The management of acute myocardial infarction (MI) has advanced substantially over the past decades, primarily due to the integration of reperfusion strategies and pharmacological therapies aimed at improving patient outcomes (1–7). In particular, for acute MI patients and reduced left ventricular systolic function, beta-blockers (BBs) are essential to reduce cardiovascular mortality and recurrent MI (8). Consequently, recent guidelines strongly recommend the application of BBs in patients with acute myocardial infarction and reduced left ventricular function, highlighting their critical role in improving patient outcomes (3, 4). Several studies have been conducted on using BBs in patients with acute MI and non-reduced left ventricular ejection fraction (LVEF) (9–13). However, the outcomes of these investigations remain controversial, partly due to the absence of large-scale randomized controlled trials. Consequently, current guidelines do not strongly recommend the long-term use of BBs in these patients (4). Furthermore, there is a notable lack of research on how long BB therapy should be continued in acute MI patients with non-reduced LVEF, leaving a significant gap in clinical practice guidance. Therefore, our study aimed to evaluate the clinical impact and determine the optimal duration of BB therapy in patients with acute MI and non-reduced LVEF by comparing the short-term effects (within 30 days) and 1-year outcomes, addressing this important gap in current clinical practice.
Methods Study population and designThe Korean Acute Myocardial Infarction Registry V (KAMIR V) is an open, prospective, observational, web-based, nationwide, multicenter acute myocardial infarction registry from 55 primary percutaneous coronary intervention (PCI) centers started in January 2016. Trained coordinators investigated clinical, laboratory, medication, and outcome data using standardized case report forms and protocols. Angiographic data were collected and assessed by the operators. Clinical data were investigated at 1, 6, and 12 months after discharge. A total of 16,831 patients initially diagnosed with acute MI were investigated. Patients with a reduced and unknown LVEF (n = 3,625) and those who did not undergo PCI (n = 987) were excluded. In addition, 118 patients who died in the hospital were excluded, leaving a total of 12,101 patients in this study. Then, participants were categorized into two groups based on the presence or absence of BB treatment at discharge, as BB use prior to discharge was not documented: the BB group (n = 9,468) and the non-BB group (n = 2,633). The flow diagram of this study is shown in Figure 1. This study followed the guidelines of the Declaration of Helsinki and was approved by the institutional review board of Yeungnam University Medical Center (YUHIRB 2016-03-017). This study was conducted after obtaining informed consent from the patients after a thorough explanation.
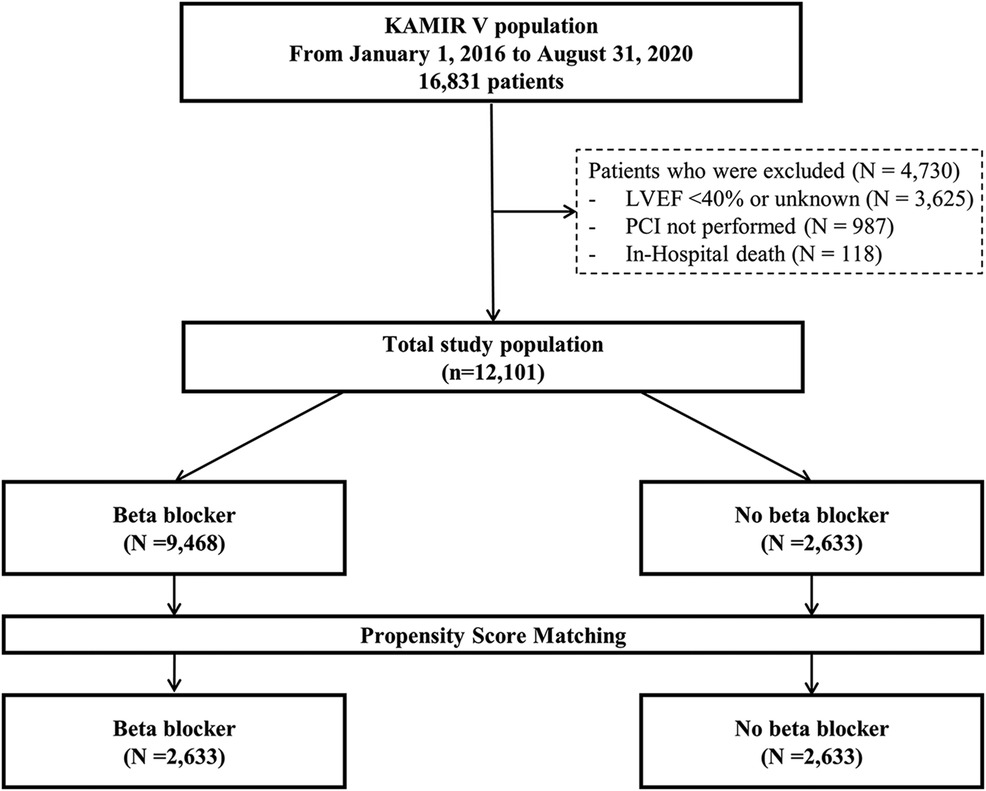
Figure 1. Flow diagram of the study population. KAMIR V, Korean Acute Myocardial Infarction Registry V; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Study endpoints and definitionsWe defined non-reduced LVEF as a concept in contrast with heart failure (HF) with reduced ejection fraction. The primary endpoint of this study was the occurrence of patient-oriented composite endpoints (POCEs), including all-cause mortality, any MI, and any revascularization, at 1-year follow-up after PCI. The secondary endpoints of this study consisted of each component of the POCE during 1-year follow-up. Chronic kidney disease (CKD) was defined as either a pre-existing diagnosis or a serum creatinine level >1.5 mg/dl. Complex PCI was defined as PCI involving the left main artery, an implanted stent length ≥38 mm, PCI for multivessel disease, or implantation of ≥3 stents (14).
Statistical analysisCategorical data were presented as absolute numbers and percentages and analyzed using the χ2 statistic or Fisher’s exact test to compare variables. Continuous data were expressed as mean ± standard deviation and compared using Student’s t-test. Survival curves were generated using the Kaplan–Meier method and compared with the log-rank test. A landmark analysis at 1 month was performed to compare the early and late effects of BB treatment. A propensity score (PS) for treatment with BB was calculated using multiple logistic regression analysis incorporating variables such as age, sex, hypertension, diabetes mellitus, dyslipidemia, heart failure, CKD, clinical presentation [ST-elevation myocardial infarction (STEMI) or non-ST-elevation myocardial infarction (NSTEMI)], complex PCI, and successful PCI that could affect clinical outcomes and treatment group assignment. PS matching was performed using a 1:1 case–control match on the PS with a hierarchical sequence until no more matches could be made. This was done to minimize selection bias and adjust for confounding factors resulting from significant differences in baseline and procedural characteristics between the two groups. In the univariate analysis, a Cox proportional hazards regression model was conducted for several clinical variables. Those variables achieving a p-value <0.1 in the univariate analysis were subsequently entered into the multivariate analysis model to determine independent predictors of POCEs. Event-free survival was analyzed using Kaplan–Meier survival curves, and differences between event-free survival curves were compared using the log-rank test. All hazard ratios (HRs) were calculated with a 95% confidence interval (CI), and p-values <0.05 were considered statistically significant. The proportional hazard assumption for the Cox regression model was tested using log(-log) survival plots and Schoenfeld residuals, with a p-value >0.05 indicating no violation of the assumption. Statistical analysis was implemented using R 4.4.1.
Results Baseline and angiographic characteristicsThe mean age was 63.3 ± 12.2 years, with 78.8% of the patients being men. Of the 12,101 patients with non-reduced LVEF, 9,468 (78.2%) received BBs at discharge, and 60.4% remained on BB therapy at 1-year follow-up. Table 1 presents the baseline characteristics of the BB and non-BB groups. Compared to the non-BB group, the BB group was younger and exhibited a lower LVEF, with a lower prevalence of previous angina pectoris, HF, atrioventricular block, cardiogenic shock, and NSTEMI. However, the BB group had a higher prevalence of hypertension, higher initial systolic/diastolic blood pressure, a higher rate of current smoking, and a greater incidence of STEMI. In terms of laboratory findings at admission, the BB group exhibited lower serum N-terminal pro-B-type natriuretic peptide levels and higher levels of serum total cholesterol, low-density lipoprotein cholesterol, and triglycerides compared to the non-BB group.
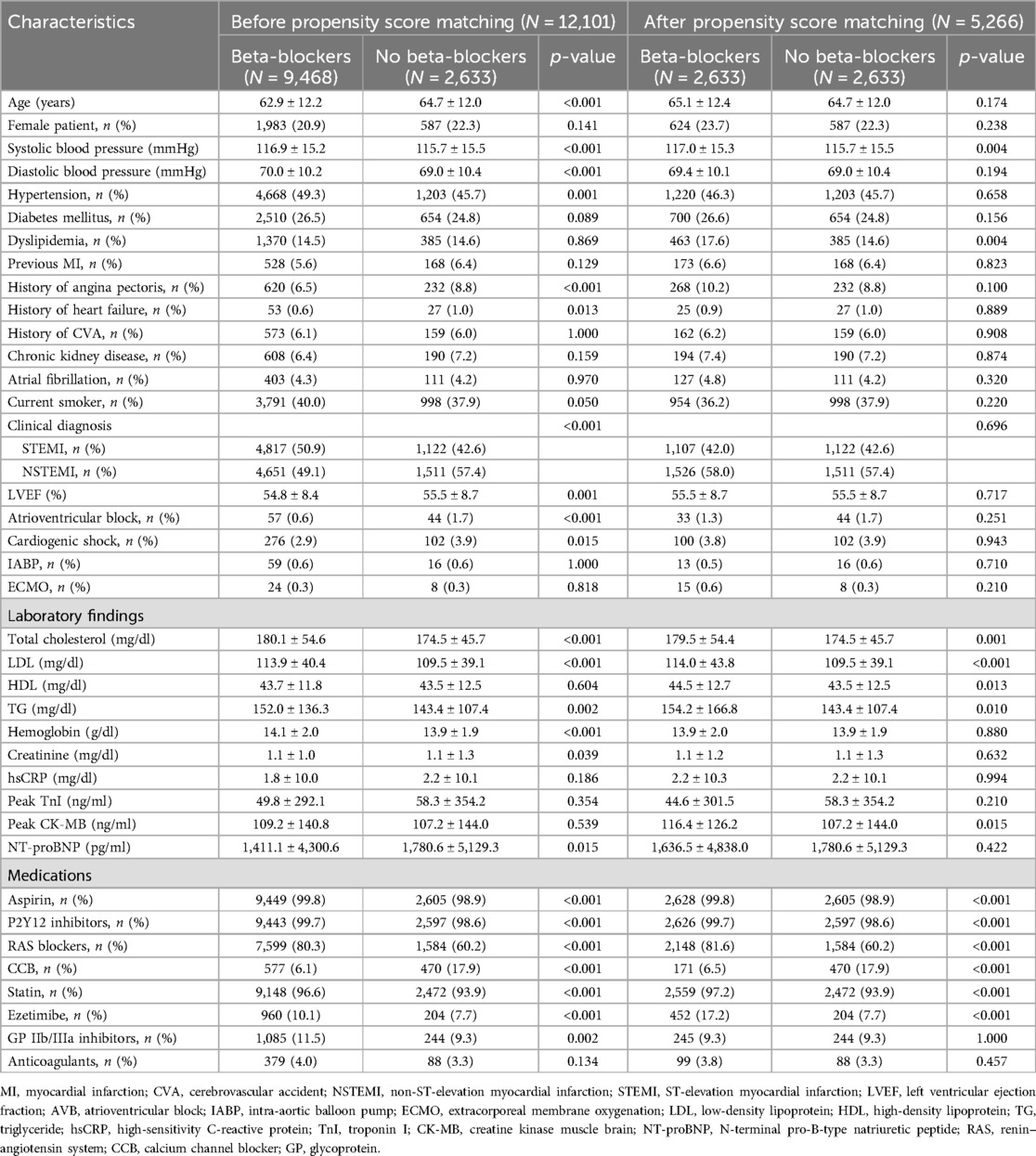
Table 1. Baseline characteristics.
Table 2 presents the angiographic characteristics of the two groups. Compared to the non-BB group, the BB group had higher rates of involvement in the left anterior descending artery, multivessel disease, lesion type C as per the American College of Cardiology/American Heart Association classification, complex PCI, and successful PCI.
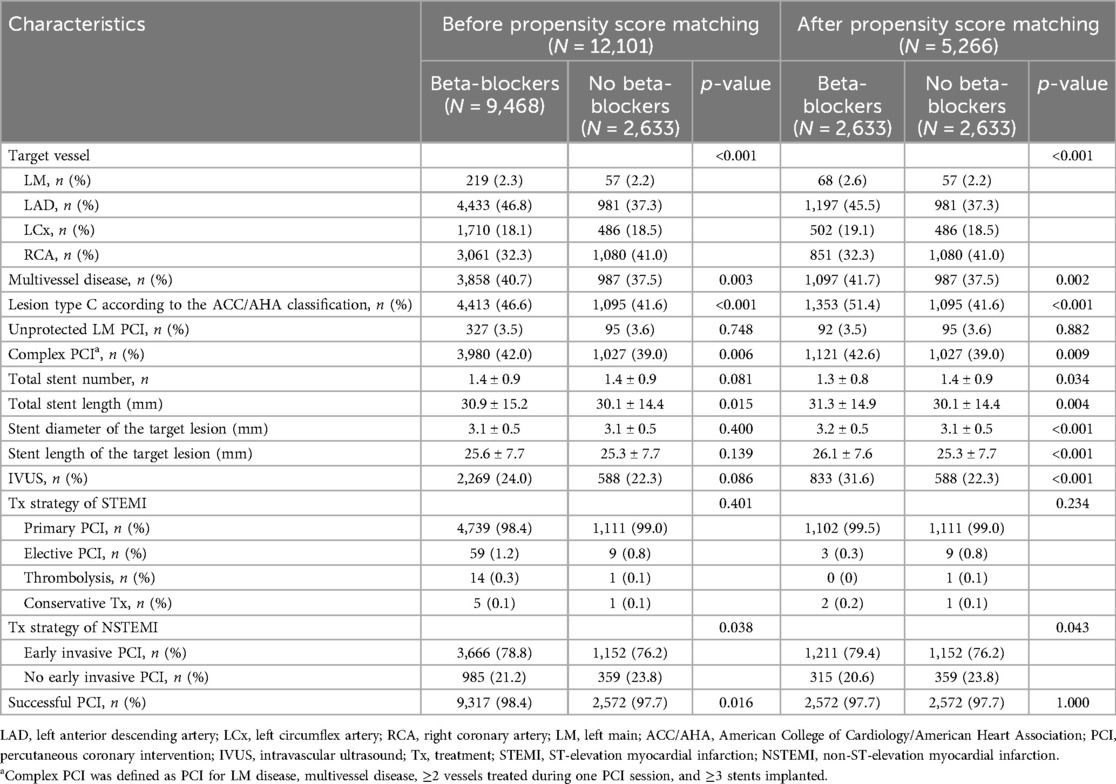
Table 2. Angiographic characteristics.
In summary, the BB group exhibited an unfavorable clinical profile and more complex lesions in the coronary artery compared to the non-BB group. To address significant differences in baseline and angiographic characteristics, PS matching was performed. After PS matching, baseline, and angiographic characteristics were well balanced.
Prescription pattern of beta-blockersIn the total population, carvedilol (45.1%) was the most commonly prescribed BB at discharge, followed by bisoprolol (29.6%), nebivolol (24.5%), and other BBs (0.8%) (Supplementary Figure S1).
Clinical endpoints according to the use of beta-blockersThe median follow-up period was 353 days (interquartile range: 194–378 days).
In the total population, at 1-year follow-up, there were no significant differences in POCEs between the BB and non-BB groups (3.1% vs. 3.4%, HR 0.86, 95% CI 0.68–1.09, p = 0.225). After PS matching, the occurrence of POCEs remained similar between the BB and non-BB groups (3.7% vs. 3.4%, HR 1.01, 95% CI 0.76–1.35, p = 0.931). No significant differences were observed between the BB and non-BB groups for individual endpoints, including all-cause mortality, any MI, and any revascularization, both before and after PS matching (Table 3 and Figures 2, 3). In the short-term outcomes within 1 month, no notable differences were found between the BB and non-BB groups regarding the primary endpoint, and the proportional hazard assumption was confirmed as not violated (p = 0.25), allowing for analysis without separating the first month from subsequent months (Supplementary Figure S2). However, there was a slight difference in the incidence of any revascularization, with the BB group showing a higher incidence (HR 3.11, 95% CI 1.01–9.54, p = 0.047). Importantly, in-hospital events, such as aggravated HF, ventricular tachycardia, and ventricular fibrillation, also showed no significant differences between the two groups.
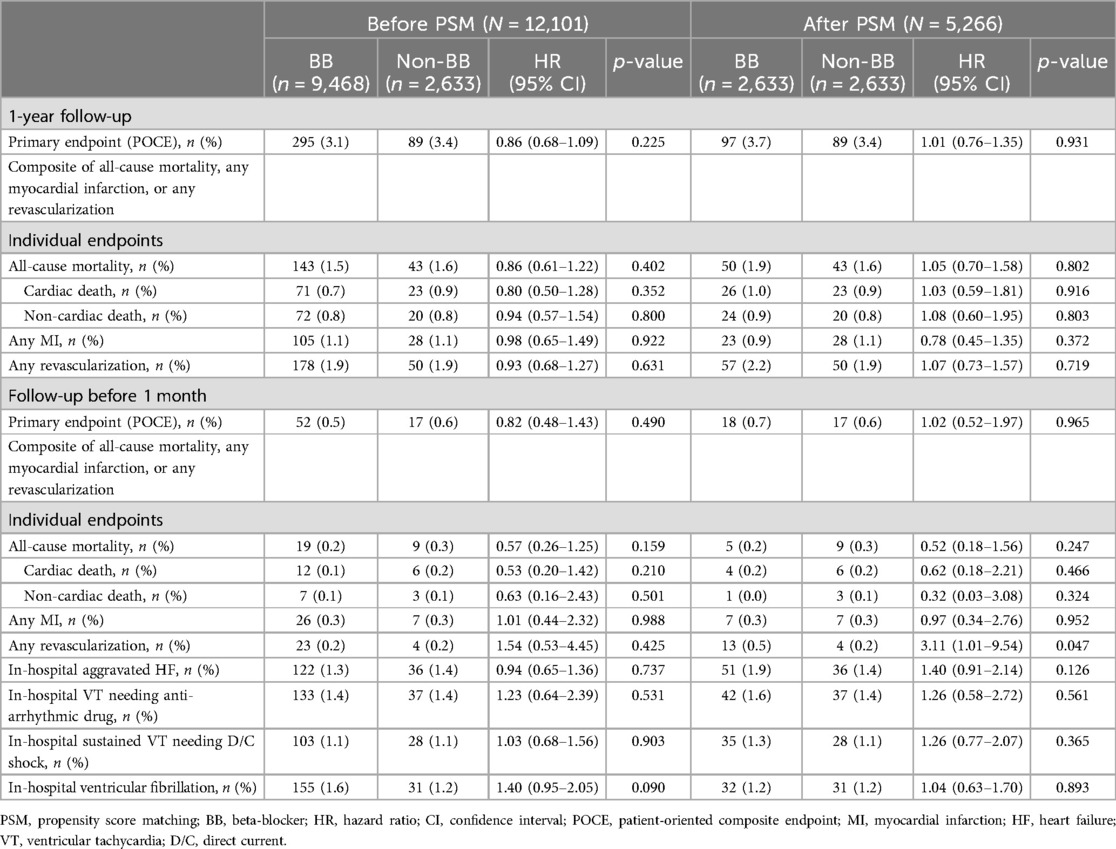
Table 3. Clinical outcomes at 1-year follow-up and before 1-month follow-up based on beta-blocker use.

Figure 2. Time-to-event curves for the primary endpoint according to beta-blocker treatment. Kaplan–Meier survival curves for POCEs at the 1-year follow-up (A) before propensity score matching and (B) after propensity score matching. POCEs, patient-oriented composite endpoints.
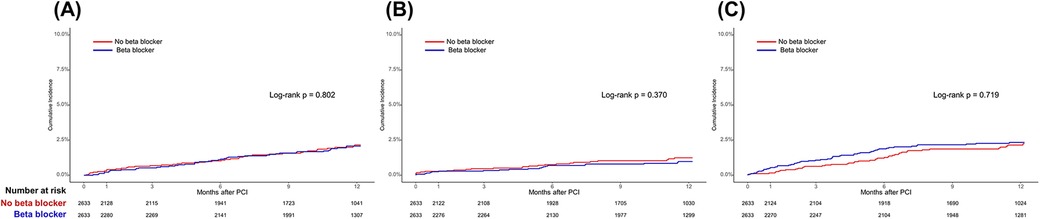
Figure 3. Time-to-event curves for the secondary endpoints according to beta-blocker use in the propensity score-matched population. Kaplan–Meier survival curves for (A) all-cause mortality, (B) any myocardial infarction, and (C) any revascularization. PCI, percutaneous coronary intervention.
Clinical outcomes of different beta-blockersIn the total population, there were no statistically significant differences in the 1-year primary endpoint based on the type of BB used (Supplementary Table S1).
Subgroup analysis for the primary endpoint in the PS-matched cohortThe association between the use of BBs and POCEs at 1-year follow-up was analyzed in prespecified subgroups, including age (<75 and ≥75), sex, hypertension, diabetes mellitus, CKD, prior MI, and type of MI (Figure 4). In the subgroup analysis, BB treatment was significantly unfavorable in patients aged 75 and older than in those under 75 (p for interaction = 0.038). In addition, BB treatment appeared more beneficial in patients with a history of MI, significantly lowering the risk of POCEs (HR 0.41, 95% CI 0.16–1.07) compared to those without prior MI (HR 1.13, 95% CI 0.83–1.53, p for interaction = 0.040). On the other hand, no significant interactions were found based on sex, diabetes mellitus, hypertension, CKD, or type of MI (STEMI/NSTEMI), suggesting that the effects of BB treatment were relatively consistent across these subgroups.
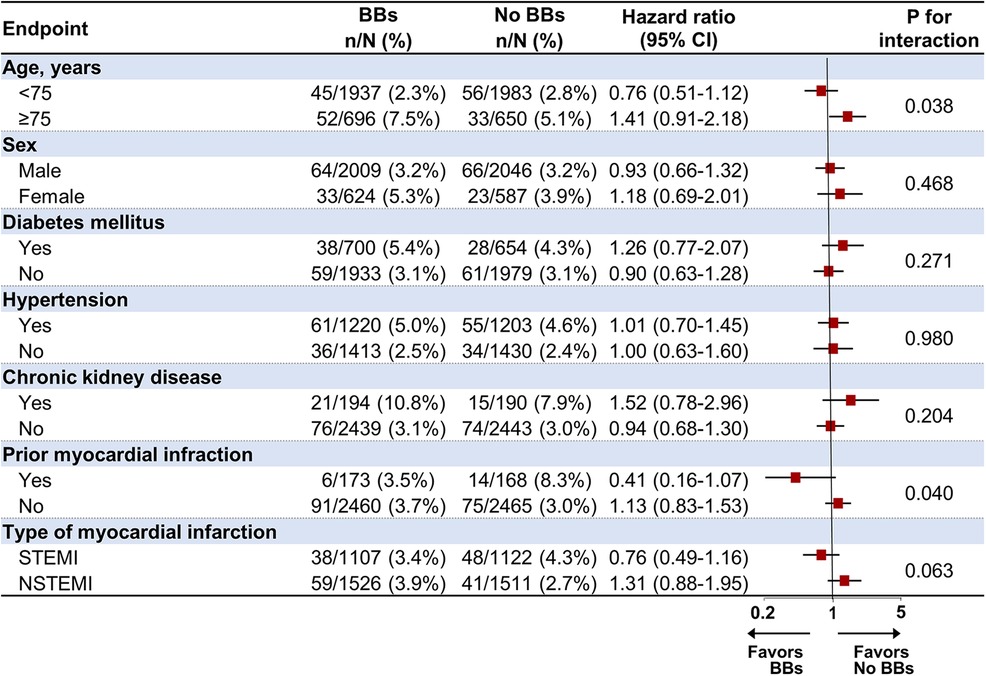
Figure 4. Subgroup analysis for the primary endpoint at 1-year follow-up in the propensity score-matched population. BB, beta-blocker; CI, confidence interval; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction.
Independent predictors for POCEs at 1-year follow-upTable 4 presents the independent predictors for POCEs at 1-year clinical follow-up. After adjusting for confounding factors, the independent predictors for POCEs were age (HR 1.02, 95% CI 1.01–1.03, p < 0.001), CKD (HR 2.47, 95% CI 1.89–3.25, p < 0.001), LVEF (HR 0.98, 95% CI 0.97–0.99, p = 0.002), and multivessel disease (HR 1.57, 95% CI 1.27–1.93, p < 0.001). The prescription of BB at discharge (HR 0.86, 95% CI 0.68–1.09, p = 0.225) was not associated with a statistically significant reduction in POCEs.
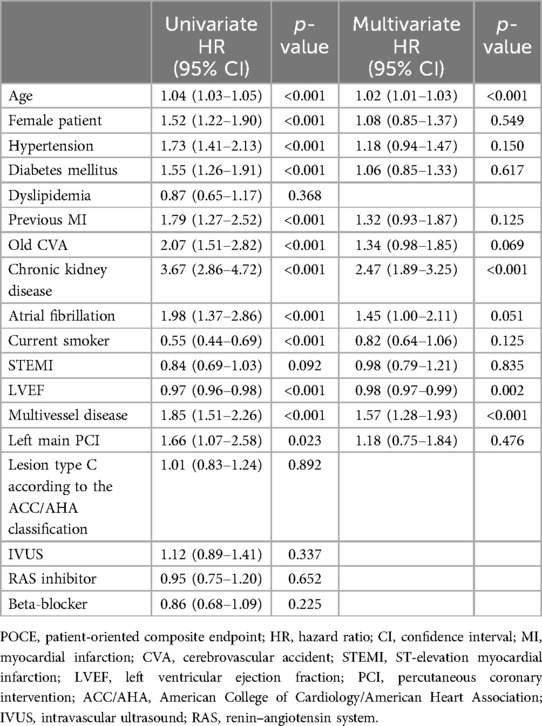
Table 4. Independent predictors of POCEs at 1-year follow-up.
DiscussionThe main findings of this study are as follows: (1) in this study, BB treatment at discharge was not associated with a significant difference in the POCE incidence in the PS-matched population at 1-year follow-up (3.7% vs. 3.4%, HR 1.01, 95% CI 0.76–1.35, p = 0.931); (2) in the short-term outcomes within 1 month, there were no notable differences between the BB and non-BB groups for the primary endpoint; and (3) independent predictors of POCEs at 1-year follow-up included age, CKD, multivessel disease, and reduced LVEF. BB use was not found to be a significant independent predictor in the multivariate analysis.
BBs have been foundational in managing acute MI, significantly benefiting patients with reduced LVEF by reducing mortality and preventing arrhythmias. The efficacy of BBs in patients with reduced LVEF is well-documented and largely undisputed, forming the basis for strong recommendations in clinical guidelines (4, 7). However, the benefit of BB treatment in patients with non-reduced LVEF, particularly in the era of advanced reperfusion therapies, remains less clear. Recently, the efficacy of BB therapy in acute MI patients with non-reduced LVEF has been extensively studied, yielding mixed outcomes. In several studies, BB therapy after MI has been found to be associated with reduced mortality and cardiovascular benefits in patients with acute MI without LV dysfunction (11, 15, 16). Conversely, in a recent prospective randomized study, the effect of beta-blockers in patients with preserved LVEF (≥50%) after MI was evaluated. The results showed that long-term beta-blocker treatment, with a median follow-up of 3.5 years, did not reduce the risk of all-cause mortality or new MI (17). In the previous randomized controlled trial of carvedilol usage in STEMI patients with non-reduced LVEF, this study did not demonstrate a significant benefit of long-term carvedilol use in these patients (10). Although this study conducted a randomized controlled trial design, the number of enrolled participants was relatively small, with a total of only 801 participants. In a nationwide cohort study involving over 30,000 MI patients without HF, no long-term effect of BB treatment on cardiovascular prognosis was observed when following the patients from 3 months to 3 years after MI admission (12). This finding aligned with the results of our study, which indicated no significant difference in 1-year outcomes between the BB and non-BB groups, suggesting that there may also be no differences in long-term outcomes.
In addition, the treatment of BBs and the duration of their usage have not been clearly studied. Two nationwide cohort studies have demonstrated that in patients without LV dysfunction undergoing PCI for acute MI, the use of BB from the first year onward did not improve cardiovascular outcomes (18, 19). Contrary to previous studies, research indicates that early use of BBs in acute MI patients without LV dysfunction could improve mortality rates at 1 month compared to non-BB users (9). Furthermore, in a larger cohort of patients with acute coronary syndrome without LV dysfunction, an initial reduction in mortality was observed at 1 month for those treated with BBs (20). However, this difference in mortality did not persist at 6 or 12 months. In contrast to earlier studies suggesting benefits from the early use of BBs, recent randomized trials have similarly shown no significant changes in outcomes within the first month or beyond 1 year (17). These findings are consistent with the results of our study, where no early (within 1 month) or long-term differences in cardiovascular outcomes were observed between the BB and non-BB groups. These results suggested that the routine use of BBs in patients with acute MI and non-reduced LVEF may not be essential. In both short-term (within 1 month) and long-term follow-ups, no notable advantages in cardiovascular outcomes were observed between those who received BBs and those who did not. This raised a question about the necessity of universally prescribing BBs in this patient population. However, while our findings aligned with recent randomized trials, further studies with longer follow-up periods are needed to fully assess the long-term impact of BB therapy in these patients.
LimitationsThis study has several limitations. First, its retrospective observational nature may introduce inherent biases. One key limitation is that, in the acute stage, patients who were more severely ill may have been less likely to receive beta-blockers due to their critical condition, which may have precluded the use of such medications. While we applied PS matching and multivariate analysis to adjust for these confounders, these statistical techniques may not have been sufficient to fully account for the baseline differences, potentially leading to an overestimation of the favorable association between early beta-blocker use and improved outcomes, especially in the non-beta-blocker group. Second, while we investigated BB prescription at discharge and its adherence during 1-year follow-up, the actual adherence to BB treatment and the exact duration of BB usage could not be definitively determined in this study. Third, there was no guarantee that the BB prescription at discharge aligned perfectly with the BB prescription at admission. Despite these limitations, this study offers significant advantages owing to its larger population size and baseline characteristics, which are more consistent with the current revascularization era compared to previous studies. Fourth, the relatively short follow-up duration may have limited our ability to fully assess the long-term effects of beta-blockers in this subset of patients. Further long-term clinical studies will be needed in patients with non-reduced LVEF.
ConclusionIn conclusion, in patients with acute MI and non-reduced LVEF, beta-blocker treatment at discharge was not associated with a significant difference in clinical cardiovascular outcomes, either within the first 30 days or at 1-year follow-up. Given the need for a more comprehensive evaluation of beta-blocker therapy, longer follow-up beyond 1 year is required to fully assess its long-term effects, warranting further investigation.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statementThe studies involving humans were approved by the institutional review board of Yeungnam University Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsJ-CJ: Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. J-IP: Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – review & editing. B-JK: Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – review & editing. H-JK: Data curation, Writing – review & editing. K-UC: Data curation, Writing – review & editing. J-HN: Data curation, Writing – review & editing. C-HL: Data curation, Writing – review & editing. J-WS: Data curation, Writing – review & editing. J-SP: Data curation, Writing – review & editing. S-HHu: Data curation, Writing – review & editing. K-YC: Data curation, Writing – review & editing. T-HA: Data curation, Writing – review & editing. M-HJ: Data curation, Writing – review & editing. S-WR: Data curation, Writing – review & editing. H-SK: Data curation, Writing – review & editing. H-CG: Data curation, Writing – review & editing. I-WS: Data curation, Writing – review & editing. K-KH: Data curation, Writing – review & editing. S-HHu: Data curation, Writing – review & editing. K-SC: Data curation, Writing – review & editing. S-KO: Data curation, Writing – review & editing. J-KC: Data curation, Writing – review & editing. UK: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1447952/full#supplementary-material
AbbreviationsBB, beta-blockers; CI, confidence interval; CKD, chronic kidney disease; CVA, cerebrovascular accident; HF, heart failure; HR, hazard ratio; KAMIR V, Korean Acute Myocardial Infarction Registry V; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; POCE, patient-oriented composite endpoint; PS, propensity score; STEMI, ST-elevation myocardial infarction.
References1. Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med. (1992) 327(4):248–54. doi: 10.1056/NEJM199207233270406
PubMed Abstract | Crossref Full Text | Google Scholar
2. Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O'Keefe J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The primary angioplasty in myocardial infarction study group. N Engl J Med. (1993) 328(10):673–9. doi: 10.1056/NEJM199303113281001
PubMed Abstract | Crossref Full Text | Google Scholar
3. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. (2022) 145(3):e4–17. doi: 10.1161/CIR.0000000000001039
PubMed Abstract | Crossref Full Text | Google Scholar
4. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44(38):3720–826. doi: 10.1093/eurheartj/ehad191
PubMed Abstract | Crossref Full Text | Google Scholar
5. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
PubMed Abstract | Crossref Full Text | Google Scholar
6. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
PubMed Abstract | Crossref Full Text | Google Scholar
7. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. (2013) 127(4):e362–425. doi: 10.1161/CIR.0b013e3182742cf6
PubMed Abstract | Crossref Full Text | Google Scholar
8. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. (2001) 357(9266):1385–90. doi: 10.1016/S0140-6736(00)04560-8
PubMed Abstract | Crossref Full Text | Google Scholar
9. Puymirat E, Riant E, Aissaoui N, Soria A, Ducrocq G, Coste P, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. Br Med J. (2016) 354:i4801. doi: 10.1136/bmj.i4801
PubMed Abstract | Crossref Full Text | Google Scholar
10. Watanabe H, Ozasa N, Morimoto T, Shiomi H, Bingyuan B, Suwa S, et al. Long-term use of carvedilol in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. PLoS One. (2018) 13(8):e0199347. doi: 10.1371/journal.pone.0199347
PubMed Abstract | Crossref Full Text | Google Scholar
11. Kim J, Kang D, Park H, Kang M, Park TK, Lee JM, et al. Long-term β-blocker therapy and clinical outcomes after acute myocardial infarction in patients without heart failure: nationwide cohort study. Eur Heart J. (2020) 41(37):3521–9. doi: 10.1093/eurheartj/ehaa376
PubMed Abstract | Crossref Full Text | Google Scholar
12. Holt A, Blanche P, Zareini B, Rajan D, El-Sheikh M, Schjerning AM, et al. Effect of long-term beta-blocker treatment following myocardial infarction among stable, optimally treated patients without heart failure in the reperfusion era: a Danish, nationwide cohort study. Eur Heart J. (2021) 42(9):907–14. doi: 10.1093/eurheartj/ehaa1058
PubMed Abstract | Crossref Full Text | Google Scholar
13. Joo SJ, Kim SY, Choi JH, Park HK, Beom JW, Lee JG, et al. Effect of beta-blocker therapy in patients with or without left ventricular systolic dysfunction after acute myocardial infarction. Eur Heart J Cardiovasc Pharmacother. (2021) 7(6):475–82. doi: 10.1093/ehjcvp/pvaa029
PubMed Abstract | Crossref Full Text | Google Scholar
14. Choi KH, Song YB, Lee JM, Lee SY, Park TK, Yang JH, et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. (2019) 12(7):607–20. doi: 10.1016/j.jcin.2019.01.227
PubMed Abstract | Crossref Full Text | Google Scholar
15. Choo EH, Chang K, Ahn Y, Jeon DS, Lee JM, Kim DB, et al. Benefit of β-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart. (2014) 100(6):492–9. doi: 10.1136/heartjnl-2013-305137
PubMed Abstract | Crossref Full Text | Google Scholar
16. Sakagami A, Soeda T, Saito Y, Nakao K, Ozaki Y, Kimura K, et al. Clinical impact of beta-blockers at discharge on long-term clinical outcomes in patients with non-reduced ejection fraction after acute myocardial infarction. J Cardiol. (2023) 81(1):83–90. doi: 10.1016/j.jjcc.2022.08.002
PubMed Abstract | Crossref Full Text | Google Scholar
17. Yndigegn T, Lindahl B, Mars K, Alfredsson J, Benatar J, Brandin L, et al. Beta-blockers after myocardial infarction and preserved ejection fraction. N Engl J Med. (2024) 390(15):1372–81. doi: 10.1056/NEJMoa2401479
PubMed Abstract | Crossref Full Text | Google Scholar
18. Ishak D, Aktaa S, Lindhagen L, Alfredsson J, Dondo TB, Held C, et al. Association of beta-blockers beyond 1 year after myocardial infarction and cardiovascular outcomes. Heart. (2023) 109(15):1159–65. doi: 10.1136/heartjnl-2022-322115
PubMed Abstract | Crossref Full Text | Google Scholar
19. Park CS, Yang HM, Ki YJ, Kang J, Han JK, Park KW, et al. Left ventricular ejection fraction 1 year after acute myocardial infarction identifies the benefits of the long-term use of β-blockers: analysis of data from the KAMIR-NIH registry. Circ Cardiovasc Interv. (2021) 14(4):e010159. doi: 10.1161/CIRCINTERVENTIONS.120.010159
PubMed Abstract | Crossref Full Text | Google Scholar
20. Abi Khalil C, Zubaid M, Mekhaimar M, Asaad N, Mahfoud Z, Al Suwaidi J. Beta-blockers and short-term cardiovascular outcomes in patients hospitalized for acute coronary syndrome and a left ventricular ejection fraction ≥40. Sci Rep. (2020) 10(1):3520. doi: 10.1038/s41598-020-60528-y
留言 (0)