The term athlete's heart (AH) refers to the state in which the heart undergoes adaptations in response to continuous high-intensity training (1, 2). Continuous training results in an increase in the chamber size and wall thickness (2). Regarding cardiac function, the left ventricular (LV) stroke volume increases, whereas LV systolic function is normal or slightly decreased in response to high-intensity training (2). AH induces structural and functional changes, and its manifestation varies depending on factors such as race, sex, age, and type and intensity of training (1, 3).
Soccer is one of the most common sports globally. As the number of soccer players increases, the number of cardiac arrests occurring during soccer games increases (1). Accordingly, the need for cardiac evaluation in soccer players is increasing. The Fédération Internationale de Football Association (FIFA) requires cardiac function evaluation, including electrocardiography and echocardiography, before international matches (1). In a recent study, elite American soccer players demonstrated several echocardiographic parameters that exceeded the normal range outlined in existing guidelines (1). However, recent studies have shown significant differences in structural and functional cardiac remodeling in male and female athletes (4). Although female athletes are known to tend to have eccentric LV hypertrophy, reports of AH in females were rare (4). Differentiating between AH and pathological changes in athletes is important because pathological diseases that exhibit a morphology similar to AH can cause sudden cardiac death (3).
We analyzed the echocardiograms of Korean female under-20 (U-20) national soccer team players to determine differences occurring in the hearts of young Asian female athletes. Young athletes are likely to display differences similar to those in adults; however, limited data are available on young athletes and early changes in AH remain unknown (3). Furthermore, data on Asian populations are lacking because most studies on AH have been conducted in Caucasian populations (5, 6). According to studies conducted in Singapore and Malaysia, cardiac remodeling in Asian athletes demonstrated similar patterns, such as large chamber size and wall thickness; however, these changes were not as severe as those in Caucasian athletes (5, 6). A recent study of Taiwanese military women showed that structural and functional differences in the heart are not characteristic of Asian female athletes (7). Notably, both studies were conducted on adults, and no study has investigated adolescent Asian athletes. Therefore, this study aimed to (1) investigate the pattern of AH in Korean adolescent female soccer players, (2) identify any differences from their non-athlete peers, and (3) determine the early changes in the heart caused by training in Asians.
2 Methods 2.1 Design and participantsThis cross-sectional observational study compared the heart structure and function between Korean female U-20 national soccer team players and healthy young adolescents. National soccer team players aged 17–20 years underwent echocardiography as part of their medical examination in July 2022 to participate in a FIFA match. The control group consisted of age-matched healthy females (aged 17–20 years) who underwent echocardiography at Kyung Hee University Hospital at Gangdong between October 2018 and August 2022. Individuals with severe systemic disease, those who did not consent to the use of their personal information, those who had previously undergone heart-related procedures or surgeries, those with underlying cardiac dysfunction, or those with congenital heart disease were excluded. Physical examination and echocardiography results were obtained for all participants. The physical examination parameters included height, weight, body mass index (BMI), body surface area (BSA), blood pressure (BP), and heart rate (HR). BSA was calculated using the Du Bois and Du Bois formula: Body Surface Area = 0.007184 × [Height(cm)0.725] × [Weight(kg)0.425]. We analyzed the differences in cardiac structure and function by comparing clinical characteristics and echocardiographic data between the two groups. The requirement for informed consent was waived because of the retrospective nature of data collection. This study was approved by the hospital's Ethics Committee (KHNMC 2023-04-010-001).
2.2 Echocardiographic examinationEchocardiography was performed with the patient in left decubitus position. Left atrium (LA) size, LV geometry and function, and ascending aorta diameter were evaluated in all participants. LA size was assessed by measuring LA diameter and volume index using M-mode and two-dimensional (2D) echocardiography. LV geometry was also evaluated using M-mode and 2D echocardiography. The LV internal dimensions at end-diastole (LVIDd), LV end-diastolic volume (LVEDV), and LV end-systolic volume (LVESV) were measured to evaluate the LV size. LV wall thickness was evaluated by measuring intraventricular septal thickness (IVSd), LV posterior wall thickness (LVPWd), LV mass index, and relative wall thickness (RWT). LV mass index was calculated using the following formula: LV mass index = LV mass/BSA, LV mass = 0.8{1.04[([LVEDD + IVSd + PWd]3 − LVEDD3)]} + 0.6. The linear dimensions of the LA and LV were measured in the parasternal long-axis view, and the volumes of the LA and LV were measured in a biplane in the apical view. LV systolic function was assessed by measuring LV ejection fraction (EF) using the biplane Simpson's technique. LV diastolic function was evaluated using early diastolic mitral inflow (E) velocity, late diastolic mitral inflow (A) velocity, septal early diastolic mitral annular (e’) velocity, lateral e’ velocity, E/A ratio, and E/e’ ratio. Pulsed-wave Doppler images of the mitral valve tip were acquired to measure peak E velocity, peak A velocity, and E/A ratio. Septal and lateral e’ velocities were measured in the basal inferoseptal and basal anterolateral walls, respectively, using tissue Doppler imaging. The E/e’ ratio was determined as the ratio of peak E velocity and peak septal e’ velocity.
2.3 Statistical analysisStatistical analysis was performed with SPSS ver 13.0. Continuous variables are expressed as mean values and standard deviations. For each variable, the differences between the athlete and control groups were compared using an independent t-test. p < 0.05 was considered statistically significant. Power analysis was performed using PASS 16.0 software to evaluate whether the sample size was sufficient.
3 Results 3.1 Study populationA total of 62 adolescents aged 17–20 years were enrolled in this study. Twenty were Korean female U-20 national soccer team players and 42 were healthy teenagers. The athletes had less than 10 years of athletic experience, with 7–10 years of experience. Although the amount of training in healthy teenagers has not been investigated, athletes, engaged in 3–4 h of training per day. The average age of participants was 19 years (Table 1). Height, weight, and BSA were higher in the athletes than in the healthy adolescents; however, no significant differences in BMI were observed (Table 1). Both BP and HR were lower in the athletic group than in the control group (Table 1).
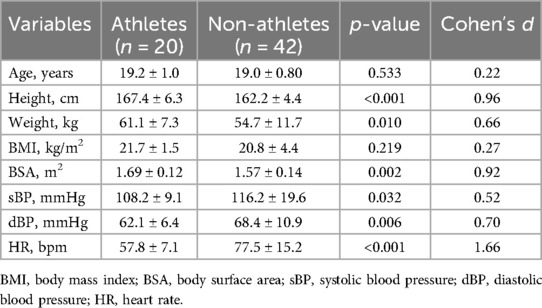
Table 1. Clinical characteristics of the study population.
3.2 EchocardiographyEchocardiographic data are presented in Table 2. Significant differences were observed in the echocardiographic parameters between the two groups. These data showed a sufficient power of over 85% for these parameters (Supplementary Table S1). In terms of overall size, the structures were larger and the diameter of the ascending aorta was dilated in the athlete group.
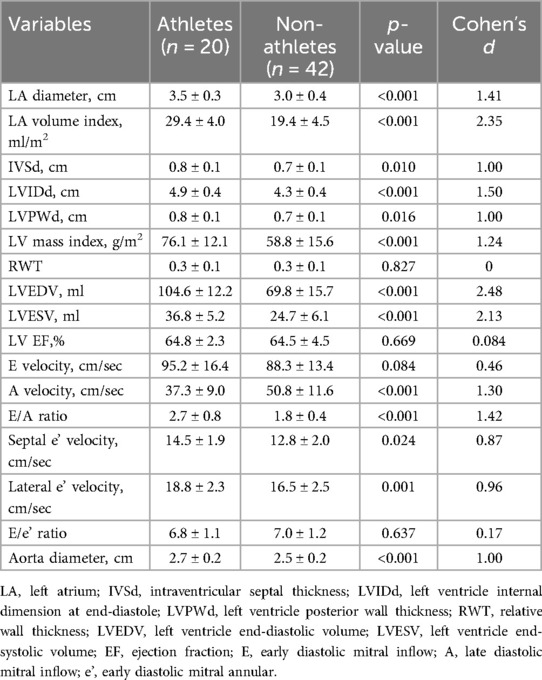
Table 2. Echocardiographic parameters in athletes and non-athletes.
Regarding the structure of the heart, the diameter of LA was large in the athlete group (3.5 ± 0.3 vs. 3.0 ± 0.4 cm; p < 0.001). Considering the LA volume index, which corrects for the effect of BSA between groups, the size of the LA in the athlete group was approximately 1.5 times more dilated than that in the control group (29.4 ± 4.0 vs. 19.4 ± 4.5 ml/m2; p < 0.001). All parameters measuring LV size were greater in the athletic group than in the control group. LVEDV and LVESV were approximately 1.5 times larger in the athletes than in the healthy controls (104.6 ± 12.2 vs. 69.8 ± 15.7 ml; p < 0.001; 36.8 ± 5.2 vs. 24.7 ± 6.1 ml; p < 0.001).
The LV wall was thicker in the athletic group than in the control group. IVSd, LVPWd, and LV mass index were also large in the athlete group (0.8 ± 0.1 vs. 0.7 ± 0.1 cm; p = 0.010; 0.8 ± 0.1 vs. 0.7 ± 0.1 cm; p = 0.016; 76.1 ± 12.1 vs. 58.8 ± 15.6 g/m2; p < 0.001). However, RWT was within the normal range with no significant difference between the two groups (0.3 ± 0.1 vs. 0.3 ± 0.1; p = 0.827).
Regarding cardiac function, the average EF in both groups was around 65%, demonstrating no significant difference between the two groups (64.8 ± 2.3 vs. 64.5 ± 4.5%; p = 0.669). In terms of diastolic function, no difference was noted in the peak E velocity between the two groups (95.2 ± 16.4 vs. 88.3 ± 13.4 cm/sec; p = 0.084). However, peak A velocity was lower in the athlete group than that in control group (37.3 ± 9.0 vs. 50.8 ± 11.6 cm/sec; p < 0.001), which resulted in the E/A ratio being higher in the athlete group (2.7 ± 0.8 vs. 1.8 ± 0.4; p < 0.001). Peak septal e’ and lateral e’ velocities were also higher in the athlete group (14.5 ± 1.9 vs. 12.8 ± 2.0 cm/sec; p = 0.024; 18.8 ± 2.3 vs. 16.5 ± 2.5 cm/sec; p = 0.001). The result produced a slightly low E/e’ ratio in the athlete group, but the difference was not significant (6.8 ± 1.1 vs. 7.0 ± 1.2; p = 0.637).
4 DiscussionThis is the first study to compare the echocardiographic findings between Korean adolescent female soccer players and healthy individuals. Many previous studies have analyzed AH in adult Caucasians; however, data on Asian, female, and young athletes are limited (5–8). Therefore, the strength of this study can be attributed to the rarity of participant groups. We aimed to analyze AH in a homogenous patient group of adolescent Asian female soccer players and compare the results with existing results. The main findings of this study are as follows: First, BP and HR were lower in the athletes than in the healthy controls. Second, the left heart and ascending aorta were dilated in the athletic group. Third, in the athlete group, the LV wall was thicker, but the RWT was within the normal range, resulting in a normal LV geometry. Fourth, the systolic function of the LV was normal, and for the LV diastolic function, the parameters indicating early diastolic filling were greater in the athletic group.
Asians have a smaller BSA than Caucasians; consequently, the degree of cardiac remodeling appears to be less pronounced in Asians compared to that in other racial groups (7, 9–12). Female athletes have smaller absolute cardiac dimensions than male athletes (9, 13, 14). As individuals age and training continuously, chamber size is gradually increases (9, 15). Based on previous studies, the changes in the chamber size and wall thickness in the athlete group in this study were expected to be relatively small.
However, the LA and LV chamber sizes in the athlete group were larger than those in the control group. The LA volume index in the athlete group was 1.5 times more than that in healthy participants, and the LVEDV in the athlete group also displayed a dilatation of 1.5 times. Although the participants included in previous Asian studies were female athletes in their 20s, their LVEDV was lower than that observed in this study (5, 6). This disparity was likely attributable to previous studies that analyzed athletes from various sports. Previous studies seem to have reported less chamber dilatation than this study because a significant number of power- and skill-type athletes were included. Traditionally, it has been known that endurance training causes changes in eccentric LV hypertrophy patterns, whereas resistance training causes concentric LV hypertrophy (9, 16, 17). However, recently published studies presented conflicting results with this hypothesis (17–19). Recent studies demonstrated that morphologic changes in the LV occur with endurance training, while resistance training does not cause changes in cardiac morphology (17, 18). Endurance training also appears to be related to both volume and pressure load on the heart (18). AH is associated with the time of training load, and it is skeptical that the AH phenotype depends on the type of training (18, 19). Supporting these findings, cardiac remodeling was noticeable in this study with more endurance-trained athletes. A previous study showing significant LV dilatation in female elite polo athletes and mild changes in female elite fitness athletes supported these findings (20). In a study analyzing adult soccer players in the United States, LV and LA chamber sizes were dilatated prominently, as observed in this study (1). This suggests that LA and LV dilatation occur in soccer players during early training loading.
LV wall thickness, as assessed by IVSd and LVPWd, got thicker in the athlete group. The LV mass index adjusted for BSA also were high in the athlete group. According to a previous study, data from adult female soccer players in the United States demonstrated that the LV mass index was an average of 91 g/cm2 (1). The results of this study revealed that, in the case of adolescent Asian athletes, the parameters were relatively small owing to differences in age and race. However, no significant difference in the RWT was observed between the athlete and control groups. In a study that serially observed adolescent cross-country skiers, after concentric remodeling occurred in the early stages of training, chamber dilatation occurred and RWT returned to normal or decreased (21).
In a study of elite judo athletes, LV dilatation was more prominent than LV wall hypertrophy (22). Similar outcomes were presumed in soccer. In this study, the thickness of the LV wall was high, but the RWT was not different from that of healthy individuals owing to the dilatation of the chamber. The athlete group showed a slightly eccentric LV pattern, but did not reach the point of eccentric hypertrophy, resulting in normal LV geometry, as in the control group. This result is consistent with recent studies demonstrating that most female athletes competing at the Olympics display normal LV geometry (23). This also matches with a recent study which showed that although the athlete cohort had higher LV mass index and wall thickness compared to the healthy cohort, the median value was in the normal range, resulting in normal LV geometry (24).
Systolic function did not differ between the athletes and healthy individuals. In previous studies, depending on the type of training, the EF of athletes was the same or slightly lower than that of healthy individuals (9). Some studies reported low EF in endurance-type athletes (9, 25). This is attributable to the reduced contraction force required to achieve the stroke volume when the LV is dilated (9, 23). This is the result of certain endurance-type athletes with eccentric LV hypertrophy (9, 26). Most athletes, including soccer players, exhibit LV systolic function similar to that of healthy people (1, 9, 23). The athletes in this study demonstrated normal LV geometry and systolic function, similar to those in the control group.
LV diastolic function in the athlete group was normal. Peak E velocity was similar between the athlete and control groups, and peak A velocity was lower in the athlete group, indicating an elevation in the E/A ratio in the athlete group. Previous studies presented that the mitral E/A ratio was higher in athletes than in healthy (27). Most studies have demonstrated normal or enhanced LV diastolic function in athletes (28, 29). As training load is continuously applied, early diastolic filling is enhanced, resulting in a decrease in the A velocity and an increase in the E/A ratio in athletes (5, 9, 29–31). According to a previous study, septal and lateral e’ velocities increased and the E/e’ ratio decreased because early relaxation of the LV increased (1, 5, 9, 29–31). Although the E/e’ ratio in this study was not significantly low in the athlete group, the septal and lateral e’ peak velocities were elevated. This indicates that the diastolic function was enhanced in the athlete group, as suggested in previous studies. As the training load continues, the change in e’ velocity, which measures myocardial velocity, is expected to become significant. A recent study analyzing adult female athletes in Asian demonstrated that the septal and lateral e’ peak velocities were higher than those in this study and the E/e’ ratio of athletes was significantly low (5). In a study analyzing adult soccer players in the United States, lateral e’ peak velocity was higher than that in this study (1). However, results regarding diastolic function based on the type and intensity of training are controversial; therefore, further studies are required (9, 32, 33).
Although the results of this study are similar to those of some previous studies, the strength and significance of our findings are as follows: First, compared to a previous study analyzing soccer players, although the athletes in this study had a small dilatation in chamber size and LV mass index, the patterns were similar. This implies that even adolescent Asian female soccer players exhibit significant differences in cardiac morphology. Second, compared to studies analyzing Asian adult athletes, the athletes in this study had greater dilatation of the LV chamber, indicating that the type and intensity of training have a significant impact on AH. Finally, in this study, the differences in the parameters measuring diastolic function were relatively small but notable. The enhancement of diastolic function appears to be at the beginning of the training.
4.1 LimitationThis study has several limitations. First, the sample size was small and there are limitations in generalizing the results because the study used data from national youth teams in a single country. However, power analysis showed that the sample size was sufficient. This suggests that significant differences existed between the groups. Second, since this was a retrospective study, the data that could be obtained were limited. Detailed echocardiographic parameters of the right heart were not obtained in most control group participants, and strain analysis was not performed. As a result, additional differences that exist between athletes and healthy individuals may not have been evaluated. Third, as this was a cross-sectional observational study, it had several limitations, including difficulty in identifying causal relationships. Multiple variables, such as diet, family history, and socioeconomic status, may affected the results. Moreover, elite athletes may have innate and genetic qualities that distinguish them from healthy people. These qualities can affect structural and functional differences in the heart. Although age, race, and sex were matched in this study, bias may have occurred because of these uninvestigated variables. To supplement the limitations of a cross-sectional study, a follow-up study with a larger sample size is required in the future.
5 ConclusionCompared with healthy adolescents, young soccer players with <10 years of athletic experience demonstrated differences in cardiac morphology, such as chamber dilatation and enhanced diastolic function. Therefore, we can assume that in female soccer players, remodeling of cardiac morphology and function occurs at the beginning of training. We expect that additional evidence will be obtained in a longitudinal study with a larger sample size.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Kyung Hee University Hospital at Gangdong. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Informed consent was waived due to the retrospective data collection.
Author contributionsNK: Writing – original draft, Writing – review & editing. HP: Writing – review & editing. IS: Writing – review & editing. I-HY: Conceptualization, Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1404780/full#supplementary-material
References1. Churchill TW, Petek BJ, Wasfy MM, Guseh JS, Weiner RB, Singh TK, et al. Cardiac structure and function in elite female and male soccer players. JAMA Cardiol. (2021) 6(3):316–25. doi: 10.1001/jamacardio.2020.6088
PubMed Abstract | Crossref Full Text | Google Scholar
2. Bjerring AW, Landgraff HEW, Leirstein S, Haugaa KH, Edvardsen T, Sarvari SI, et al. From talented child to elite athlete: the development of cardiac morphology and function in a cohort of endurance athletes from age 12 to 18. Eur J Prev Cardiol. (2021) 28(10):1061–7. doi: 10.1177/2047487320921317
PubMed Abstract | Crossref Full Text | Google Scholar
3. Rodriguez-Lopez AM, Javier G, Carmen P, Esteban P, Luisa GC, Tomas F, et al. Athlete heart in children and young athletes. Echocardiographic findings in 331 cases. Pediatr Cardiol. (2022) 43(2):407–12. doi: 10.1007/s00246-021-02736-5
PubMed Abstract | Crossref Full Text | Google Scholar
5. Roslan A, Stanislaus R, Yee Sin T, Aris FA, Ashari A, Shaparudin AA, et al. Echocardiography and strain analysis in Malaysian elite athletes versus young healthy adults. Int J Cardiol Heart Vasc. (2023) 47:101242. doi: 10.1016/j.ijcha.2023.101242
PubMed Abstract | Crossref Full Text | Google Scholar
6. Yeo TJ, Wang M, Grignani R, McKinney J, Koh LP, Tan FHY, et al. Electrocardiographic and echocardiographic insights from a prospective registry of Asian elite athletes. Front Cardiovasc Med. (2022) 8:799129. doi: 10.3389/fcvm.2021.799129
PubMed Abstract | Crossref Full Text | Google Scholar
7. Liu MY, Liu PY, Tsai KZ, Lima JAC, Lavie CJ, Lin GM. Asian female athlete’s heart: the chief heart study. Acta Cardiol Sin. (2023) 39(6):888–900. doi: 10.6515/ACS.202311_39(6).20230306F
PubMed Abstract | Crossref Full Text | Google Scholar
8. Oxborough D, Augustine D, Gati S, George K, Harkness A, Mathew T, et al. A guideline update for the practice of echocardiography in the cardiac screening of sports participants: a joint policy statement from the British society of echocardiography and cardiac risk in the young. Echo Res Pract. (2018) 5(1):G1–G10. doi: 10.1530/ERP-17-0075
PubMed Abstract | Crossref Full Text | Google Scholar
9. Flanagan H, Cooper R, George KP, Augustine DX, Malhotra A, Paton MF, et al. The athlete’s heart: insights from echocardiography. Echo Res Pract. (2023) 10(1):15. doi: 10.1186/s44156-023-00027-8
PubMed Abstract | Crossref Full Text | Google Scholar
10. Ma JZ, Dai J, Sun B, Ji P, Yang D, Zhang JN. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death in China. J Sci Med Sport. (2007) 10(4):227–33. doi: 10.1016/j.jsams.2006.07.001
PubMed Abstract | Crossref Full Text | Google Scholar
11. Kervio G, Pelliccia A, Nagashima J, Wilson MG, Gauthier J, Murayama M, et al. Alterations in echocardiographic and electrocardiographic features in Japanese professional soccer players: comparison to African-Caucasian ethnicities. Eur J Prev Cardiol. (2013) 20(5):880–8. doi: 10.1177/2047487312447905
PubMed Abstract | Crossref Full Text | Google Scholar
12. Nagashima J, Musha H, Takada H, Murayama M. New upper limit of physiologic cardiac hypertrophy in Japanese participants in the 100-km ultramarathon. J Am Coll Cardiol. (2003) 42(9):1617–23. doi: 10.1016/j.jacc.2003.06.005
PubMed Abstract | Crossref Full Text | Google Scholar
13. Giraldeau G, Kobayashi Y, Finocchiaro G, Wheeler M, Perez M, Kuznetsova T, et al. Gender differences in ventricular remodeling and function in college athletes, insights from lean body mass scaling and deformation imaging. Am J Cardiol. (2015) 116(10):1610–6. doi: 10.1016/j.amjcard.2015.08.026
PubMed Abstract | Crossref Full Text | Google Scholar
14. Pelliccia A, Borrazzo C, Maestrini V, D'Ascenzi F, Caselli S, Lemme E, et al. Determinants of lv mass in athletes: the impact of sport, constitutional traits and cardiovascular risk factors. Eur J Appl Physiol. (2023) 123(4):769–79. doi: 10.1007/s00421-022-05098-9
PubMed Abstract | Crossref Full Text | Google Scholar
15. Galanti G, Toncelli L, Tosi B, Orlandi M, Giannelli C, Stefani L, et al. Evaluation of left ventricular remodelling in young afro-Caribbean athletes. Cardiovasc Ultrasound. (2019) 17(1):20. doi: 10.1186/s12947-019-0169-8
PubMed Abstract | Crossref Full Text | Google Scholar
17. Lewis EJ, McKillop A, Banks L. The morganroth hypothesis revisited: endurance exercise elicits eccentric hypertrophy of the heart. J Physiol. (2012) 590(12):2833–4. doi: 10.1113/jphysiol.2011.226217
PubMed Abstract | Crossref Full Text | Google Scholar
18. Haykowsky MJ, Samuel TJ, Nelson MD, La Gerche A. Athlete’s heart: is the morganroth hypothesis obsolete? Heart Lung Circ. (2018) 27(9):1037–41. doi: 10.1016/j.hlc.2018.04.289
PubMed Abstract | Crossref Full Text | Google Scholar
19. Naylor LH, George K, O'Driscoll G, Green DJ. The athlete’s heart: a contemporary appraisal of the ‘morganroth hypothesis’. Sports Med. (2008) 38(1):69–90. doi: 10.2165/00007256-200838010-00006
PubMed Abstract | Crossref Full Text | Google Scholar
20. Doronina A, Edes IF, Ujvari A, Kantor Z, Lakatos BK, Tokodi M, et al. The female athlete’s heart: comparison of cardiac changes induced by different types of exercise training using 3D echocardiography. Biomed Res Int. (2018) 2018:1. doi: 10.1155/2018/3561962
PubMed Abstract | Crossref Full Text | Google Scholar
21. Bjerring AW, Landgraff HE, Stokke TM, Murbraech K, Leirstein S, Aaeng A, et al. The developing athlete’s heart: a cohort study in young athletes transitioning through adolescence. Eur J Prev Cardiol. (2019) 26(18):2001–8. doi: 10.1177/2047487319862061
PubMed Abstract | Crossref Full Text | Google Scholar
22. Milovancev A, Petrovic M, Miljkovic T, Ilic A, Mudrinic TR, Miljkovic A, et al. The elite judo female athlete’s heart. Front Physiol. (2022) 13:990142. doi: 10.3389/fphys.2022.990142
PubMed Abstract | Crossref Full Text | Google Scholar
23. D'Ascenzi F, Biella F, Lemme E, Maestrini V, Di Giacinto B, Pelliccia A. Female athlete’s heart. Circ Cardiovasc Imaging. (2020) 13(12):e011587. doi: 10.1161/CIRCIMAGING.120.011587
Crossref Full Text | Google Scholar
24. Lundin M, Heiberg E, Nordlund D, Gyllenhammar T, Steding-Ehrenborg K, Engblom H, et al. Prognostic utility and characterization of left ventricular hypertrophy using global thickness. Sci Rep. (2023) 13(1):22806. doi: 10.1038/s41598-023-48173-7
PubMed Abstract | Crossref Full Text | Google Scholar
25. Abergel E, Chatellier G, Hagege AA, Oblak A, Linhart A, Ducardonnet A, et al. Serial left ventricular adaptations in world-class professional cyclists. J Am Coll Cardiol. (2004) 44(1):144–9. doi: 10.1016/j.jacc.2004.02.057
PubMed Abstract | Crossref Full Text | Google Scholar
26. Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, et al. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol (1985). (2008) 104(4):1121–8. doi: 10.1152/japplphysiol.01170.2007
PubMed Abstract | Crossref Full Text | Google Scholar
27. Hedman K, Tamas E, Henriksson J, Bjarnegard N, Brudin L, Nylander E. Female athlete’s heart: systolic and diastolic function related to circulatory dimensions. Scand J Med Sci Sports. (2015) 25(3):372–81. doi: 10.1111/sms.12246
PubMed Abstract | Crossref Full Text | Google Scholar
28. Rodrigues AC, de Melo Costa J, Alves GB, Ferreira da Silva D, Picard MH, Andrade JL, et al. Left ventricular function after exercise training in young men. Am J Cardiol. (2006) 97(7):1089–92. doi: 10.1016/j.amjcard.2005.10.055
PubMed Abstract | Crossref Full Text | Google Scholar
29. D’Andrea A, Palermi S, Dweck M, La Gerche A, Cameli M. Chapter 4—echocardiogram in athlete’s heart. In: D’Andrea A, Bossone E, editors. Athlete’s Heart. Academic Press (2023). p. 77–101. doi: 10.1016/B978-0-323-95221-7.00006-9
Crossref Full Text | Google Scholar
30. Brown B, Somauroo J, Green DJ, Wilson M, Drezner J, George K, et al. The complex phenotype of the athlete’s heart: implications for preparticipation screening. Exerc Sport Sci Rev. (2017) 45(2):96–104. doi: 10.1249/JES.0000000000000102
PubMed Abstract | Crossref Full Text | Google Scholar
31. George KP, Naylor LH, Whyte GP, Shave RE, Oxborough D, Green DJ. Diastolic function in healthy humans: non-invasive assessment and the impact of acute and chronic exercise. Eur J Appl Physiol. (2010) 108(1):1–14. doi: 10.1007/s00421-009-1233-0
PubMed Abstract | Crossref Full Text | Google Scholar
32. Brown B, Millar L, Somauroo J, George K, Sharma S, La Gerche A, et al. Left ventricular remodeling in elite and sub-elite road cyclists. Scand J Med Sci Sports. (2020) 30(7):1132–9. doi: 10.1111/sms.13656
PubMed Abstract | Crossref Full Text | Google Scholar
33. Kleinnibbelink G, Panhuyzen-Goedkoop N, Hulshof H, van Dijk A, George K, Somauroo J, et al. Exercise training induces left- but not right-sided cardiac remodelling in Olympic rowers. Int J Sports Med. (2022) 43(2):151–60. doi: 10.1055/a-1524-2611
留言 (0)