Laparoscopic appendectomy (LA) is the gold standard for the surgical care of appendicitis, with this minimally invasive approach allowing patients to typically spend less time in hospital and promptly return to normal life activities (1). It is deemed an ideal training opportunity for young surgeons owing to the high volume of patients who present requiring this surgery (2), most cases are deemed technically straightforward, and it is not associated with high morbidity or mortality rates (3).
Despite all of this, key opinion leaders give this procedure high priority for evaluating resident competency in a simulation-based setting (4). Standardized procedural checklists within an organised training environment, involving both trainers and residents, have been shown to be useful training paradigms (5). Alongside this process, simulation-training models enable learners to rehearse critical procedural steps and develop the necessary psychomotor skills in a safe learning environment.
Although cost-effective biological model equivalents are useful, there are limitations on their biological application in laboratories that aren't licensed to use such specimens (6). In contrast, virtual reality simulators (7), and to a lesser extent, single-use commercially made synthetic training models may carry a significant financial burden on the delivery of these training programmes. Silicone is a moldable material that can be used to effectively replicate soft tissue. It is available in a range of shore hardness levels, from soft to hard. There is a growing need to create simulation-training models of low cost and high-fidelity, to enable construction and subsequent use in low resource settings (8).
We describe a high fidelity, low cost, sustainable, simulation-based training model for LA that can be replicated at large scale, using different representations of variegated silicone as a proof of concept.
MethodConcepts from design-based research (DBR) were used to guide the evolutionary design and model development process over a period of 6 months (November 2023—May 2024) (9). Engineering methods that constitute evaluative and incremental improvement elements are becoming more prevalent in simulation-based education; with the aim of implementing continuous improvement cycles to effectively address learner needs (10). The main goal of this LA model development was to create a model that could be used for experiential learning (11), specifically for large cohorts of surgical residents to engage in deliberate practice (12).
As a framework template, a predetermined procedural checklist was also used to ensure the model replicated the majority of the previously specified procedural steps (5). A team dedicated to developing this surgical simulation-training model was established, equipped with the clinical knowledge and model engineering expertise. This comprised three simulation-based researchers (AR, GD, COC), a consultant general surgeon (NMcC), a junior general surgical registrar (WD) and a simulation-based educationalist (CC). Figure 1 outlines the critical steps of this process.
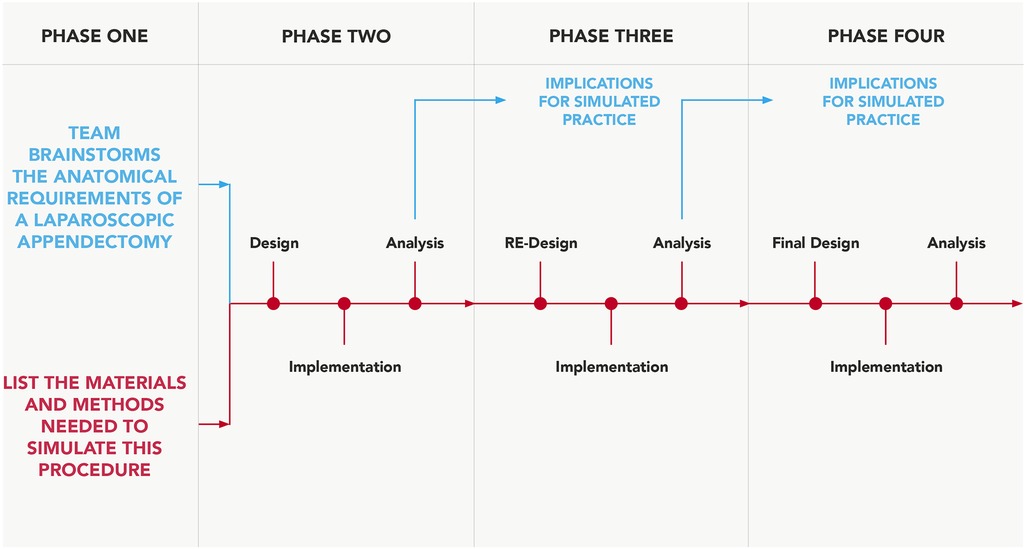
Figure 1. Design-based approach. Process adapted and modified for simulation-model development, from Fraefel (13).
Phase one design was a singularly occurring event, led by AR, GD, COC and CC, who have extensive experience utilising cadaveric visceral tissue for simulation-based training and have considerable expertise in replicating surgical anatomy with synthetic materials (14–16). This incorporated an anatomical review of appendix anatomy (5, 17), subsequent brainstorming and documentation around potential materials to use for physical model creation. Arising from a review of the literature and brainstorming with colleagues, it was agreed to utilise various shore hardness and colorants of silicone to make the model in its entirety for phase two testing, as previous research has reported success in utilising silicone to mimic deep tissue biomechanics (18). The fact that silicone does not adhere well to most other synthetic materials besides itself was also a reason for this selection; the use of a single material type would enable efficacious model composition. It was decided not to obtain clinical input until phase two onwards in order to avoid feedback saturation.
The remaining testing and feedback phases comprised task execution and analysis, with surgical input from NMcC and WD at separate intervals, in order for the remaining team to evaluate and collate model usability feedback from both surgeon attempts in isolation. Both surgeons have carried out multiple laparoscopic appendectomies as the primary operator, NMcC (>800), WD (≥) 50. An objective approach was taken to procedural execution for task assessment, utilising some of the steps outlined in a previous study (5), to also include clipping of the appendicular artery. Reflection and detailed analysis on the models usability followed task assessment from both user attempts combined.
The author team could not find any validated rubrics in the literature, which seek to evaluate the usability of simulation models; therefore, we devised a traffic light system evaluation method, which records and maps the progression of qualitative user feedback from all iterative feedback phases (Table 1). This evaluation method focused on three main areas: (1) tensile qualities, (2) tissue discernibility and (3) anatomical scale and location. Both surgeons would need to be in unanimous agreement that functional fidelity (19) has been achieved for each of the seven components before green light status can be achieved.
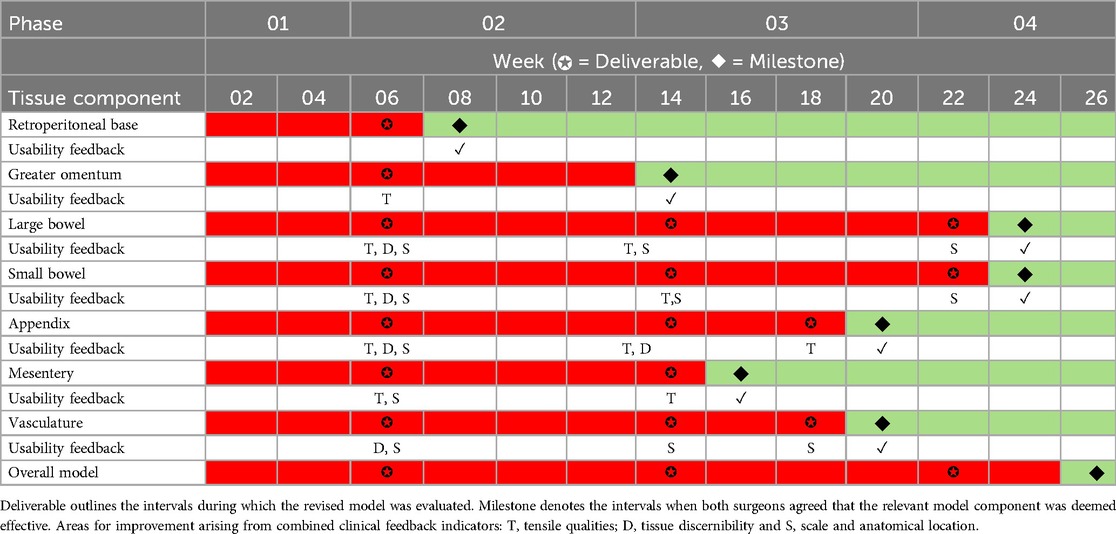
Table 1. Overview of development timeline for each component of the simulation-model.
One simulation-based researcher (AR) led the documentation process, and convened with the remaining research team to discuss user feedback and map the terrain for further model development. The majority of the surgical input for each component's improvement was based largely around anatomy that was either too thin or thick, incorrect colour, improper fixation or tissue adjoining to the incorrect anatomical location. Our LA training model underwent four stages of model development prior to unified stakeholder consensus that this model was deemed effective and suitable for integration into formative surgical simulation curricula, thus all components obtaining green traffic light status. Additional surgical evaluation occurred during phase three in order to somewhat expedite the model development process.
ResultsOur simulation training-model effectively allows residents to execute most of the critical steps associated with performing an LA. The visceral anatomy sits on top of a hard red silicone retroperitoneal base. The small and large intestines are adhered to different areas of the base in order to prevent full lift off throughout practice. The entire model is inserted into a laparoscopic box trainer; since the trainer has already established abdominal access, it is not possible to practice abdominal entry through pneumoperitoneum execution. The model design approach is scalable to accommodate different sized box trainers (Figure 2). Appendix 1 contains a video of one author (NMcC) performing procedural tasks on this model; Appendix 2 provides step-by-step instructions on how to build this model.
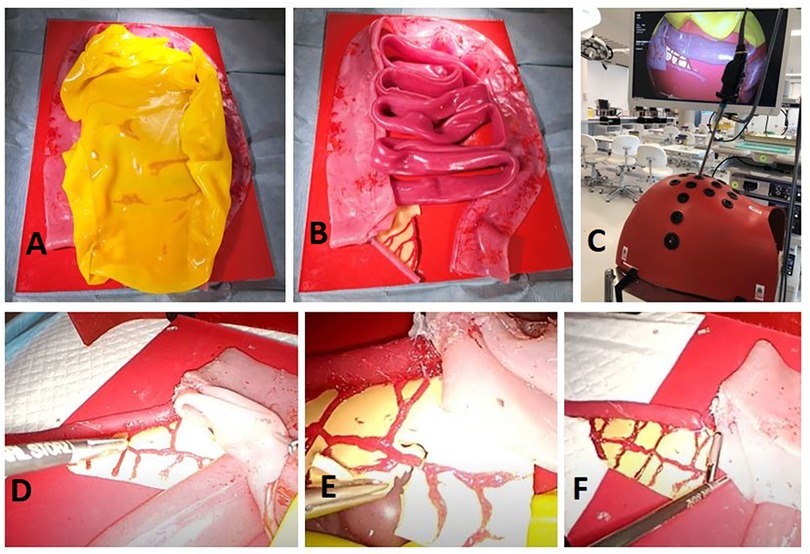
Figure 2. Entire model fixed to retroperitoneal base covered in a sheet of greater omentum (A) appendix and mesoappendix visibly connected to caecum and ileum (B) model view from inside laparoscopic box trainer (C) retraction of caecum to isolate appendix (D) division of mesoappendix and clipping of appendicular artery (E) endoloop placement prior to division of appendix (F).
Of the intestinal anatomy, only the caecum and ileum are physically required for task execution in this model. We decided to recreate the entire small and large bowel tracts since they are reusable, inexpensive to construct, and gives residents an opportunity to immerse themselves in the task as a whole through representation of additional intestinal anatomy. Representation of the greater momentum adds another step to the procedural process, as residents must retract this prior to task progression.
Mesoappendix and surrounding vasculature are represented with anatomical accuracy in this model, in terms of discernibility, anatomical placement and tensile qualities. This allows residents to effectively divide the mesentery, clip the appendicular artery and continue with the appendix division. One limitation of the model, though, is that, unlike in reality, where surgeons usually separate the mesentery using diathermy, the mesentery is not reactable with energy devices. However, it does allow for practice of careful tissue handling with a laparoscopic scissors.
Crucially, the silicone used to make the appendix is more durable, in order to give a realistic tensile feel, and without being too soft that endoloop tightening would cause an ill-timed division. This was given a lot of consideration throughout the model development process. The appendix is attached to the caecum using a button fastener, meaning the only single use aspect of this model are the appendix components, which are time and cost effective to replace. This model allows for different representations of the task, as the appendix can be positioned retrocaecul, retroileal and in the normal lying free position. A single model in its entirety costs €9.67 to create, and single use appendix cost €1.22 to build thereafter. This, however, does not take into account hidden costs that are difficult to quantify, such as the time technicians require to assemble the model and laparoscopic equipment, such as instruments, box trainer, light source, camera and monitor.
DiscussionLimited information is available in the literature regarding the description of low-cost synthetic simulation-training models for LA. Due to the wide range of conceivable silicone stiffness properties, every anatomical region in our model is fabricated with tensile qualities that resemble real tissue as closely as possible. Using iterative qualitative feedback from experts at critical phases in the model development process, we were able to produce a high-fidelity, low-cost training model for LA that serves as a useful training tool for junior surgeons.
The European Association of Endoscopic Surgery (EAES) recommend that surgical residents need to perform 20 surgeries during the learning curve phase, in order to be able to perform these surgeries independently (20). Through deliberate practice (12), simulation-training utilising this LA simulation model can enhance performance automation, which supports transfer of skills to the operating room (OR) environment. Enhanced task performance through simulation reduces cognitive load in the OR, enabling residents to have greater use of nonrecurrent skills such as decision-making and problem-solving (21). As a result, residents are better equipped to deal with uncertainty and cope with other issues as they arise throughout operating.
Simulation-based education can be costly. Our LA simulation model is economically manufactured, but as previously stated, it cannot be used in isolation; additional equipment and consumables are required for complete laparoscopic practice. As seen in Figure 2, laparoscopic equipment of a clinical standard is prohibitively more expensive, rendering it unaffordable and unattainable in many institutions and jurisdictions. However, a host of affordable laparoscopic setups are available for home creation, as previously described (22, 23). Another study describes a simple laparoscopic setup that uses supplies that can be purchased at a hardware store (24), the only expensive aspect being instrumentation. However, we were able to obtain a catalogue of cost-effective laparoscopic instruments ranging from €22.65, from internet-based shops, which can mitigate costs associated with purchasing costly instruments. By combining our LA model with a cost effective and impactful laparoscopic simulation training setup, residents in the western world and in low resource settings (25) can engage in affordable and meaningful practice of these skills.
Our model has some limitations. In the event of vascular perforation, the vasculature in our model does not bleed. Future designs of the model should address this issue. As previously described, our model is not reactable with energy devices. While some synthetic materials, such as hydrogel, do function in this way, their cost is far higher. Further research should focus on utilising established methods (26, 27) to collate validity evidence to evaluate the model further in order to quantifiably determine whether the simulation-model performs as intended. Previous studies have showed positive correlational outcomes between iterative model design and collating sufficient validation metrics to deem a model effective (13, 28).
ConclusionSimulation-based education provides a useful platform for surgical residents to develop psychomotor skills in a safe learning environment. We describe a model for LA, which allows learners to develop their skill proficiency in this area under expert supervision.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributionsAR: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. GD: Conceptualization, Data curation, Investigation, Project administration, Writing – review & editing. NM: Data curation, Investigation, Validation, Writing – review & editing. WD: Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing. AD: Investigation, Project administration, Supervision, Writing – review & editing. TL: Data curation, Project administration, Writing – review & editing. CO: Data curation, Formal Analysis, Writing – review & editing. CC: Data curation, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Destek S, Kundakcioglu H, Bektasoglu HK, Kunduz E, Yigman S, Tak AY, et al. Comparison of open and laparoscopic techniques in the surgical treatment of acute appendicitis. North Clin Istanb. (2023) 22:704–10. doi: 10.14744/nci.2022.08941
Crossref Full Text | Google Scholar
2. Ahmed O, Mealy K, Sorensen J. Exploring geographic variation in acute appendectomy in Ireland: results from a national registry study. BMJ Open. (2019) 9(8). doi: 10.1136/bmjopen-2018-025231
Crossref Full Text | Google Scholar
3. Bregendahl S, Nørgaard M, Laurberg S, Jepsen P. Risk of complications and 30-day mortality after laparoscopic and open appendectomy in a Danish region, 1998–2007; a population-based study of 18,426 patients. Pol Przegl Chir. (2013) 85(7):395–400. doi: 10.2478/pjs-2013-0060
PubMed Abstract | Crossref Full Text | Google Scholar
4. Toale C, Morris M, Konge L, Nayahangan LJ, Roche A, Heskin L, et al. Generating a prioritized list of operative procedures for simulation-based assessment of general surgery trainees through consensus. Ann Surg. (2024) 1:900–5. doi: 10.1097/SLA.0000000000006118
Crossref Full Text | Google Scholar
5. Skjold-Odegaard B, Ersdal HL, Assmus J, Nedrebo BSO, Sjo O, Soreide K. Development and clinical implementation of a structured, simulation-based training programme in laparoscopic appendectomy: description, validation and evaluation. BMJ Simul Technol Enhanc Learn. (2021) 7:517–23. doi: 10.1136/bmjstel-2020-000728
PubMed Abstract | Crossref Full Text | Google Scholar
6. O’Connor A, Paraoan M. An inexpensive novel training model for simulated laparoscopic appendicectomy training. Ann R Coll Surg Engl. (2021) 103:621–2. doi: 10.1308/rcsann.2021.0085
Crossref Full Text | Google Scholar
7. Andreatta PB, Woodrum DT, Birkmeyer JD, Yellamanchilli RK, Doherty GM, Gauger PG, et al. Laparoscopic skills are improved with LapMentor training: results of a randomized, double-blinded study. Ann Surg. (2006) 243(6):854–63. doi: 10.1097/01.sla.0000219641.79092.e5
PubMed Abstract | Crossref Full Text | Google Scholar
8. Obayemi JE, Donkersloot J, Kim E, Thelander K, Byrnes M, Kim GJ. A needs assessment for simulation in African surgical education. Surg Endosc. (2024) 38(3):1654–61. doi: 10.1007/s00464-023-10665-y
PubMed Abstract | Crossref Full Text | Google Scholar
9. Cowling M, Birt J. Pedagogy before technology: a design-based research approach to enhancing skills development in paramedic science using mixed reality. Information. (2018) 9:29. doi: 10.3390/info9020029
Crossref Full Text | Google Scholar
10. Villanueva PJ, Sugiyama T, Villanueva BM, Rodriguez HI, Arciénaga A, Cherian I. Using engineering methods (Kaizen and micromovements science) to improve and provide evidence regarding microsurgical hand skills. World Neurosurg. (2024) 189:e380–90. doi: 10.1016/j.wneu.2024.06.075
PubMed Abstract | Crossref Full Text | Google Scholar
11. Engels PT, de Gara C. Learning styles of medical students, general surgery residents, and general surgeons: implications for surgical education. BMC Med Educ. (2010) 10(1). doi: 10.1186/1472-6920-10-51
PubMed Abstract | Crossref Full Text | Google Scholar
12. Bhatti NI, Ahmed A. Improving skills development in residency using a deliberate-practice and learner-centered model. Laryngoscope. (2015) 125(S8):1–14. doi: 10.1002/lary.25434
Crossref Full Text | Google Scholar
13. Fraefel U. Professionalization of pre-service teachers through university-school partnerships. In: WERA Focal Meeting; Edinburgh. (2014). doi: 10.13140/RG.2.1.1979.5925
Crossref Full Text | Google Scholar
14. Roche AF, Voborsky M, Meighan V, O’Connor G, Eppich WJ, Condron CM. Developing a clamshell thoracotomy training model to support hybrid teaching in simulation-based education. Emerg Med Australas. (2024) 36(3):482–4. doi: 10.1111/1742-6723.14398
PubMed Abstract | Crossref Full Text | Google Scholar
15. Roche AF, Eppich WJ, O’Keeffe D, O’Riordan JM, Condron C. Developing a simulation training model for abdominal wall opening and closure. Int J Healthc Simul. (2023):66–9. doi: 10.54531/vlpw2676
Crossref Full Text | Google Scholar
16. Roche AF, Redmond T, Zilani G, Healy V, Condron CM. Developing a high fidelity, low cost simulation model for retrosigmoid craniotomy and microvascular decompression of the trigeminal nerve. Br J Neurosurg. (2024):1–4. doi: 10.1080/02688697.2024.2391858
PubMed Abstract | Crossref Full Text | Google Scholar
17. Hodge BD, Kashyap S, Khorasani-Zadeh A. Anatomy, abdomen and pelvis: appendix. In: Ackley WB, editor. StatPearls. Treasure Island, FL: StatPearls Publishing; (2024).
18. Sparks JL, Vavalle NA, Kasting KE, Long B, Tanaka ML, Sanger PA, et al. Use of silicone materials to simulate tissue biomechanics as related to deep tissue injury. Adv Skin Wound Care. (2015) 28(2):59–68. doi: 10.1097/01.ASW.0000460127.47415.6e
PubMed Abstract | Crossref Full Text | Google Scholar
20. Neugebauer E, Troidl H, Kum CK, Eypasch E, Miserez M, Paul A. The E.A.E.S. consensus development conferences on laparoscopic cholecystectomy, appendectomy and hernia repair. Consensus statements—September 1994. The educational committee of the European Association for Endoscopic Surgery. Surg Endosc. (1995) 9:550–63. doi: 10.1007/BF00206852
PubMed Abstract | Crossref Full Text | Google Scholar
21. Frerejean J, van Merriënboer JJG, Condron C, Strauch U, Eppich W. Critical design choices in healthcare simulation education: a 4C/ID perspective on design that leads to transfer. Adv Simul (Lond). (2023) 8(1):5. doi: 10.1186/s41077-023-00242-7
PubMed Abstract | Crossref Full Text | Google Scholar
22. Adrales GL, Chu UB, Hoskins JD, Witzke DB, Park AE. Development of a valid, cost-effective laparoscopic training program. Am J Surg. (2024) 187(2):157–63. doi: 10.1016/j.amjsurg.2003.11.020
PubMed Abstract | Crossref Full Text | Google Scholar
24. Łysak JM, Lis M, Więckowski PR. Low-cost laparoscopic simulator—viable way of enabling access to basic laparoscopic training for medical students? Surgeon. (2023) 21(6):351–5. doi: 10.1016/j.surge.2023.03.005
Crossref Full Text | Google Scholar
25. Traynor MD Jr, Owino J, Rivera M, Parker RK, White RE, Steffes BC, et al. Surgical simulation in east, central, and Southern Africa: a multinational survey. J Surg Educ. (2021) 78(5):1644–54. doi: 10.1016/j.jsurg.2021.01.005
PubMed Abstract | Crossref Full Text | Google Scholar
26. Messick S. Standards of validity and the validity of standards in performance assessment. Educ Meas Issues Pract. (1995) 14:5–8. doi: 10.1111/j.1745-3992.1995.tb00881.x
Crossref Full Text | Google Scholar
27. Van Nortwick SS, Lendvay TS, Jensen AR, Wright AS, Horvath KD, Kim S. Methodologies for establishing validity in surgical simulation studies. Surgery. (2010) 147(5):622–30. doi: 10.1016/j.surg.2009.10.068
PubMed Abstract | Crossref Full Text | Google Scholar
28. Roche AF, Kavanagh D, McCawley N, O’Riordan JM, Cahir C, Toale C, et al. Collating evidence to support the validation of a simulated laparotomy incision and closure-training model. Am J Surg. (2024) 233:84–9. doi: 10.1016/j.amjsurg.2024.02.020
PubMed Abstract | Crossref Full Text | Google Scholar
Appendix Appendix 1 Video recording of expert performance of laparoscopic appendectomy using this modelExpert video recorded performance https://www.youtube.com/watch?v=IKgowAlIDGs.
Appendix 2 Step-wise instructions on how to build the model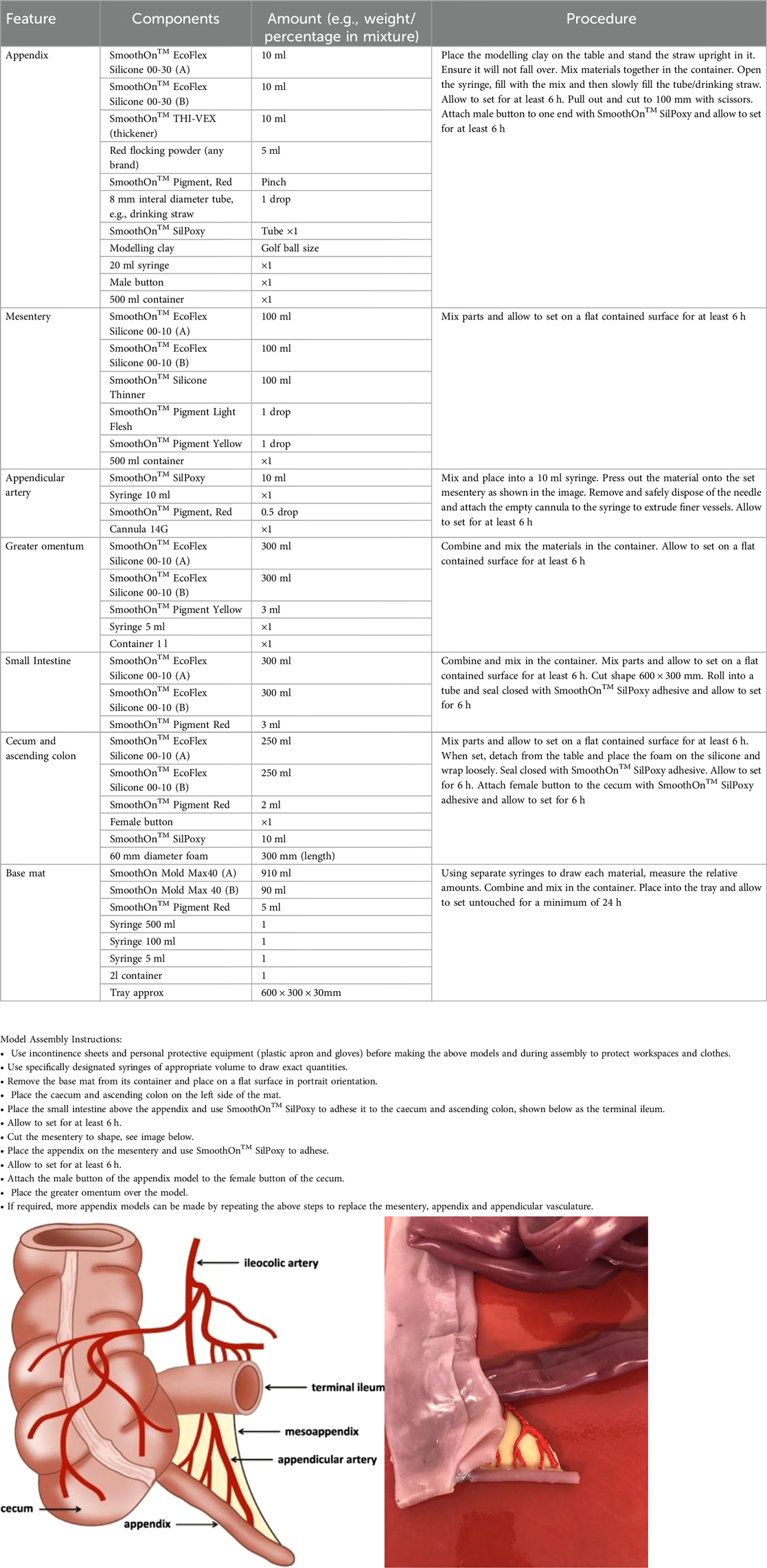
留言 (0)