Endometrial cancer (EC) is the most prevalent gynecological malignancy of the female reproductive system (1, 2). The incidence rate of EC is increasing annually, and due to lifestyle changes, its population becomes progressively younger. The average age at diagnosis for EC patients is 61 years, with postmenopausal vaginal bleeding being the most common clinical presentation, accelerating the early diagnosis of EC (3). However, the presence of atypical symptoms such as menstrual irregularities could mask the condition, making early diagnosis challenging for young EC patients.
Early-onset EC refers to individuals under 50 years diagnosed with EC (4, 5). Previous studies have shown that compared to late-onset EC (age ≥ 50 years), patients with early-onset EC have better tumor differentiation and prognosis (6). However, recurrence remains a leading cause of reduction in survival rates among this specific group, with a five-year overall survival from 15% to 17% (7). This highlights the imminent need to develop novel assessment modalities to predict recurrence of EC and improve the prognosis outcomes for this population of patients. Recently, the Cancer Genome Atlas (TCGA) Research Project unveiled a genomic reclassification of EC with four distinct subtypes: DNA polymerase epsilon catalytic subunit (POLE) ultramutated, microsatellite instability (MSI), copy-number low (CNL) and cope-number high (CNH) (7, 8). The molecular subtype demonstrates good prognostic value for EC patients, but its high cost of detection and demanding requirements for equipment and detection technology levels make it challenging to be widely promoted and applied in many regions and countries in a short period of time. Immunohistochemistry remains a fast, cost-effective, and reliable postoperative assessment method, allowing it to be easily integrated into the clinical management of EC and widely used in clinical practice.
Currently, the management of young patients, especially the risk assessment of recurrence in early-onset EC patients, is a contentious issue. Therefore, a comprehensive consideration of clinical features and immunohistochemical markers to understand the main factors influencing the recurrence-free survival (RFS) of early-onset EC patient could help to predict, prevent recurrence, or at least improve therapy.
Herein, we analyzed 628 early-onset EC patients from multiple centers to identify independent prognosis factors associated with RFS. Subsequently, we established a nomogram model to accurately predict RFS, allowing patients to receive earlier prognosis information, and facilitating optimization of treatment planning.
2 Materials and methods2.1 Patients and databaseThis retrospective large-sample multicenter study included the First Affiliated Hospital of Chongqing Medical University (FAHCQMU, n = 458 cases), Women and Children’s Hospital of Chongqing Medical University (WCHCQMU, n = 140 cases), and Chongqing Yubei District Maternal and Child Health Center (CYMCHC, n = 30 cases). A total of 628 patients who diagnosed with early-onset EC in three medical centers from October 20, 2014 to May 20, 2021 were included.
The inclusion criteria were as follows: (1) primary EC patients with age less than 50 years; (2) no other malignant tumors; (3) comprehensive clinical and postoperative pathological information. The criteria for exclusion were as follows: (1) patients with age ≥ 50 years old; (2) administration of preoperative adjuvant therapy; (3) incomplete medical records; (4) no standard surgical treatment; (5) loss to follow-up after surgery. The schematic representation of the study flow is illustrated in Figure 1.
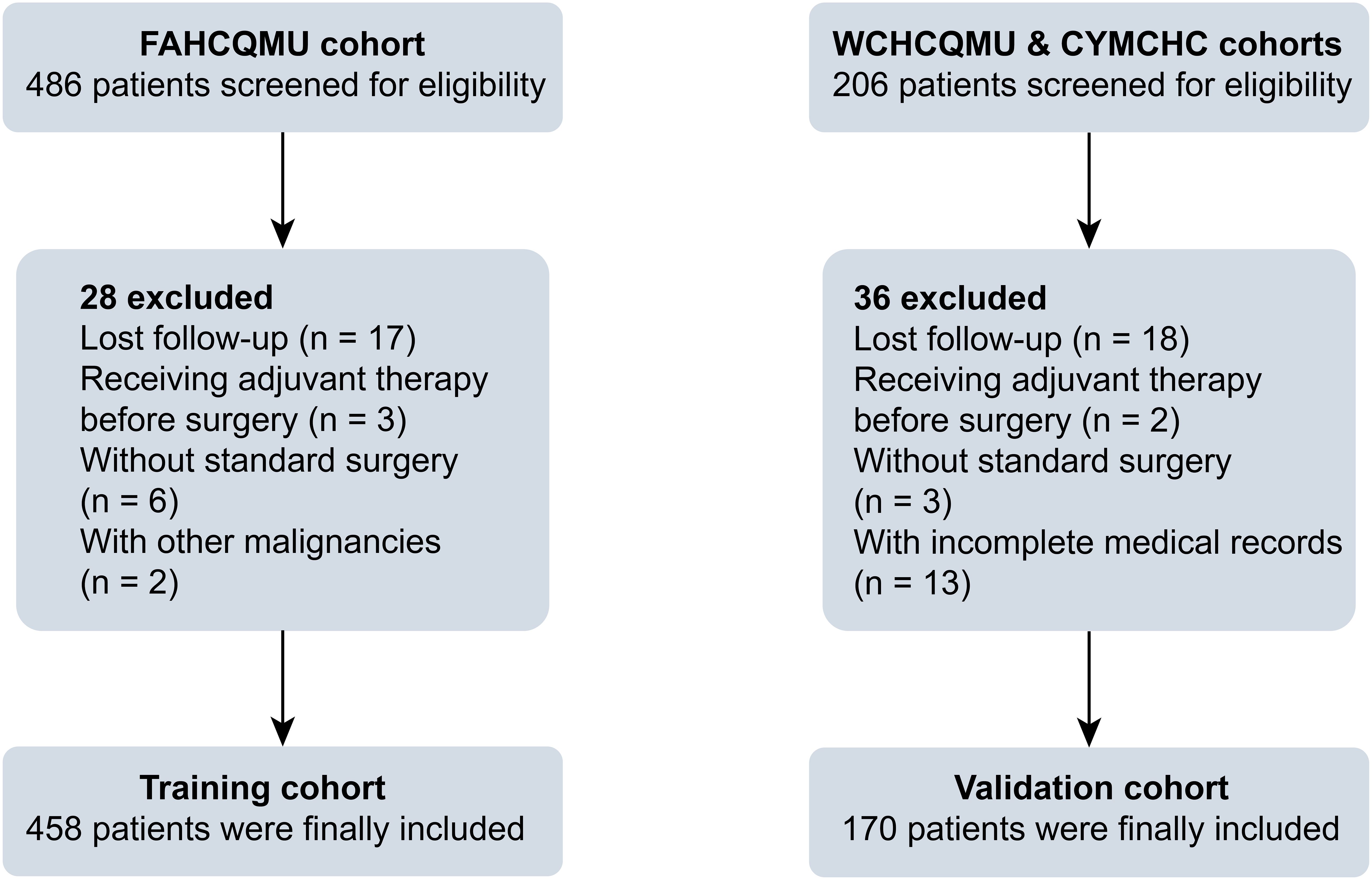
Figure 1. Flow chart for early-onset EC patient inclusion.
2.2 Surgical procedures and postoperative adjuvant treatmentAll patients underwent a surgical staging procedure, including total hysterectomy, bilateral salpingo-oophorectomy, with or without lymph node staging (including sentinel lymph node ± pelvic lymph node ± para-aortic lymphadenectomy) (2, 9). After comprehensive surgery and pathological staging, EC patients will be advised to consider observation or various postoperative adjuvant treatments based on international guidelines and the assessment of risk factors (10, 11). When molecular classification is known, specific recommendations exist for POLE mutations (POLEmut) and P53 abnormal (P53abn) (see ESGO/ESTRO/ESP Guidelines) (12). Radiotherapy mainly consisted of vaginal brachytherapy (total radiation dose ranges from 22 to 24 Gy, administered in 4 fractions of 5.5-6 Gy, 2 fractions per week) or pelvic external beam radiotherapy total radiation dose ranges from 45 to 50 Gy, administered in 25 fractions of 1.8-2 Gy, 5 fractions per week), administered within 12 weeks post-operative. The chemotherapy regimen is mainly based on the TP regimen (carboplatin combined with paclitaxel) administered every 3 weeks for 6-8 cycles (13).
2.3 Patients characteristics collection and follow-up investigationClinical pathological and follow-up data were collected by well-trained assistants and recorded using standardized data collection forms, and reviewed by a senior doctor. Specifically, clinicopathological data and surgical details were meticulously collected from medical records, including age at diagnosis, body mass index (BMI), International Federation of Gynecology and Obstetrics (FIGO) stage, histological subtype (categorized as type I or type II), depth of myometrial invasion (categorized as <1/2 or ≥1/2), cervical stromal invasion, lymphovascular space invasion (LVSI, categorized as none, focal, or substantial LVSI) (Supplementary Table S1) (14, 15). In the present study, the optimal age cut-off point of early-onset EC was determined based on RFS using X-tile software (Supplementary Figure S1) (16). The follow-up was performed every 3 months for the first 2 years, every 6 months for the next 3-5 years, and once per year thereafter.
2.4 RecurrenceRecurrences were diagnosed by physical examination and/or imaging, and were confirmed histologically. RFS was calculated as the duration from the initial surgery to the confirmation of the first recurrence or the last follow-up date, with the follow-up deadline scheduled for July 19, 2024.
2.5 Histology, MMR and P53 immunohistochemistryImmunohistochemistry (IHC) was performed on formalin-fixed paraffin-embedded specimens. All pathology slides underwent analysis by experienced pathologists. P53 IHC results were categorized as normal (1-80% of tumor cell nuclei staining positive), or abnormal (no tumor cell nuclear staining; at least 80% of tumor cell nuclei staining positive) (17). The loss of nuclear expression of at least one mismatch repair (MMR) proteins (MLH1, MSH2, MSH6, and PMS2) was defined as MMR-deficiency (dMMR); and positive nuclear staining of all four MMR proteins was defined as proficient MMR (pMMR) (18–20). Representative images of IHC staining for P53 and MMR proteins in early-onset EC tissues are shown in Figure 2.
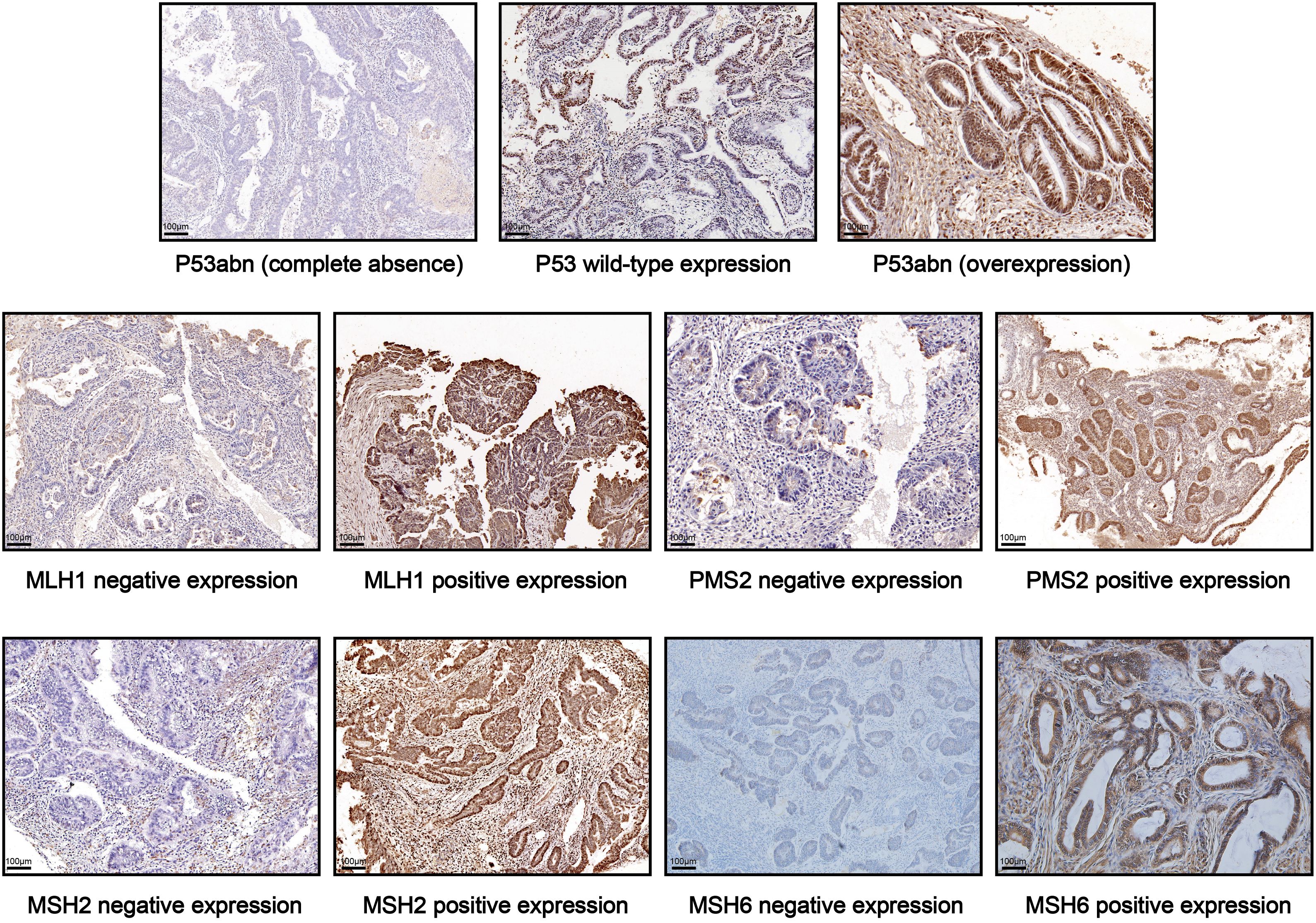
Figure 2. Representative IHC images of P53 and MMR protein expression in EC tissues.
2.6 Statistical analysisStatistical analysis was performed using R software (version 4.0.3) and SPSS software (version 22.0). Categorical data were presented as frequencies with percentages, and statistical comparison for categorical variables were calculated using the χ2 or Fisher exact test. Continuous variables were reported as means with standard deviations, and compared using a two-tailed Student’s t test (21). RFS probabilities were estimated using the Kaplan-Meier curves, and group comparisons were analyzed by the log-rank test. Patients from FAHCQMU cohort were included in developing the nomogram model, while patients from WCHCQMU & CYMCHC cohorts were enrolled as the external validation cohort. X-tile software (version 3.6.1) was utilized to determine the diagnostic thresholds for nomogram scores. Subsequently, patients were divided into low- and high-risk groups based on the risk score threshold (16). P-values < 0.05 were considered as statistically significant.
3 Results3.1 Demographic and clinical characteristicsA total of 628 young women diagnosed with EC (age less than 50 years) from three major medical institutions were included in this study. More than one-half (524/628, 83.4%) of patients were diagnosed with EC in the age range of 40-49 years, and majority of early-onset EC patients were diagnosed between the ages of 45 and 49 years (368/628, 58.6%, Supplementary Figure S2). The clinicopathological features of the training cohort (FAHCQMU cohort, n = 458), and the validation cohort (WCHCQMU & CYMCHC cohorts, n = 170) are shown in Table 1. The mean age of onset for early-onset EC was 44.1 ± 5.0 years, and most of the uterine tumors found were histological type I (76.1%), FIGO I (69.3%), and LVSI negative (77.4%). IHC analysis highlighted abnormal P53 expression and dMMR status in 145/628 (23.1%) and 172/628 (27.4%) cases, respectively. Additionally, 10.8% of the cases experienced relapse during the follow-up period. The distribution of clinicopathological characteristics among early-onset EC in training cohort was similar to that of validation cohort (P > 0.05).
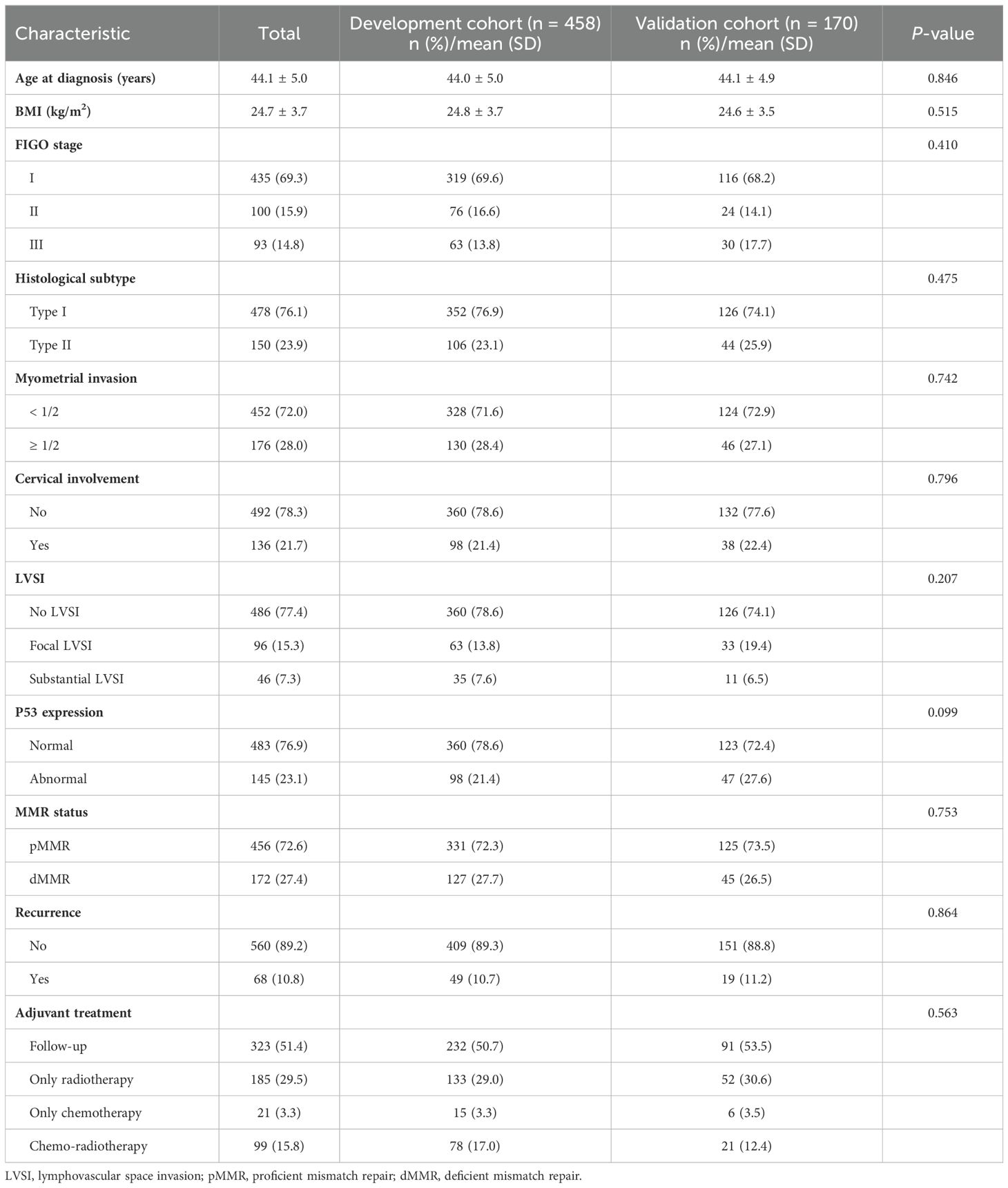
Table 1. Demographics and clinical characteristics of the study population.
In the training cohort, a total of 49 patients relapsed, with a median RFS of 51.0 months (range, 3-91); while in the validation cohort, 19 patients relapsed, with a median RFS of 53.0 months (range, 4-90). The recurrence sites were similar between the two cohorts (P > 0.05). Detailed information on recurrence characteristics was summarized in Table 2.
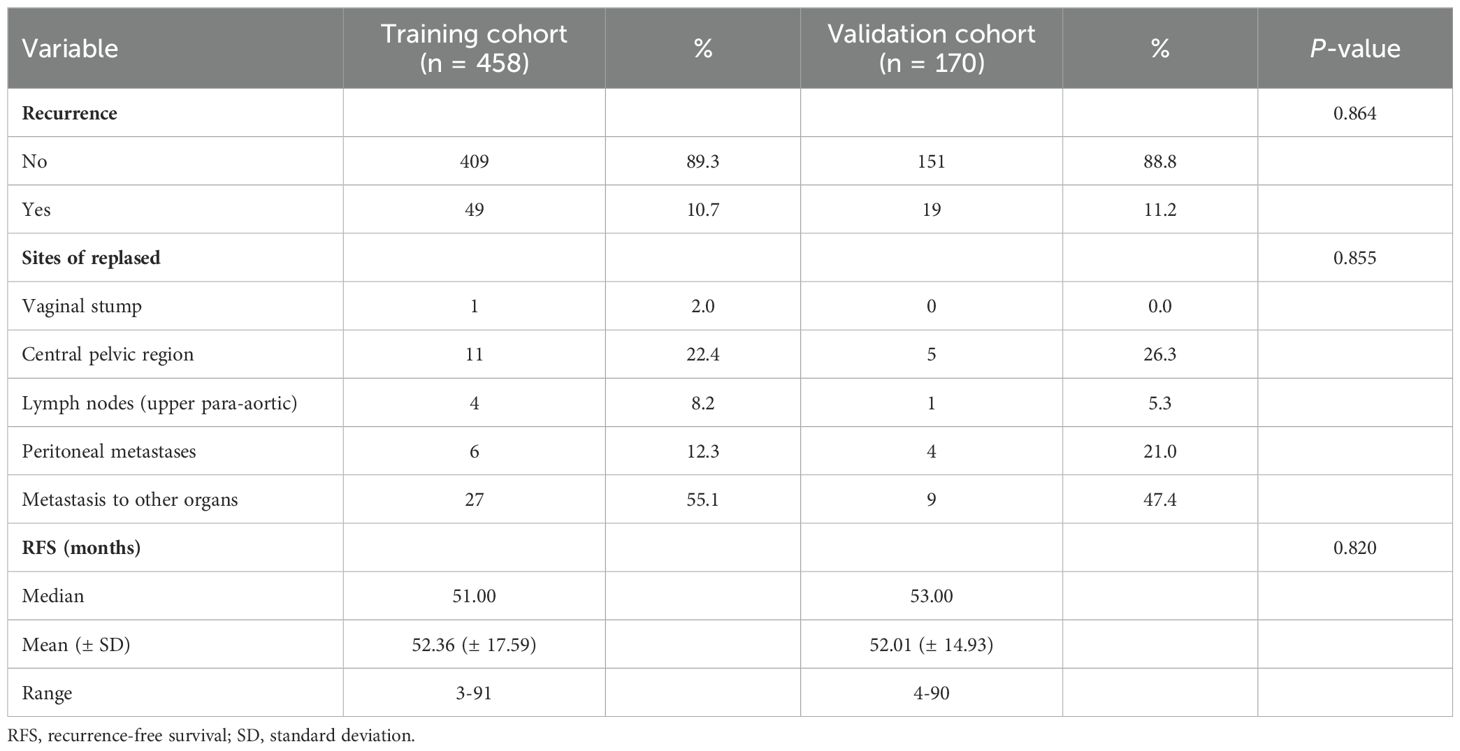
Table 2. Recurrence characteristics of early-onset EC.
3.2 Independent prognostic factors of RFSWithin the training cohort, both clinicopathologic features and immunohistochemical markers were included in the univariate and multivariate analysis. Multivariate Cox analysis showed that histological type II (HR 2.122, 95% CI 1.119-4.022, P = 0.021), FIGO stage III (HR 3.088, 95% CI 1.469-6.491, P = 0.003), deep myometrial invasion (HR 2.063, 95% CI 1.036-4.108, P = 0.039), substantial LVSI (HR 2.591, 95% CI 1.291-5.198, P = 0.007), and abnormal P53 expression (HR 3.350, 95% CI 1.848-6.073, P < 0.001), dMMR status (HR 1.863, 95% CI 1.026-3.382, P = 0.041) were identified as independent risk factors for RFS in patients with early-onset EC (Table 3).
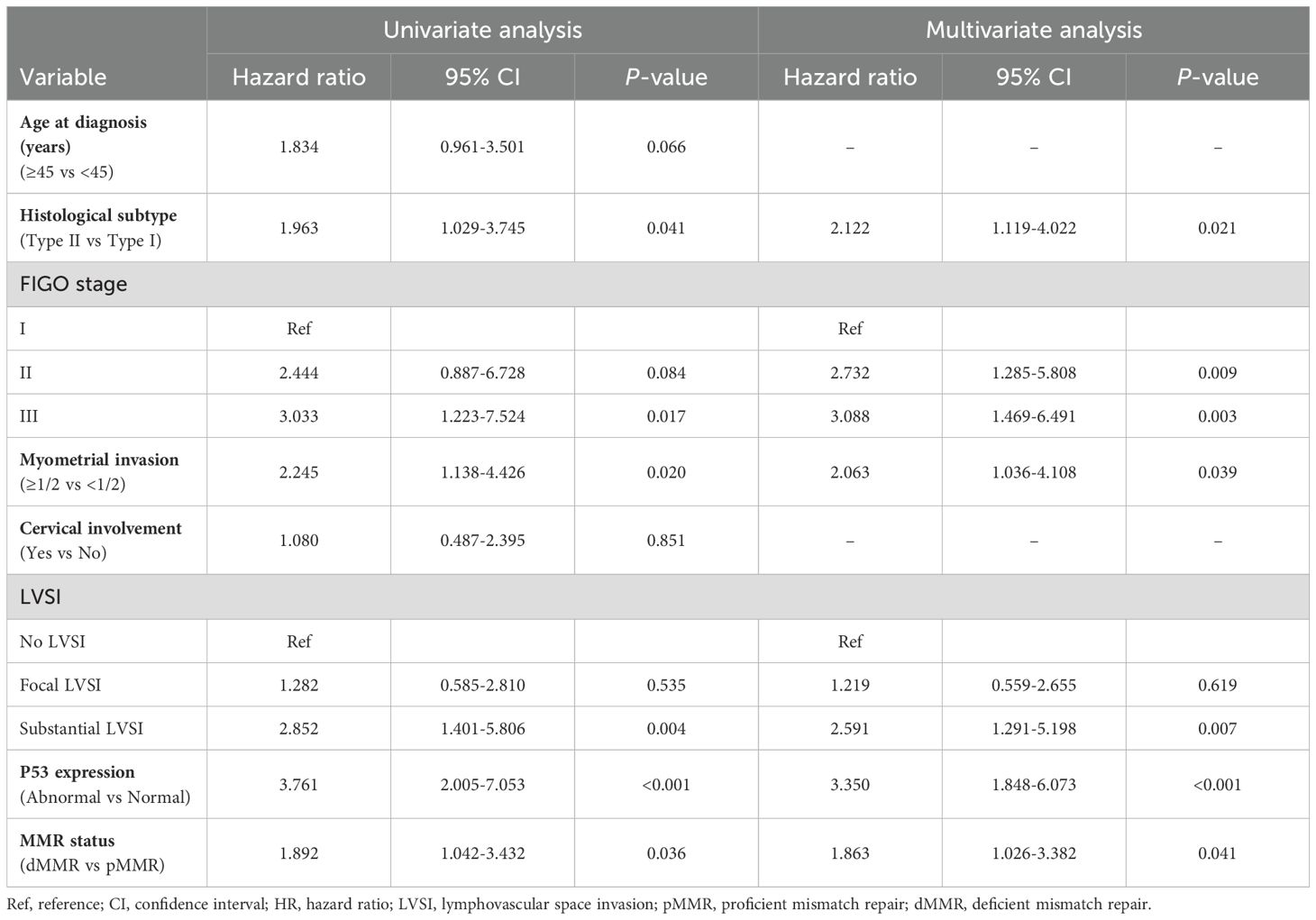
Table 3. Factors predicting the RFS of early-onset EC by univariate and multivariate Cox regression analysis.
3.3 Establishing and validation of the nomogramBased on the multivariate Cox regression analysis, we constructed a nomogram model for personalized prediction of RFS by calculating each patient’s weighted score (Figure 3). According to the degree of contribution of each predictor to the resulting events (RFS), the corresponding points (the first axis) were obtained. Then, the points of each predictor were summed to predict the 3- and 5-year RFS probability of early-onset EC patients. The C-index for nomogram of RFS was 0.844 (95% CI 0.795-0.893) in the training cohort and 0.876 (95% CI 0.807-0.945) in the validation cohort, outperforming the use of clinical parameters and immunohistochemical markers individually (Supplementary Table S2). In addition, the ROC curve was utilized to calculate the AUC of the nomogram, revealing that AUC values of both the training cohort and validation cohort were greater than 0.85 (Figure 4). The calibration curves revealed an excellent coherence between predicted 3- and 5-year RFS rates and actual RFS rates, indicating the predictive accuracy of the nomogram model (Figure 5).
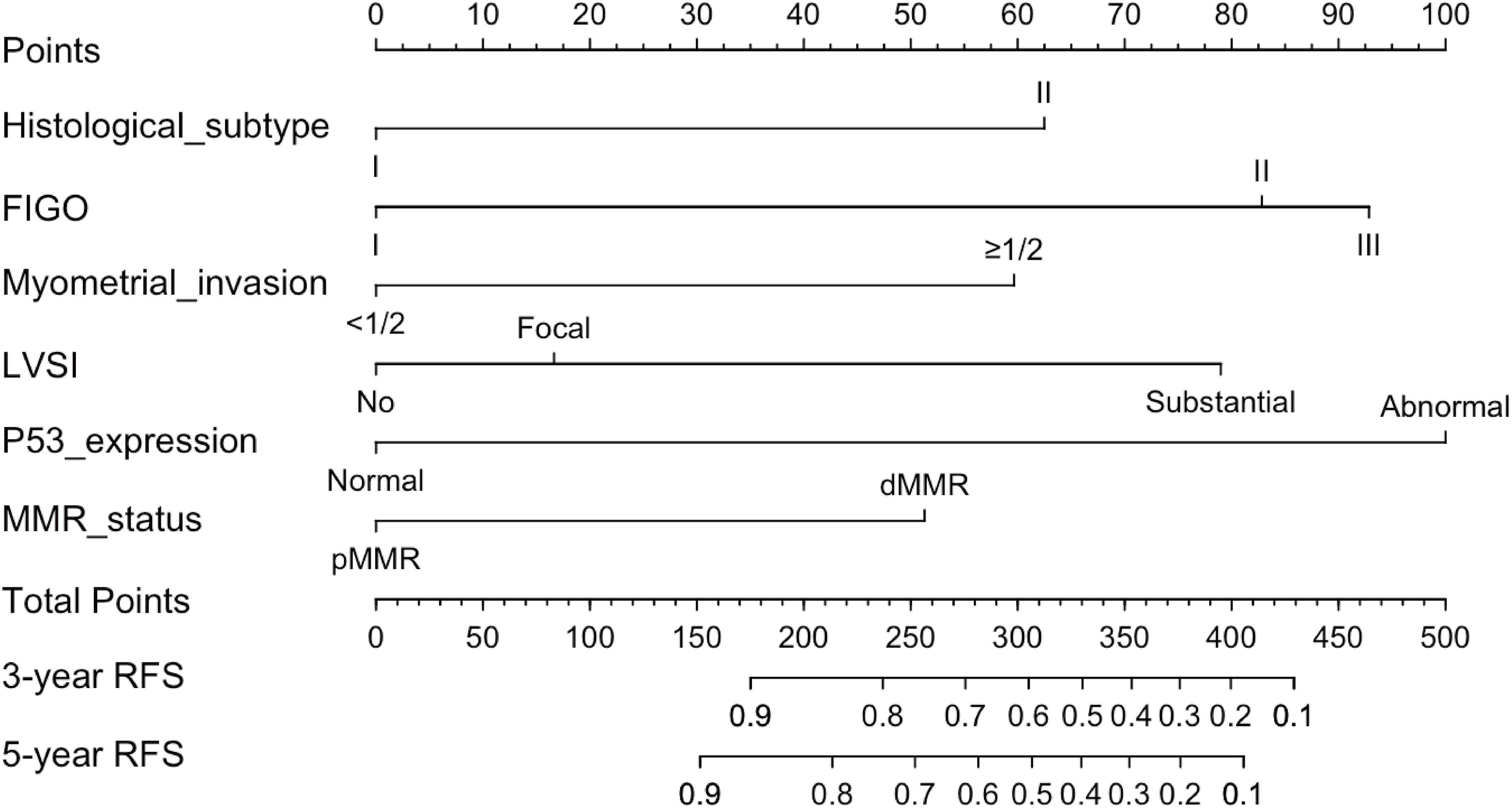
Figure 3. The nomogram was established to predict the 3-, and 5-year RFS of early-onset EC after hysterectomy.
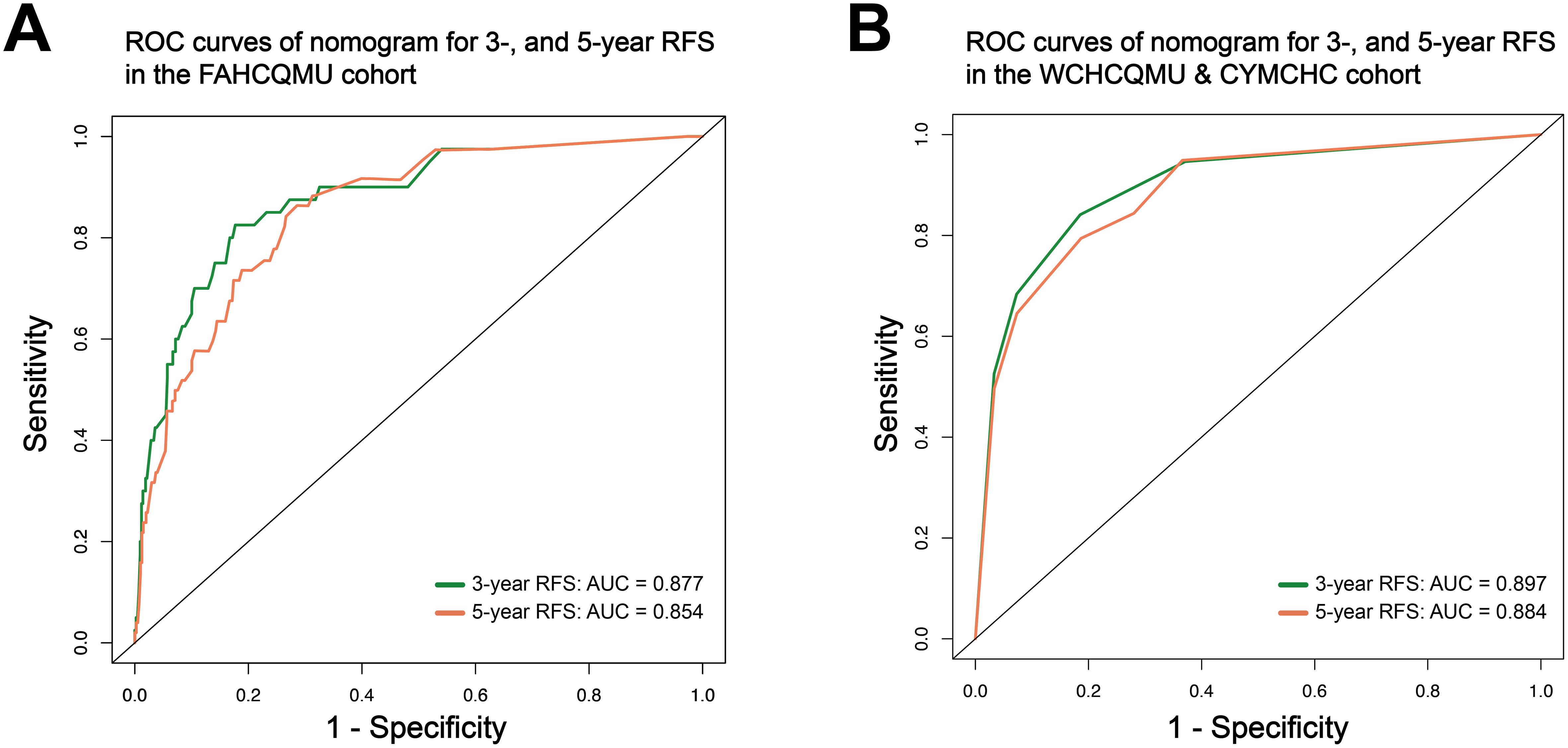
Figure 4. The ROC curve of the nomogram to predict 3-, and 5-year RFS rates in the training cohort (A); and in the validation cohort (B).
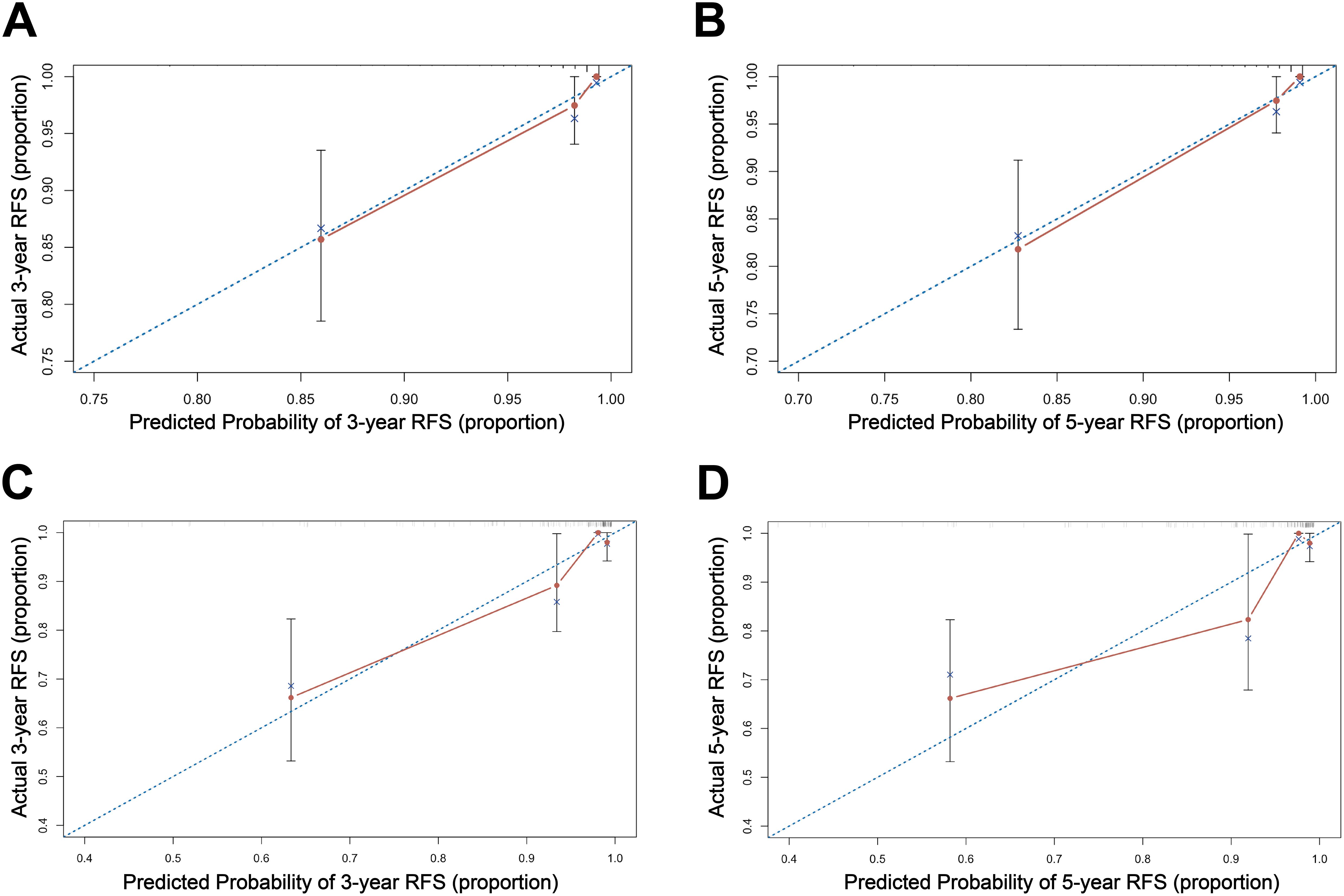
Figure 5. The calibration curve of the nomogram for 3-, and 5-year RFS in the training cohort (A, B); and in the validation cohort (C, D).
3.4 Influence of various variables on the RFS of early-onset EC patientsKaplan-Meier survival analysis was used to evaluate the RFS among early-onset EC patients in the training set. Young EC patients with histological type II, FIGO stage III, and deep myometrial invasion had a worse RFS. Additionally, in young patients, postoperative pathology showing substantial LVSI, abnormal P53 expression and dMMR status indicated a higher probability of postoperative recurrence and shorter RFS (P < 0.001, Figure 6).
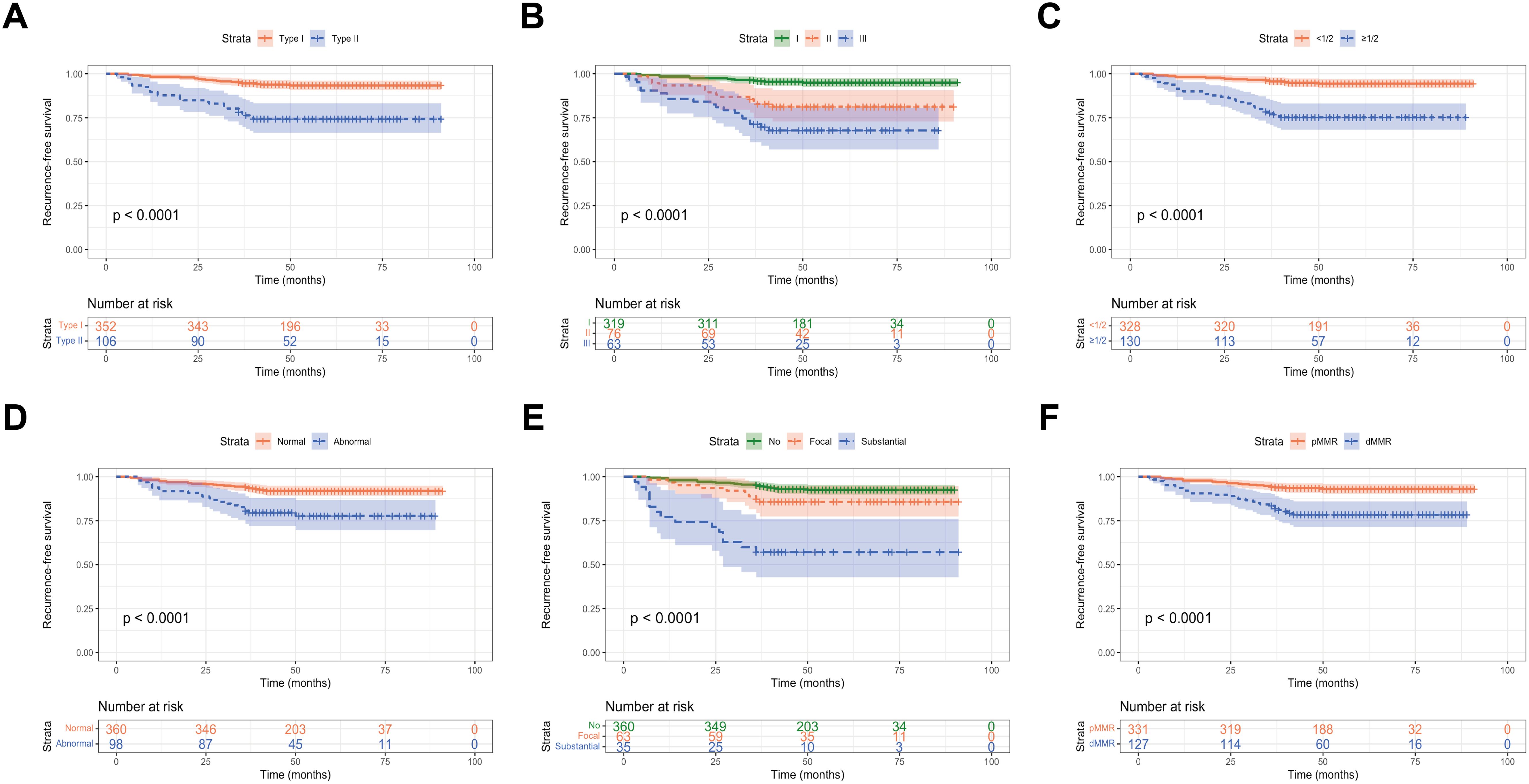
Figure 6. RFS of early-onset EC according to (A) histological subtype; (B) FIGO stage; (C) myometrial invasion; (D) P53 expression; (E) LVSI; and (F) MMR expression.
3.5 Performance of the nomogram in stratifying riskIn order to further validate our prediction model, patients from the training cohort and validation cohort were stratified into low- and high-risk groups based on the nomogram-generated scores (NGS) for RFS. The cut-off value of 249.1 was determined using X-tile (Supplementary Figure S3). Kaplan-Meier curves indicated that patients classified as high-risk had significantly worse RFS and OS outcomes compared to those in the low-risk group (P < 0.001, Figure 7). Furthermore, the 3- and 5-year RFS and OS rates of patients in the high-risk group were much lower than those in the low-risk group in both cohorts (P < 0.001, Table 4).

Figure 7. Kaplan-Meier curves of the low-risk groups and high-risk group. (A, B) RFS curves of patients with early-onset EC stratified by the risk scores in the training cohort and external validation cohorts; (C, D) OS curves of patients with early-onset EC stratified by the risk scores in the training cohort and external validation cohorts.
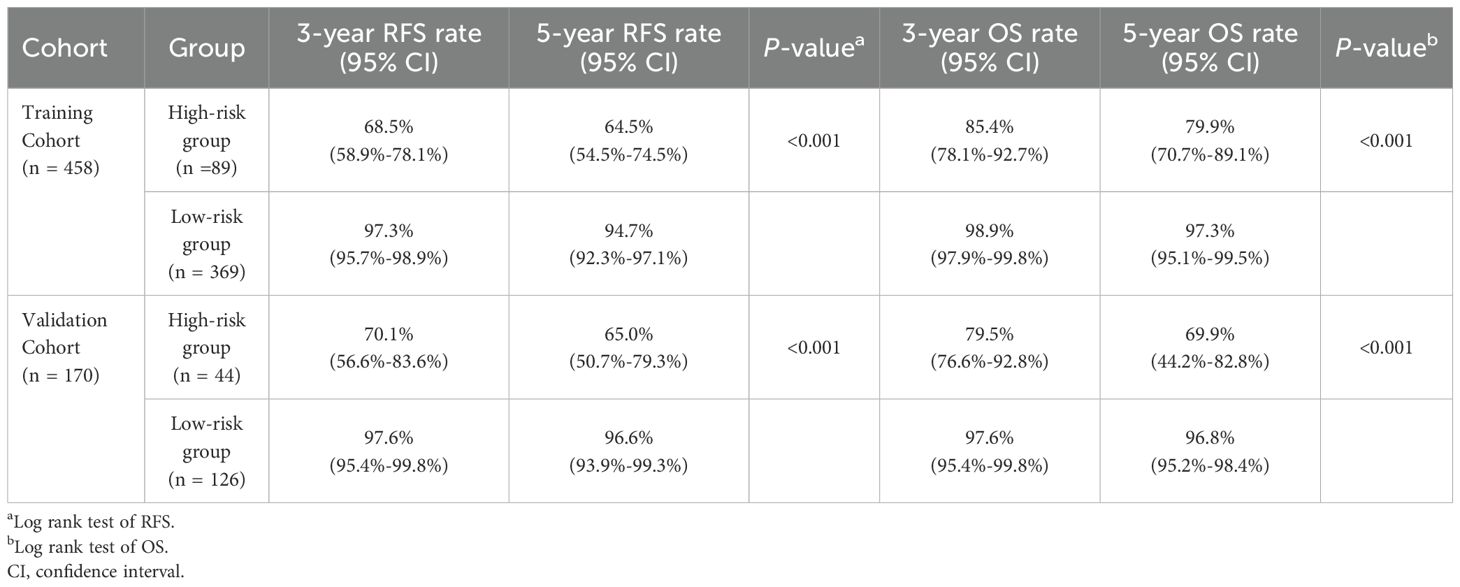
Table 4. Analysis of survival differences between high- and low-risk groups in training cohort and validation cohort.
To further assess the potential of risk stratification in guiding postoperative adjuvant therapy, we compared the two groups to identify individuals who could benefit from adjuvant therapy. In the low-risk group, there was no significant difference in RFS and OS between patients who received adjuvant therapy and those who did not (P > 0.05, Supplementary Figure S4). Among patients in the high-risk group, to our surprise, individuals who received postoperative adjuvant therapy had significantly better RFS and OS than those who received no adjuvant therapy (P < 0.05, Figure 8).
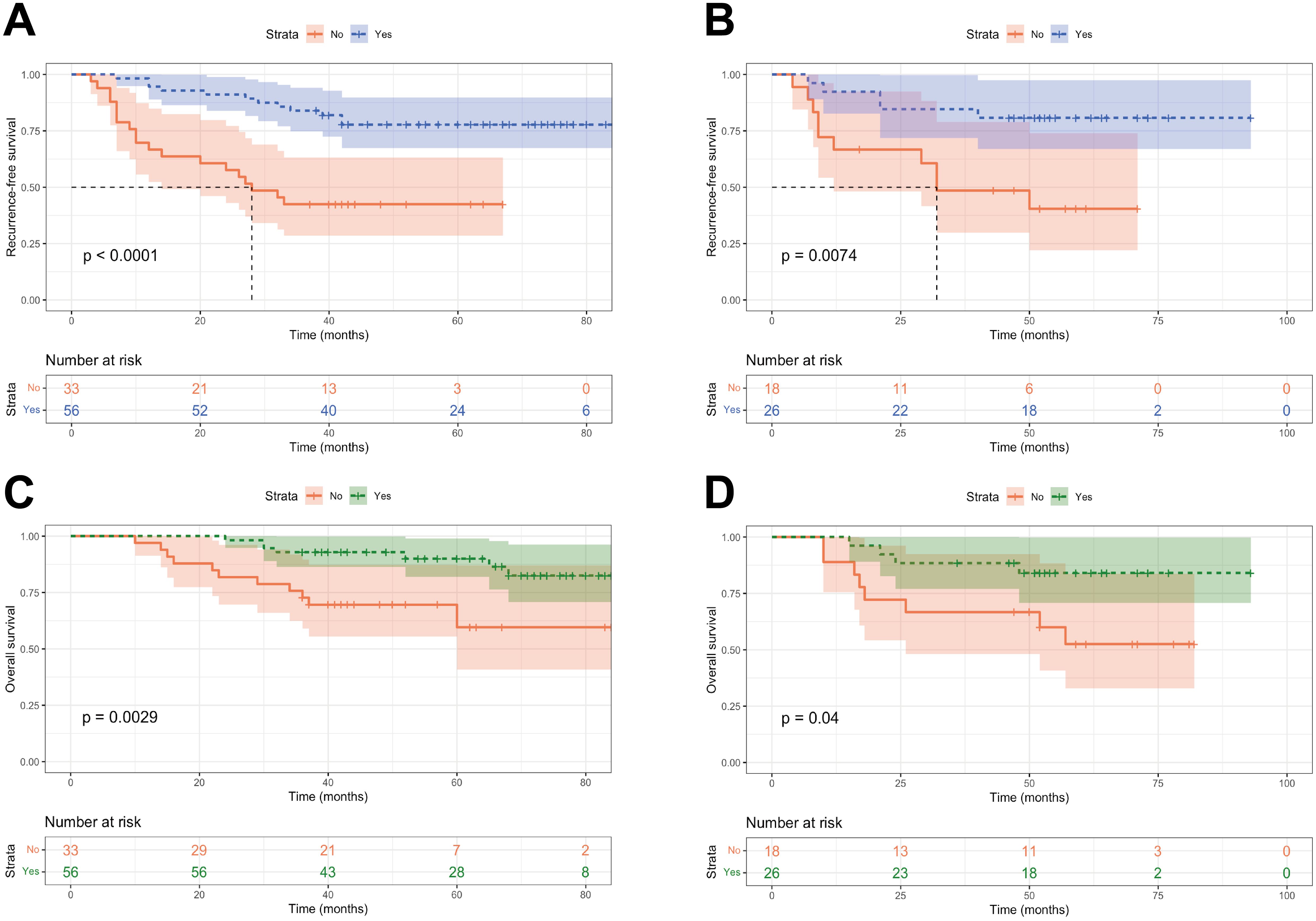
Figure 8. Kaplan-Meier curves of patients with or without adjuvant treatment in high-risk group. (A, B) RFS curves of patients with or without adjuvant treatment in high-risk group in the training and validation cohorts; (C, D) OS curves of patients with or without adjuvant treatment in high-risk group in the training and validation cohorts.
4 DiscussionThe increasing prevalence of early-onset EC has resulted in the development of a distinct and unique subset among EC patients, characterized by differences in disease-free survival rates and tumor characteristics (22, 23). Our previous studies have compared the RFS of young EC patients who underwent various fertility-sparing treatments and hysterectomy, showing that surgical intervention could improve the prognosis of early-onset EC patients. Consequently, the present study focuses primarily on early-onset EC patients receiving surgical treatment (Supplementary Table S3; Supplementary Figures S5, S6). In our study, the initial diagnosis age of early-onset EC is concentrated between 45 and 49 years. Therefore, appropriately screening EC patients aged 45-49 years may help in the early detection and diagnosis of EC in individuals. Although previous studies have indicated a favorable prognosis for early-onset EC, our research still reports a 10.8% postoperative recurrence rate in early-onset EC patients. Therefore, accurate and effective prediction of recurrence and prognosis in this subgroup contributes to guiding clinical practice and treatment decisions.
In this study, we identified six independent risk factors for predicting RFS of early-onset EC patients. These factors include histological subtype, FIGO stage, myometrial invasion, LVSI, P53 expression, and MMR expression. Based on these clinical parameters and immunohistochemical markers, we developed a robust nomogram to predict the 3- and 5-year RFS rates of early-onset EC patients. By utilizing weighted scores, early-onset EC patients can be divided into high- and low-risk group, providing accurate RFS for each patient and guiding clinical treatment.
Histological subtypes are one of the key features of the revised 2023 FIGO staging, which includes two different types. Type I is composed of low-grade (grades 1 and 2) endometrioid adenocarcinoma (EEC), resulting from atypical hyperplasia due to chronic estrogen stimulation, and typically exhibit non-aggressive characteristics. Type II comprises high-grade (grade 3) EEC and non-endometrioid cancers, which often have a worse prognosis (2). Given the poor prognosis of advanced EEC and non-endometrioid EC, as well as the significant association between histological subtypes and recurrence in EC patients, we incorporated histological subtypes as a variable in this study. Through multivariable Cox regression analysis, histological type II was identified as an independent risk factor for early-onset EC. Additionally, early-onset EC patients with high-grade EEC or non-endometrioid cancer have a poorer RFS compared to low-grade EEC. Recent studies suggested different histological subtypes have distinct molecular characteristics and precursor lesions, playing essential role in risk stratification of EC, which is consistent with our research (24).
In this study, we demonstrated that LVSI is a significant prognostic variable, and based on NGS, LVSI is identified as one of the primary factors influencing RFS in early-onset EC. Previous studies have confirmed that LVSI is an independent negative prognostic factor for recurrence, which is consistent with our findings (25). A pooled analysis of the PORTEC 1 and 2 trials revealed that substantial LVSI is the strongest predictor for pelvic regional recurrence and distant metastasis, indicating that early-stage patients with substantial LVSI should consider receiving adjuvant EBRT and/or chemotherapy (15). However, most studies have explored the impact of LVSI on recurrence and survival prognosis in EC patients. In comparison to these studies, this research focuses on the significant role of LVSI in recurrence among early-onset EC patients. Our study also indicates that early-onset EC patients with substantial LVSI have shorter RFS and worse prognosis compared to no/focal LVSI patients. Furthermore, our study demonstrates that myometrial invasion ≥ 50% is another independent risk factor for recurrence in early-onset EC. For the assessment of prognosis in early-onset EC, account needs to be taken of deep myometrial invasion. Therefore, combining histological subtypes, FIGO staging, myometrial invasion, and LVSI can significantly enhance the predictive ability of RFS in early-onset EC patients.
The clinical management of high-risk and advanced EC is transitioning from histological subtype to molecular classifications. However, the high cost and technical requirements of genetic testing limit its widespread use as a routine diagnostic tool in many developing regions. By identifying the molecular characteristics of tumors, immunohistochemistry can serve as a good transition and complement to genetic testing, providing clinicians with a more comprehensive understanding of the tumor. Current studies have not specifically reported on the impact of immunohistochemical markers on the recurrence of early-onset EC patients. In fact, relying solely on clinical features cannot effectively assess the prognosis of early-onset EC patients. Therefore, further incorporating immunohistochemical molecular markers for a comprehensive evaluation of the prognosis of early-onset EC patients is of significant importance. Our study analyzed commonly used immunohistochemical markers and ultimately identified abnormal P53 expression and complete loss of MMR protein expression as risk factors for recurrence in early-onset EC patients. MMR deficiency or MSI is common in EC, affecting 20% to 40% of patients, and impacting risk stratification, treatment decisions, and Lynch syndrome (LS) screening (26–28). With increasing emphasis on molecular classification in EC, MMR status has emerged as a crucial biomarker in ongoing clinical trials (GINECOEN105b, ClinicalTrials.gov identifier, NCT05201547; RAINBO, ClinicalTrials.gov identifier NCT05255653). While the value of MMR status in assessing recurrence and prognosis in EC patients remains controversial, our study indicated that MMR status is one of the independent predictors of recurrence in early-onset EC patients. In addition to the potential value of MMR status in refining risk stratification, this result may be partly related to the higher proportion of LS carriers among early-onset EC patients. Therefore, screening for LS in young populations or specific high-risk groups is of paramount importance (29, 30). The aim of screening is to detect atypical hyperplasia or EC at the earliest stage to improve chances of cure and minimize treatment-related morbidity. Reducing mortality from this disease and enhancing cancer screening and prevention among family members is challenging and requires further high-quality research. Aberrant P53 protein expression was typically associated with aggressive histology and clinical course (31, 32). For clinical outcomes prediction, a previous study showed EC patients with TP53 missense mutations and P53 protein over-expression exhibited comparable impacts on progression-free survival and overall survival. Furthermore, the concordance between TP53 next-generation sequencing (NGS) and P53 IHC was 88%; this percentage increased to 92% when cases with TP53 mutations accompanied by POLEmut or dMMR were excluded (17).
Noteworthy, factors such as age and cervical stromal invasion were not identified as predictors for RFS. This could be explained by the “collinearity” among different risk factors and the specific population included in present study. Hence, we established a nomogram based on above six readily available variables to assist clinicians in assessing patients’ RFS. Additionally, we further stratified early-onset EC patients based on NGS for risk assessment. It is worth noting that adjuvant therapy reduced loco-regional recurrence in high-risk cases and demonstrated significantly better RFS and OS compared to those who received no adjuvant therapy. In the low-risk group, RFS and OS of patients who received postoperative adjuvant therapy were comparable to those who did not, suggesting that patients in the high-risk group may require more proactive postoperative management than those in the low-risk group. Therefore, risk stratification for early-onset EC patients is essential for the risk-benefit analysis of adjuvant therapy in the context of patient individualization. The nomogram integrating multiple clinical variables and immunohistochemical molecular markers demonstrates superior predictive performance in assessing the RFS of early-onset EC patients compared to using clinical variables and immunohistochemical markers separately.
In light of the above, there remains a significant opportunity and unmet need for novel diagnosis approaches or prediction models in early-onset EC to improve their prognosis. To our knowledge, this is the first multicenter study that integrates clinicopathological parameters and immunohistochemical markers to analyze risk factors for RFS in early-onset EC patients and develop a predictive model, although there are still limitations. One limitation of our study is the inability to include POLE mutation status in the analysis due to small number of such cases. This resulted in a small portion of patients harboring multiple molecular subtypes, such as POLEmut and P53abn, being classified as the P53abn subtype, potentially contributing to an overestimation of the risk of recurrence. In fact, previous studies have shown that patients with POLEmut accounted for a relatively low proportion (about 5-8%) among the TCGA molecular subtypes of all EC patients, while the proportion of patients harboring multiple molecular subtypes simultaneously is even lower, approximately 3%-5% (33–35). Therefore, the model established in this study overestimates the risk of recurrence in high-risk patients to a relatively low extent. Furthermore, another limitation of this study is its reliance on retrospective analysis. Due to the early diagnosis and low post-surgery recurrence rate of most early-onset EC patients, the study included a small number of recurrent cases relative to the prognostic factors involved in the multivariate analysis, which may cause statistical bias to a certain extent. Therefore, necessitating further validation of the universality of this model through further prospective clinical trials.
5 ConclusionIn summary, this study developed and verified a highly accurate nomogram for the prediction of RFS in early-onset EC patients after surgery. This novel risk stratification system can serve as an important supplement to FIGO staging and molecular subtyping in predicting RFS of early-onset EC patients, providing personalized treatment guidance for this specific subgroup and improving clinical decision-making.
Data availability statementThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to Rui Yuan, yrui96@hospital.cqmu.edu.cn.
Ethics statementThe studies involving humans were approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of Chongqing Medical University (IRB number: K2024-137-01) & the Women and Children’s Hospital of Chongqing Medical University (IRB number: 2023-002) & Chongqing Yubei Maternity & Child Healthcare Hospital (IRB number: 2024-K21-1). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsYZ: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. QS: Data curation, Writing – original draft, Writing – review & editing. FY: Data curation, Formal analysis, Writing – review & editing. JW: Data curation, Formal analysis, Writing – review & editing. QZ: Data curation, Supervision, Writing – review & editing. RH: Data curation, Supervision, Writing – review & editing. PJ: Conceptualization, Methodology, Writing – review & editing. RY: Conceptualization, Methodology, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Chongqing under Grant (No. CSTB2022NSCQ-MSX0071; No. CSTB2024NSCQ-MSX1240); the Chongqing Municipal Science and Health Joint Medical Research Project under Grant (No. 2023MSXM116); the Chongqing Municipal Health Appropriate Technology Promotion Program under Grant (No. 2022jstg018); the China Postdoctoral Science Foundation (CPSF) under Grade (No. 2024M753872); and the Postdoctoral Fellowship Program of CPSF under Grade (No. GZC20242141).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1442489/full#supplementary-material
References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
2. Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, et al. FIGO staging of endometrial cancer: 2023. J Gynecol Oncol. (2023) 34:e85. doi: 10.3802/jgo.2023.34.e85
PubMed Abstract | Crossref Full Text | Google Scholar
3. Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2011) 22 Suppl 6:vi35–39. doi: 10.1093/annonc/mdr374
PubMed Abstract | Crossref Full Text | Google Scholar
4. Choi J, Holowatyj AN, Du M, Chen Z, Wen W, Schultz N, et al. Distinct genomic landscapes in early-Onset and late-Onset endometrial cancer. JCO Precis Oncol. (2022) 6:e2100401. doi: 10.1200/PO.21.00401
PubMed Abstract | Crossref Full Text | Google Scholar
5. Walsh MD, Cummings MC, Buchanan DD, Dambacher WM, Arnold S, McKeone D, et al. Molecular, pathologic, and clinical features of early-onset endometrial cancer: identifying presumptive Lynch syndrome patients. Clin Cancer Res. (2008) 14:1692–700. doi: 10.1158/1078-0432.CCR-07-1849
PubMed Abstract | Crossref Full Text | Google Scholar
6. Crissman JD, Azoury RS, Barnes AE, Schellhas HF. Endometrial carcinoma in women 40 years of age or younger. Obstet Gynecol. (1981) 57:699–704.
PubMed Abstract | Google Scholar
8. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. (2013) 497:67–73. doi: 10.1038/nature12113
PubMed Abstract | Crossref Full Text | Google Scholar
9. Hruda M, Sehnal B, Halaška MJ, Drozenová J, Robová H, Pichlík T, et al. New staging of endometrial carcinoma - FIGO 2023. Ceska Gynekol. (2024) 89:120–7. doi: 10.48095/cccg2024120
PubMed Abstract | Crossref Full Text | Google Scholar
10. Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. (2016) 27:16–41. doi: 10.1093/annonc/mdv484
PubMed Abstract | Crossref Full Text | Google Scholar
11. Bendifallah S, Ilenko A, Daraï E. High risk endometrial cancer: Clues towards a revision of the therapeutic paradigm. J Gynecol Obstet Hum Reprod. (2019) 48:863–71. doi: 10.1016/j.jogoh.2019.06.003
PubMed Abstract | Crossref Full Text | Google Scholar
12. Concin N, Creutzberg CL, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch. (2021) 478:153–90. doi: 10.1007/s00428-020-03007-z
PubMed Abstract | Crossref Full Text | Google Scholar
13. Jiang P, Wang J, Gong C, Yi Q, Zhu M, Hu Z. A nomogram model for predicting recurrence of stage I-III endometrial cancer based on inflammation-immunity-nutrition score (IINS) and traditional classical predictors. J Inflammation Res. (2022) 15:3021–37. doi: 10.2147/JIR.S362166
PubMed Abstract | Crossref Full Text | Google Scholar
14. Fujimoto T, Nanjyo H, Fukuda J, Nakamura A, Mizunuma H, Yaegashi N, et al. Endometrioid uterine cancer: histopathological risk factors of local and distant recurrence. Gynecol Oncol. (2009) 112:342–7. doi: 10.1016/j.ygyno.2008.10.019
PubMed Abstract | Crossref Full Text | Google Scholar
15. Bosse T, Peters EE, Creutzberg CL, Jürgenliemk-Schulz IM, Jobsen JJ, Mens JW, et al. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer–A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer. (2015) 51:1742–50. doi: 10.1016/j.ejca.2015.05.015
PubMed Abstract | Crossref Full Text | Google Scholar
16. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. (2004) 10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713
PubMed Abstract | Crossref Full Text | Google Scholar
17. Thiel KW, Devor EJ, Filiaci VL, Mutch D, Moxley K, Alvarez Secord A, et al. TP53 sequencing and p53 immunohistochemistry predict outcomes when bevacizumab is added to frontline chemotherapy in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. (2022) 40:3289–300. doi: 10.1200/JCO.21.02506
PubMed Abstract | Crossref Full Text | Google Scholar
18. Zong L, Mo S, Sun Z, Lu Z, Chen J, Yu S, et al. Incorporating molecular classification when stratifying the survival risk of patients with high-Grade endometrial carcinomas. J Clin Med. (2023) 12:530. doi: 10.3390/jcm12020530
留言 (0)