Gestational diabetes mellitus (GDM), characterized by glucose intolerance first occurring during pregnancy (Buchanan et al., 2007), is a significant complication affecting 7.0–27.6% of pregnant women worldwide, and its prevalence is increasing (Zhu and Zhang, 2016; Wang et al., 2022). Previous studies found that GDM can lead to adverse pregnancy outcomes, such as obstructed labor, hypertensive disorders of pregnancy, macrosomia and so on (Metzger et al., 2008; Mitanchez, 2010; Billionnet et al., 2017). Furthermore, it significantly raises the risk of cardiovascular diseases, type 2 diabetes (T2D), and mental disorders in both affected pregnant women and their offspring (Dabelea and Pettitt, 2001; Bellamy et al., 2009). Despite extensive researches, the precise pathogenesis of GDM remains poorly understood. Thus, investigating innovative perspectives on the mechanisms is critical for enhancing its prevention and treatment.
The human gut microbiome, comprising bacteria, fungi, viruses, archaea, and protozoa, plays a crucial role in maintaining internal environmental balance and promoting health (Qin et al., 2012). Emerging evidence shows that the human gut microbiome is closely related to GDM. Ma et al. (2020) reported that the gut microbial genera Tyzzerella 4 and Eisenbergiella were positively correlated with fasting blood glucose levels in GDM patients. Furthermore, Mendelian randomization analyses have confirmed the causal relationships involving gut microbiota, their metabolites, and GDM (Wu et al., 2023). Recent studies have also investigated the association between the gut virome and T2D, which shares mechanistic similarities with GDM (Crusell et al., 2018). Rasmussen et al. (2020) found impaired glucose tolerance in mice on a high-fat diet that received fecal virus transplantation. In another study, Fan et al. (2023) assessed the intestinal microorganism profiles of 90 individuals with T2D and 49 healthy controls, revealing significant decreases in Flavobacterium phage and Cellulophaga phaga in the T2D group. The combined use of gut viral and bacterial markers showed promising diagnostic potential for T2D (Fan et al., 2023).
Viruses, as crucial contributors to intestinal homeostasis, likely play a role in regulating blood glucose metabolism through the gut microbiome (Reyes et al., 2012). Using the mouse model, researchers found that gut virome can activate host immune responses via intestinal mucosa and intestinal immune cells (Gogokhia et al., 2019), which are involved in the pathogenesis of obesity-related insulin resistance and T2D (Esser et al., 2014). Furthermore, phages can also influence the metabolic activity of their hosts through prophage integration and horizontal gene transfer (de Jonge et al., 2022). However, existing studies have some limitations. Firstly, most studies have focused on bacteria, and there has been no research on the relationship between the gut virome and GDM to date. Secondly, animal experiments struggle to replicate the complex environment of the human intestinal tract where diverse microorganisms coexist. Furthermore, most existing findings derive from observational studies, and the temporal relationship between exposure (gut microorganisms) and outcome (GDM) remains unclear for making causal inferences (Smith and Ebrahim, 2002).
In this study, we conducted a prospective nested case-control study to analyze the gut virome of GDM patients in early pregnancy and explore the cross-kingdom correlation between gut viruses and gut bacteria in GDM. Based on the gut microbial features and clinical indices, a new prediction model of early pregnancy GDM was constructed. Our findings could enhance strategies for preventing and treating GDM by considering the microorganisms, and offer novel insights into its pathogenesis.
Materials and methods Study populationBetween March 2017 and December 2018, pregnant women from the early pregnancy cohort at the Maternity and Child Health Hospital of Hunan Province were included in our study (no.ChiCTR1900020652). Participants were enrolled during early pregnancy and followed up until 42 days postpartum. Inclusion criteria comprised: (1) singleton pregnancy through natural conception; (2) absence of pre-existing diabetes, hypertension, or thyroid disease prior to pregnancy; (3) no acute infections and no recent antibiotic usage within the preceding 2 weeks. According to the International Association of Diabetes and Pregnancy Study Groups Standard (Metzger et al., 2010), pregnant women were diagnosed with GDM if one or more of the following applied glucose levels were elevated at 24 ∼ 28 weeks in pregnancy (fasting ≥ 5.1 mmol/L, 1 h ≥ 10.0 mmol/L, 2 h ≥ 8.5 mmol/L).
This cohort initially comprised 744 subjects followed from early pregnancy to postpartum, with a dropout rate of 6.0%. After excluding individuals with incomplete clinical data, inadequate stool samples, or unsuccessful metagenomic sequencing, 51 pregnant women diagnosed with GDM were selected for the case group. A control group of 51 pregnant women with normal blood glucose levels was randomly chosen from the original cohort. All participants provided informed written consent. The study was approved by the Maternity and Child Health Hospital of Hunan Province (no. EC201624), and all procedures adhered to applicable guidelines and regulations.
DNA extraction and metagenomics sequencingMicrobiome DNA was extracted from 180 to 200 mg of feces using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Germany). Extracted DNA were checked by the NanoDrop2000 and Qubit 4.0 for concentration and purity, and stored at −20°C until use. Sequencing libraries were prepared using the NEBNext Ultra™ DNA Library Preparation Kit (Illumina, USA) according to the manufacturer’s instructions. Clustering of index-coded samples was conducted on a cBot Cluster Generation System, followed by shotgun metagenomics sequencing on the Illumina HiSeq platform (paired-end; insert size, 350 bp; read length, 150 bp).
Raw reads were first processed with fastp version 0.23.2, which trimmed reads if adapter-contaminated, N-containing, shorter than 90 bp, or bases with quality low than 15 were more than 50% of reads to get the high quality data (Chen, 2023). Then, reads aligned to the human reference genome (GRCh38/hg38) were removed using Bowtie2 version 2.5.3 to get the clean data (Langmead and Salzberg, 2012). To enhance sequencing depth of virus sequences per sample, virus-related reads in each sample were screened using Kraken2 version 2.1.3 and cross-assembled via MEGAHIT version 1.1.2 with a k-mer ranging from 27 to 127 to construct contigs from all samples (Li et al., 2016; Wood et al., 2019). Contigs longer than 1000 bp were retained then assessed using CheckV version 1.0.3 to test whether their viral gene count was more than the number of microbial genes (Nayfach et al., 2021). Contig coverage, measured in Reads Per Kilobase per Million mapped reads (RPKMs), was calculated using Bowtie2 version 2.5.3 with default parameters. This measurement was normalized based on both contig length and the number of mapped reads in each specimen (Han et al., 2018). The average RPKM for each virus was calculated and normalized across all samples to derive the abundance profile. Additionally, the details on taxonomic identification and abundance profiling of gut bacteria can be found in Supplementary material.
Statistical analysesAlpha diversity was measured by Shannon and Simpson indices. Principal coordinates analysis (PCoA), Permutational multivariate analysis of variance (ADONIS), and Analysis of Similarities (ANOSIM) were utilized to cluster and visualize the samples as well as quantify the differences in beta diversity between groups.
Multivariate linear regression analysis (MaAsLin2) was conducted to explore the differential microbial features (gut viruses and bacteria) between groups (Morgan et al., 2012). Taxa with P < 0.05 and corrected P-value (Q-value) < 0.25 were ultimately considered (Glickman et al., 2014). Spearman’s rank correlation assessed correlations between differential gut viruses, clinical indicators, and bacteria, visualized using the corrplot package. To determine the co-occurrence network of bacterial and viral communities in healthy controls and GDM patients, the top 50 most abundant gut viruses and bacteria at the species level were selected, and virus-bacteria linkages were further examined. Spearman correlation analysis was carried out to determine these associations with the P-value corrected for multiple testing utilizing the Benjamini-Hochberg FDR method. The cutoffs of the correlation coefficient and the FDR-corrected P-value were set at 0.5 and 0.05, respectively. The igraph package was applied to calculate degree distribution, node betweenness, and network natural connectivity.
Two different methods including random forest and logistic regression models were used to predict GDM in early pregnancy, with samples randomly split into training (70%) and validation sets (30%). Random forest model was set as prime method and model performance was evaluated using metrics such as area under curve (AUC), accuracy, recall, precision, F1 score, sensitivity, and specificity and visualized using the ROCR package to generate receiver operating characteristic curves (ROC). These analyses were conducted utilizing R version 4.2.2, with the significance threshold set at P < 0.05.
Results Characteristics of the subjectsThis study included 51 GDM patients and 51 controls with normal blood glucose levels during pregnancy. Baseline data showed that the average age of GDM patients was 30.58 ± 3.70 years and 30.66 ± 3.83 years for controls, without significant difference. The BMI was significantly higher in the GDM group compared to the control group (P = 0.018). Significant differences existed between groups in height, systolic blood pressure (SBP), hemoglobin (HGB), glucose (GLU), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) (P < 0.05). Furthermore, logistic regression was employed for multivariate analysis to explore determinants related to GDM based on univariate analysis results. Height, HGB, and GLU were associated with GDM occurrence (P < 0.05). Additional subject characteristics and results of logistic regression analysis are presented in Table 1 and Supplementary Table 1.
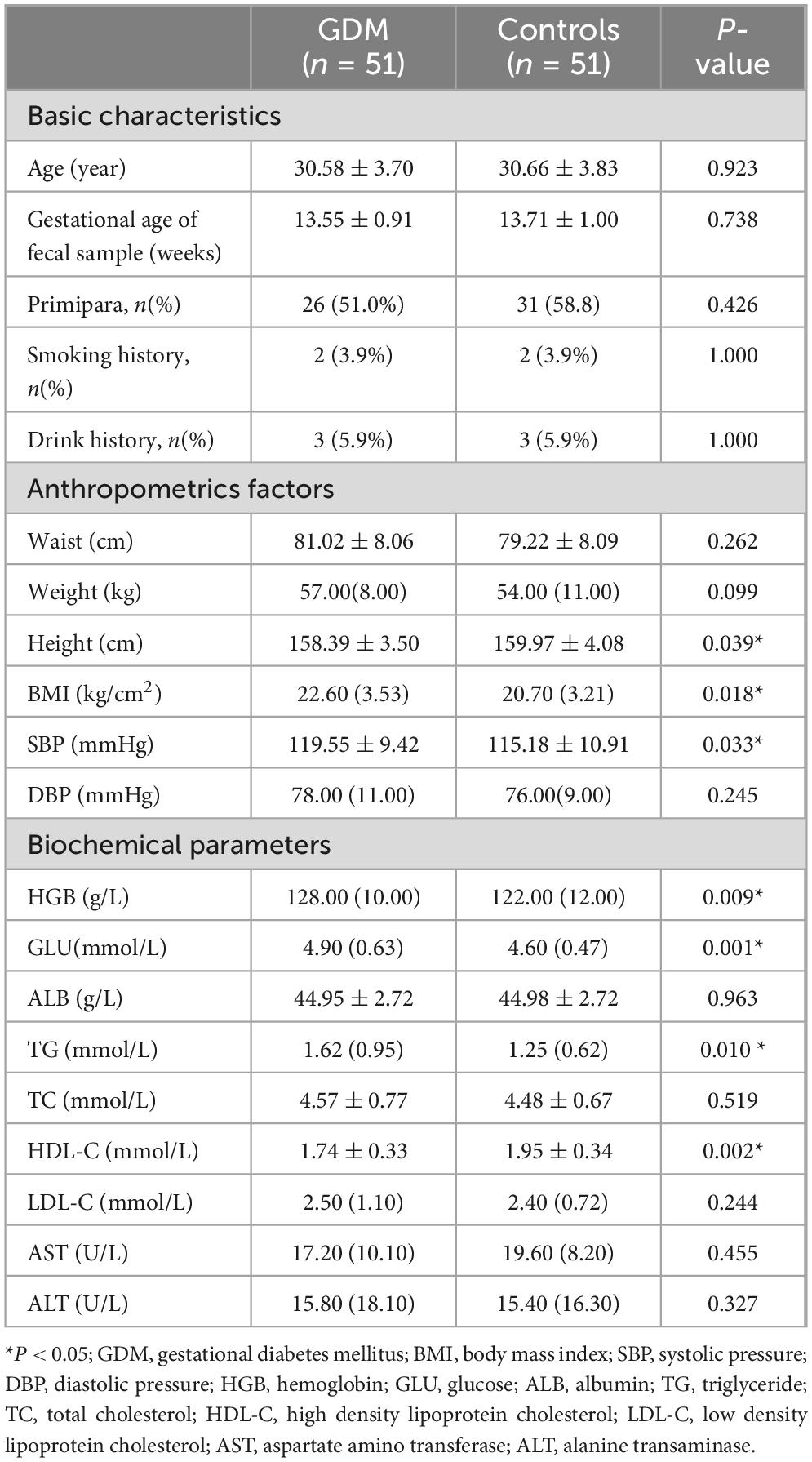
Table 1. Characteristics of the subjects.
Metagenomics sequencing analysisIn this study, we obtained a total of 778.96 gigabytes of raw data, averaging 82,525 reads per sample after sequencing. The number of reads per sample ranged from 39,822,166 to 137,574,946. Quality control was performed using fastq, resulting in 5,089,345,182 high-quality reads collected for further analysis. After cross-assembling reads from all samples, we obtained a total of 312,465 contigs, averaging 3,063 bp per sample, ranging from 1,000 to 213,528 bp. Based on the quality estimation using the CheckV algorithm (Nayfach et al., 2021), 13.7% of these contigs were evaluated as complete viral genomes, 20.4, 25.1, and 40.8% of them were high-, medium-, and low-quality viruses, respectively (Figure 1A). Rarefaction curves indicated that the sequencing depth adequately reflected the microbial diversity of the samples (Figure 1B).
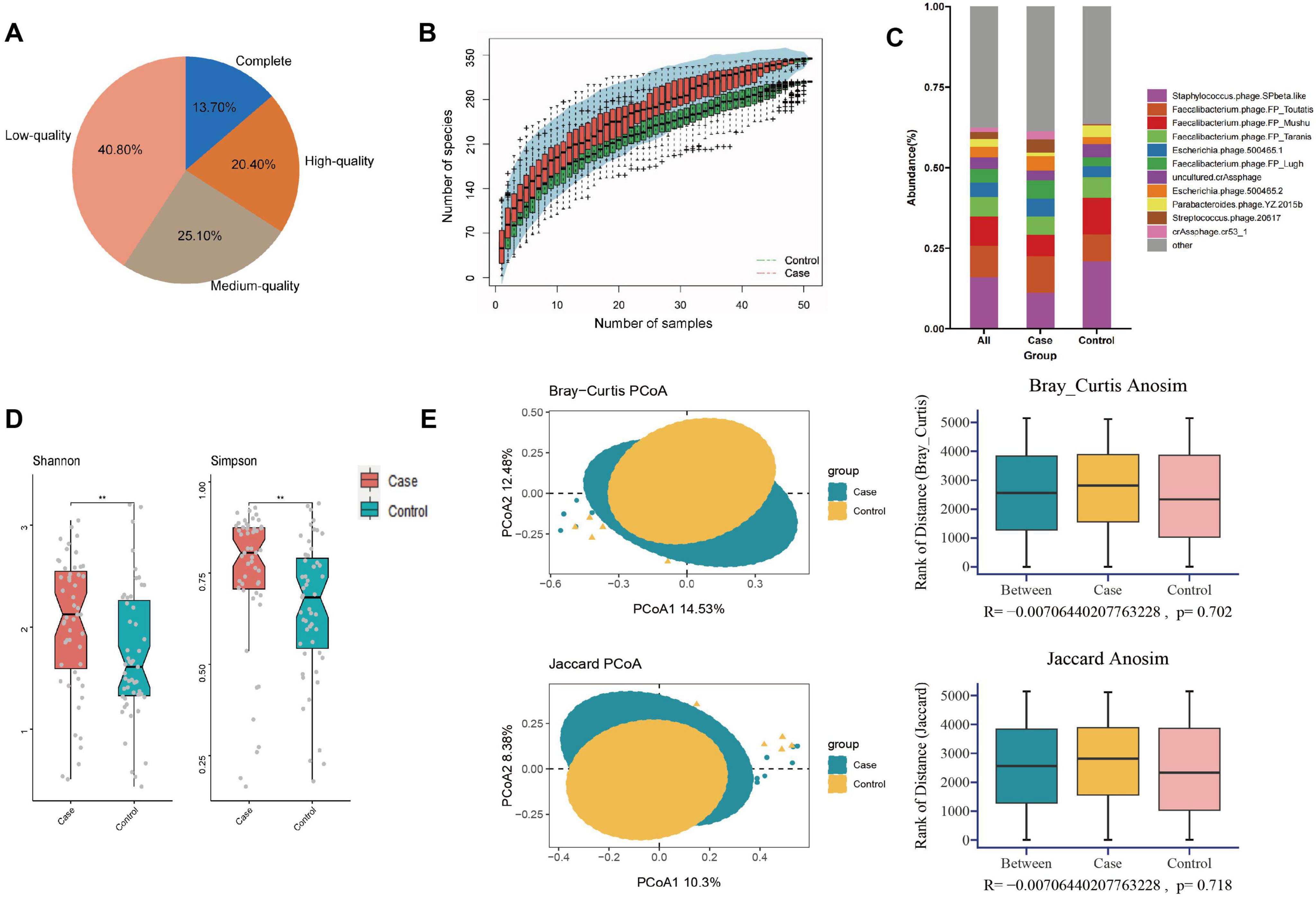
Figure 1. Composition and diversity analysis of gut virome in different groups. (A) Pie plot of the quality assessment; (B) rarefaction curve; (C) composition analysis; (D) alpha diversity; (E) the PCOA and ANOSIM analysis on beta diversity. **P < 0.01.
Composition and diversity analysisThe contigs of all samples were annotated to viruses at different classification levels (8 orders, 43 families, 176 genera, 328 species). The average number of viruses per sample at different classification levels were 5.22 ± 1.20 orders, 29.67 ± 4.55 families, 98.23 ± 9.25 genera, and 148.34 ± 12.37 species, respectively.
At the species level, the five most abundant taxa included Staphylococcus phage SPbeta-like (16.10%), Faecalibacterium phage FP Toutatis (9.78%), Faecalibacterium phage FP Mushu (8.99%), Faecalibacterium phage FP Taranis (6.11%), Escherichia phage 500465-1 (4.44%), collectively constituting about 45.42% of all gut viruses. Figure 1C demonstrated the top 10 viruses among all participants, including the GDM and control groups. To facilitate subsequent cross-kingdom analysis and prediction model construction, we additionally annotated the gut bacteria of all participants. A total of 1,303 species of bacteria were found at the species level, of which 62 species were common with relative abundance greater than 0.10%, accounting for 61.02% of the total gut bacteria (Wang and Jia, 2016).
Analysis of alpha diversity revealed that women who later developed GDM exhibited significantly higher diversity (higher Shannon, P = 0.007; Simpson, P = 0.003) compared to controls (Figure 1D and Supplementary Table 2). PCoA plots based on Bray-Curtis and Jaccard distances were constructed to detect the differences in beta diversity, while both ADONIS and ANOSIM analyses indicated no differences in the overall composition of the gut virome between the two groups (Figure 1E and Supplementary Table 3).
Differential gut microbiome and correlation analysisThe MaAsLin2 analysis was conducted to investigate differences in the gut microorganisms between GDM patients and healthy controls. For gut viruses, there were increased abundance of Escherichia phage SH2026Stx1 (MaAsLin coefficient = 16.72, Q = 0.23), Enterobacteria phage mEp043 c-1 (MaAsLin coefficient = 15.41, Q = 0.21), crAssphage cr50_1 (MaAsLin coefficient = 11.52, Q = 0.21), Enterobacteria phage phi80 (MaAsLin coefficient = 7.07, Q = 0.21), and Escherichia phage HK106 (MaAsLin coefficient = 5.23, Q = 0.23) in GDM patients. While, for gut microbiota, there were less Eubacterium eligens (MaAsLin coefficient = −3.35, Q = 0.04) and Escherichia phage HK106 (MaAsLin coefficient = −2.75, Q = 0.04) in GDM patients. Even after adjusting for potential confounding factors such as age and gestational age, significant differences in these species persisted between the two groups (Figure 2A and Supplementary Table 4).
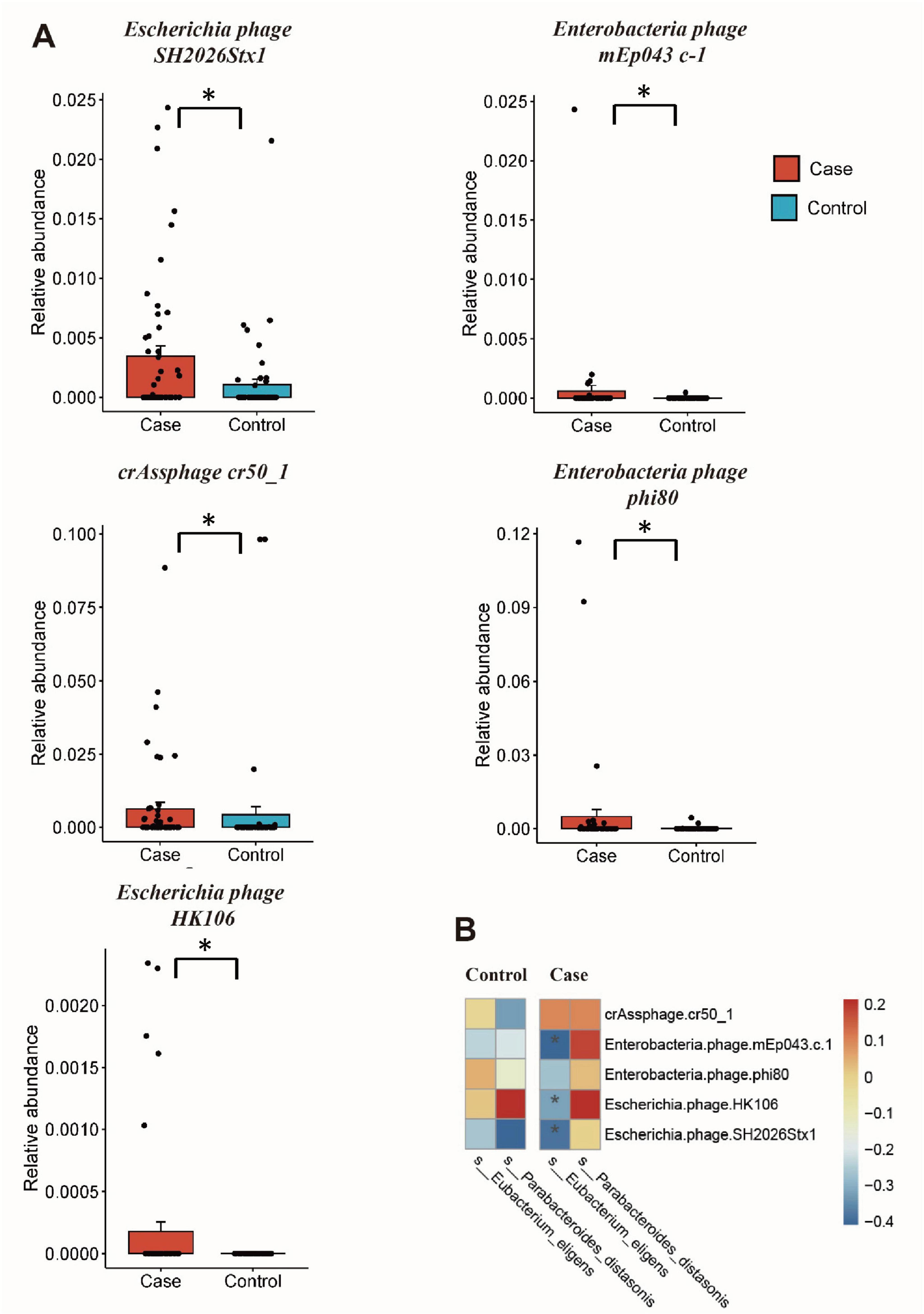
Figure 2. Differential gut microbiome and correlation analysis. (A) The relative abundance of differential gut viruses using MaAsLin2 analysis; (B) cross-kingdom correlation analysis of the differential viruses and bacteria. *Q < 0.25 and P < 0.05 in panel (A), P < 0.05 in panel (B).
Spearman’s rank correlation showed that the relative abundance of Enterobacteria phage phi80 is negatively correlated with GLU (r = −0.217, P = 0.028), while other differential gut viruses showed no statistically associations with clinical factors (Supplementary Figure 1 and Supplementary Table 5). Subsequently, the correlation between differential viruses and bacteria was analyzed, revealing a negative correlation between Eubacterium eligens and three bacteriophages (Escherichia phage SH2026Stx1, Enterobacteria phage mEp043 c-1, and Escherichia phage HK106) in GDM group (r < 0, P < 0.05) (Figure 2B and Supplementary Table 6). However, no such correlation was observed in the control group.
Virus-bacteria co-occurrence network analysisCo-occurrence network analysis was used to evaluate the cross-kingdom correlation between gut viruses and bacteria. We observed that Escherichia phages, including Enterobacteria phage phi80, Escherichia phage SH2026Stx1, and Escherichia phage HK106, were positively correlated with Prevotella copri, which suggested that Prevotella copri could infect these species (Figure 3A). We also found that Clostridium phage phiSM101 and Clostridium phage vB CpeS were more abundant in control group, and multiple viruses, including Escherichia phage D6, were negatively associated with them (Figure 3B). The results showed the predominance of positive correlations over negative ones in both groups (P < 0.05).
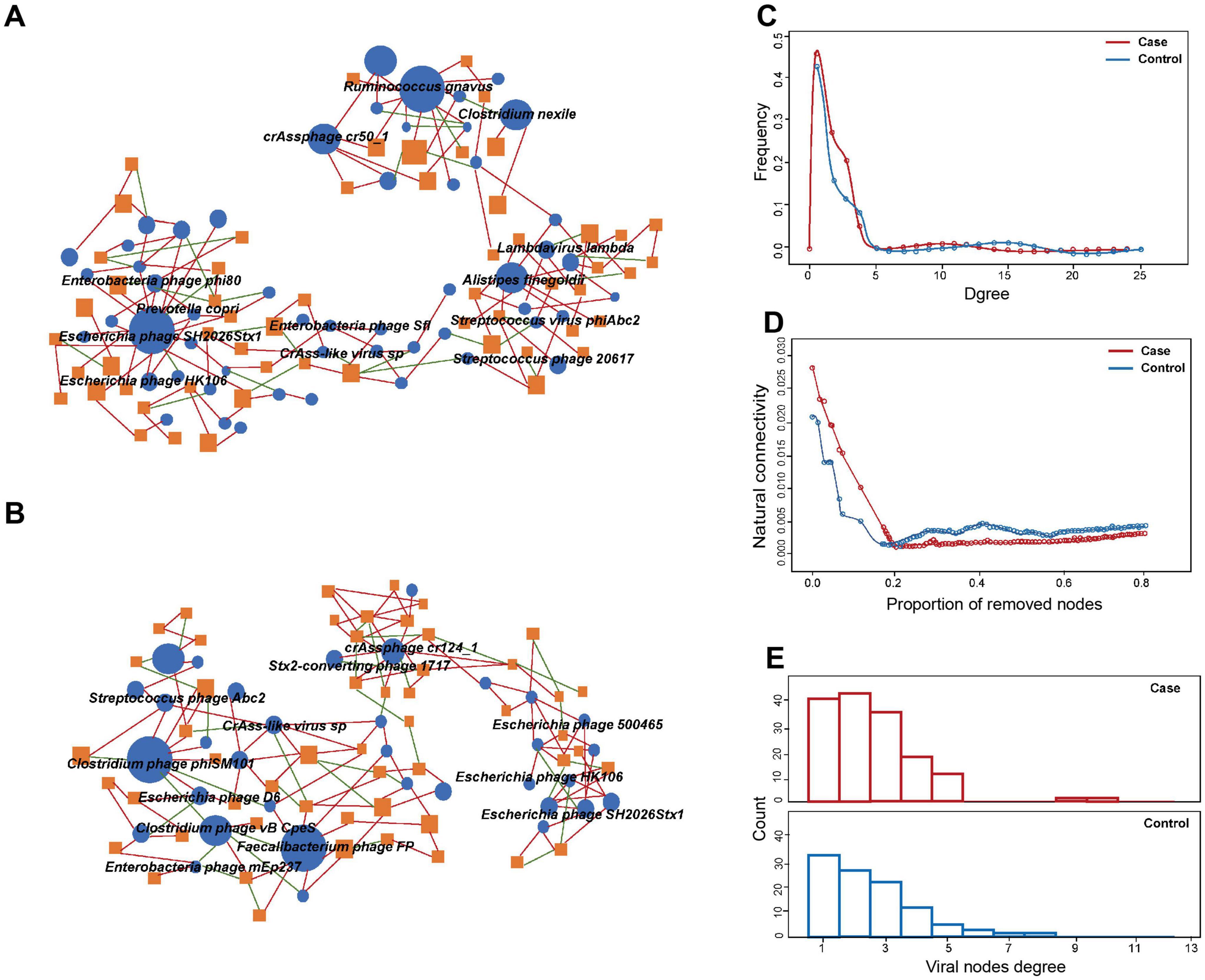
Figure 3. Comparison of virus-bacteria co-occurrence networks between two groups. Virus-bacteria co-occurrence networks for panels (A) case and (B) control groups. The nodes are viruses and bacterial species. Blue ellipses and orange rectangles represent viruses and bacteria, respectively. Edges represent positive associations (red) and negative associations (green) between virus and bacteria at the species level. (C) Degrees of distribution of virus-bacteria co-occurrence networks. (D) Alternations of virus-bacteria co-occurrence networks based on the proportion of removed modes. (E) Degrees of viral nodes degree in virus-bacteria co-occurrence networks.
Structurally, the network of GDM cases comprised 98 nodes and 163 edges, while that of controls consisted of 77 nodes and 135 edge. The densities of the networks in these two groups were 0.0283 and 0.0237, respectively. Degree distribution of the GDM and healthy control network was different (Figure 3C). Despite the higher number of nodes and edges in the network of GDM cases, the network natural connectivity of which was more fragile than the control group with up to 25% nodes removed (Figure 3D). Specifically, virus-bacteria linkages increased from 33 in control group to 40 in case group, suggesting the linkage is more pervasive in GDM patients than healthy pregnant women (Figure 3E).
GDM prediction modelsWe employed both random forest and logistic regression methods to build prediction models for GDM, integrating differential clinical indicators, gut viruses, and gut bacteria. 70% of the subjects were used as the training set, and 30% were used as the validation set for model rehearsal to select the optimal model.
Model 1: We first constructed a predictive model for GDM by using the significantly different clinical indicators. For random forest method, the ROC curve indicated an AUC of 0.742 (95% CI: 0.528 ∼ 0.912). Importance analysis was performed on the model’s included indicators using the Mean Decrease Gini coefficient. The result showed that GLU, HGB, and height were ranked in order of decreasing importance. As for logistic regression model, the ROC curve indicated an AUC of 0.772 (95% CI: 0.663 ∼ 0.891).
Model 2: On the basis of Model 1, two differential gut bacteria in early pregnancy were incorporated into Model 2 as predictive indicators. The ROC curve indicated an AUC of 0.733 (95% CI: 0.515 ∼ 0.902). The ranking of importance was as follows: Eubacterium eligens, GLU, Parabacteroides distasonis, HGB, and height.
Model 3: Based on Model 1, five differential gut viruses present in early pregnancy were incorporated into Model 3 as predictive indicators. The ROC curve indicated an AUC of 0.769 (95% CI: 0.473 ∼ 0.931). The ranking of importance was as follows: GLU, HGB, height, crAssphage cr50_1, Escherichia phage SH2026Stx1, Enterobacteria phage phi80, Enterobacteria phage mEp043 c-1, and Escherichia phage HK106.
Model 4: Based on Model 1, seven differential gut microbial species were all added into the Model 4 as predictive indicators. The order of importance ranking was GLU, Eubacterium eligens, HGB, Parabacteroides distasonis, crAssphage cr50_1, height, Escherichia phage SH2026Stx1, Enterobacteria phage phi80, Enterobacteria phage, and Escherichia phage HK106mEp043 c-1, indicated an AUC of 0.893 (95% CI: 0.736 ∼ 0.990). The accuracy (0.878), recall (0.941), precision (0.800), and F1 score (0.865) were higher than those of Models 1, 2, and 3. As for logistic regression model, the ROC curve indicated an AUC of 0.930 (95% CI: 0.872 ∼ 0.991). Additionally, the accuracy (0.872), sensitivity (0.861), and specificity (0.891) were higher than those of Models 1, 2, and 3 (Figure 4, Table 2, and Supplementary Figure 2).
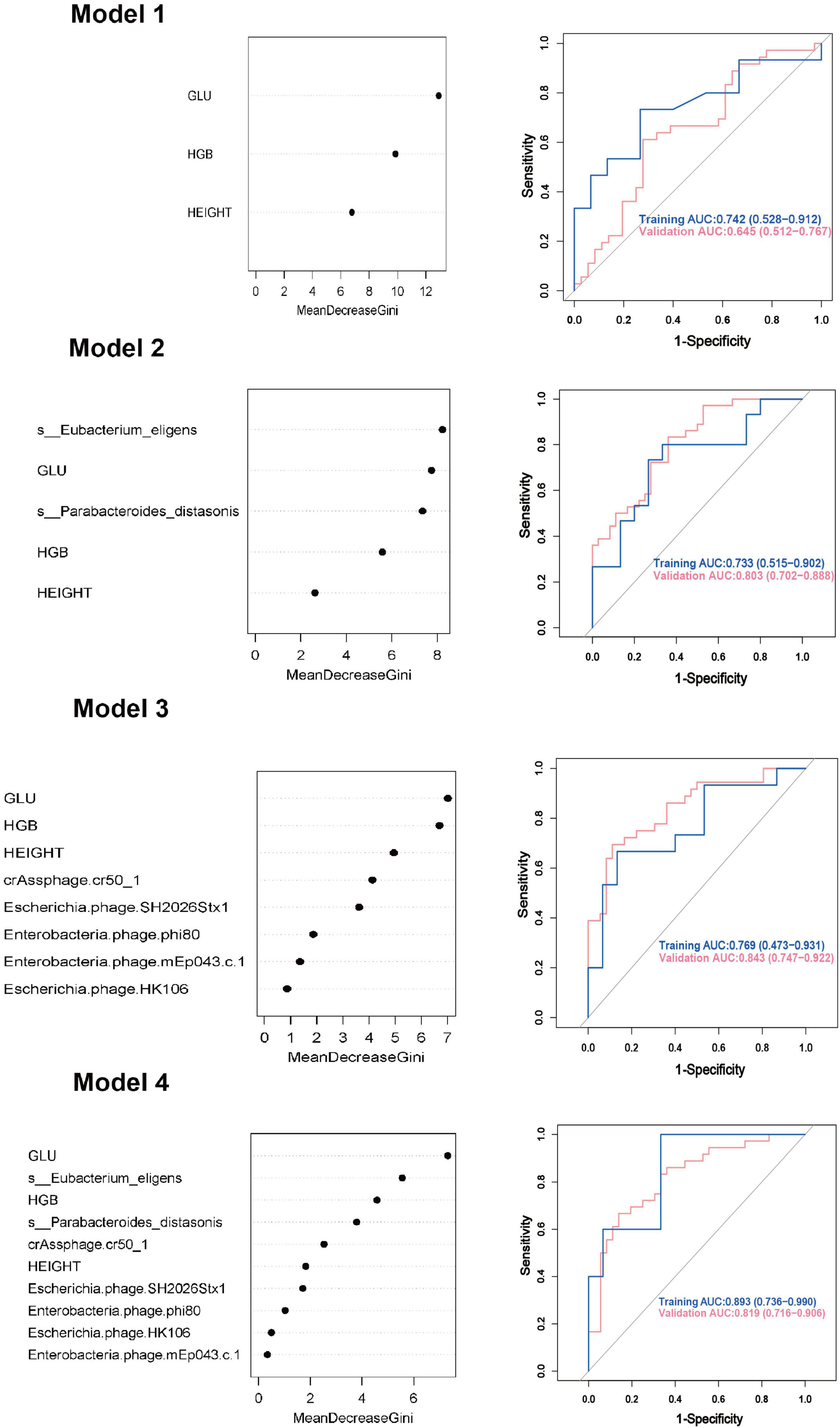
Figure 4. Gestational diabetes mellitus prediction models in early pregnancy using random forest method. GLU, glucose; HGB, hemoglobin; AUC, area under curve; CI, confidence interval.
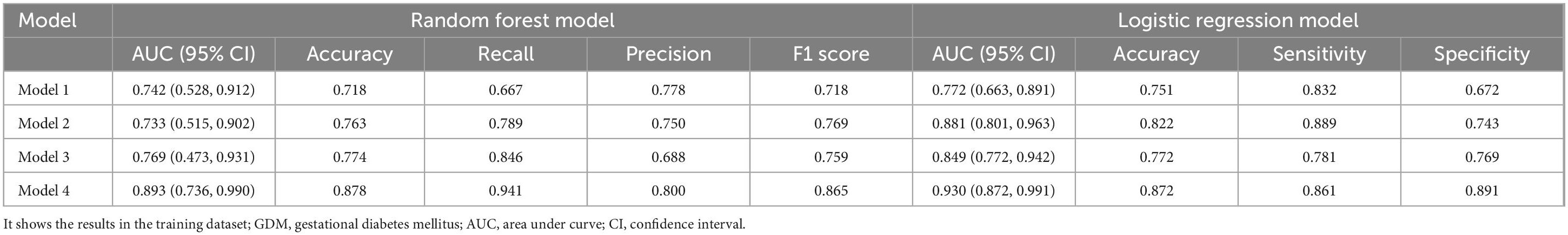
Table 2. Gestational diabetes mellitus prediction models in early pregnancy.
DiscussionIn this nested case–control study, we conducted shotgun metagenomics sequencing of a total of 102 stool samples to characterize and analyze the differences in the composition of gut virome between GDM patients and healthy controls. We investigated the cross-kingdom correlation between gut viruses and bacteria, and developed a novel prediction model for early pregnancy GDM using gut microbial features and clinical indexes, demonstrating robust predictive performance.
In our study, the alpha diversity of gut virome in GMD was higher than that of the control group, consistent with the finding of Ma et al. (2018). However, beta diversity did not show significant differences, suggesting that it may not be the entire composition of the gut virome but rather specific taxa that affect GDM. To explore this possibility, we performed MaAsLin2 analysis and identified five differentially abundant virus taxa in pregnant GDM patients, including four Enterobacteria phages and crAssphage cr50_1. Our result showed that after adjusting for confounding factors such as age and gestational age, Enterobacteria phage phi80 was enriched in the GDM patients. A similar difference was observed in patients with colorectal cancer (Zuo et al., 2022). Certain intestinal microbes may increase intestinal permeability and the likelihood of harmful intestinal metabolites entering the bloodstream, thereby affecting systemic inflammation (Abdullah et al., 2021). The inflammatory environment may also promote insulin resistance and β-cell dysfunction, contributing to the onset of GDM (Pinto et al., 2023). Another case-control study identified significant increases in Enterobacter and its bacteriophages in patients with T2D, along with elevated levels of serum LPS, IL-6 and TNF-α (Chen et al., 2020). Enrichment of Enterobacteria phage phi80 might increase circulating LPS levels, thereby inducing systemic subclinical inflammation and affecting insulin sensitivity, potentially contributing to GDM (Mehta et al., 2010).
Additionally, we found significantly elevated levels of Escherichia phage HK10 and Enterobacteria phage mEp043 c-1 in the GDM group and those of which were also negatively correlated with the relative abundance of gut bacterium Eubacterium eligens. As a short-chain fatty acid (SCFA) butyrate-producing bacteria, Eubacterium has been reported to play an important role in maintaining the integrity of intestinal barrier, blood glucose response, and cholesterol homeostasis (Forslund et al., 2015). SCFA can improve insulin sensitivity and satiety by inhibiting HDAC-mediated reprogramming of pancreatic β-cells (Khan and Jena, 2015). This implies that increased levels of Escherichia phage HK106 and Enterobacteria phage mEp043 c-1 may reduce the levels of Eubacterium eligens (Shuwen and Kefeng, 2022), leading to butyrate depletion in the intestine and initiating a cascade of pro-inflammatory reactions, thus impacting the blood glucose metabolism of pregnant women.
Consistent with our results, Ma et al. (2018) and Chen et al. (2020) reported the increased cross-kingdom correlations between enteric viruses and bacteria in T2D patients. Furthermore, the positive correlations are significantly more than the negative correlations. Yang et al. (2021) found the intensive cross-kingdom correlations in lean controls compared to obese subjects with T2D, which contradicted our findings. The conflicting results are likely due to the limited sample size and sample heterogeneity. The evidence suggests that gut viruses and bacteria are likely to exhibit synergistic or mutually reinforcing relationships, influencing glucose and lipid metabolism. However, additional functional experiments and randomized controlled trials are needed to confirm their association with GDM.
We first developed the model to predict early pregnancy GDM using clinical indicators associated with GDM and achieved an AUC of 0.724, similar to other published GDM prediction models (AUC range: 0.672 ∼ 0.784) (Zheng et al., 2019; Gao et al., 2020; Duo et al., 2023). At present, most of the prediction models for GDM were based on clinical indicators and maternal characteristics. However, recent studies increasingly suggest the potential of gut viral and bacterial markers in diagnosing T2D (Fan et al., 2023; Dash et al., 2023). Adding seven GDM-related gut microbial features (five gut viruses and two bacteria) to the initial model increased the AUC to 0.895 and recall rate to 0.941, respectively. Pinto et al. (2023) established a GDM prediction model incorporating early pregnancy gut microbiome composition, cytokine levels, medical history, and dietary factors, achieving an AUC of 0.830. Ma et al. (2020) and Hu et al. (2021) also constructed the model with AUC of 0.771 and 0.736, respectively. Comparing to the exsiting models only using gut microbiota and other indicators, our study is the first to include gut viruses in the GDM prediction model and demonstrated better prediction performance, thus indicating a potential association between gut viruses and GDM occurrence in early pregnancy. At the same time, our study used two different methods (random forest and logistic regression methods) to construct the model together with training and validation datasets to perform internal validation. The consistent results ensure the stability of our research. Furthermore, all variables included in this model are in the first trimester of pregnancy. Therefore, this model could predict the probability of GDM better in early pregnancy, helping to target high-risk people. Early intervention in these high-risk people may reduce the risk of GDM and have better application value.
Our study has several strengths. Firstly, it is the first to investigate the relationship between the gut virome and GDM, and to analyze interactions between gut viruses and bacteria. Secondly, the nested case-control design ensures that exposure (gut virome) precedes outcome (GDM). Thirdly, our findings were reinforced by a GDM prediction model incorporating early pregnancy gut viruses, gut bacteria, and clinical indicators, which showed good predictive capability.
There are several limitations in our study. Firstly, the cohort only included pregnant women from Hunan Province, limiting the generalizability of our findings to other populations. Secondly, due to an incomplete database and the lack of standardized procedures for handling virus sequences, some metagenomic sequencing data could not be annotated, which affected the robustness and reliability of our findings. Thirdly, while we observed the relationship between the gut virome and GDM, the function and underlying mechanisms remain unclear. Nevertheless, this study is exploratory and aims to guide future research on gut virome functions and gut virus-bacteria interactions in health and disease.
In conclusion, by conducting a nested case-control study, we characterized the gut virome of GDM patients in early pregnancy and explored the correlation between gut viruses and gut bacteria across kingdoms. Using clinical indicators and gut microbial features, we developed a new predictive model for GDM that demonstrated good predictive performance. Our findings have implications for both the prevention and treatment of GDM, offering valuable insights into its pathogenesis. In the future, the gut viruses and bacteria associated with GDM discovered in this study would be validated through function annotation and mouse experiments. Additionally, metabolites derived from gut microbiome would be analyzed to clarify the connections between metagenomics, metabolomics, and GDM, with the goal of thoroughly investigating its pathogenesis and creating early prediction models using a multi-omics approach.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in this article/Supplementary material.
Ethics statementThe studies involving humans were approved by the Maternity and Child Health Hospital of Hunan Province (no. EC201624). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsXW: Conceptualization, Funding acquisition, Writing – original draft, Writing – review and editing. XL: Software, Visualization, Writing – original draft. WX: Investigation, Writing – original draft. WC: Investigation, Writing – original draft. ZZ: Investigation, Writing – original draft. HT: Conceptualization, Supervision, Writing – review and editing. TX: Formal analysis, Software, Visualization, Writing – original draft.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82360665), the Natural Science Foundation of Hunan Province (2024JJ7406 and 2024JJ9043), the Research Project on Teaching Reform of Ordinary Colleges and Universities in Hunan Province (202401000932), and the Degree and Graduate Education Reform Research Project in Hunan Province (2024JGYB206).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1461259/full#supplementary-material
ReferencesAbdullah, M., Sukartini, N., Nursyirwan, S. A., Pribadi, R. R., Maulahela, H., Utari, A. P., et al. (2021). Gut microbiota profiles in early- and late-onset colorectal cancer: A potential diagnostic biomarker in the future. Digestion 102, 823–832. doi: 10.1159/000516689
PubMed Abstract | Crossref Full Text | Google Scholar
Bellamy, L., Casas, J. P., Hingorani, A. D., and Williams, D. (2009). Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 373, 1773–1779. doi: 10.1016/s0140-6736(09)60731-5
PubMed Abstract | Crossref Full Text | Google Scholar
Billionnet, C., Mitanchez, D., Weill, A., Nizard, J., Alla, F., Hartemann, A., et al. (2017). Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 60, 636–644. doi: 10.1007/s00125-017-4206-6
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, Q., Ma, X., Li, C., Shen, Y., Zhu, W., Zhang, Y., et al. (2020). Enteric phageome alterations in patients with type 2 diabetes. Front. Cell Infect. Microbiol. 10:575084. doi: 10.3389/fcimb.2020.575084
PubMed Abstract | Crossref Full Text | Google Scholar
Crusell, M. K. W., Hansen, T. H., Nielsen, T., Allin, K. H., Rühlemann, M. C., Damm, P., et al. (2018). Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6:89. doi: 10.1186/s40168-018-0472-x
PubMed Abstract | Crossref Full Text | Google Scholar
Dabelea, D., and Pettitt, D. J. (2001). Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J. Pediatr. Endocrinol. Metab. 14, 1085–1091. doi: 10.1515/jpem-2001-0803
PubMed Abstract | Crossref Full Text | Google Scholar
Dash, N. R., Al Bataineh, M. T., Alili, R., Al Safar, H., Alkhayyal, N., Prifti, E., et al. (2023). Functional alterations and predictive capacity of gut microbiome in type 2 diabetes. Sci. Rep. 13:22386. doi: 10.1038/s41598-023-49679-w
PubMed Abstract | Crossref Full Text | Google Scholar
de Jonge, P. A., Wortelboer, K., Scheithauer, T. P. M., van den Born, B. H., Zwinderman, A. H., Nobrega, F. L., et al. (2022). Gut virome profiling identifies a widespread bacteriophage family associated with metabolic syndrome. Nat Commun. 13:3594. doi: 10.1038/s41467-022-31390-5
PubMed Abstract | Crossref Full Text | Google Scholar
Duo, Y., Song, S., Qiao, X., Zhang, Y., Xu, J., Zhang, J., et al. (2023). A simplified screening model to predict the risk of gestational diabetes mellitus in pregnant chinese women. Diabetes Ther. 14, 2143–2157. doi: 10.1007/s13300-023-01480-8
PubMed Abstract | Crossref Full Text | Google Scholar
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J., and Paquot, N. (2014). Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105, 141–150. doi: 10.1016/j.diabres.2014.04.006
PubMed Abstract | Crossref Full Text | Google Scholar
Fan, G., Cao, F., Kuang, T., Yi, H., Zhao, C., Wang, L., et al. (2023). Alterations in the gut virome are associated with type 2 diabetes and diabetic nephropathy. Gut Microbes 15:2226925. doi: 10.1080/19490976.2023.2226925
PubMed Abstract | Crossref Full Text | Google Scholar
Forslund, K., Hildebrand, F., Nielsen, T., Falony, G., Le Chatelier, E., Sunagawa, S., et al. (2015). Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266. doi: 10.1038/nature15766
PubMed Abstract | Crossref Full Text | Google Scholar
Gao, S., Leng, J., Liu, H., Wang, S., Li, W., Wang, Y., et al. (2020). Development and validation of an early pregnancy risk score for the prediction of gestational diabetes mellitus in Chinese pregnant women. BMJ Open Diabetes Res. Care 8:e000909. doi: 10.1136/bmjdrc-2019-000909
PubMed Abstract | Crossref Full Text | Google Scholar
Glickman, M. E., Rao, S. R., and Schultz, M. R. (2014). False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 67, 850–857. doi: 10.1016/j.jclinepi.2014.03.012
PubMed Abstract | Crossref Full Text | Google Scholar
Gogokhia, L., Buhrke, K., Bell, R., Hoffman, B., Brown, D. G., Hanke-Gogokhia, C., et al. (2019). Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 25, 285–299.e8. doi: 10.1016/j.chom.2019.01.008
PubMed Abstract | Crossref Full Text | Google Scholar
Hu, P., Chen, X., Chu, X., Fan, M., Ye, Y., Wang, Y., et al. (2021). Association of gut microbiota during early pregnancy with risk of incident gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 106, e4128–e4141. doi: 10.1210/clinem/dgab346
PubMed Abstract | Crossref Full Text | Google Scholar
Khan, S., and Jena, G. (2015). The role of butyrate, a histone deacetylase inhibitor in diabetes mellitus: Experimental evidence for therapeutic intervention. Epigenomics 7, 669–680. doi: 10.2217/epi.15.20
PubMed Abstract | Crossref Full Text | Google Scholar
Li, D., Luo, R., Liu, C. M., Leung, C. M., Ting, H. F., Sadakane, K., et al. (2016). MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 102, 3–11. doi: 10.1016/j.ymeth.2016.02.020
PubMed Abstract | Crossref Full Text | Google Scholar
Ma, S., You, Y., Huang, L., Long, S., Zhang, J., Guo, C., et al. (2020). Alterations in gut microbiota of gestational diabetes patients during the first trimester of pregnancy. Front. Cell Infect. Microbiol. 10:58. doi: 10.3389/fcimb.2020.00058
PubMed Abstract | Crossref Full Text | Google Scholar
Ma, Y., You, X., Mai, G., Tokuyasu, T., and Liu, C. (2018). A human gut phage catalog correlates the gut phageome with type 2 diabetes. Microbiome 6:24. doi: 10.1186/s40168-018-0410-y
PubMed Abstract | Crossref Full Text | Google Scholar
Mehta, N. N., McGillicuddy, F. C., Anderson, P. D., Hinkle, C. C., Shah, R., Pruscino, L., et al. (2010). Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59, 172–181. doi: 10.2337/db09-0367
PubMed Abstract | Crossref Full Text | Google Scholar
Metzger, B. E., Gabbe, S. G., Persson, B., Buchanan, T. A., Catalano, P. A., Damm, P., et al. (2010). International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682. doi: 10.2337/dc09-1848
PubMed Abstract | Crossref Full Text | Google Scholar
Metzger, B. E., Lowe, L. P., Dyer, A. R., Trimble, E. R., Chaovarindr, U., Coustan, D. R., et al. (2008). Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 358, 1991–2002. doi: 10.1056/NEJMoa0707943
留言 (0)