Hospital-acquired infections (HAIs) can increase morbidity and mortality, increase health care cost due to prolonged stay, and contribute to increased microbial resistance due to the widespread occurrence of multi-drug resistant (MDR) pathogens in health facilities (1–3). Approximately, one-third of neonatal deaths annually (680,000) caused by infections (4) The share of HAIs to this remains uncertain, but earlier studies have shown that rates of neonatal infections among hospital-born children in low-income countries are 3–20 times higher than those in higher income countries (5). Most of these infections were present soon after birth and were resistant to antibiotics. Inadequate water, sanitation and hygiene (WASH) and the low adherence to infection control protocols, unsafe waste management, exacerbated by the overcrowding of health facilities increase the risk for HAIs (6, 7).
Recent estimates suggest that HAIs affect about 8% of patients in regular wards and more than half of patients admitted in intensive care units (ICU) in low income settings (8–11). A recent study from Jimma University Medical Center reported a prevalence of HAIs of 19%, and the risk was significantly higher in those that received surgical procedures (12). A study conducted in rural health care facilities in Ethiopia, Kenya, Mozambique, Rwanda, Uganda and Zambia, reported that less than 50% of the surveyed facilities had access to: improved water sources on their premises, improved sanitation, hand washing facilities with constant access to water and soap (6). In Ethiopia, only an estimated 55% of health facilities have access to basic water services (3, 13). However, such data is scarce for lower level health facilities such as woreda (district) health centers and health posts, where the problem may be even more significant.
The global burden of HAIs has increased due to antibiotic-resistant bacteria, raising risk to health, particularly in developing countries (14). The WHO African region estimated 1.05 million deaths associated with bacterial antimicrobial resistance (AMR) in 2019 (14, 15). A recent study revealed that 23.5% of the patients had HAIs, with surgical site infections (SSI) being the most common, and primarily acquired for preventive purposes. From this, Ethiopia used 698.2 tons of antibiotics in 2018, according to the country’s most recent national data, with a per capita usage of 5.8grams, where the one antibiotic product that completely explained the 20.8% consumption; level of beta-lactamase-resistant (16). The Ethiopian policy brief and the regional state reports of Oromia and SNNPs regions primarily indicate that the need of cooperation with the long-term investment of a lasting solution, as well as the necessity of WASH response to avoid cholera outbreaks (17). Understanding the magnitude of nosocomial pathogens and their AMR would help design interventions that improve WASH and infection prevention control in health care facilities, but will also contribute to improving the quality of health care delivered. Therefore, the present study aimed to assess WASH facilities and practices, and levels of nosocomial pathogens in hand-touch sites in selected health facilities in Oromia and SNNPs Regions.
2 Materials and methods 2.1 Study area and designA facility based observational cross-sectional study design was employed for the WASH compliance and survey of pathogens occurrence from random spots in the health facilities of two regions. This study is reporting on a baseline assessment conducted in health facilities of Bidre town in Bale zone from Oromia Region, Bulle town in Gedeo zone and Doyogena in Kembata-Tembaro zone both from SNNPs region. The WASH assessments included all health facilities that were functional at the time of the survey (i.e., health post, health centers, and hospitals).
2.2 Sample size and sampling procedureTo select appropriate sample size, the current study involved all hospitals from 3 districts and 30% of health facilities sample from the WASH program implementation of UNICEF-Ethiopia in 2 specific regions randomly. The study site was selected based on the list provided by UNICEF-Ethiopia and the possibility to transfer microbial samples in time (in 24 h) was considered. From the list of health facilities, we selected a sub-sample, stratified by type of facility. From the 31 health care facilities, we selected a subset of 12 health facilities from which sample was collected. We excluded pharmacies and clinics and focused on health posts, health centers and hospitals.
2.3 Assessment of WASH in health facilitiesTo assess the facilities of WASH and practices, the observational checklist of core questions for infection prevention and control (IPC) and WASH common indicators is developed based on international standards—WHO/UNICEF (18). All questionnaires and checklists were translated into Amharic/Oromifaa and were pretested prior to the interviews. The checklist allowed the collection of information on the prevailing sanitary conditions, access to water and hand-washing facility, as well as hand-washing and waste disposal practices. The WHO protocol on monitoring fulfilment of opportunities for hand-hygiene was used to assess the health personnel’s adherence to hand- hygiene guidelines (19) from June 2021 to July 2021.
2.4 Surface and water sample collectionSample collection was performed on August 2021 following the United States Center for Disease Control and Prevention (CDC) and Public health England guidelines (20, 21). Surface and water sample primarily collected from hospitals and health centers. Surface sample collection was performed using sterile cotton swabs. The swabs were first moist in sterile normal saline solution. The samples were collected from surfaces including beds, door handles, walls, gowns, autoclaves, tables, and chairs. The sampling areas included out-patients departments, different wards, pharmacy, laboratories, receptions, toilets and cafeterias in the health facilities.
Water samples were collected from sources from which the health facilities obtain water for washing, drinking and other activities in the healthcare settings. A total of 14 water sample is collected and delivered for analysis from delivery wards, medical ward, tanker, and bore-hole and rainwater collection systems. Overall, 59 water samples were collected from all health facilities, including health centers and health posts.
2.5 Sample handling and transportationThe collected surface samples were immediately put in Amies transport media and kept in pre-cooled ice box and transported to SNNPs region Public Health Institute laboratory. On arrival at the laboratory, the surface samples were transferred to the nutrient broth and enriched overnight at 37°C. After an overnight incubation, the samples were inoculated on blood agar and MacConkey agar plates and put overnight at 37°C. In case of no growth after an overnight culture, the plates were incubated for an additional 24 h.
The water samples were assessed for their safety using modified Method 9,215 to enumerate heterotrophic bacteria and membrane filtration technique for Gram-negative bacteria (22). To enumerate heterotrophic bacteria, 1 mL of each water sample was pipetted into a sterile petri dish. After thoroughly mixing, the melted MacConkey agar was poured into the dish. The melted medium was mixed thoroughly with the sample and solidified. The plates were incubated for 48 h at 37°C.
The Gram-negative bacteria were counted by filtrating 100 mL water samples through 0.45 μm pore size-47 mm, and cellulose nitrate membranes using the modified ISO 9308-1 protocol (23). The samples were incubated on MacConkey agar for 24 h at 37°C. All results of Gram-negative bacteria were expressed as colony forming units per 100 mL water. The bacterial colonies were collected and put in Trypticase Soy Broth containing 20% glycerol and were transported to the National bacteriology and mycology Reference Laboratory (NRL) at the Ethiopian Public Health Institute, where they were stored in deep-freeze until further analyses.
2.6 Bacterial isolation and identificationThe bacteria were refreshed by culturing on three different culture media: (i) 5% sheep blood agar plate, (ii) MacConkey agar plate, and (iii) Mannitol salt agar plate. Colony appearance on culture plates, microscopic examination, and biochemical tests were used to identify Gram-positive and Gram-negative bacteria.
2.6.1 Identification of gram-positive cocciThe common Gram-positive cocci are Staphylococcus spp. and Streptococcus spp. We used Blood agar and Mannitol salt agar media for isolation of Staphylococcus spp: The culture plates were incubated in air at 37°C for 24 h. Colony morphology on culture plates and microscopic examination for Gram-positive cocci in clusters were used for initial Staphylococcus spp. identification. Catalase and coagulase tests were used to classify Staphylococcus spp. into Staphylococcus aureus and coagulase negative Staphylococcus. All Staphylococci are Catalase positive and only S. aureus is coagulase positive.
Streptococcus spp. were identified based on colony morphology on: (i) blood agar plates (beta hemolytic, alpha hemolytic and non-hemolytic), (ii) microscopic examination for Gram-positive in chin, and (iii) different biochemical tests. Negative catalase test differentiated Streptococcus spp. from Staphylococci Bacillus spp.
Blood agar with 5% sheep blood media was used for the bacteria isolation. Colony morphology on the culture plates and gram stain were used for the bacterial identification. To differentiate Bacillus cereus from other Bacillus species we used citrate test which is only positive for B. cereus.
2.6.2 Identification of gram-negative bacilliThe common gram negative bacteria are generally divided into two major categories: Fermenters and non-fermenters. Fermenters gram-negative bacilli utilize lactose and become pink color colonies on MacConkey agar while non-fermenters cannot utilize lactose and they are colorless colonies on MacConkey agar plate. Biochemical tests such as Triple Sugar Iron Agar (TSI), urea, citrate, Sulfide Indole Motility (SIM) medium, growth in Lysine Iron Agar (LIA), and oxidase were additionally used to identify Gram-negative bacteria.
2.7 Antimicrobial susceptibility testingThe antimicrobial Susceptibility Tests (AST) were performed based on the Kirby–Bauer disk diffusion method on Mueller-Hinton agar (MHA) as recommended by clinical and laboratory standard Institute (CLSI) for all Gram-negative bacteria and Staphylococcus species (24). Well-isolated three to four colonies were emulsified in a tube containing sterile normal saline and the turbidity adjusted to 0.5 McFarland standards. The emulsified bacterial suspension was uniformly streaked on MHA plates using sterile cotton swabs, on which the antibiotic disks were applied and incubated for 18–24 h at 37°C. The antibiotic agents tested in this study were ampicillin (10 μg), amoxicillin-clavulanic acid (20/10 μg), pepracillin/ tazobactum, cefazolin (30 μg), cefuroxime (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), cefepime (30 μg), cefoxitin (30 μg), ciprofloxacin (5 μg), amikacin (30 μg), meropenem (10 μg), chloramphenicol, tetracycline, cotrimoxazole, and penicillin. Penicillin and cefoxitin were tested only for Staphylococcus species and the result of oxacillin was determined from cefoxitin breakpoint. Antibiotic susceptibility results were interpreted according to the CLSI zone size interpretive standards (24). Intermediate results were considered resistant.
Multidrug resistance (MDR) was defined according to guidelines compiled by the European Center for Disease prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) (25). Accordingly, bacterial isolates that were resistant to at least one agent in three different antimicrobial categories were considered as MDR.
2.8 Quality assuranceAll media, biochemical reagents, gram stain reagents and antibiotic disks were checked for their quality using standards ATCC strains. Standard ATCC quality strains used for this study were S. aureus ATCC® 25923, E. coli ATCC® 2592, P. aeruginosa ATCC 27853.
2.9 Data analysisEpi-info was used for data entry and the data was subsequently exported to Microsoft Excel and SPSS version 26 for data cleaning and further analysis. The frequencies of bacterial isolates and antimicrobial susceptibility were calculated. Mean and frequencies (percentage) were used to present descriptive data.
2.10 EthicsEthical clearance was obtained from the Institutional Review Board of the College of Natural and Computational Sciences of Addis Ababa University (Ref. No: IRB/04/14/2021). Additionally, the research was ethically approved by letter of support is sent to Oromia and SNNPs regional Health Bureaus with the letter of minute no. (ምሳኒፕ/453/13/21). The Oromia and SNPP’s Regional Health Bureau Ethics review committee also reviewed and approved the research for the implementation. Prior to the collection of data, the informed consent was obtained from staff and the administration of the each health facilities. Every task and procedures was completed in accordance with the WHO guidance, rule and regulations.
3 ResultsWASH assessments were conducted in 26 health facilities in Bulle and Doyogena (SNNPs Region) and in Bidre (Oromia Region). The assessments included hospitals (n = 3), health posts (n = 13), clinics (n = 8), and health centers (n = 2; Table 1). A great majority of the health facilities relied on tanker trucks for their water supply (Table 2). At the time of the survey, piped water supply was available in only 11 of the 26 health facilities. Open pit latrines (14/26) were the commonest type of toilet and only in 8 out of the 26 facilities, the toilets were accessible for people with limited mobility. Infectious waste was primarily dumped into an open/protected pit, incinerated, and added to other wastes. Sharp waste was mostly collected for off-site disposal, autoclaved, or incinerated. Only 10 of the 26 assessed health facilities had guidelines on standard precautions for IPC. Only six had cleaning protocols available, and only in one health facility, the staff responsible for cleaning received training. Environmental disinfectant was only available in only 8 of the 26 health facilities.
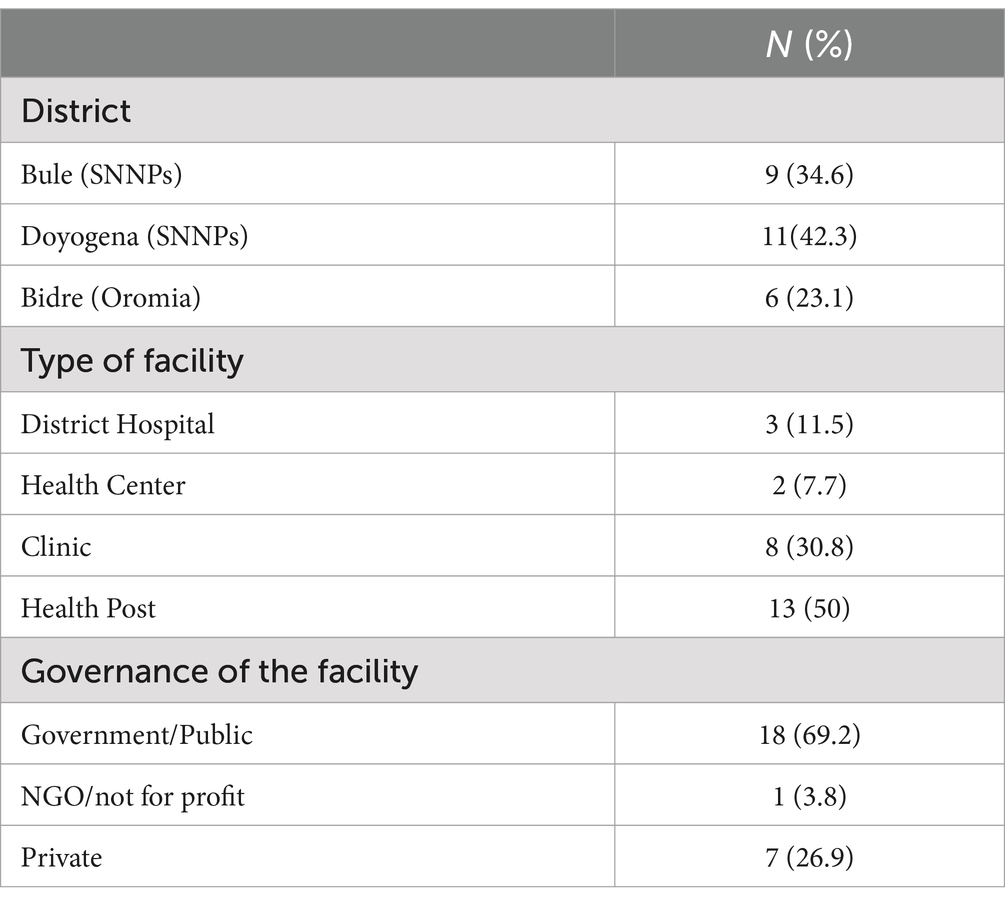
Table 1. Characteristics of the health facilities.
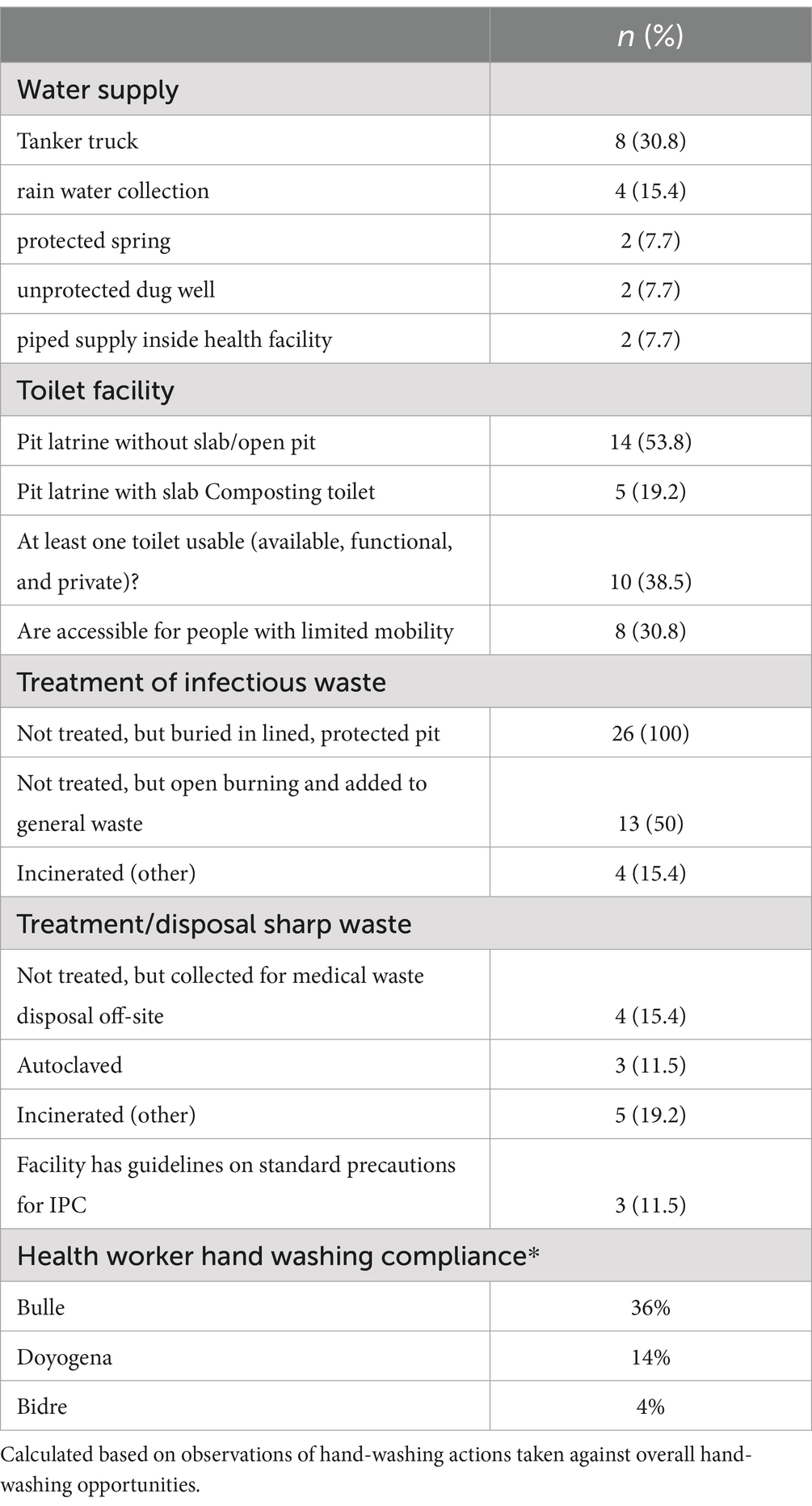
Table 2. Water, sanitation, and hygiene of the assessed health facilities.
Hand-hygiene opportunities were directly observed (1,194 ± 326 min) and evaluated using the WHO checklist to assess compliance (Table 2). Hand-hygiene opportunities were: (i) before touching a patient; (ii) before a procedure; (iii) after body fluid exposure/risk; (iv) after touching a patient; (v) after touching a patient’s surrounding. Hand-hygiene compliance was overall low, but varied by site. The lowest compliance was for Bidre (4%), followed by Doyogena (14%), and Bulle (36%).
A total of 90 surface swabs and 14 water samples were collected from which a number of bacteria (n = 224) were identified (Table 3). Over 70% of the identified bacteria were from four categories: Staphylococcus spp., Bacillus spp., E. coli, and Klebsiella spp. These bacteria were the most widely distributed and were also found in high-risk locations including neonatal intensive care units, delivery and surgical rooms (Table 4). More details on the identified bacteria by study sites, location and sample source can be found in the Supplementary Tables S1–S3 and Supplementary Figure S1.
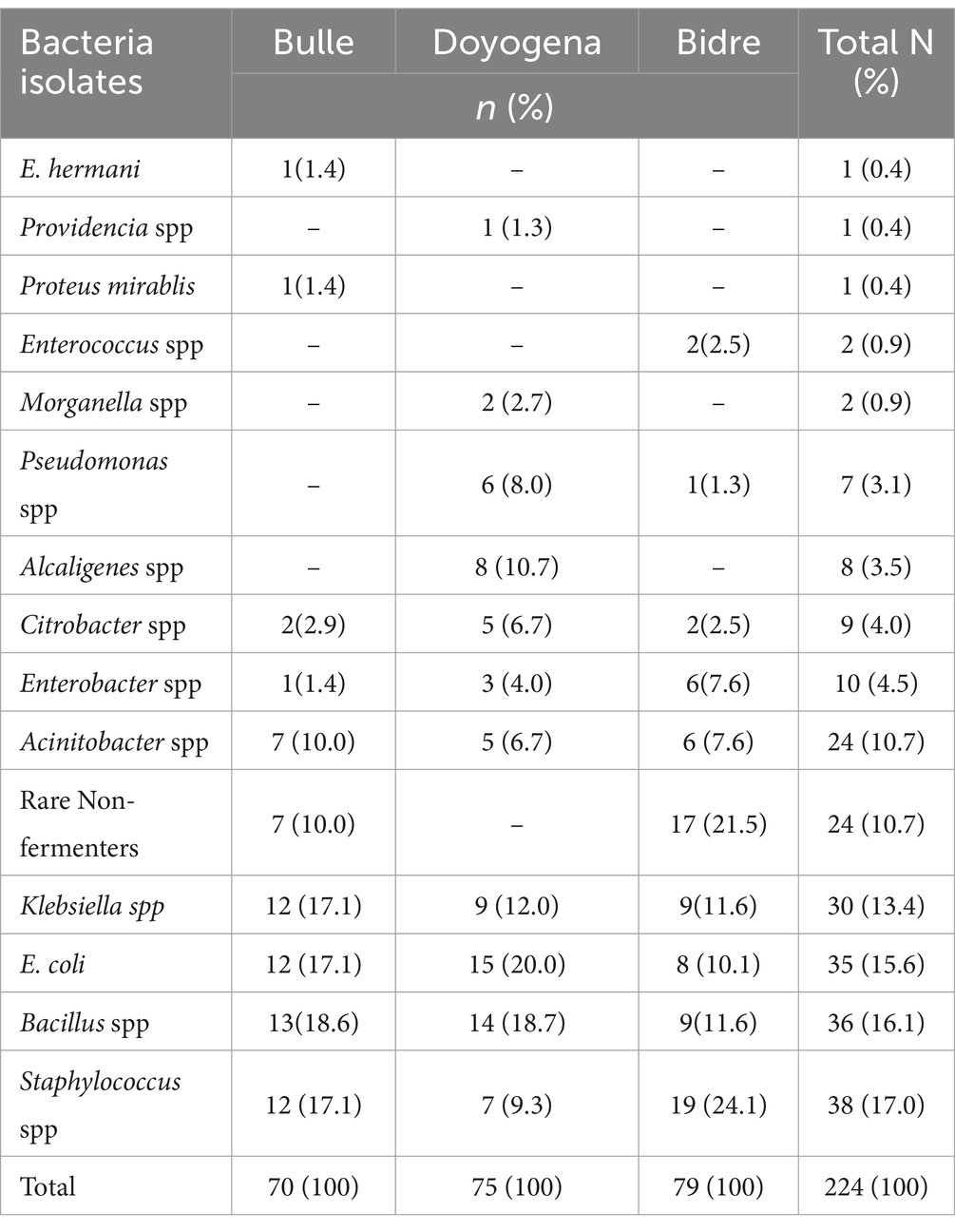
Table 3. Identification of bacteria from surface swabs and water samples.
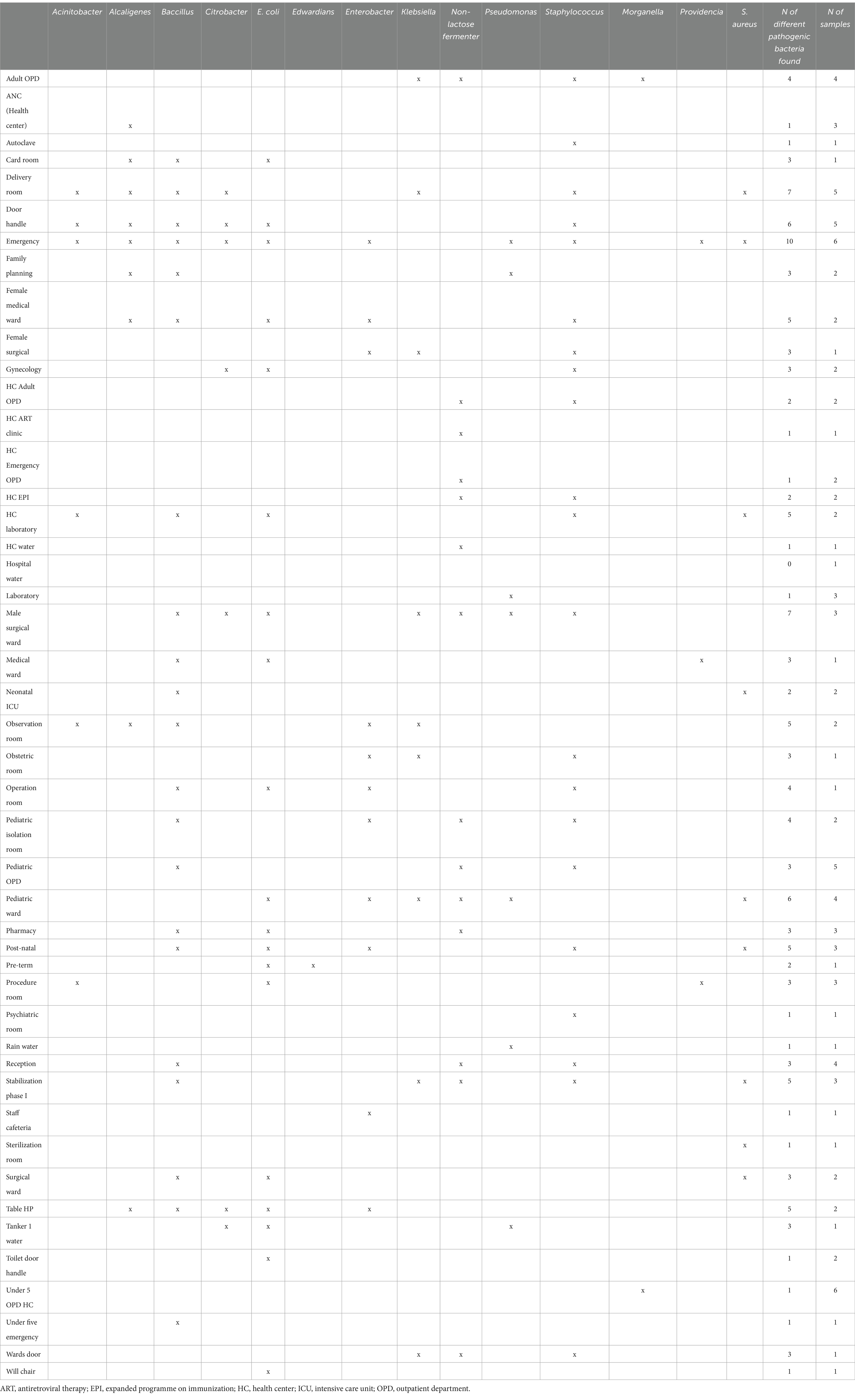
Table 4. Identified bacteria by sample source/location.
Figure 1 presents the antimicrobial resistance of the identified bacterial isolates. Antimicrobial susceptibility was detected in 50% or more of the isolates for penicillin, cefazolin, ampicillin, oxacillin, and cotrimoxazole. More than 50% of the isolates displayed multi-drug resistance, defined as resistant to at least one agent in three different antimicrobial categories.
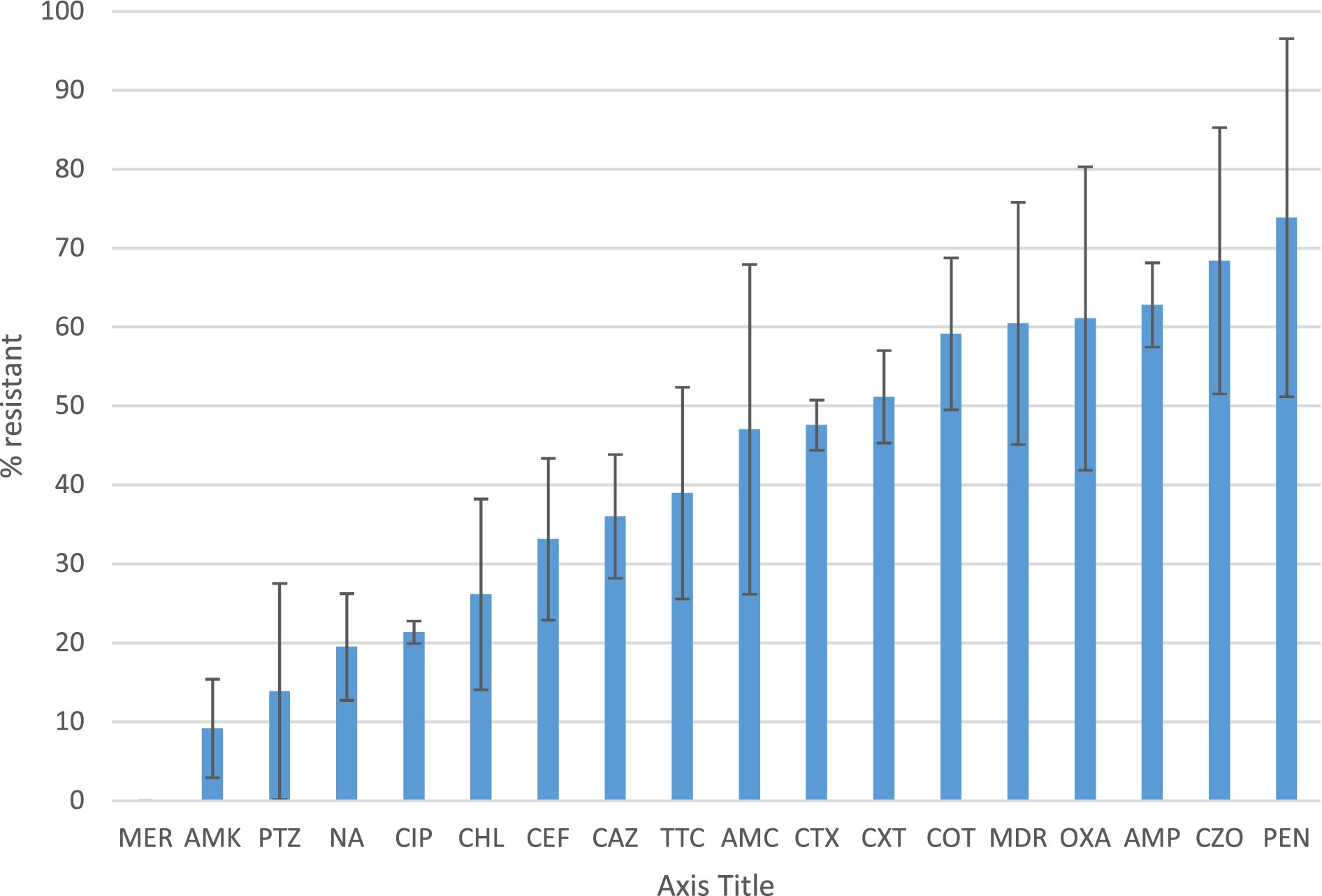
Figure 1. Antibiotic susceptibility profile and multidrug resistance of the identified bacteria Percentage of resistance of bacterial isolates identified from healthcare surface environmental and water samples according to the CLSI disk diffusion breakpoints. Resistance was defined as isolates with intermediate resistance and complete resistance inhibition zone size. Antibiotics tested were ampicillin (AMP), amoxicillin-clavulanate (AMC), pepracillin with tazobactum (PTZ), cefazolin (CZO), cefuroxime (CXT), ceftazidime (CAZ), cefepime (CEF), cefotaxime (CTX), ciprofloxacin (CIP), nalidixic acid(NA), chloramphenicol (CHL), Cotrimoxazole (COT) Amikacin (AMK), Meropenem (MER),tetracycline (TTC), Penicillin (PEN) and oxacillin (OXA). MDR is to indicate the rate of Multidrug resistant bacterial isolates.
4 DiscussionWater supply, availability of clean and accessible toilets, as well as infection prevention measures were found suboptimal. Hand hygiene practice by health workers was very low. Consequently, surface swabs and water samples revealed high bacterial contamination, with some of the identified bacteria known for their pathogenicity. These bacteria were also found in highly sensitive areas like surgical rooms, delivery rooms, and neonatal intensive care units (ICUs).
Our findings highlight the need to invest in safely managed water supply, provision of safely managed sanitation services, but also strict hygiene and environmental cleaning in health facilities. Earlier studies assessing 1,318 health facilities in multiple African countries including Ethiopia showed that less than 50% of the facilities had access to improved water sources on premises, improved sanitation, and consistent access to water and soap for handwashing (6). A recent meta-analyses of studies on health workers’ handwashing practice in Ethiopia also estimated that 57.87% (95% CI: 44.14–71.61) practiced hand-washing (26), a figure that is higher than estimates from the current study. This difference may be explained by the rather rigorous evaluation of hand-hygiene practice in this study assessed using the more systematic WHO’s protocol of hand-hygiene opportunities. It can as well suggest that the selected sites have more significant WASH constraints, further justifying their selection for WASH and IPC improvements by the planned intervention.
The poor WASH and IPC conditions observed in the health facilities can greatly impact the quality of the health care provided. First, satisfaction with WASH and IPC conditions can be associated with lower job satisfaction as reported from a recent multi-country study (27). Second, health facilities with suboptimal WASH and IPC procedures increase the risk for nosocomial infections. Indeed, a recent meta-analyses pooling results from 18 studies in Ethiopia (28), estimated the prevalence of nosocomial infections to be as high as 17% (95% CI 14.10–19.82). This prevalence can be even higher when considering vulnerable sub-groups like neonates, infants and young children. Indeed, studies have shown that HAIs contribute significantly to neonatal infections and mortality in low income countries like Ethiopia (5).
A number of pathogenic bacteria associated with nosocomial infections have been identified from highly sensitive locations like surgical rooms, delivery room, and neonatal ICU. Klebsiella spp., E. coli, Acinetobacter spp., bacillus spp. and Staphylococcus spp. were identified in high number of samples collected from various locations. Poor hand-hygiene and bacterial contamination with AMR was a common feature of health facilities in all the three sites. More concerning is that a large number of the identified bacteria displayed antibiotic resistance and these same species were reported to be the major pathogens identified in bloodstream isolates (n = 11,471) of hospital-acquired neonatal infections (5). A recent global study showed that most of the bacterial isolates identified in our study were responsible for high rates of deaths associated with AMR, particularly in sub-Saharan African (SSA) countries (29). This study might need further investigation of evidence with the recent finding of an estimation that Ethiopia used the lowest dose of antibiotics (28%) among central SSA (4.2 billion DDD, i.e., 42%) in 2018 (30).
The present study has a number of limitations that need to be considered when interpreting our findings. First, this is a cross-sectional study and thus only provides a snapshot of the situation at the time of the survey. Second, the survey happened during the COVID-19 pandemic that in principle would have increased awareness on hand-hygiene because of the nation-wide campaigns. Third, this is a baseline assessment of health facilities selected for WASH/IPC intervention and thus may not be representative. However, evidence from our WASH data is in line with previous assessments and thus can be indicative of situations in similar settings in Ethiopia.
5 ConclusionThe health facilities assessed were confronted with serious problems related to WASH. Compliance to hand-hygiene practice by the health care workers was very low. Analyses of environmental and water samples revealed high levels of bacterial contamination. Most of the identified bacteria displayed AMR. Beyond increasing access to health coverage, emphasis should be put to improving infrastructure and services. This requires safely managed water supply, provision of safely managed sanitation services, but also strict hygiene and environmental cleaning in health facilities. Ensuring the supply chain of critical consumables such as soap, chlorine and decontaminants or disinfectants is key, but this will also need to be accompanied by behavioral change on hand hygiene and environmental cleaning practices. A critical element of strengthening health systems should also focus on antibiotic stewardship.
The current study employed a cross-sectional study design to evaluate the present situation or existing facilities, and inadequacy of WASH in health settings and assess the risk factors focusing on antimicrobial resistance (AMR). The reason for the selection of the current study design, is suitable for capturing a snapshot of current WASH conditions and related health risks. However, the study design cannot establish a causal relationship between inadequate WASH facilities and heightened AMR infection rates. Therefore, we suggested that a longitudinal study design would have more comprehensive insights into and establish the long-term impacts of WASH improvements on the AMR, which offers evidence that is more robust over time. In addition, the need for continuous monitoring is required to understand how the WASH improvements might influence health outcomes.
The recommendation emphasizes the need for urgent advocacy for policies requiring health facilities to adhere to WHO recommendations of WASH standards and improvement as well as infection control protocols. This might include the provision of safe water, sanitation, and adequate hygiene facilities for clients in order to reduce the risk of HAIs and AMR. The healthcare facilities, particularly the hospitals, should establish routine monitoring of NIs through established protocols and reporting of NIs to identify potential and critical hazards in infection rates over time. Additionally, promoting the regular training programs, particularly continuous professional developments (CPD) on infection control and AMR prevention for healthcare workers is crucial.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementEthical clearance was obtained from the Institutional Review Board of the College of Natural and Computational Sciences of Addis Ababa University. Informed consent was obtained prior to the collection of data.
Author contributionsTBE: Data curation, Software, Supervision, Writing – original draft, Writing – review & editing, Formal analysis, Visualization. AN: Investigation, Validation, Writing – review & editing. LV: Conceptualization, Methodology, Visualization, Writing – review & editing. AFD: Methodology, Supervision, Validation, Writing – review & editing. TA-M: Investigation, Supervision, Visualization, Writing – review & editing. KG: Investigation, Supervision, Validation, Visualization, Writing – review & editing. KB: Conceptualization, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this study was received from UNICE Ethiopia.
AcknowledgmentsThe facilitation offered by the Oromia and SNNPs Health Bureaus and the Hospitals of Bidre, Doyo Gena and Bulle is duly acknowledged. We would like to extend our acknowledgement to Addis Ababa University College of Natural and Computational Science for reviewing the protocol, approved for implementation. The authors also like to acknowledge the Ethiopian Public Health Institute (EPHI) microbiological laboratory team of experts and SNNPs regional laboratory.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1478906/full#supplementary-material
References1. Feleke, T, Eshetie, S, Dagnew, M, Endris, M, Abebe, W, Tiruneh, M, et al. Multidrug-resistant bacterial isolates from patients suspected of nosocomial infections at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Res Notes. (2018) 11:1–7. doi: 10.1186/s13104-018-3709-7
Crossref Full Text | Google Scholar
2. Manoukian, S, Stewart, S, Graves, N, Mason, H, Robertson, C, Kennedy, S, et al. Bed-days and costs associated with the inpatient burden of healthcare-associated infection in the UK. J Hosp Infect. (2021) 114:43–50. doi: 10.1016/j.jhin.2020.12.027
PubMed Abstract | Crossref Full Text | Google Scholar
3. World Health Organization. WHO global water, sanitation and hygiene: annual report. Geneva: World Health Organization (2022).
4. UNIGME. Levels and trends in child mortality: Report 2021. New York, NY: United Nations Inter-Agency Group for Child Mortality Estimation (2021).
5. Zaidi, AK, Huskins, WC, Thaver, D, Bhutta, ZA, Abbas, Z, and Goldmann, DA. Hospital-acquired neonatal infections in developing countries. Lancet. (2005) 365:1175–88. doi: 10.1016/S0140-6736(05)71881-X
Crossref Full Text | Google Scholar
6. Guo, A, Bowling, JM, Bartram, J, and Kayser, G. Water, sanitation, and hygiene in rural health-care facilities: a cross-sectional study in Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia. Am J Trop Med Hyg. (2017) 97:1033–42. doi: 10.4269/ajtmh.17-0208
PubMed Abstract | Crossref Full Text | Google Scholar
7. Schwab, F, Meyer, E, Geffers, C, and Gastmeier, P. Understaffing, overcrowding, inappropriate nurse: ventilated patient ratio and nosocomial infections: which parameter is the best reflection of deficits? J Hosp Infect. (2012) 80:133–9. doi: 10.1016/j.jhin.2011.11.014
PubMed Abstract | Crossref Full Text | Google Scholar
8. Alp, E, Cookson, B, Erdem, H, Rello, J, Akhvlediani, T, Akkoyunlu, Y, et al. Infection control bundles in intensive care: an international cross-sectional survey in low- and middle-income countries. J Hosp Infect. (2018) 101:248–56. doi: 10.1016/j.jhin.2018.07.022
PubMed Abstract | Crossref Full Text | Google Scholar
9. Antonelli, A, Ales, ME, Chiecca, G, Dalla Valle, Z, de Ponti, E, Cereda, D, et al. Healthcare-associated infections and antimicrobial use in acute care hospitals: a point prevalence survey in Lombardy, Italy, in 2022. BMC Infect Dis. (2024) 24:632. doi: 10.1186/s12879-024-09487-7
Crossref Full Text | Google Scholar
10. Nejad, S.B., Allegranzi, B., Syed, S.B., Ellisc, B., and Pittetd, D. (2011). Infections liées aux soins de santé en afrique: Une étude systématique. Bull World Health Organization. 89:757–65. doi: 10.2471/BLT.11.088179
Crossref Full Text | Google Scholar
11. Vincent, J-L, Rello, J, Marshall, J, Silva, E, Anzueto, A, Martin, CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. (2009) 302:2323–9. doi: 10.1001/jama.2009.1754
PubMed Abstract | Crossref Full Text | Google Scholar
12. Ali, S, Birhane, M, Bekele, S, Kibru, G, Teshager, L, Yilma, Y, et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect Control. (2018) 7. doi: 10.1186/s13756-017-0298-5
PubMed Abstract | Crossref Full Text | Google Scholar
13. Global Waters Strategy Country Plan (GWSCP). Progress on WASH in health care facilities 2000–2021: special focus on WASH and infection prevention and control (launch version). Ethiopia: (2023).
14. Sartorius,, Benn,, et al. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. The Lancet Glob Health. (2024) 12:e201–e216.
15. Auer, GK, Oliver, PM, Rajendram, M, Lin, T-Y, Yao, Q, Jensen, GJ, et al. Bacterial swarming reduces Proteus mirabilis and Vibrio parahaemolyticus cell stiffness and increases β-lactam susceptibility. MBio. (2019) 10:19. doi: 10.1128/MBIO.00210-19
PubMed Abstract | Crossref Full Text | Google Scholar
16. Gutema, G., Ali, S., Engidawork, E., Kaba, M., and Toverud, E. (2021). WITHDRAWN: Ethiopia’s Antibiotic Footprint: Estimate of National Antibiotic Consumption Using 2018 Data. Research Square. doi: 10.21203/rs.3.rs-186995/v1
Crossref Full Text | Google Scholar
17. National Information Platforms for Nutrition (NIPN). Improving adolescent undernutrition in Ethiopia: a rapid review evidence brief. Addis Ababa: Ethiopian Public Health Institute (2021) Addis Ababa, Ethiopia.
19. WHO. Hand hygiene technical reference manual: to be used by health-care workers, trainers and observers of hand hygiene practices. Geneva: World Health Organization (2009).
20. CDC. Guidelines for environmental infection control in health-care facilities. CDC: (2017).
21. Willis, C, Nye, K, Aird, H, Lamph, D, and Fox, A. Examining food, water and environmental samples from healthcare environments. Microb Guid Pub Health Engl. (2013):1–48.
23. ISO. Water quality-enumeration of Escherichia Coli and coliform Bacteria International Organization for Standardization (2014).
24. CLSI. Performance standards for antimicrobial susceptibility testing. CLSI: (2017).
25. Magiorakos, AP, Srinivasan, A, Carey, RB, Carmeli, Y, Falagas, ME, Giske, CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
PubMed Abstract | Crossref Full Text | Google Scholar
26. Gedamu, H, Wgiorgis, T, Tesfa, G, Tafere, Y, Genet, M, Tesfa, G, et al. Hand washing practice among health care workers in Ethiopia: systemic review and meta-analysis, 2020. Heliyon. (2021) 7:e06972. doi: 10.1016/j.heliyon.2021.e06972
PubMed Abstract | Crossref Full Text | Google Scholar
27. Fejfar, D, Guo, A, Kelly, E, Tidwell, JB, Ochieng, O, and Cronk, R. Healthcare provider satisfaction with environmental conditions in rural healthcare facilities of 14 low-and middle-income countries. Int J Hyg Environ Health. (2021) 236:113802. doi: 10.1016/j.ijheh.2021.113802
PubMed Abstract | Crossref Full Text | Google Scholar
28. Alemu, AY, Endalamaw, A, and Bayih, WA. The burden of healthcare-associated infection in Ethiopia: a systematic review and meta-analysis. Tropic Med Health. (2020) 48:77. doi: 10.1186/s41182-020-00263-2
PubMed Abstract | Crossref Full Text | Google Scholar
29. Murray, CJ, Ikuta, KS, Sharara, F, Swetschinski, L, Robles Aguilar, G, Gray, A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
PubMed Abstract | Crossref Full Text | Google Scholar
30. Browne, AJ, Chipeta, MG, Haines-Woodhouse, G, Kumaran, EPA, Hamadani, BHK, Zaraa, S, et al. Global antibiotic consumption and usage in humans, 2000-18: a spatial modelling study. Lancet Planet Health. (2021) 5:e893–904. doi: 10.1016/S2542-5196(21)00280-1
留言 (0)