decline in gait speed between the first and the last minute during the 6MWT compared to the NFall cohort. In our study population, the majority of the Fall cohort experienced multiple falls, with some serious injuries resulting in loss of ambulation. Individuals who reported falls were more likely to own and use a wheelchair for community distances than individuals who did not report falls. This may indicate an increased reliance on assistive devices for mobility in those with a history of falls to reduce risk of injury. Further investigation should focus on analyzing gait and fatigue to assess for changes in ambulation that may require assistive devices or intervention to ensure safe ambulation.
4.1 Gait speed and fall riskIn various neurological conditions, there has been a well-established association between gait speed and fall risk. In diagnoses such as Huntington’s disease (11, 12), Parkinson’s disease (13, 14), and stroke (15), a gait speed under 1.0 m/s correlated with having a higher risk for falls. Our data showed similar trends. The majority of the patients in our Fall cohort had gait speeds less than 1.0 m/s, while all but one individual in the NFall cohort had gait speeds >1.0 m/s as shown in Figure 2C. A larger sample size would allow for further exploration of this finding.
This observed trend highlights the importance of continuing to monitor gait speed over time to assess a patient’s decline in mobility. Our findings align with other publications in the neuromuscular disease population, which also report slower gait speeds compared to healthy controls (16, 17).
We noted that one individual in our Fall cohort reported multiple falls but demonstrated gait speeds >1.0 m/s. This suggests that working past the point of fatigability may contribute to fall risk. On the other hand, one individual reported no falls but demonstrated gait speeds <1.0 m/s, which might be related to higher fear of falling, leading to extra caution with walking. This raises the question should we be assessing fear of falling and other psychosocial factors to assess risk of falls? Falls are often a multifactorial event as a result of weakness, balance impairment, unforeseen perturbation, environmental context, perception of fatigue, fatigability, cognitive awareness, or equipment malfunction. Therefore, though a useful measure to identify a trend, gait speed alone should not be used as a sole predictor of fall risk. Future research should investigate other variables regarding psychometric and fall history measures as well as gait components. To assess for overall risk of falls, collecting a falls history, fear of falling assessment such as Falls Efficacy Scale-International, a balance assessment such as the Activity-Specific Balance Confidence Scale, and a general strength assessment would be recommended.
There are also multiple ways to assess gait speed. During the 6MWT, gait speed is approximated and averaged over time by dividing the total distance traveled by 360 s. Other studies have begun using wearable devices to capture real time information regarding stride length, step length, medial and lateral sway, tibial advancement, and gait speed. These devices have been proven to be more accurate and a reliable means of assessing gait, but researchers can be limited by cost and accessibility of the device. Future research should consider using wearable devices with a larger population to further investigate the association between gait speed and other gait parameters with predicting fall risk.
4.2 Fatigability, gait variability, and fallsThis study attempted to capture fatigability by assessing the fatigability or change in speeds between the minutes of the 6MWT. Both the NFall and Fall cohorts demonstrated a notable decline in gait speed between minutes 1 and 2, 1 and 6, and 2 and 6. Healthy adults were found to have a natural decline in gait speed by 1.4% between minutes 1 and 6 (28). In contrast, our study found that those who fall had a 13.01% decline in gait speeds between minutes 1 and 6, which is almost two and a half times greater than those who did not fall (5.16%). These findings suggest that both cohorts, regardless of fall history, have greater fatigability than healthy controls. A previous study found a 17% decline in speed between the first minute and the last minute of the 6MWT in individuals with SMA (9). However, that study did not separate individuals who did and did not report falls, making our finding relevant as they highlight the possible effect of fatigability on fall risk.
In our study, one of the individuals in the Fall cohort demonstrated a 66.37% decline in gait speed between minutes 1 and 6 of the 6MWT, while the greatest change in gait speeds between the same time points in the NFall cohort was 26.45%. This highlights that fatigability may be a contributing factor in the decline in gait speeds. This aligns with Montes et al. in 2011 finding that fatigability shortens stride length resulting in slower gait speed (27). In addition, 7 of 10 patients in the Fall cohort reported multiple falls between clinic visits.
These findings also reflect greater gait variability among fallers compared to non-fallers. In a study assessing gait in ALS using wearable devices, patients displayed significantly higher gait variability specifically in swing and stance time compared to healthy controls (36). This study also investigated the affects of mild cognitive impairment on gait variability in single-and dual-task conditions (36). As expected, the magnitude of variability was greater during the dual-task condition compared to single-task (36). However, this study suggests that cognitive impairment effects gait variability and is independently associated with fall risk (36). In our study of SMA in a single-task environment, the change in gait speeds across the timed test represent gait variability. Participants in the Fall cohort expressed greater percent change in speed between minutes 1 and 6 (0.67–66.37%) when compared to the NFall cohort (1.79–26.45%) in Table 1. Future studies should assess gait variability in SMA by capturing not only gait speed but also stride length, stability index, cadence, stance time, and swing time with wearable devices. Additionally, performing a gait analysis in the context of a dual-task environment may better predict fall risk in individuals with SMA as it may more accurately depict a community environment.
Future research should continue to use objective data, such as changes in gait speeds during 6MWT, with a larger population to identify a possible standard percent decline that would classify individuals as being at high risk of falls. In addition to objective measures, future research should also consider obtaining perceptions of fatigue such as using the Ratings of Perceived Exertion (RPE) scale, NeuroQol Fatigue Computer Adaptive Test (CAT), or Fatigue Impact Scale (FIS). Since fatigue contains both mental and physical components, utilizing subjective and objective measures would more accurately capture holistic fatigue.
4.3 LimitationsDue to the retrospective nature of the study, we were not able to capture the exact number of falls each participant experienced as we relied on the documentation from the physician and physical therapy notes. The small sample size impacted the power and significance of our statistical analysis. Also, we did not account for the potential influence of the different therapies, such as Nusinersen or Risdiplam, which could impact ambulation, though 13 out of 14 were on Nusinersen. When assessing fatigability, this study only considered changes in gait speed over time and did not account for subjective fatigue or muscle strength. Despite these limitations, the noted trends warrant further investigation to provide more meaningful conclusions in respect to guiding clinical practice and predicting fall risk.
5 ConclusionOur study assessed fall risk in adults with SMA by examining gait speed and fatigability. A trend emerged indicating that lower gait speeds might increase an individual’s risk of falls. The data also suggested a more pronounced decline in gait speed over the course of the 6MWT among fallers, highlighting fatigability as a possible determinant of fall risk that requires further evaluation. Overall, the findings emphasize the importance of continuing to assess gait speed and fatigability in this population to better predict fall risk. Future research should not only continue to assess gait speed but also expand to include other gait parameters in real time using wearable devices.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the Ohio State University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsKJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AJ: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. MA: Data curation, Investigation, Writing – review & editing. SZ: Formal analysis, Software, Writing – review & editing. KK: Writing – review & editing. WA: Formal analysis, Visualization, Writing – review & editing. BE: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors acknowledge the contributions of the individuals whose data were included in this analysis.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Butchbach, MER. Genomic variability in the survival motor neuron genes (SMN1 and SMN2): implications for spinal muscular atrophy phenotype and therapeutics development. Int J Mol Sci. (2021) 22:7896. doi: 10.3390/ijms22157896
PubMed Abstract | Crossref Full Text | Google Scholar
3. Sugarman, EA, Nagan, N, Zhu, H, Akmaev, VR, Zhou, Z, Rohlfs, EM, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet. (2012) 20:27–32. doi: 10.1038/ejhg.2011.134
PubMed Abstract | Crossref Full Text | Google Scholar
4. Verhaart, IEC, Robertson, A, Wilson, IJ, Aartsma-Rus, A, Cameron, S, Jones, CC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J Rare Dis. (2017) 12:124. Published 2017 Jul 4. doi: 10.1186/s13023-017-0671-8
PubMed Abstract | Crossref Full Text | Google Scholar
5. Sun, J, Harrington, MA, and Porter, B. Sex difference in spinal muscular atrophy patients – are males more vulnerable? J Neuromuscul Dis. (2023) 10:847–67. doi: 10.3233/JND-230011
PubMed Abstract | Crossref Full Text | Google Scholar
6. Cuscó, I, Bernal, S, Blasco-Pérez, L, Calucho, M, Alias, L, Fuentes-Prior, P, et al. Practical guidelines to manage discordant situations of SMN2 copy number in patients with spinal muscular atrophy. Neurol Genet. (2020) 6:e530. doi: 10.1212/NXG.0000000000000530
PubMed Abstract | Crossref Full Text | Google Scholar
7. Dosi, C, and Masson, R. The impact of three SMN2 gene copies on clinical characteristics and effect of disease-modifying treatment in patients with spinal muscular atrophy: a systematic literature review. Front. Neurology. (2024) 15:1308296. doi: 10.3389/fneur.2024.1308296
Crossref Full Text | Google Scholar
8. Maggi, L, Bello, L, Bonanno, S, Govoni, A, Caponnetto, C, Passamano, L, et al. Adults with spinal muscular atrophy: a large-scale natural history study shows gender effect on disease. J Neurol Neurosurg Psychiatry. (2022) 93:329320. doi: 10.1136/jnnp-2022-329320
Crossref Full Text | Google Scholar
9. Montes, J, Mcisaac, TL, Dunaway, S, Kamil-Rosenberg, S, Sproule, D, Garber, CE, et al. Falls and spinal muscular atrophy: exploring cause and prevention. Muscle Nerve. (2012) 47:118–23. doi: 10.1002/mus.23656
PubMed Abstract | Crossref Full Text | Google Scholar
10. Pieterse, A, Luttikhold, T, de Laat, K, Bloem, B, van Engelen, B, and Munneke, M. Falls in patients with neuromuscular disorders. J Neurol Sci. (2006) 251:87–90. doi: 10.1016/j.jns.2006.09.008
PubMed Abstract | Crossref Full Text | Google Scholar
11. Browning, S, Holland, S, Wellwood, I, and Bilney, B. Spatiotemporal gait parameters in adults with Premanifest and manifest Huntington’s disease: a systematic review. J Mov Disord. (2023) 16:307–20. doi: 10.14802/jmd.23111
PubMed Abstract | Crossref Full Text | Google Scholar
12. Grimbergen, YAM, Knol, MJ, Bloem, BR, Kremer, BPH, Roos, RAC, and Munneke, M. Falls and gait disturbances in Huntington’s disease. Mov Disord. (2008) 23:970–6. doi: 10.1002/mds.22003
Crossref Full Text | Google Scholar
13. Paker, N, Bugdayci, D, Goksenoglu, G, Demircioğlu, DT, Kesiktas, N, and Ince, N. Gait speed and related factors in Parkinson’s disease. J Phys Ther Sci. (2015) 27:3675–9. Available from: doi: 10.1589/jpts.27.3675
PubMed Abstract | Crossref Full Text | Google Scholar
14. Mak, MKY, and Pang, MYC. Parkinsonian single fallers versus recurrent fallers: different fall characteristics and clinical features. J Neurol. (2010) 257:1543–51. Available from: doi: 10.1007/s00415-010-5573-9
PubMed Abstract | Crossref Full Text | Google Scholar
15. Regan, E, Middleton, A, Stewart, JC, Wilcox, S, Pearson, JL, and Fritz, S. The six-minute walk test as a fall risk screening tool in community programs for persons with stroke: a cross-sectional analysis. Top Stroke Rehabil. (2019) 27:118–26. doi: 10.1080/10749357.2019.1667657
PubMed Abstract | Crossref Full Text | Google Scholar
16. Õunpuu, S, Garibay, E, Solomito, M, Bell, K, Pierz, K, Thomson, J, et al. A comprehensive evaluation of the variation in ankle function during gait in children and youth with Charcot–Marie–tooth disease. Gait Posture. (2013) 38:900–6. Available from: doi: 10.1016/j.gaitpost.2013.04.016
PubMed Abstract | Crossref Full Text | Google Scholar
17. Kennedy, RA, De Valle, K, Adams, J, Ryan, MM, Fitzgerald, AK, and Carroll, K. Characterising gait in paediatric neuromuscular disorders: an observational study of spatio-temporal gait in a clinical cohort. Disabil Rehabil. (2021) 44:7023–9. doi: 10.1080/09638288.2021.1977399
PubMed Abstract | Crossref Full Text | Google Scholar
19. Fritz, S, and Lusardi, M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. (2009) 32:2–5. doi: 10.1519/00139143-200932020-00002
Crossref Full Text | Google Scholar
20. Hausdorff, JM, Lertratanakul, A, Cudkowicz, ME, Peterson, AL, Kaliton, D, and Goldberger, AL. Dynamic markers of altered gait rhythm in amyotrophic lateral sclerosis. J Appl Physiol. (2000) 88:2045–53. doi: 10.1152/jappl.2000.88.6.2045
PubMed Abstract | Crossref Full Text | Google Scholar
21. Wu, Y, and Krishnan, S. Computer-aided analysis of gait rhythm fluctuations in amyotrophic lateral sclerosis. Med Biol Eng Comput. (2009) 47:1165–71. doi: 10.1007/s11517-009-0527-z
PubMed Abstract | Crossref Full Text | Google Scholar
22. Wu, Y, and Shi, L. Analysis of altered gait cycle duration in amyotrophic lateral sclerosis based on nonparametric probability density function estimation. Med Eng Phys. (2011) 33:347–55. doi: 10.1016/j.medengphy.2010.10.023
PubMed Abstract | Crossref Full Text | Google Scholar
23. Lukac, M, Luben, H, Martin, AE, Simmons, Z, and Geronimo, A. Spatial-temporal analysis of gait in amyotrophic lateral sclerosis using foot-worn inertial sensors: an observational study. Digit Biomark. (2023) 8:22–9. doi: 10.1159/000530067
PubMed Abstract | Crossref Full Text | Google Scholar
25. Liu, X, Chen, L, Ye, S, Liu, X, Zhang, Y, and Fan, D. Postural instability and lower extremity dysfunction in upper motor neuron-dominant amyotrophic lateral sclerosis. Front Neurol. (2024):15:1406109. doi: 10.3389/fneur.2024.1406109
Crossref Full Text | Google Scholar
26. Rodriguez-Torres, RS, Uher, D, Gay, EL, Coratti, G, Dunaway Young, S, Rohwer, A, et al. Measuring fatigue and fatigability in spinal muscular atrophy (SMA): challenges and opportunities. J Clin Med. (2023) 12:3458. doi: 10.3390/jcm12103458
PubMed Abstract | Crossref Full Text | Google Scholar
27. Dittner, AJ, Wessely, SC, and Brown, RG. The assessment of fatigue. J Psychosom Res. (2004) 56:157–70. doi: 10.1016/S0022-3999(03)00371-4
Crossref Full Text | Google Scholar
28. Gollie, JM, Patel, SS, Harris-Love, MO, Cohen, SD, and Blackman, MR. Fatigability and the role of neuromuscular impairments in chronic kidney disease. Am J Nephrol. (2022) 53:253–63. doi: 10.1159/000523714
PubMed Abstract | Crossref Full Text | Google Scholar
29. Noto, Y, Misawa, S, Mori, M, Kawaguchi, N, Kanai, K, Shibuya, K, et al. Prominent fatigue in spinal muscular atrophy and spinal and bulbar muscular atrophy: evidence of activity-dependent conduction block. Clin Neurophysiol. (2013) 124:1893–8. doi: 10.1016/j.clinph.2012.12.053
PubMed Abstract | Crossref Full Text | Google Scholar
30. Dunaway Young, S, Montes, J, Kramer, SS, Podwika, B, Rao, AK, and De Vivo, DC. Perceived fatigue in spinal muscular atrophy: a pilot study. J Neuromuscul Dis. (2019) 6:109–17. doi: 10.3233/JND-180342
PubMed Abstract | Crossref Full Text | Google Scholar
31. Chaudhuri, A, and Behan, PO. Fatigue in neurological disorders. Lancet. (2004) 363:978–88. doi: 10.1016/S0140-6736(04)15794-2
Crossref Full Text | Google Scholar
32. Montes, J, Dunaway, S, Montgomery, MJ, Sproule, D, Kaufmann, P, De Vivo, DC, et al. Fatigue leads to gait changes in spinal muscular atrophy. Muscle Nerve. (2011) 43:485–8. doi: 10.1002/mus.21917
PubMed Abstract | Crossref Full Text | Google Scholar
33. Andersen, LK, Knak, KL, Witting, N, and Vissing, J. Two-and 6-minute walk tests assess walking capability equally in neuromuscular diseases. Neurology. (2016) 86:442–5. doi: 10.1212/WNL.0000000000002332
PubMed Abstract | Crossref Full Text | Google Scholar
34. Dunaway Young, S, Montes, J, Kramer, SS, Marra, J, Salazar, R, Cruz, R, et al. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve. (2016) 54:836–42. doi: 10.1002/mus.25120
PubMed Abstract | Crossref Full Text | Google Scholar
36. Dubbioso, R, Spisto, M, Hausdorff, JM, Aceto, G, Iuzzolino, VV, Senerchia, G, et al. Cognitive impairment is associated with gait variability and fall risk in amyotrophic lateral sclerosis. Eur J Neurol. (2023) 30:3056–67. doi: 10.1111/ene.15936
留言 (0)