A rare group of tumors known as thymic epithelial tumors (TETs) typically appears in the anterior mediastinum (1, 2). Epithelial cells and lymphocytes are the sources of thymic epithelial tumors (3). The average age of occurrence is similar in men and women, averaging 5th and 6th decades (3). Thymoma (Type A, AB, B1, B2, B3), thymic carcinoma (Type C), and thymic neuroendocrine tumor (NET) constitute subgroups of thymic epithelial tumors (2, 4, 5). The literature reports a thymoma incidence of 0.13–0.19 per 100,000 (4, 6, 7). Estimates place the incidence rate for neuroendocrine tumors at 0.02 per 100,000 and for thymic carcinomas at 0.07 per 100,000 (4, 7, 8). Although thymic epithelial tumors are rare, they are the most common tumor group in the anterior mediastinum (4, 6). The literature has not clarified the etiology, but it has not identified smoking, ionizing radiation, or alcohol as risk factors (9). The higher incidence of thymic epithelial tumors in Asians, African Americans, and Pacific Islanders supports the hypothesis of hereditary disease etiology (9). Some specific genomic changes have been detected in TETs; various chromosome deletions, translocations, and duplications can be observed according to subtypes (10).
Although they are usually clinically asymptomatic, paraneoplastic syndromes may occur in some patients (3, 11). Among the paraneoplastic autoimmune diseases associated with thymic epithelial tumors, hypogammaglobulinemia, pure red cell aplasia, Cushing's syndrome, systemic lupus erythematosus, and polymyositis, myasthenia gravis is the most common, although it occurs less frequently (11–13).
Thymomas have an overall 5-year survival of 90%, whereas distant metastases accompany thymic carcinomas, which have an overall 5-year survival of about 55% (9). TETs require long-term follow-up (e.g., 10 years) to evaluate overall survival and detect possible recurrences (9).
The 8th TNM staging is used in the anatomical staging of thymic epithelial tumors, but the 9th TNM criteria have also entered the literature (14–16). Table 1 displays TNM staging. In the 9th TNM staging, dividing the tumor size by 5 cm in the T evaluation is a newly added criterion (14, 16).Mediastinal pleural invasion was removed from the T classification and included as an additional histological factor (15, 16). Lung and phrenic nerve invasion were reduced to the T2 stage (14, 16). No changes were made in the N and M stages (14, 16) (Table 1).
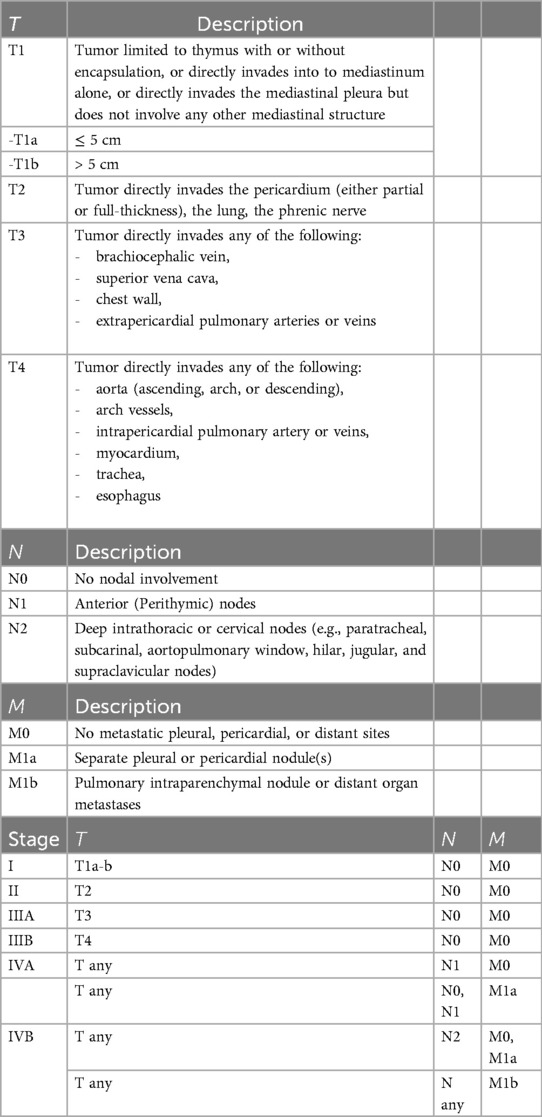
Table 1. The 9th TNM staging criteria for thymic epithelial tumors.
Masaoka et al. staged thymic epithelial tumors based on capsule invasion and invasion into the surrounding fatty tissue (17). Koga and his colleagues also made significant contributions to the literature by developing the Masaoka-Koga staging, which focused on the invasion of surrounding tissues, lymphatic spread, and hematogenous spread (18, 19). Table 2 provides the Masaoka-Koga staging for thymic epithelial tumors (18, 19). In clinical use, TNM staging and Masaoka-Koga staging are used together (14, 19) (Tables 1, 2).

Table 2. The table shows the masaoka-koga staging for thymic epithelial tumors.
Thymic epithelial tumor treatment varies according to subpathological type and stage (20). Clinical practice currently uses the 8th TNM in treatment options, but the increasing use of the 9th TNM will shift treatment to the 9th TNM in the coming years (15, 20, 21). The surgical margin negativity is the most important prognostic factor in the surgical treatment of thymic epithelial tumors (20, 21). For this reason, neoadjuvant treatments are used to achieve R0 resection (20, 22). Patients considered surgically inoperable benefit from the combination of chemotherapy and radiotherapy treatments, which also detect distant metastases (20, 22).
If myasthenia gravis is also present, the surgical treatment recommendation for thymoma, when evaluated according to subtypes, is total resection (extended thymectomy) (21, 23). Although minimally invasive approaches have advantages, open sternotomy remains the standard (21, 24).
Neoadjuvant radiotherapy and systemic treatment are not suitable for thymoma patients (21, 23). In TNM staging, the main treatment for the stage 1 thymoma group is surgery (21, 24). In medically inoperable stage 1 thymoma patients, chemotherapy or radiotherapy is recommended instead of chemoradiotherapy (21, 24). Extended thymectomy remains the gold standard treatment for stage 2 thymoma patients (21, 24). Despite the thought of neoadjuvant radiotherapy options being beneficial due to various invasions (pericardium, lung, phrenic nerve), we do not recommend neoadjuvant radiotherapy (21, 24). However, if R0 resection cannot be achieved, adjuvant radiotherapy options should be considered (21, 23). Systemic treatment is not recommended in stage 2 patients, and radiotherapy options are primarily recommended in medically inoperable patients (21, 23).
Surgery or direct surgery is the best way to treat stage 3 and stage 4 thymomas that can be removed after neoadjuvant treatment for R0 resection (21, 23). Neoadjuvant treatment is recommended as chemoradiotherapy or chemotherapy; in chemotherapy, cisplatin-based combination chemotherapy is recommended (21, 23). If it is possible to resect pleural and pericardial metastases in stage 4 thymoma, surgical treatment is recommended (21, 23). Open surgery (sternotomy) is superior to minimally invasive methods, and total resection is superior to partial resections (21). Unilateral phrenic nerve excision may be tolerated during surgery, but bilateral phrenic nerve excision is a contraindication (21). Adjuvant RT may be recommended if neoadjuvant RT has not been received, but there is no routine adjuvant RT recommendation (21, 23). In un-resectable stage 3 and 4 patients, radiotherapy and/or concurrent chemotherapy regimens are recommended (21, 23). In patients with recurrent thymoma, resection is recommended if there is a tumor in the thorax. Radiotherapy and platinum-based systemic chemotherapy treatments are parts of multimodal treatment (21, 23).
Patients with stage 1 and 2 thymic carcinoma should not receive neoadjuvant chemotherapy or radiotherapy; instead, they should undergo total resection with open surgery (21, 25). Adjuvant chemotherapy also has no place in treatment (21, 25). Radiotherapy is recommended for medically inoperable patients; chemotherapy has no place in the treatment (21, 25). It should not be forgotten that patients with stage 3 and 4 thymic carcinoma have locally advanced disease and multimodal treatment options (21, 25, 26). For R0 resection, surgical or direct surgical treatment options can be applied after neoadjuvant therapy (21, 26). Open surgery (sternotomy) is superior to minimally invasive methods, and total resection is superior to partial resections (21, 26). Unilateral phrenic nerve excision may be tolerated during surgery, but bilateral phrenic nerve excision is a contraindication (21). If neoadjuvant chemotherapy or radiotherapy is not given, they should be considered as adjuvant treatment options (21, 26). In un-resectable stage 3 and 4 patients, radiotherapy and/or concurrent chemotherapy regimens are recommended (21, 25, 26). When a tumor in the thorax is present in patients with recurrent thymic carcinoma, similar to thymoma, multimodal treatment includes radiotherapy and platinum-based systemic chemotherapy treatments (21, 25, 26).
Surgery is the first treatment option for stage 1–2 NETs, and R0 resection is the main treatment target (21, 27). If R0 resection cannot be achieved, radiotherapy and systemic chemotherapy (cisplatin + etoposide, carboplatin + etoposide) modalities should be added to the treatment (21, 27). For symptom control in Stage 3 and 4 NETs, consider adding local treatment options such as endobronchial therapy and ablation (21). Octreotide, lanreotide, everolimus, temozolomide ± capecitabine, or RT options are effective in low-grade typical carcinoids. In moderately atypical carcinoids, treatment options of cisplatin + etoposide, carboplatin + etoposide, temozolomide ± capecitabine, octreotide, lanreotide, and everolimus simultaneously with RT are effective (21).
In recent years, immunotherapy has emerged as a treatment option with increasing frequency. The literature reports a wide range of PD-L1 expression, including 25%–90% in thymomas and 40%–80% in thymic carcinomas (28–30). In this context, immune regulatory agents (Pembrolizumab and Avelumab) that block the PD-1/PD-L1 pathway may be a treatment option for patients with resistant thymoma and thymic carcinoma (28). However, immunotherapies are not standard treatments, and patients should exercise caution to avoid immune response-related side effects during treatment processes (28).
1.2 Myasthenia GravisMyasthenia gravis is a heterogeneous autoimmune disease (31). It is a neuromuscular junction disease that develops through antibody-dependent T lymphocytes (32). It has a general incidence of 0.3–2.8/100,000 years (33). Women between the ages of 20 and 30 are more likely to experience it, and it affects both men and women equally in middle and older ages (34). Approximately 10% of myasthenia gravis patients also have thymoma (33, 34). Myasthenia gravis manifests various clinical symptoms, with ptosis and diplopia being the most common ones (31, 35). Dysphagia, dyspnea, and extremity involvement are less common (31, 35). The clinical classification of myasthenia gravis uses the Osserman classification (31).
There are various clinical spectrums associated with myasthenia gravis, such as ocular involvement or generalized muscle involvement (31). The age of onset may be early or late (34, 36, 37). There may be single antibody positivity, multiple antibody positivity, or seronegativeness (36, 37) (Table 8). Patients with myasthenia gravis often exhibit various antibody positivities, with the most common being anti-acetylcholine receptor (Anti-AChR) positive, a condition where antibodies develop against the acetylcholine receptor (38–40). Less comm0only, patients may also exhibit antibodies against muscle-specific kinase (Anti-MuSK) and antibody positivity against lipoprotein receptor-related protein 4 (Anti-LRP4) (38–40).
Myasthenia gravis treatment varies according to clinical severity and classification (41). The most commonly used agent in the symptomatic treatment of mild myasthenia gravis is pyridostigmine, an acetylcholinesterase inhibitor (42). In moderate-to-severe myasthenia gravis, immunosuppressive drugs are added to the treatment (41, 42). Apply nonsteroidal immunosuppressive treatments when the daily dose of minimum effective prednisone exceeds 7.5 mg, as corticosteroid side effects are common (42). Azathioprine, tacrolimus, mycophenolate mofetil, methotrexate, and cyclosporine are among the nonsteroidal immunosuppressive agents used in the treatment of myasthenia gravis (42).
Antibodies activate the classical complement pathway (34). As a result of the systematically progressing complement system, C5b6789 (membrane attack complex (MAC)/terminal complement component (TCC)) is formed (34, 43). The membrane attack complex (MAC) destroys the postsynaptic membrane (34). The complement pathway's C5, C3, and C1 components are intermediate steps that enable the complement pathway to start and continue. Inhibitory agents for C5, C3, and C1 components provide improvement in clinical symptoms (34). Eculizumab, ravulizumab, and zilucoplan, developed against the C5 unit of complement, appear to be the most effective and successful agents in clinical practice (34, 43).
Developing B lymphocytes have the transmembrane protein CD20 on their surface, which regulates calcium flux (44). Progenitor cells and mature plasma cells do not express it (44). Rituximab is a monoclonal antibody that targets the CD20 protein, causing cell death upon binding to CD20 (44). When B lymphocytes die, the production of new antibodies ceases, leading to improvement in the myasthenia gravis clinic (44). Cell death occurs through antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, the caspase pathway, and lysosomal activation (44).
Neonatal Fc receptor (FcRn) prolongs serum half-life by affecting immunoglobulin G (IgG) transport, distribution, and persistence (45). Traditional treatment methods for myasthenia gravis do not include FcRn inhibitors (45). However, the FcRn inhibitor efgartigimod has received FDA approval (45).
Drug-resistant cases can utilize the plasmapheresis method to stabilize the patient during the perioperative period (46). Agents such as intravenous immunoglobulin and subcutaneous immunoglobulin can aid in the maintenance treatment of myasthenia gravis (47). Aerobic exercises and respiratory muscle training are effective in treatment by increasing functional capacity (48) Thymectomy is among the effective treatment methods for patients with myasthenia gravis (49).
1.3 Surgical treatmentThymus tissue originates embryologically from the third pharyngeal pouch and is anatomically located in the anterior mediastinum (50, 51). Thymus tissue consists of two lobes separated by septa and continues to grow until adolescence (50, 52). The inferior thyroid artery, the internal mammary artery, the internal thoracic artery, the pericardiophrenic artery, and the intercostal arteries all bring blood to the thymus (50). The counterparts of the arteries and the left innominate vein provide venous drainage (50). Lymphatic drainage occurs through parasternal, tracheobronchial, and brachiocephalic lymph nodes (50).
The thymus is a primary lymphoid organ that contributes to cellular immunity (50, 53). Thymus tissue consists of the cortex and medulla, with thymic lymphocytes located in the cortex (50, 54). Lymphocytes formed in the bone marrow come to the thymus tissue, where they undergo antigen-mediated induction and maturation of T lymphocytes into cytotoxic cells (50, 53).
Thymectomy indications are most commonly caused by thymoma and myasthenia gravis (50, 53). Thymectomy is also indicated in clinical conditions such as thymic carcinoma, neuroendocrine tumors, thymic cysts, and ectopic parathyroid glands located in the thymus (50, 55).
Before surgery, myasthenia gravis patients should receive clinical stabilization with cholinesterase inhibitors, intravenous immunoglobulin, and plasmapheresis agents to prevent myasthenic crises (50, 56, 57). Myasthenia gravis patients also need surgical anesthesia, and they should avoid using calcium channel blockers and magnesium (56). Excessive use of acetylcholinesterase inhibitors may lead to cholinergic crises (56, 57). The depressant effects of benzodiazepines and opiates on respiratory functions appear more clearly in myasthenia gravis patients (56). Myasthenia gravis patients exhibit resistance to depolarizing neuromuscular blocking agents (e.g., succinylcholine) and sensitivity to non-depolarizing neuromuscular blocking agents (e.g., rocuronium) due to the decrease in acetylcholine, indicating caution during the induction of anesthesia (56).
Patients with non-thymomatous anti-AChR-positive myasthenia gravis can benefit more after having a thymectomy (58–61).Wolfe and colleagues reported that, female gender, earlier onset of disease (<40 years of age) were associated with better outcome after thymectomy (58). Patients with myasthenia gravis over 50 years old who exhibit juvenile-onset or purely ocular symptoms were found to have limited treatment success with thymectomy (62). Extended thymectomy has been recommended in order to remove all of the thymus tissue in patients with myasthenia gravis or a thymic epithelial tumor (24, 60). An extended thymectomy may provide complete removal of the thymus tissue and the surrounding fatty tissue between the right and left phrenic nerves (63, 64). As with cancer, the tumor tissue must be completely removed and there must be no remaining thymus tissue to lower the rate of recurrence (24, 60).
Although sternotomy was initially the standard procedure for thymectomy surgery, technological advancements and the use of minimally invasive methods have demonstrated significant benefits in both the perioperative and postoperative course (50, 59). Minimally invasive methods consist of video-assisted thoracic surgery (VATS) and robotic-assisted thoracic surgery (RATS) (50, 59, 60). Even though minimally invasive methods stand out in terms of less pain, early discharge, cost, and aesthetics, they should not be preferred if oncological principles cannot be preserved (24, 50, 59, 65).
In general, contraindications to thymectomy include clinical conditions such as the patient's inability to tolerate general anesthesia, hemodynamic instability, and coagulopathy (50). However, once clinical improvement is achieved with medical treatment for the contraindication, thymectomy may be reconsidered (50). The complications of thymectomy can be listed as bleeding, pericardial injury, phrenic nerve damage, chylothorax, and pneumothorax (50, 65).
Myasthenia gravis is an autoimmune disease with different clinical spectrums (31, 34). Myasthenia gravis may also be associated with thymoma (35). Various antibodies, most commonly anti-acetylcholine receptor antibodies, may be present in patients with myasthenia gravis (38–40). Early-onset myasthenia gravis patients with anti acetylcholine antibody positivity are the most likely to benefit from surgery (58, 59). Extended thymectomy has been defined as the gold standard in myasthenia gravis surgery (63, 64). In an extended thymectomy, the thymus tissue between the right and left phrenic nerves and the fat tissue around them are completely cut out (63, 64). Applying simple thymectomy instead of extended thymectomy in myasthenia gravis patients results in recurrence after surgery (63, 64). Although simple thymectomy is preferred in patients with early-stage thymoma and no myasthenia gravis due to its shorter surgical time and fewer complications, extended thymectomy maintains its place in this field because simple thymectomy has worse results in terms of overall survival, 5-year survival, and recurrence-free survival (66). While minimally invasive methods (VATS/RATS) yield superior results in the postoperative period, patients with large size and invasion into surrounding tissues should also opt for open surgery (sternotomy) (59, 60).
Staudies have shown that while surgical results for stage IVA thymic tumors are acceptable, it is more accurate to make a multidisciplinary surgical decision due to the lack of data for stage 4B thymic tumors (21, 23, 24, 67). The surgical margin for advanced-stage thymic tumors is to provide R0 resection (21, 23, 24, 67). The stage IVA group, a heterogeneous group that includes both pleural and pericardial metastases, can resect pleural metastases, but pericardial metastases require careful evaluation (67, 68). Another difference in the stage IVA group is that recurrent and new pleural nodules were both staged the same way. However, thymic tumors that were found to be stage IV were more aggressive (68). Surgery for local control rather than adjuvant treatment is recommended for patients with recurrent pleural metastases (68).Re-operative surgery plays a critical role in the management of stage IV thymomas, particularly in cases where recurrence occurs after initial treatment. Stage IV thymomas, characterized by metastasis either to pleural or pericardial surfaces(IVA) or distant organs (stage IVB), often require multimodal treatment, including surgery, chemotherapy, and radiotherapy (67, 68). While complete surgical resection remains the cornerstone of treatment for early-stage thymomas, re-operative surgery can offer a significant survival benefit in advanced cases, especially for patients with localized recurrences or limited metastatic disease (68). Studies have shown that re-operative surgery can help achieve prolonged disease control and improve overall survival, particularly in patients who are candidates for resection of recurrent pleural nodules or other resectable metastases. However, the decision for re-operation must be individualized, taking into account the patient's overall condition, the extent of disease, and prior treatments. Combining surgery with other modalities like chemotherapy or radiotherapy can further enhance outcomes in this challenging patient population.
Cytoreductive Surgery Followed by Intraoperative Hyperthermic Chemotherapy (HITHOC) is a method applied after extended thymectomy in advanced stage thymomas with pleural spread, and its success in terms of local control has been demonstrated (69). In HITHOC, the intrathoracic temperature is raised to 42.5°C after surgery, and the procedure is carried out with a chemotherapeutic agent for about an hour (69). This method is used for both initial abdominal and pleural cancers (69). Research has demonstrated that this method enhances overall survival in cases of pleural recurrence and advanced-stage thymic epithelial tumors (69).
2 ConclusionAlthough myasthenia gravis is an autoimmune disease, surgery has an important role in its treatment (31, 49). Extended thymectomy is the gold standard for surgical treatment of myasthenia gravis (24, 60, 63, 64). Removing the thymus and peripheral fatty tissue between the bilateral phrenic nerves prevents relapses (24, 60, 63, 64, 70).
Surgical treatment is important for early-stage thymomas (24, 60). Staging is of great importance in determining the best possible treatment (15, 20, 21). In this regard, it is recommended that the N component of staging should not be overlooked and it is recommended to perform lymph node dissection carefully exclusively in type B thymomas and thymic carcinomas (21–24).
Author contributionsGO: Methodology, Writing – original draft, Writing – review & editing. AT: Conceptualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. (2008) 44(1):123–30. doi: 10.1016/j.ejca.2007.11.004
PubMed Abstract | Crossref Full Text | Google Scholar
2. Shao Y, Tang M, Fang L, Wei S, Gao X, Liu W. Prognostic value of tumor size in thymic epithelial tumors: a systematic review and meta-analysis. Medicine (Baltimore). (2022) 101(46):e31741. doi: 10.1097/MD.0000000000031741
PubMed Abstract | Crossref Full Text | Google Scholar
3. Zhou Q, Ke X, Man J, Zhang B, Wang F, Zhou J. Predicting Masaoka-Koga clinical stage of thymic epithelial tumors using preoperative spectral computed tomography imaging. Front Oncol. (2021) 11:631649. doi: 10.3389/fonc.2021.631649
PubMed Abstract | Crossref Full Text | Google Scholar
5. Ao YQ, Gao J, Wang S, Jiang JH, Deng J, Wang HK, et al. Immunotherapy of thymic epithelial tumors: molecular understandings and clinical perspectives. Mol Cancer. (2023) 22(1):70. doi: 10.1186/s12943-023-01772-4
PubMed Abstract | Crossref Full Text | Google Scholar
7. Hsu C-H, Chan JK, Yin C-H, Lee C-C, Chern C-U, Liao C-I. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PLoS One. (2020) 14(12):e0227197. doi: 10.1371/journal.pone.0227197
PubMed Abstract | Crossref Full Text | Google Scholar
9. Xu C, Zhang Y, Wang W, Wang Q, Li Z, Song Z, et al. Chinese Expert consensus on the diagnosis and treatment of thymic epithelial tumors. Thorac Cancer. (2023) 14(12):1102–17. doi: 10.1111/1759-7714.14847
PubMed Abstract | Crossref Full Text | Google Scholar
10. Shin DW, Cho JH, Ha J, Jung KW. Trends in incidence and survival of patients with thymic epithelial tumor in a high-incidence Asian country: analysis of the Korean central cancer registry 1999 to 2017. J Thorac Oncol. (2022) 17(6):827–37. doi: 10.1016/j.jtho.2022.02.001
PubMed Abstract | Crossref Full Text | Google Scholar
11. Hirai K, Usuda J. The characteristics and prognostic role of thymic epithelial tumors with paraneoplastic autoimmune syndromes. Transl Lung Cancer Res. (2019) 8(Suppl 4):S321–2. doi: 10.21037/tlcr.2019.11.21
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ruffini E, Huang J, Cilento V, Goren E, Detterbeck F, Ahmad U, et al. The international association for the study of lung cancer thymic epithelial tumors staging project: proposal for a stage classification for the forthcoming (ninth) edition of the TNM classification of malignant tumors. J Thorac Oncol. (2023) 18(12):1655–71. doi: 10.1016/j.jtho.2023.09.002
PubMed Abstract | Crossref Full Text | Google Scholar
16. Okumura M, Marino M, Cilento V, Goren E, Ruffini E, Dibaba D, et al. The international association for the study of lung cancer thymic epithelial tumor staging project: proposal for the T component for the forthcoming (ninth) edition of the TNM classification of malignant tumors. J Thorac Oncol. (2023) 18(12):1638–54. doi: 10.1016/j.jtho.2023.08.024
PubMed Abstract | Crossref Full Text | Google Scholar
17. Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. (1981) 48(11):2485–92. doi: 10.1002/1097-0142(19811201)48:11%3C2485::AID-CNCR2820481123%3E3.0.CO;2-R
PubMed Abstract | Crossref Full Text | Google Scholar
18. Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. (1994) 44:359–67. doi: 10.1111/j.1440-1827.1994.tb02936.x
PubMed Abstract | Crossref Full Text | Google Scholar
19. Chiappetta M, Lococo F, Pogliani L, Sperduti I, Tabacco D, Bria E, et al. Masaoka-Koga and TNM staging system in thymic epithelial tumors: prognostic comparison and the role of the number of involved structures. Cancers (Basel). (2021) 13(21):5254. doi: 10.3390/cancers13215254
PubMed Abstract | Crossref Full Text | Google Scholar
20. Falkson CB, Vella ET, Ellis PM, Maziak DE, Ung YC, Yu E. Surgical, radiation, and systemic treatments of patients with thymic epithelial tumors: a systematic review. J Thorac Oncol. (2023) 18(3):299–312. doi: 10.1016/j.jtho.2022.10.016
PubMed Abstract | Crossref Full Text | Google Scholar
21. Baudin E, Caplin M, Garcia-Carbonero R, Fazio N, Ferolla P, Filosso PL, et al. Lung and thymic carcinoids: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2021) 32(4):439–51. doi: 10.1016/j.annonc.2021.01.003
PubMed Abstract | Crossref Full Text | Google Scholar
22. Du J, Zhou XJ. Precise diagnosis and treatment of thymic epithelial tumors based on molecular biomarkers. Crit Rev Oncog. (2017) 22(5-6):507–14. doi: 10.1615/CritRevOncog.2017020577
PubMed Abstract | Crossref Full Text | Google Scholar
26. Yang X, Zhuo M, Shi A, Yang S, Wang Z, Wu M, et al. Optimal first-line treatment for advanced thymic carcinoma. Thorac Cancer. (2019) 10(11):2081–7. doi: 10.1111/1759-7714.13181
PubMed Abstract | Crossref Full Text | Google Scholar
29. Padda SK, Riess JW, Schwartz EJ, Tian L, Kohrt HE, Neal JW, et al. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol. (2015) 10:500–8. doi: 10.1097/JTO.0000000000000429
PubMed Abstract | Crossref Full Text | Google Scholar
30. Yokoyama S, Miyoshi H, Nakashima K, Shimono J, Hashiguchi T, Mitsuoka M, et al. Prognostic value of programmed death ligand 1 and programmed death 1 expression in thymic carcinoma. Clin Cancer Res. (2016) 22:4727–34. doi: 10.1158/1078-0432.CCR-16-0434
PubMed Abstract | Crossref Full Text | Google Scholar
35. Osserman KE, Genkins G. Critical reappraisal of the use of edrophonium (tensilon) chloride tests in myasthenia gravis and significance of clinical classification. Ann N Y Acad Sci. (1966) 135(1):312–34. doi: 10.1111/j.1749-6632.1966.tb45479.x
PubMed Abstract | Crossref Full Text | Google Scholar
38. Comacchio GM, Marulli G, Mammana M, Natale G, Schiavon M, Rea F. Surgical decision making: thymoma and myasthenia Gravis. Thorac Surg Clin. (2019) 29(2):203–13. doi: 10.1016/j.thorsurg.2018.12.007
PubMed Abstract | Crossref Full Text | Google Scholar
39. Vakrakou AG, Karachaliou E, Chroni E, Zouvelou V, Tzanetakos D, Salakou S, et al. Immunotherapies in MuSK-positive myasthenia Gravis; an IgG4 antibody-mediated disease. Front Immunol. (2023) 14:1212757. doi: 10.3389/fimmu.2023.1212757
PubMed Abstract | Crossref Full Text | Google Scholar
40. Pascuzzi RM, Bodkin CL. Myasthenia Gravis and Lambert-eaton myasthenic syndrome: new developments in diagnosis and treatment. Neuropsychiatr Dis Treat. (2022) 18:3001–22. doi: 10.2147/NDT.S296714
PubMed Abstract | Crossref Full Text | Google Scholar
41. Vu T, Ortiz S, Katsuno M, Annane D, Mantegazza R, Beasley KN, et al. Ravulizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis. J Neurol. (2023) 270(6):3129–37. doi: 10.1007/s00415-023-11617-1
PubMed Abstract | Crossref Full Text | Google Scholar
43. Vu T, Wiendl H, Katsuno M, Reddel SW, Howard JF Jr. Ravulizumab in myasthenia Gravis: a review of the current evidence. Neuropsychiatr Dis Treat. (2023) 19:2639–55. doi: 10.2147/NDT.S374694
PubMed Abstract | Crossref Full Text | Google Scholar
45. Zhu LN, Hou HM, Wang S, Zhang S, Wang GG, Guo ZY, et al. Fcrn inhibitors: a novel option for the treatment of myasthenia gravis. Neural Regen Res. (2023) 18(8):1637–44. doi: 10.4103/1673-5374.363824
PubMed Abstract | Crossref Full Text | Google Scholar
46. Beloor Suresh A, Asuncion RMD. Myasthenia Gravis. StatPearls. Treasure Island, FL: StatPearls Publishing (2023). p. 7–8.
47. Pasnoor M, Bril V, Levine T, Trivedi J, Silvestri NJ, Phadnis M, et al. Phase 2 trial in acetylcholine receptor antibody-positive myasthenia gravis of transition from intravenous to subcutaneous immunoglobulin: the MGSCIg study. Eur J Neurol. (2023) 30(5):1417–24. doi: 10.1111/ene.15745
PubMed Abstract | Crossref Full Text | Google Scholar
48. Chen S, Li X, Wu Y, Li Y, Cao P, Yin Y, et al. Preoperative respiratory muscle training combined with aerobic exercise improves respiratory vital capacity and daily life activity following surgical treatment for myasthenia gravis. J Cardiothorac Surg. (2023) 18(1):160. doi: 10.1186/s13019-023-02283-5
PubMed Abstract | Crossref Full Text | Google Scholar
49. Khawaja I. Effect of thymectomy on outcomes of myasthenia Gravis patients: a case-control study at a tertiary care hospital. Cureus. (2023) 15(4):e37584. doi: 10.7759/cureus.37584
PubMed Abstract | Crossref Full Text | Google Scholar
50. Bennett B, Rentea RM. Thymectomy. StatPearls. Treasure Island, FL: StatPearls Publishing (2023). p. 2–6.
51. Zhu LF, Zhang LM, Zuo CJ, Sun TY, Jiang B. Robot versus video-assisted thoracoscopic thymectomy for large thymic epithelial tumors: a propensity-matched analysis. BMC Surg. (2023) 23(1):330. doi: 10.1186/s12893-023-02228-8
PubMed Abstract | Crossref Full Text | Google Scholar
53. Tang M, Shao Y, Dong J, Gao X, Wei S, Ma J, et al. Risk factors for postoperative myasthenia gravis in patients with thymoma without myasthenia gravis: a systematic review and meta-analysis. Front Oncol. (2023) 13:1061264. doi: 10.3389/fonc.2023.1061264
PubMed Abstract | Crossref Full Text | Google Scholar
56. Neuman A, Granlund B. Anesthesia for patients with myasthenia Gravis. StatPearls. Treasure Island, FL: StatPearls Publishing (2022). p. 2–5.
57. Hien VV, Tu NH, Thu ND. Propofol TCI or sevoflurane anesthesia without muscle relaxant for thoracoscopic thymectomy in myasthenia gravis patients: a prospective, observational study. BMC Anesthesiol. (2023) 23(1):349. doi: 10.1186/s12871-023-02296-6
PubMed Abstract | Crossref Full Text | Google Scholar
58. Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. Randomized trial of thymectomy in myasthenia Gravis. N Engl J Med. (2016) 375(6):511–22. doi: 10.1056/NEJMoa1602489
留言 (0)