Globally, cervical cancer is the fourth most prevalent cancer in women, with 604,127 incidences and 341,843 deaths recorded in 2020, and 84–90% of cases occurring in low- and middle-income countries (LMICs) (1–5). High-risk (hr) strains of human papillomavirus (HPV), particularly HPV-16 and HPV-18, are responsible for nearly 70% of cervical cancer cases, which can be detected through nucleic acid testing (6–12). Although prevention methods like vaccination and screening are available, the uptake of HPV screening in LMICs remains low (approximately 27%) due to various personal, social, and systemic challenges (13–16). This low rate of screening uptake is alarming, given that 84–90% of cervical cancer cases occur in these regions, underscoring a profound public health challenge (1–5). Despite the availability of preventive methods, several factors contribute to this gap in screening.
Personal, social, and systemic barriers significantly hinder HPV screening uptake in LMICs, where cervical cancer rates are disproportionately high (13–16). Many women in LMICs lack awareness of the link between HPV and cervical cancer, and fear, embarrassment, and stigma surrounding the screening process further reduce participation (15). Social and cultural factors, such as taboos around reproductive health and gender dynamics limiting women’s autonomy, also play a role (15). Additionally, programmatic challenges, including shortages of trained healthcare workers, limited access to facilities, and the high costs of provider-screening, exacerbate the problem (15). Considering these barriers, HPV self-sampling presents a promising alternative. This method offers a private, non-invasive, and cost-effective approach that reduces reliance on healthcare professionals and infrastructure, while empowering women to take control of the sample collection process (17). It thereby addresses personal, social, and systemic challenges to screening.
Traditional cytology testing has been the standard method for cervical cancer screening; however, it relies heavily on trained professionals for sample collection, which can pose challenges in resource-limited settings and reduce cancer screening uptake (18, 19). In contrast, HPV self-sampling has emerged as a promising alternative, offering women a convenient and private method for collecting samples (19, 20). Recent studies have indicated that self-sampled HPV tests demonstrate higher detection rates for cervical intraepithelial neoplasia grade 2 and above (CIN2+) compared to cytology (19). Notably, self-sampling has shown detection rates comparable to those of HPV and cytology co-testing, as evidenced by one non-randomised study and a randomised controlled trial (RCT) (19). Furthermore, findings from recent research revealed that repeated self-sampled HPV tests were associated with a two-fold increase in CIN2+ detection rates compared to cytology (19). HPV self-sampling provides conclusive results that enable the development of a streamlined protocol with well-defined management options (21).
HPV self-sampling not only demonstrates higher detection rates for CIN2+ compared to traditional cytology, but it also offers a more accessible and private alternative for women in various settings. This accessibility is largely due to the simplicity of the self-sampling process, which allows participants to collect samples with minimal clinical support. HPV self-sampling typically involves participants receiving a kit, collecting the sample, and sending it to a laboratory for testing, whether the sampling occurs at home, in a clinic, or another healthcare setting (17). Participants are provided with instructions for use, which may be verbal or written (17). The self-sampling process usually involves the participant collecting a vaginal swab, either with or without supervision (17). The swab is inserted into the vagina, and the participant collects the sample by moving the swab in a circular motion (17). After collection, the swab is placed into a sample collection tube, which may or may not contain a transportation liquid (17). Variations exist regarding sample transportation across different contexts including use of postal services, delivery companies, or local health services (16, 17).
Provider-sampling has been the conventional strategy for HPV testing and considered more accurate as it is collected by trained medical professionals (22, 23). However, concerns have been reported because of the sample collection’s invasive nature, cost and access limitations (24, 25). Self-sampling, a relatively newer approach, has been reported to be widely accepted compared to provider-sampling, particularly in LMICs (17). This method addresses many acceptability concerns associated with provider-sampling, including invasiveness, discomfort, cultural sensitivities and embarrassment (26–30). It may also be preferable in resource-limited regions because of its cost-effectiveness, relative feasibility and sustainability (31). It provides an opportunity for increased screening coverage and early detection of HPV, especially in LMIC settings where access to healthcare facilities and trained health workers is limited (16).
Globally, current research examining the accuracy of HPV self-sampling vs. provider-sampling presents conflicting findings (4, 16, 22, 32, 33). A primary study by Feng et al., 2022 demonstrated benefits in the accuracy of HPV self-sampling; however, another meta-analysis reported that provider-sampling was superior in accuracy (4, 32). Some studies state that self and provider-sampling have similar accuracy effects (16, 22, 33). Most of these studies were carried out in high-income countries, covering approximately 63%, which results in knowledge gaps in LMICs (15). Staley et al. (34), conducted a Cochrane review and meta-analysis in 2021 on interventions targeting women to encourage the uptake of cervical cancer screening. They included 69 trials in the analysis and found that most evidence (97%) was reported from high-income countries (34). Therefore, evidence on the uptake of cervical cancer screening through self-sampling in LMIC remains unclear.
Mekuria et al. (30) is a systematic review and meta-analysis that included six studies from Uganda, Nigeria, Ethiopia, Mexico, Brazil and Argentina (30). It reported that HPV self-sampling increased uptake of cervical cancer screening, particularly in low-income countries (30). While Mekuria et al. (30) focused on examining the effect of HPV self-sampling on screening uptake and estimating associated costs in LMICs, their review did not address other critical measures such as accuracy, acceptability, and equity. Additionally, their analysis included fewer studies—six, leaving a gap in the comprehensive evaluation of HPV self-sampling compared to provider-sampling (30). While HPV self-sampling is a promising solution, there is still a lack of comprehensive data on its accuracy, acceptability, cost, and the impact of equity factors such as socioeconomic factors on cervical cancer screening uptake in LMICs. This scoping review aimed to address these gaps by providing a comprehensive evaluation of HPV self-sampling compared to provider-sampling in LMICs. It focused on critical dimensions such as test acceptability, accuracy, cost, and the impact of health equity factors on the test’s uptake. By doing so, this study sought to inform public health strategies to improve cervical cancer screening in resource-limited settings.
2 MethodsThis scoping review was conducted according to the framework by Arksey and O’Malley (35). Our review followed the guidance by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews (PRISMA-Scry) (36). See Supplementary Annex 1 for the PRISMA-ScR checklist (36). Our protocol was submitted to the OSF registries.
2.1 Eligibility criteriaEligibility was defined by the population, concept, context (PCC) framework as follows:
Population and setting: Studies conducted on female participants at risk of HPV, regardless of age, gender, HIV status or cervical cancer screening status within LMICs were included. Studies involving populations confirmed to have invasive cancer were excluded from our review because HPV self-sampling is primarily designed for early detection and screening of HPV infection, rather than for individuals already diagnosed with invasive cancer.
Concept: Studies reporting on the outcomes of cost, acceptability, health equity, and uptake of HPV self-sampling were included regardless of whether they compared self-sampling to provider sampling. However, studies focusing on accuracy were restricted to those that specifically compared HPV self-sampling to provider sampling. Accuracy was defined by both; test sensitivity—the ability of the self-sampling method to correctly identify individuals who have an HPV infection and test specificity— ability of the self-sampling method to correctly identify individuals who do not have an HPV infection (37). Cost was defined as the estimated expense (in US dollars for HPV testing using self-sampling or provider-sampling (20). Acceptability was defined by how well HPV self-sampling was received by the target population, including their willingness, comfort and overall satisfaction using the test (38). Uptake was defined as how many eligible individuals chose to participate in the self-sampling process, as a percentage of those that were eligible to participate (39). Health equity was defined as fair access to HPV self-sampling regardless of socioeconomic or demographic factors to reduce disparities in access to testing (40). Studies reporting alternative cervical cancer screening or testing strategies to self-sampling were excluded.
Context: Studies conducted in at least one of the LMICs were included based on the World Bank classification (41). Those conducted in high-income countries were excluded for falling outside the scope of this review (Supplementary Annex 2).
Types of studies: Published studies written in English or those with accessible English translations, regardless of their randomisation unit, the presence of a control or multiple comparators were identified. Quantitative (experimental and observational), qualitative and mixed-methods studies were considered. Studies reporting on accuracy data or two-by-two tables were limited to those evaluating self-sampling compared to provider-sampling, regardless of the reference test option. Modelling studies reporting on cost and conference abstracts published in conference proceedings with their corresponding full-text papers were also considered. Conference abstracts only, traditional literature reviews, editorials with insufficient information, case reports and opinion papers were excluded because of the increased risk of bias within these article types.
2.2 Study identificationCochrane’s information specialist (VL) performed the electronic searches. The following databases were searched from 1946 to 10 July 2023: MEDLINE (via OVID), EMBASE (via OVID), CINAHL (via EBSCOhost), SCOPUS, Web of Science and Global Index Medicus. The following clinical trial registries were also searched: CENTRAL (Cochrane Central Register of Controlled Trials), ClinicalTrials.gov, WHO Trials Register—International Clinical Trials Registry Platform (ICTRP) and the pre-print server for health sciences-MedRxiv.
Other searches were conducted through the reference lists of the relevant secondary studies and grey literature from the following websites: WHO, International AIDS Society (IAS) and the International Agency for Research on Cancer (IARC). The following search terms alongside their synonyms were applied during the search: “human papillomavirus (HPV),” “self-collected sample,” “provider-collected sample” “cost benefit analysis,” “healthcare access,” “health equity,” “socioeconomic parameters,” “patient acceptance of health care,” and “preferences.” See Supplementary Annex 3 for the full search strategy.
2.3 Study selectionTwo independent reviewers (any pair from JO, LW, EEO, and MN) screened the titles and abstracts of all eligible studies using Covidence—a systematic review management software to identify relevant studies (42). After that, two independent reviewers (any pair from JO, LW, EEO, and MN) retrieved and screened full texts of the remaining studies against our eligibility criteria. All disagreements were resolved by discussion between the reviewers (JO, LW, EEO, and MN) or by consulting senior reviewers (MM and EO) for consensus. To minimise bias, senior reviewers (MM and EO) checked a random sample (20%) of the included studies.
2.4 Data chartingTwo independent reviewers (any pair from JO, LW, EEO, and MN) piloted the data extraction form of five eligible studies. However, data extraction was done by at most one reviewer (either JO, LW, EEO, or MN) using a pre-designed data extraction form in Covidence (42). A senior reviewer (MM) conducted quality checks on a random sample (10%) of the extracted data. Discrepancies were resolved by discussion between the reviewers (JO, LW, EEO, and MN) or by consulting a senior reviewer (MM and EO) for a consensus.
Data was charted on the following aspects:
General study details: title, objectives, lead author’s surname, country, WHO region (Supplementary Annex 2)—and year of publication.
General study characteristics: study type, design, participant description (number and age), target disease, index test (HPV self-sampling) and comparator/reference test (HPV provider-sampling) particulars (assay, sample type, diagnostic test, collection device, sample transportation mode and manufacturer), outcomes (accuracy—sensitivity and specificity, acceptability, cost-effectiveness, financial costs and uptake), health facility setting and facility ownership.
Relevant PROGRESS-Plus factors across all studies, as guided by the PROGRESS-Plus framework for Health Equity, that is, Place of residence, Race/ethnicity/culture/language, Occupation, Gender identity, Religion, Education, Socioeconomic status and Social capital (40). The Plus factors included age, disability and comorbidity. See Supplementary Annex 4 for a detailed definition of the PROGRESS-Plus equity factors.
2.5 Quality appraisalThe methodological quality or risk of bias in all included studies was not assessed, as the scoping review guidance does not recommend it and our review solely aimed to map the existing literature (35). Nevertheless, two independent reviewers (any pair from JO, LW, EEO, and MN) appraised the quality of included diagnostic accuracy studies to guide the interpretation of findings during narrative synthesis. The appraisal was based on the reporting of the 10 items across the four domains of the QUADAS-2 tool (43).
2.6 Data synthesisSTATA version 17 (Stata Corp LLC, College Station, TX) was utilised to descriptively summarise quantitative data on diagnostic accuracy, acceptability, cost, uptake, and health equity factors. Qualitative data was synthesised using thematic analysis and reported in narrative form. Findings were presented using tables and graphs. Recommendations for future research and implications for policy and practice were provided based on the identified evidence gaps.
3 Results 3.1 Study selectionOur search yielded 3,739 articles, of which 1,179 duplicates were excluded. Screening involved 2,560 titles and abstracts, resulting in the exclusion of 2,245 studies. We screened 315 full-text articles from which we excluded 191 (Figure 1 and Supplementary Annex 5) and included 124 primary studies.
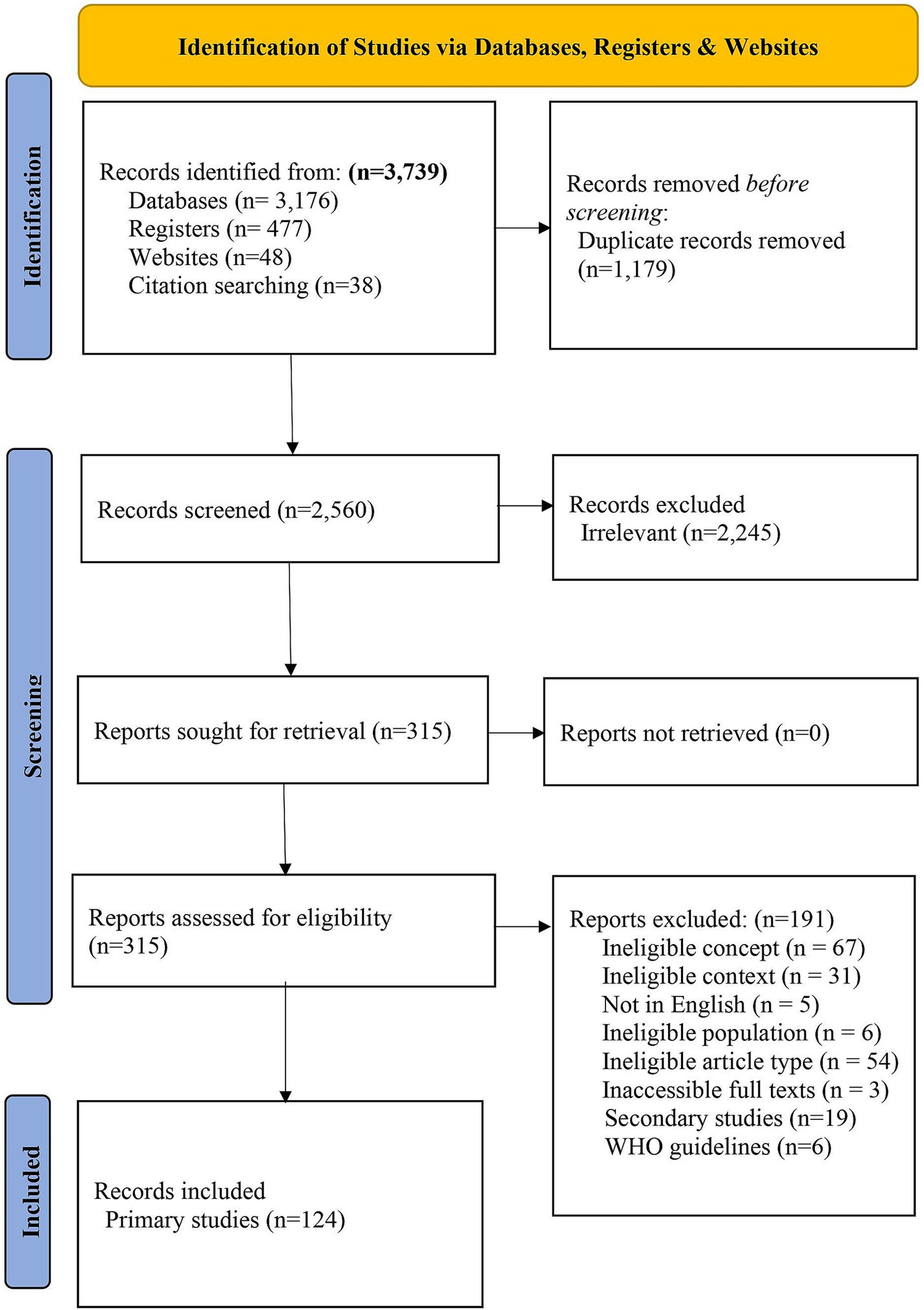
Figure 1. The PRISMA 2020 flow chart showing study selection process.
3.2 General characteristics of included studies and guidelinesThe included studies (n = 124, 100%) were published between 2000 and 2023 (Supplementary Annex 6). Most studies (n = 123, 99.2%) were done on women aged between 15 and 88 years, except for one on transgender men—individuals identifying as male who were assigned female sex at birth (44). Many studies were conducted within the African region (n = 61, 49.2%). No studies were identified from the European or Eastern Mediterranean regions. By country, the highest number of studies were conducted in China (n = 13, 10.5%) and South Africa (n = 12, 9.7%), both of which are considered upper-middle-income countries.
A large proportion of studies were conducted in healthcare facilities (n = 97, 78.2%), with some taking place in community health posts (n = 31, 25.0%) and fewer in-home settings (n = 8, 6.5%). The majority were quantitative studies (n = 111, 89.9%), followed by qualitative (n = 7, 5.6%) and mixed methods (n = 6, 4.8%). Among the quantitative studies, cross-sectional designs were the most common (n = 90, 81.1%), followed by cohort studies (n = 9, 8.1%), randomised trials (n = 5, 4.5%), modelling (n = 4, 3.6%), micro-costing (n = 2, 1.8%), and non-randomised crossover trials (n = 1, 0.9%).
Studies primarily reported on acceptability (n = 79, 63.7%, 71,418 participants), followed by accuracy (n = 51, 41.1%, 73,618 participants), cost (n = 7, 5.6%, 11,593 participants), and uptake (n = 7, 5.6%, 17,784 participants). Although no studies assessed equity as an outcome, the majority (n = 106, 85.5%) mentioned at least one equity factor. Nearly all studies (n = 118, 95.2%) were conducted in single-country settings, with a few (n = 6, 4.8%) involving multiple countries (Mexico, Peru, Malawi, South Africa, Argentina, and Brazil).
All studies (n = 124, 100.0%) conducted HPV DNA for the self-collected samples. For HPV provider-sampling, most studies (n = 58, 46.8%) assessed HPV DNA testing, with few studies (n = 6, 4.8%) reported on HPV messenger ribonucleic acid (mRNA) testing. The remaining 60 studies did not report on diagnostic tests for provider-sampling. The Digene HC2 (n = 23, 33.3%), careHPV and Xpert (each n = 11, 15.9%) and Aptima (n = 5, 7.2%) assays were the most frequently reported tests used for both self-sampling and provider-sampling. Most studies reported using brushes for specimen collection (n = 71, 57.3%), followed by swabs (n = 44, 35.5%) and nine did not report on the collection device. Of all studies included (n = 62, 50.0%) used wet and (n = 17, 13.7%) used dry self-collected sample transportation. Forty-five studies did not report on the mode of sample transportation. Only one study directly compared the sensitivity and specificity of dry vs. wet self- and provider-collected sample transportation modes, respectively.
3.3 Summary of findings 3.3.1 AcceptabilityMost studies (n = 66, 84.6%) reported quantitative outcomes on HPV self-sampling (Table 1) of which (n = 53, 80.3%) reported that more than half of the participants were willing to obtain the samples themselves (57.7–100.0%). A few quantitative studies (n = 12, 18.2%) reported that participants preferred provider-sampling (54.8–83.8%). One study reported that the acceptability of self-sampling was 95.6% in rural areas and 79% in urban areas (45). Studies reporting qualitatively on HPV self-sampling acceptability were (n = 11, 13.9%). The central theme of these qualitative studies revolved around the acceptability of HPV self-sampling, giving rise to eight sub-themes as follows: (Supplementary Annex 7).
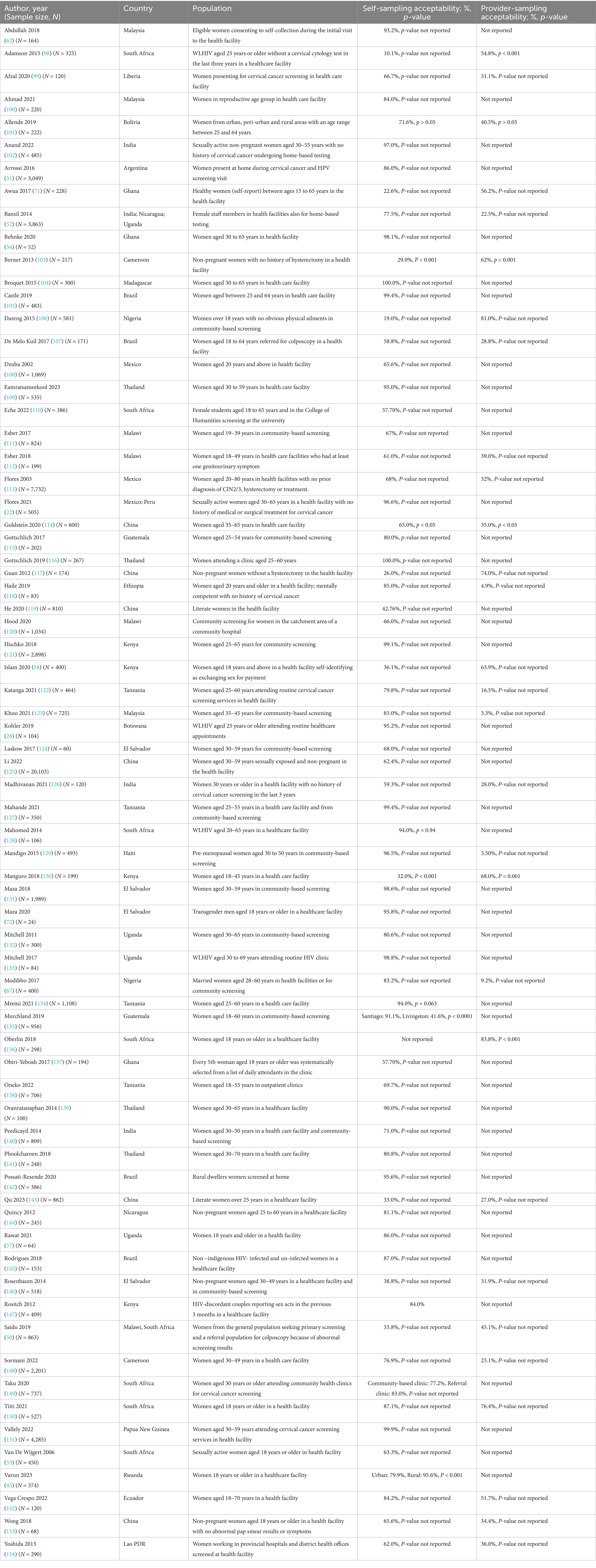
Table 1. Acceptability of HPV self-sampling in LMICs.
Preference: Eight studies presented this sub-theme, of which seven studies showed evidence supporting participants’ preferences of self-sampling to provider-sampling (46–52). Many women expressed a strong preference for self-sampling over provider-sampling due to the increased privacy and the ability to avoid the discomfort of being examined by a healthcare worker (46–52). The convenience and autonomy that self-sampling provided at home were key motivators for women, allowing them to perform the procedure on their terms, in their own time, and without needing to visit a healthcare facility (46–52). In another study, women preferred provider- to self-sampling because healthcare workers could examine and see any other abnormalities that would instead go unnoticed (50).
Provider’s role in self-sampling: Five studies reported under this sub-theme (46, 48, 52–54). Two of them demonstrated a lack of trust among the participants in their capacities to perform self-sampling correctly (46, 53). Additionally, the role of the providers in offering guidance and assurances was highlighted by the participants in two other studies (52, 54). In one study, women expressed a preference for having a healthcare provider present while they were performing self-sampling for medical tests (48).
Privacy: Five studies reported on this sub-theme (46, 48, 50, 53, 55). They noted that the participants felt self-sampling was private and less embarrassing than provider-sampling.
Barriers to self-sampling acceptance: Four studies reported barriers to the acceptability of self-sampling, amongst which one demonstrated participants’ fear of hurting themselves while obtaining the self-samples (52). In another study, self-sampling conducted at clinics was found to be more convenient than at-home settings for the participants (50). In the other two studies, participants reported the lack of need to screen for HPV due to a lack of symptoms and lack of immediate treatment initiation, assuming the sample was taken at home (29, 56).
Social influence: Four studies were on social impact, two of which showcased the challenges related to social stigmatisation that individuals face when attending health screenings (48, 56). Those attending the screenings feared that the community members perceived them as being ill (48, 56). Additionally, these studies report that the participants often require approval from their partners before taking self-samples (57). Issues regarding lack of confidentiality among the local healthcare providers who would openly discuss the test results were also reported (51).
Perception: Two studies demonstrated that participants had limited understanding and poor perception regarding self-sampling which contributed to low prioritization of cervical (55, 56). Cultural obligations regarding modesty impacted some women’s perceptions of self-sampling (56).
Motivation for provider-sampling: A study reported that participants were motivated to choose provider-sampling because they valued the expertise of healthcare workers, believing that these professionals were more capable of identifying complications and knowing where to refer more complex cases (55). The study highlighted that provider’s engagement in the sampling process, offered better guidance and reassurance, ultimately fostering greater acceptance and understanding among patients (55).
Religious influence: In one study, women, particularly wives to religious leaders, argued that self-sampling interfered with their religious beliefs and practices (56).
3.3.2 AccuracyStudies reported a wide range of sensitivity (37.5 to 96.8%) and specificity (41.6 to 100.0%) for HPV self-sampling compared to provider sampling (Table 2). Only one study directly reported the sensitivity and specificity of dry self-sampling vs. wet provider-sampling (58). In wet transportation, self-sampling had 85.0% sensitivity (95% CI: 66.0–96.0%) and 66.0% specificity (95% CI: 61–71%), while in dry transportation, sensitivity was 78.0% (95% CI: 58.0–91.0%) and specificity was 71.0% (95% CI: 66–76%) (58).
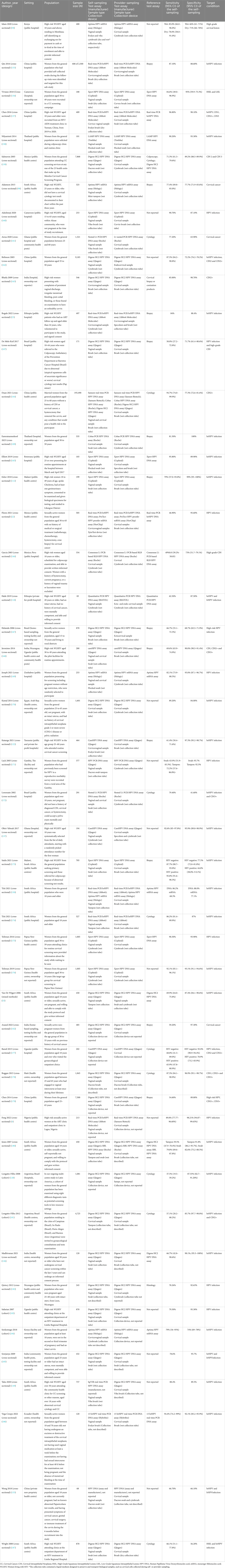
Table 2. Accuracy of HPV self-sampling in LMICs across included quantitative studies stratified by sample transportation mode comparisons between the index and the comparator test.
3.3.3 CostMost studies of the seven reporting on costs (n = 5, 71.4%) focused on the expenditures associated with either self- or provider-sampling (Table 3). Laboratory processing costs (associated with sample processing and analysis) were identical [$5.75 (United States dollars)] for both self and provider-collected samples (59). However, another study estimated a higher laboratory processing cost for the provider-collected samples ($7.10) (60). The round-trip transportation cost for provider-sampling was $0.76 (60). The mean cost per woman screened was slightly lower for self-sampling [$37.1 (range $27.6–$54.0)] compared to provider-sampling [$37.7 (range $26.4–$52.0)] (61). The self-sampling test kit’s reported price range was $11.24–$14.15 (62, 63). For the clinician’s time, the cost was $20.06 for provider-sampling (63). Self-sampling had a lower final aggregated direct medical cost (all expenses incurred throughout the testing process—overall costs) of $7.49 compared to $7.95 for provider-sampling (59).
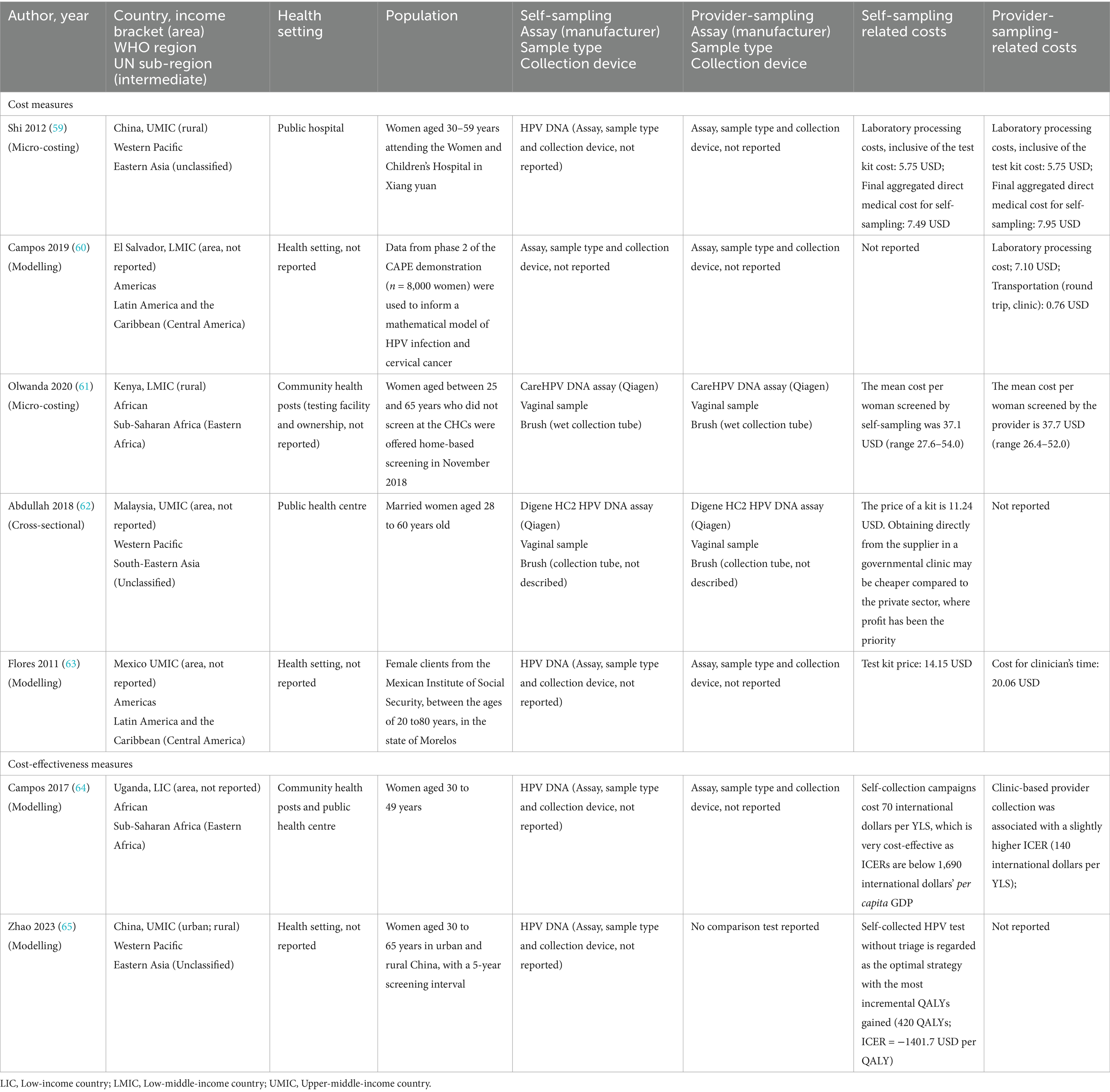
Table 3. Cost of HPV self-sampling and provider-sampling.
Two studies (28.6%) reported on the following cost-effectiveness measures: incremental quality-adjusted life years (QALYs) and total cost-effectiveness ratio (ICER) per year of life saved (YLS) (64, 65). Self-sampling was associated with lower costs of 70 international dollars per YLS, with ICERs below 1,690 international dollars (64). Compared with current strategies (physician-HPV with genotype or cytology triage), all screen-and-treat strategies were cost-effective and self-HPV without triage was optimal with the most incremental QALYs gained (220–440) (65).
3.3.4 UptakeFour randomised studies of the seven reporting on uptake (57.1%) reported that the uptake of HPV self-sampling ranged between 84.1 and 99.2% (Table 4) (66–69). Test uptake was measured based on participants’ consent to the sampling method offered within randomised studies. Participants randomised to self-sampling were 4.02 times more likely to consent than those in the provider-sampling group (95% CI: 3.44–4.71) (69). The remaining three studies (42.9%) offered participants a choice between self and provider-sampling (52, 54, 70). Where a choice was offered (n = 3), uptake of self-sampling ranged between (52.7–96%) in comparison to where participants were randomised (n = 4) (84.5–99%).
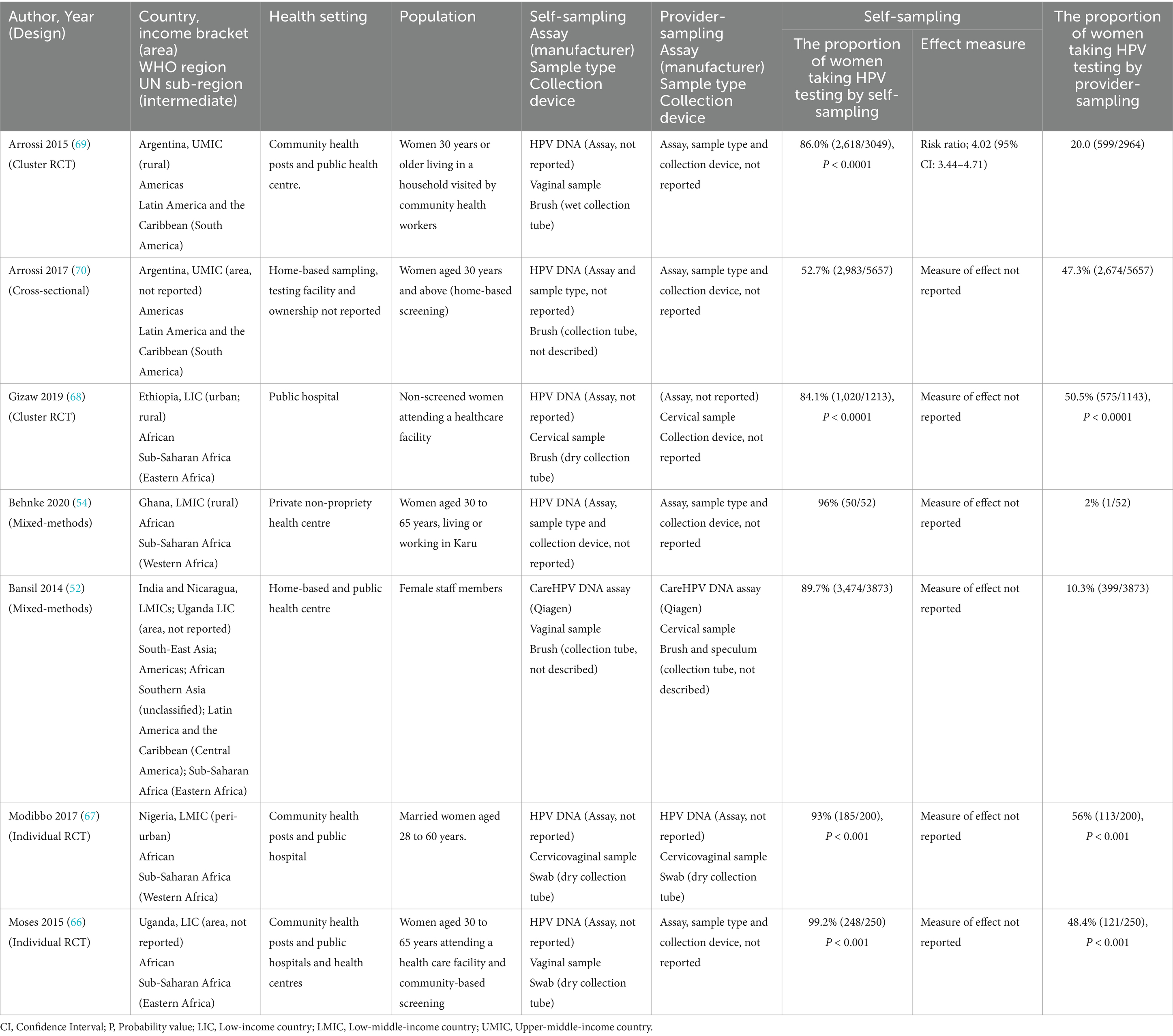
Table 4. Uptake of HPV self-sampling.
3.3.5 Health equity factorsStudies mentioned 10 of the 11 PROGRESS-Plus health-important equity factors but none of them evaluated their impacts on HPV self-sampling (Figure 2 and Supplementary Annex 8) (40). No study mentioned social capital. The most frequently mentioned equity factors were age (n = 69, 65.1%), education (n = 68, 64.2%) and place of residence (n = 59, 55.6%). The least mentioned equity factors were disability (n = 2, 1.9%) and gender identity (n = 1, 0.9%) (69, 71, 72).
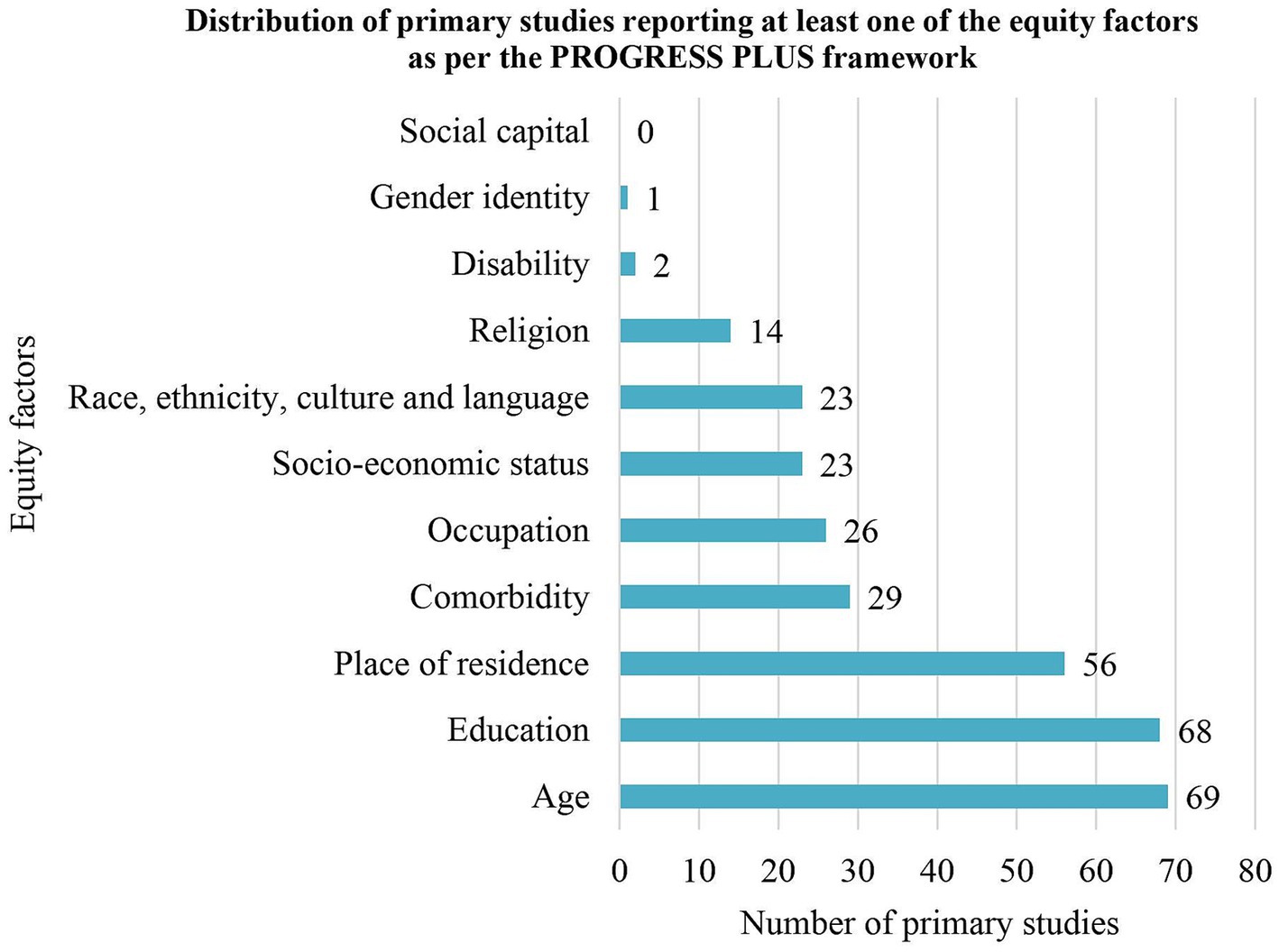
Figure 2. Distribution of studies reporting at least one of the equity factors as per the PROGRESS PLUS framework.
3.4 Quality appraisalQuality appraisal findings of the included accuracy studies using the QUADAS-2 tool are available in Figure 3 (43). Most accuracy studies reported an appropriate interval between the index and the reference test (n = 40, 78.4%) and that all patients received the same reference standard (n = 31, 60.8%). Most of the accuracy studies avoided inappropriate exclusions (n = 28, 54.9%) case–control designs (n = 24, 47.1%) and used suitable reference standards (n = 26, 50.9%). However, only (n = 22, 43.1%) studies included all patients in their analyses. Few studies (n = 16, 31.3%) adopted random or consecutive sampling and (n = 12, 23.5%) used pre-specified thresholds. Fewer studies (n = 6, 11.8%) blinded the interpretation of the reference and the index (n = 5, 9.8%) test results.
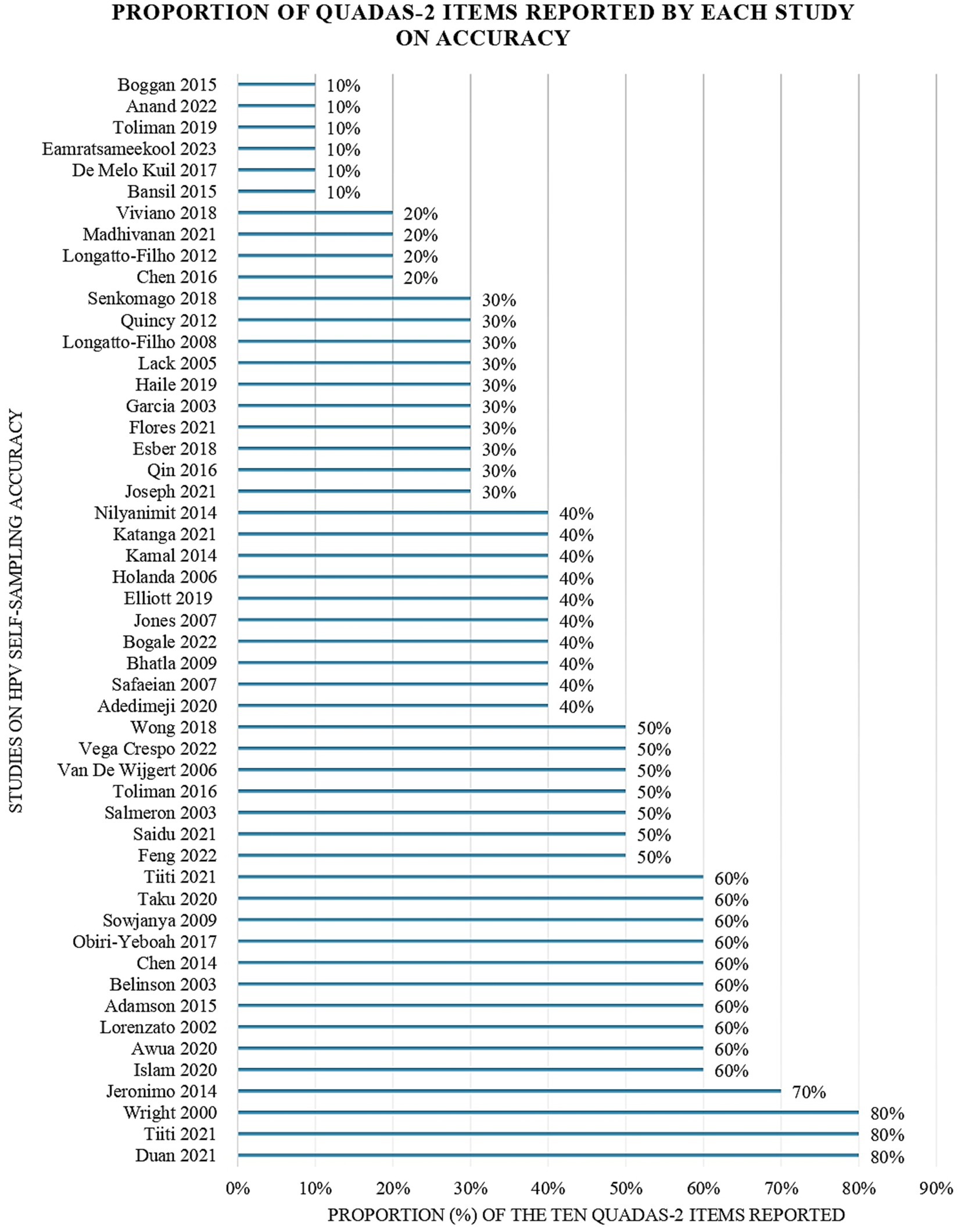
Figure 3. Proportion of QUADAS-2 items reported by each study evaluating the accuracy of HPV self-sampling.
4 Discussion 4.1 Summary of the key findingsIn this scoping review, we mapped and summarised the existing evidence and identified gaps in HPV self-sampling vs. provider-sampling for cervical cancer screening in LMICs. The majority/most studies were from sub-Saharan Africa and upper-middle-income countries indicating minimal evidence from other LMIC settings such as South Asia, Southeast Asia and Latin America. Participants included had a wide age range (15–88 years). WHO recommends HPV screening at 30 years of age for non-HIV-infected individuals and 25 years for persons living with HIV with regular screening after every 5–10 years (73). While most accuracy studies used wet sample transportation modes, few included dry sample transportation modes. Direct comparisons were limited to one study, which suggested that dry sample transportation performed similarly to wet sample transportation for detecting high-grade squamous intraepithelial lesions (HSIL) (58). Our findings revealed HPV self-sampling’s importance for improving cervical cancer screening uptake and highlighted the need for further research on equity considerations (66–69). Most studies found HPV self-sampling highly acceptable although the reported ranges were broad (46–52). Laboratory processing costs were similar, but final aggregated costs were lower for self-collected samples (59). Most studies reported higher uptake with self-collected samples when blind randomisation of participants took place (66–69). Regarding equity factors, most studies mentioned place of residence, education, and age while few mentioned disabilities, and only one reported on gender identity (69, 71, 72).
4.2 Comparisons with other studiesSimilar to our findings on the acceptability of self-sampling, Morgan et al. (74) reported wide ranges (64.7–93.0%). The reported wide ranges could be attributed to variations in sample sizes across studies, study populations, cultural differences and the settings in which the studies were conducted (74). In our study, these variations could reflect underlying social and cultural dynamics, especially in LMICs, where health-seeking behaviours and perceptions of reproductive health are shaped by societal norms and access to healthcare. For instance, cultural taboos and embarrassment around cervical cancer screening may explain the lower acceptability rates that were reported in certain settings. Additionally, Camara et al. (75) also reported that most women favoured self-sampling to evade feelings of embarrassment, although some opted for provider-sampling because of issues related to trust and self-confidence. These findings suggest that trust in the healthcare system and self-efficacy in performing medical procedures play crucial roles in screening uptake. Efforts to build trust and increase confidence in self-sampling could therefore help address these challenges. Other barriers like limited access to healthcare, religious and cultural beliefs, and time constraints due to daily responsibilities are important factors that contribute to lower acceptance rates of self-sampling among women (16, 17, 30, 74, 76–80). These barriers not only affect acceptability but also the feasibility of scaling up HPV self-sampling programs in regions where healthcare resources are scarce. Addressing these factors is critical for improving uptake and ensuring the sustainability of self-sampling initiatives in such settings.
In accordance with our results, three systematic reviews encompassing studies done in Africa and another review of studies done in Asia reported higher sensitivities and specificities of self-sampling (81–84). Two of these reported higher sensitivities for CIN2+ but low specificities for CIN2+ (82, 84). Sy et al. (83), assessed the accuracy of self-sampling compared to provider-sampling, including 21 studies for meta-analysis. They reported sensitivities close to 80% and specificities close to 90% of self-sampling to detect high-risk HPV in reference to provider-sampling (83). They also investigated inter-study heterogeneity, finding variabilities in populations, settings and testing methods, likewise our study (83). Our findings revealed that the sensitivity and specificity of self-sampling ranged from 37.5 to 96.8% and 41.6 to 100.0%, respectively. However, the wide range of accuracy measures presented in this review could be attributed to variations in factors such as the sampled population, transportation method, sample-collection device and sample type across included studies (83). These variations underscore the importance of standardising protocols and ensuring reliable sample transport, especially in LMICs, where infrastructure challenges may affect diagnostic accuracy. Self-sampling using dry collection devices was comparable to provider-sampling using wet collection devices for the detection of HSIL (58). While promising, these findings would require further validation as Islam et al. (58) were limited in sample size (400 participants). Additionally, despite the good quality of this study (by reporting 60% of the QUADAS II tool items) interpretations regarding these findings should be made with caution.
Our findings, indicating that HPV self-sampling yielded population coverage gains over provider-sampling and increased cervical cancer screening attendance because of its cost-effectiveness, align with three other systematic reviews focusing on the cost and effectiveness of HPV self-sampling (30, 31, 85). However, evidence on the cost-effectiveness of HPV self-sampling was limited to only two studies (64, 65). While our findings suggest that self-sampling is generally more cost-effective than provider-sampling, the cost-effectiveness of HPV self-sampling in LMICs remains highly context-dependent. Differences in healthcare infrastructure, resource allocation, and local economic factors can significantly affect costs, such as transportation and clinician time, which were shown to vary across studies. Its cost-effectiveness should be evaluated within the specific economic and healthcare contexts of individual regions, acknowledging the uncertainties and limitations that may affect its broader applicability.
The uptake of cervical cancer screening in low and middle-income countries (LMICs) increased when women were offered self-sampling options (30, 86–88). In a scoping review comprising 27 studies, Serrano et al. (88) found that, as of 2022, only a few countries globally (n = 17, 12%) recommended HPV self-sampling. This finding aligns with ours, as most of the evidence on self-sampling uptake originated from just seven countries (Argentina, Ethiopia, Ghana, India, Nicaragua, Nigeria and Uganda). Uptake of self-sampling was high when participants were randomised, but when they had a choice between self- and provider-sampling, there was a large range of uptake reported across studies. This variability could be attributed to differences in sample sizes between randomised studies (200–3,049 participants) and non-randomised studies (52 to 5,657 participants). In a meta-analysis that included 26 high-income countries (HICs), Yeh et al. (86) also reported that uptake was two times higher among participants randomised. Understanding these underlying mechanisms and tailoring strategies accordingly could enhance the effectiveness of self-sampling interventions within LMICs.
Six WHO guidelines published between 2014 and 2020 recommended HPV self-sampling on women aged 30–60 years (89–94). Two of these strongly advocated for HPV self-sampling for DNA testing (89, 92). However, concerning HPV mRNA testing, the guidelines acknowledged that the available low-certainty evidence suggested an inferior performance of self-samples (90). Therefore, provider sampling is recommended for mRNA testing (91). Further studies comparing the outcomes between self-sampling and provider-sampling strategies for DNA and mRNA testing are essential to addressing this gap.
4.3 Gaps and implications for practice, policy and future researchEase of use may necessitate increased uptake of HPV self-sampling (20). More research on the effects of the population sampled, transportation mode, type of swabs, type of samples and cadres of providers on test accuracy would inform decision-making on the uptake, especially with the noted variation in test sensitivity and specificity. Reference tests, comparator tests, type of tests (mRNA vs. DNA), and sampling devices vary in studies requiring more research on their effects on the test accuracy of HPV self-sampling across multiple settings. It is imperative to conduct a systematic review and meta-analysis that compares the accuracy and acceptability of self-sampling in LMICs versus high-income countries. This will help identify gaps in evidence and provide crucial guidance for implementation decisions. While one study showcased that there are no differences in the accuracy of self-sampling depending on the transportation mode, interpretations should be drawn with caution (58). Further research is necessary to confirm these findings and enhance generalizability.
Examining the impact of health equity factors on self-sampling is crucial for shaping policy and reducing disparities (95). Policymakers can utilise the evidence provided to guide policy and guideline development, allocate resources and plan implementation strategies. Therefore, studies may incorporate health equity factors within their evaluations—encompassing participants’ characteristics, future relationships with other settings, and time-dependent relationships (96). Adhering to reporting guidelines on health equity, such as those available on the Equator Network, can improve the quality of implementation studies on HPV self-sampling and enhance applicability (97).
4.4 Strengths and limitationsThe strength of our review included conducting an extensive search of the literature, minimising bias by having two review authors conduct study selection independently and having two senior reviewers conduct quality control by double-checking all excluded studies. Studies reporting on the diagnostic accuracy of HPV self-sampling were also subjected to quality assessment by checking on the reporting of the 10 items across the four domains of the QUADAS-2 tool. Our review also gives an overall view of all relevant factors for any diagnostic intervention with the public health lens. Included studies utilized data from a range of contexts including small pilot studies such as community-based initiatives and local screening programs encompassing multiple regions within the respective nations. This review provides an overview of all critical pieces (accuracy, acceptability, cost, uptake and equity) of self-sampling needed for policy recommendation at both national and global levels.
One of the limitations of our review was that data extraction for each study was done by a single reviewer independently and not by two reviewers independently. Studies that compared the sensitivity and specificity of dry vs. wet transportation modes were limited. While our search terms were kept broad, our review could have missed a few studies, especially those focusing on equity concepts using other related terms; however, we mitigated that by searching the reference lists of relevant secondary studies. Since the scope of this review was broad, differences in study populations, concepts, outcomes and methodologies within included studies may have limited the comparisons across studies.
5 ConclusionThe acceptability of HPV self-sampling was high across most studies, with 80.3% of participants willing to perform the procedure themselves, citing increased privacy, convenience, and autonomy as key motivators. However, barriers such as concerns over proper self-sampling technique, fear of discomfort, and cultural or religious beliefs influenced the preferences of some participants, highlighting the need for targeted interventions to address these concerns. However, this review showed that self-sampling tests varied widely in how accurately they detected HPV. Transportation of self-collected vaginal samples using swabs stored in liquid media incurs additional costs and might create testing barriers for women. Dry transport of samples has the advantage of lower cost and ease of handling. Evidence on comparisons between self-collected vaginal samples using the dry swab and those transported in liquid media is limited, particularly in LMICs. The evidence on cost-effectiveness varies across regions, and further research is needed to determine its broader applicability and address context-specific limitations. More research evaluating variable outcomes for accuracy and uptake, comparisons of transportation modes and comparisons with high-income countries will effectively inform cervical screening uptake. Impact evaluations of health equity factors on HPV self-sampling can improve the applicability and guide the development of policies and programmes.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributionsJO: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LW: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing. MN: Investigation, Methodology, Resources, Writing – review & editing. EaO: Investigation, Methodology, Resources, Writing – review & editing. MM: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. XS: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. MK: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. JM: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. AM: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. ElO: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This review was funded by FIND, with support from Canada’s Department of Foreign Affairs, Trade and Development (DFATD) and the UK MRC African Research Leaders award (MR/T008768/1). The UK MRC African African Researchers Leaders award is jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO) under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union.
AcknowledgmentsWe acknowledge the information specialist, Vittoria Lutje, for designing the electronic search strategies and conducting literature searches.
Conflict of interestThe authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1439164/full#supplementary-material
Footnotes References1. Vu, M, Yu, J, Awolude, OA, and Chuang, L. Cervical cancer worldwide. Curr Probl Cancer. (2018) 42:457–65. doi: 10.1016/j.currproblcancer.2018.06.003
Crossref Full Text | Google Scholar
2. Singh, D, Vignat, J, Lorenzoni, V, Eslahi, M, Ginsburg, O, Lauby-Secretan, B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the who global cervical cancer elimination initiative. Lancet Glob Health. (2023) 11:e197–206. doi: 10.1016/s2214-109x(22)00501-0
PubMed Abstract | Crossref Full Text | Google Scholar
3. LaVigne, AW, Triedman, SA, Randall, TC, Trimble, EL, and Viswanathan, AN. Cervical cancer in low and middle income countries: addressing barriers to radiotherapy delivery. Gynecol Oncol Rep. (2017) 22:16–20. doi: 10.1016/j.gore.2017.08.004
PubMed Abstract | Crossref Full Text | Google Scholar
4. Feng, N, Ezechi, O, Uwandu, M, Abimbola, BS, Vincent, GD, Idigbe, I, et al. Self-collected versus medic-collected sampling for human papillomavirus testing among women in Lagos, Nigeria: a comparative study. BMC Public Health. (2022) 22:1922. doi: 10.1186/s12889-022-14222-5
PubMed Abstract | Crossref Full Text | Google Scholar
5. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
留言 (0)