Transoral penetrating craniocerebral injuries are a rare complication of neurosurgery, and their primary treatment is craniotomy due to the presence of a foreign body. Craniocerebral CT is the main imaging method for this condition, and cranial reconstruction is an important basis for formulating relevant surgical protocols. Because the incidence of postoperative intracranial infection is extremely high, antibiotics are necessary to prevent and treat infection.
Case descriptionA 20-year-old male patient was admitted to the Neurotrauma Surgery Department of Jilin University First Hospital due to attempted suicide by shooting an arrow through his mandible into his skull approximately 7 h prior. He had a 2-year history of depression, which was moderately controlled with regular oral medication. On physical examination upon admission, the patient was conscious, with a GCS score of E3VTM6. The arrow penetrated the skin and was visible in the mandibular region, and the tail of the arrow was outside the body (Figure 1A). The diameter of both pupils was 3.0 mm, and his reflexes to direct and indirect light were slow; his limb muscle strength was grade 5, his muscular tone was normal, and he had negative bilateral Babinski signs. Preoperative craniocerebral CT revealed tubular high-density shadows in the brain tissue, with the upper margin reaching the frontal bone and the lower margin extending to the right nasal region through the skull base (Figures 2, 3). Maxillofacial CT and 3D reconstruction revealed that a thin tubular foreign body had penetrated the submentum, tongue, oral cavity, right nasal cavity and ethmoid sinus, extending intracranially (Figure 4). During surgery, craniotomy was performed with a milling cutter at the transverse midline of the frontotemporal flap on the affected side. After the dura mater was exposed, local damage to the dura mater was observed, with the arrow exposed and dark red venous blood overflowing along the edge of the arrow. Bleeding was completely stopped by rinsing with hydrogen peroxide and gentamicin, after which the dura mater was cut radially. The dura mater was incised, and the subarachnoid space was opened to release cerebrospinal fluid and therefore allow slow decompression. After the collapse of the brain tissue comprising the frontal lobe, the hematoma and brain tissue damaged by the arrow were removed, the section of the arrow that extended intracranially was gradually exposed, the arrow was fixed with a bone rongeur, and the external shaft of the arrow was gently rotated until the head of the arrow was completely separated (Figure 1B) from the shaft of the arrow (Figure 1C). The head of the arrow was removed, and the shaft of the arrow was extracorpotruly retracted until it was completely removed from the skull (Figure 1D). After the damaged area of the skull base was fully exposed and the surrounding brain tissue was protected, the temporal fascia and artificial dura mater were removed to repair the damaged area of the skull base. After the skull base was completely repaired, active bleeding in the1 surgical area was 1absent, and the bone flap was also reset. The otolaryngologist subsequently continued to perform surgery to treat the nasopharyngeal injury. Postoperative craniocerebral CT reexamination revealed that the intracranial foreign body was completely removed, without residue or secondary intracranial hemorrhage (Figure 5). After surgery, the patient was administered cefminol sodium to prevent intracranial infection. He recovered well and did not exhibit signs of neurological sequelae at the 6-month follow-up visit.
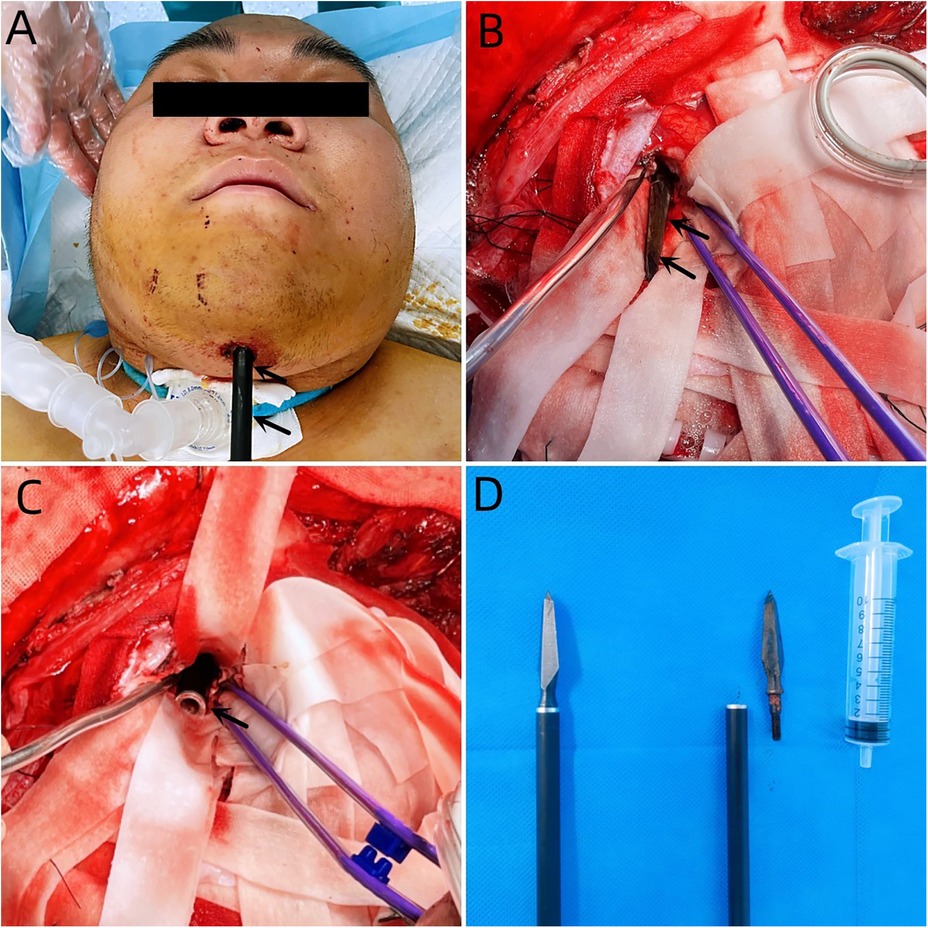
Figure 1. (A) Penetration of the skin of the mandibular region with an arrow, with its tail outside the body. (B) The black arrow indicates the head of the arrow. (C) The black arrow indicates the tail of the arrow. (D) The right image shows the intraoperative removal of the arrow, and the left image shows the arrow.
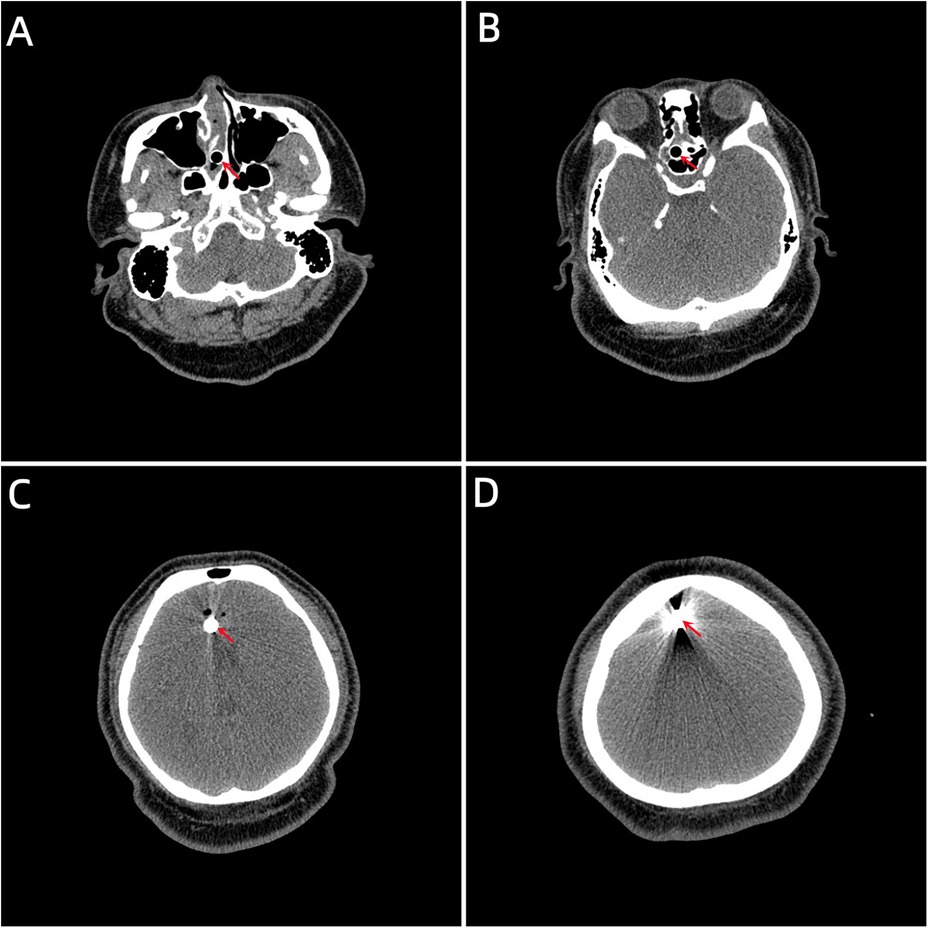
Figure 2. (A) The lower margin extending to the right nasal region through the skull base. (B, C) Tubular high-density shadow in the brain tissue. (D) The upper margin reaching the frontal bone. (the red arrow indicates the location of the foreign body location).

Figure 3. (A) Coronal view of the foreign body. (B) Sagittal view of the foreign body.
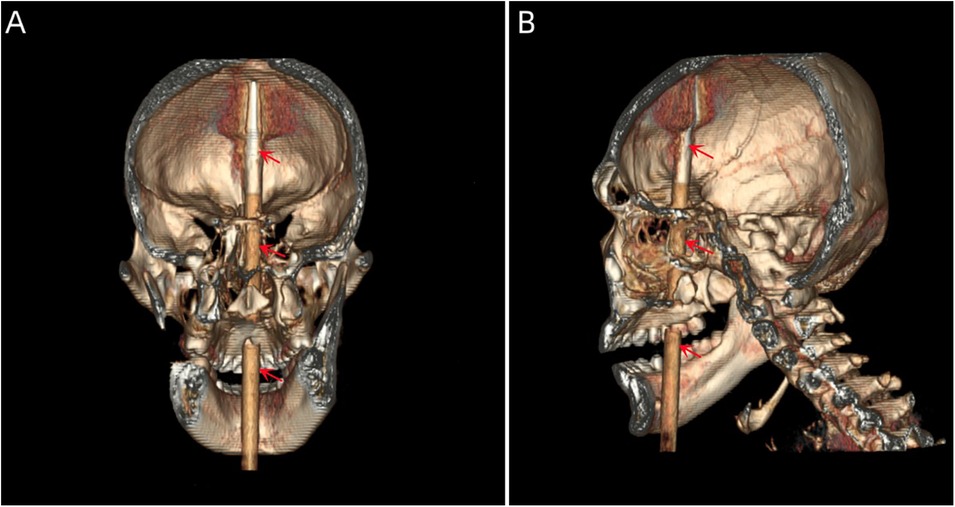
Figure 4. (A, B) 3D reconstructed images showing the location of the foreign body within the skull (the red arrow indicates the foreign body).
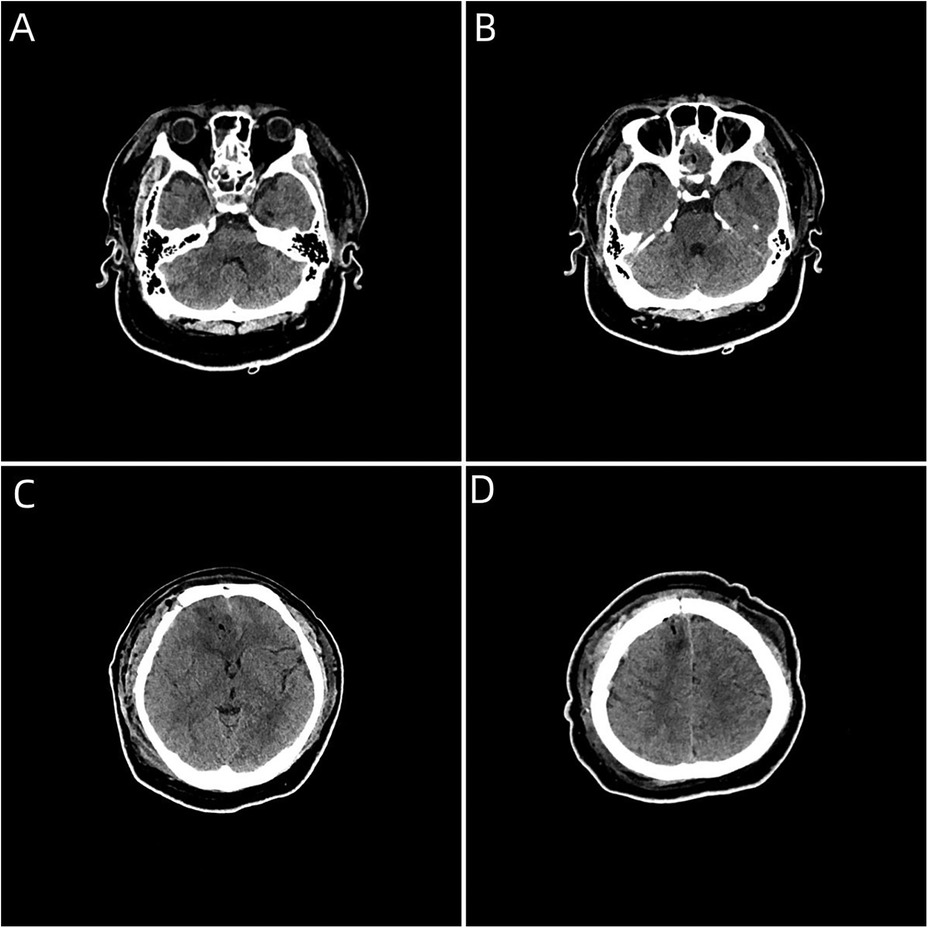
Figure 5. (A–D) Postoperative craniocerebral CT image showing complete removal of the intracranial foreign body, without foreign body residue or secondary bleeding complications.
DiscussionPenetrating brain injuries are serious and have high mortality and morbidity rates (1). Arrow-induced intracranial injury is rare, and injuries caused by such weapons have historically often been accidental. However, the number of self-inflicted injuries has increased; these injuries have varying patterns and a relatively high mortality rate (1–3). PBIs caused by objects with impact velocities <100 m/s, such as metal, wood, and plastic objects, are referred to as nonfirearm penetrating brain injuries. The mechanism of craniocerebral dynamic injury is direct tearing of the injury tract (4).
Although penetrating brain injories are rarer than closed brain injuries, their prognosis is usually poor (5). These injuries mostly occur in males aged 6–55 years, with the most common cause being accidents during exercise and the second most common cause being suicide attempts (1–3, 6–8). These two causes often vary in entry location and trajectory. In accidental injuries, the arrow usually enters the skull from the frontal or occipital region. A transoral penetrating injury is a common injury associated with violent suicide (2). This type of injury is caused by gunfire (9, 10), whereas craniocerebral injury is caused by means other than gunfire (e.g., low-velocity penetrating craniocerebral injury) and is extremely rare (3). According to previous reports, 75% (12/16) of such injuries are related to suicide (Table 1). In suicide attempts, arrows usually enter through the submental or submandibular region of the mouth (1). The trajectory of the arrow is directly associated with the location of the brain injury—an injury to a major artery or the venous sinus is associated with an increased risk of vascular complications (1).
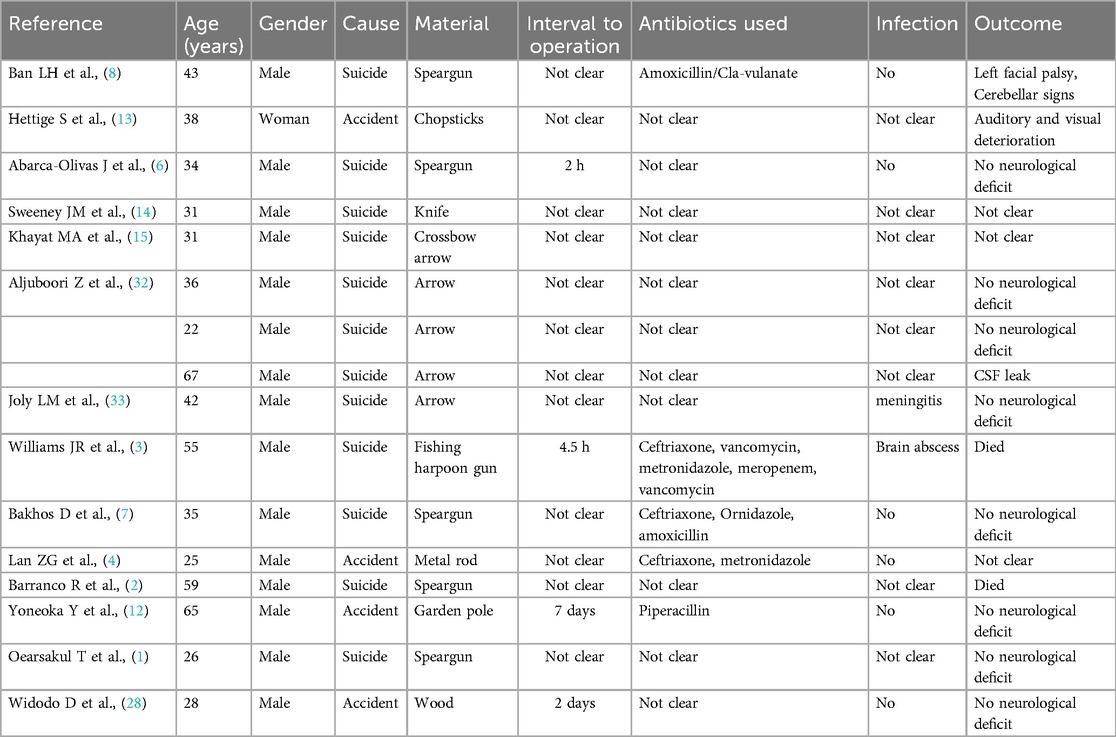
Table 1. Reported cases of transoral penetrating craniocerebral injury.
Imaging examination is essential for determining the shape, size and trajectory of the penetrating foreign body, as well as to correctly diagnose the injury and select the appropriate surgical protocol (16–21). The missed detection rate of CT for nonmetallic foreign bodies is 42% (10). Several cases missed detections on CT images have been reported (16, 22, 23). The density of woody foreign bodies increases over time, possibly due to granulomas around the foreign bodies or calcium deposits inside the wood (16). Therefore, the density of foreign bodies is similar to that of brain tissue, which hinders their identification on CT. Accordingly, metallic foreign bodies are more easily diagnosed with CT, whereas MRI has great value for the identification of woody foreign bodies. Specifically, MRI helps distinguish the woody foreign body from the surrounding air and fat tissue (24). Notably, MRI may cause the movement of metal foreign bodies and may require a longer examination time. Because our patient's intracranial foreign body was made of metal (arrowhead), the patient was unable to undergo a brain MRI examination. Cerebral angiography is recommended for diagnosing transoral penetrating craniocerebral injury after ruling out cerebrovascular injury, and the association between the foreign body and the intracranial vasculature can be examined (16). Unfortunately, cerebral angiography was not performed because the equipment in our hospital was damaged on that day. CT angiography should be performed to investigate the cerebrovascular injuries either by the location or trajectory of the foreign body after an intracranial penetrating trauma. CT angiography is accurate in detecting most traumatic intracranial aneurysms, dissections, and occlusions or for revealing the location of hematomas.
Currently, clinical data are insufficient to provide an appropriate antibiotic regimen for penetrating brain injuries. Intracranial infections following penetrating brain injuries are generally associated with an increased risk of mortality (5). The rates of local wound infection, meningitis and brain abscess are elevated among patients with PBI secondary to contamination from foreign objects and skin and bone fragments introduced into the brain parenchyma along the projectile track. Before the advent of antibiotics, the rate of these infectious complications was reported to be as high as 58.8% in the military population (5). According to our review of previous cases, all but 12 patients, whose data were not explicitly recorded, were treated with antibiotics (Table 1). The infection rate was 28.6% (2/7) (9 patients did not explicitly claim to be infected) (Table 1).
Broad-spectrum antibiotics are most commonly used for treatment (16, 25). The method to select the most suitable prophylactic antibiotic regimen for patients with penetrating brain injuries varies. Cephalosporins are the most commonly used antibiotics (26). Esposito and Walker recommended treating penetrating brain injury patients with intravenous ceftriaxone, vancomycin and metronidazole for at least 6 weeks (27). Previous studies clearly indicate that all prophylactic antibiotics are broad-spectrum antibiotics (Table 1). Notably, the overuse of antibiotics can increase the risk of fungal infections. Widodo D therefore recommended the commencement of this therapeutic regimen as soon as possible after craniocerebral injury and its continuation for 5 days postoperatively (27). However, given the rarity of this condition, relevant reports are scarce; therefore, the various antibiotic treatment regimens still need further research.
The application of antiepileptic drugs has also been studied in this context. Today, the use of anticonvulsant drugs within the first 7 days of this injury is believed to be beneficial (3). However, the preventive use of anticonvulsant drugs for periods longer than 7 days is controversial. Nevertheless, the latest evidence shows that given the high incidence of epilepsy during the course of the disease, the benefits of continued use of anticonvulsant drugs are greater than their adverse effects (3).
The goal of surgical treatment for a transoral penetrating craniocerebral injury is to reduce the space-occupying effect of the foreign body and reduce the risk of infectious complications (5). Surgery is the major strategy for treating transoral penetrating craniocerebral injury (3, 26, 28, 29). Surgical indications for this injury include fracture displacement, cerebrospinal fluid leakage, foreign body residue, vascular injury and intracranial hemorrhage (3, 28, 29). However, the amount of foreign body residue or intracranial hemorrhage indicated for surgery have not been reported. Some small foreign bodies or those adjacent to important exposed structures, such as the brainstem, can be treated conservatively, and surgery should be performed within 12 h of injury (28). However, active debridement of deep fragments should be avoided because this procedure increases the risks of disability and mortality (3, 28, 29).
Early complications include vascular injury, ischemic injury, cerebral contusion hemorrhage, cerebral edema and infection, whereas late complications include hydrocephalus, cerebrospinal fluid leakage and infection (30). Early infections are associated with debris (foreign bodies, bone fragments, and hair or skin penetrating the brain) (30). Infections, including extracranial and intracranial infections such as wound infection, cranial osteomyelitis, meningitis and brain abscess, account for 5%–23% of complications. These infections are often linked to cerebrospinal fluid leakage, debris impingement and sinus wounds (30, 31). Cerebrospinal fluid leakage usually occurs as a result of dura mater tears that fail to heal. They most commonly occur at the entry wound site (or at the exit in cases of bullet injury), are present in up to 9% of patients and are highly predictive of intracranial infections (11, 30). Intranasal topical application of fluorescein is conducive to detecting cerebrospinal fluid leakage, and autologous transplantation of epifascial adipose tissue can be performed to repair cerebrospinal fluid leakage. The use of a fat-on-fascia plug shortens the surgical time, thereby reducing the risk of aerosolization during surgery (12). Specifically, minimal intranasal submucosal dissection reduces the operative time and the risk of virus “aerosolization”. In addition, the described fat-on-fascia plug method does not require the placement of a mucosal pedicle flap, which also reduces the operative time and the risk of virus aerosolization.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by The First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsHX: Formal Analysis, Writing – original draft, Writing – review & editing. WL: Writing – review & editing. WZ: Writing – review & editing. LS: Writing – review & editing. YL: Writing – review & editing. HY: Funding acquisition, Resources, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Oearsakul T, Kaewborisutsakul A, Jantharapattana K, Khumtong R, Puetpaiboon A, Sangthong B. Multidisciplinary management of a penetrating cerebellar injury by a fishing speargun: a case study and literature review. Surg Neurol Int. (2021) 12:391. doi: 10.25259/SNI_506_2021
PubMed Abstract | Crossref Full Text | Google Scholar
2. Barranco R, Caputo F, Pinto SL, Drommi M, Ventura F. Unusual suicide by a speargun shot: case report. Medicine (Baltimore). (2020) 99(49):e22308. doi: 10.1097/MD.0000000000022308
PubMed Abstract | Crossref Full Text | Google Scholar
3. Williams JR, Aghion DM, Doberstein CE, Cosgrove GR, Asaad WF. Penetrating brain injury after suicide attempt with speargun: case study and review of literature. Front Neurol. (2014) 5:113. doi: 10.3389/fneur.2014.00113
PubMed Abstract | Crossref Full Text | Google Scholar
4. Lan ZG, Richard SA, Li J, Yang C. Nonprojectile penetrating iron rod from the oral cavity to the posterior cranial fossa: a case report and review of literature. Int Med Case Rep J. (2018) 11:41–5. doi: 10.2147/IMCRJ.S157237
PubMed Abstract | Crossref Full Text | Google Scholar
6. Abarca-Olivas J, Concepción-Aramendía LA, Baño-Ruiz E, Caminero-Canas MA, Navarro-Moncho JA, Botella-Asunción C. Perforating brain injury from a speargun. A case report. Neurocirugia (Astur). (2011) 22(3):271–5. doi: 10.1016/S1130-1473(11)70025-2
PubMed Abstract | Crossref Full Text | Google Scholar
8. Ban LH, Leone M, Visintini P, Blasco V, Antonini F, Kaya JM, et al. Craniocerebral penetrating injury caused by a spear gun through the mouth: case report. J Neurosurg. (2008) 108(5):1021–3. doi: 10.3171/JNS/2008/108/5/1021
PubMed Abstract | Crossref Full Text | Google Scholar
9. Nouma Y, Krimi S, Regaieg K. Unusual suicide by a speargun shot through the mouth: a case report. Am J Forensic Med Pathol. (2018) 39(1):73–7. doi: 10.1097/PAF.0000000000000359
PubMed Abstract | Crossref Full Text | Google Scholar
12. Yoneoka Y, Aizawa N, Nonomura Y, Ogi M, Seki Y, Akiyama K. Traumatic nonmissile penetrating transnasal anterior skull base fracture and brain injury with cerebrospinal fluid leak: intraoperative leak detection and an effective reconstruction procedure for a localized skull base defect especially after coronavirus disease 2019 outbreak. World Neurosurg. (2020) 140:166–72. doi: 10.1016/j.wneu.2020.05.236
PubMed Abstract | Crossref Full Text | Google Scholar
14. Sweeney JM, Lebovitz JJ, Eller JL, Coppens JR, Bucholz RD, Abdulrauf SI. Management of nonmissile penetrating brain injuries: a description of three cases and review of the literature. Skull Base Rep. (2011) 1(1):39–46. doi: 10.1055/s-0031-1275257
PubMed Abstract | Crossref Full Text | Google Scholar
15. Khayat MA, Khayat H, Alhantoobi MR, Aljoghaiman M, Sommer DD, Algird A, et al. Traumatic penetrating head injury by crossbow projectiles: a case report and literature review. Surg Neurol Int. (2024) 15:35. doi: 10.25259/SNI_574_2023
PubMed Abstract | Crossref Full Text | Google Scholar
16. Wu Y, He W, Yang Y, Chen J. A rare case of orbitocranial penetrating injury with intracranial wooden foreign body residue. Medicina (Kaunas). (2022) 58(12):1832. doi: 10.3390/medicina58121832
PubMed Abstract | Crossref Full Text | Google Scholar
17. Wang CB, Wang H, Zhao JS, Wu ZJ, Liu HD, Wang CJ, et al. Right-to-Left displacement of an airgun lead bullet after transorbital entry into the skull complicated by posttraumatic epilepsy: a case report. J Korean Neurosurg Soc. (2023) 66(5):598–604. doi: 10.3340/jkns.2022.0234
PubMed Abstract | Crossref Full Text | Google Scholar
18. Buttram SD, Garcia-Filion P, Miller J, Youssfi M, Brown SD, Dalton HJ, et al. Computed tomography vs magnetic resonance imaging for identifying acute lesions in pediatric traumatic brain injury. Hosp Pediatr. (2015) 5(2):79–84. doi: 10.1542/hpeds.2014-0094
PubMed Abstract | Crossref Full Text | Google Scholar
20. Muehlschlegel S, Ayturk D, Ahlawat A, Izzy S, Scalea TM, Stein DM, et al. Predicting survival after acute civilian penetrating brain injuries: the SPIN score. Neurology. (2016) 87(21):2244–53. doi: 10.1212/WNL.0000000000003355
PubMed Abstract | Crossref Full Text | Google Scholar
21. Vakil MT, Singh AK. A review of penetrating brain trauma: epidemiology, pathophysiology, imaging assessment, complications, and treatment. Emerg Radiol. (2017) 24(3):301–9. doi: 10.1007/s10140-016-1477-z
PubMed Abstract | Crossref Full Text | Google Scholar
22. Wilson MH, Collins TR, Revington PJ. Orbitocranial wooden foreign body retrieved by transcranial and superior orbitotomy. Br J Oral Maxillofac Surg. (2016) 54(9):1050–1. doi: 10.1016/j.bjoms.2016.02.040
PubMed Abstract | Crossref Full Text | Google Scholar
23. Maruya J, Yamamoto K, Wakai M, Kaneko U. Brain abscess following transorbital penetrating injury due to bamboo fragments–case report. Neurol Med Chir (Tokyo). (2002) 42(3):143–6. doi: 10.2176/nmc.42.143
PubMed Abstract | Crossref Full Text | Google Scholar
25. Litvack ZN, Hunt MA, Weinstein JS, West GA. Self-inflicted nail-gun injury with 12 cranial penetrations and associated cerebral trauma. Case report and review of the literature. J Neurosurg. (2006) 104(5):828–34. doi: 10.3171/jns.2006.104.5.828
PubMed Abstract | Crossref Full Text | Google Scholar
26. Prasetyo E, Oley MC, Sumual V, Faruk M. Transorbital-penetrating intracranial injury due to a homemade metal arrow: a case report. Ann Med Surg (Lond). (2020) 57:183–9. doi: 10.1016/j.amsu.2020.07.049
PubMed Abstract | Crossref Full Text | Google Scholar
27. Esposito DP, Walker JB. Contemporary management of penetrating brain injury. Neurosurg Q. (2009) 19:249–54. doi: 10.1097/WNQ.0b013e3181bd1d53
Crossref Full Text | Google Scholar
28. Widodo D, Perkasa F, Al-'Abqary R, Sjukur KJ, Faruk M. Combined transcranial and transnasal endoscopic approach in transnasal-penetrating intracranial injury: a rare case report. Int J Surg Case Rep. (2022) 97:107422. doi: 10.1016/j.ijscr.2022.107422
PubMed Abstract | Crossref Full Text | Google Scholar
29. Zhang D, Chen J, Han K, Yu M, Hou L. Management of penetrating skull base injury: a single institutional experience and review of the literature. BioMed Res Int. (2017) 2017:2838167. doi: 10.1155/2017/2838167
PubMed Abstract | Crossref Full Text | Google Scholar
30. Somrani K, Gader G, Badri M, Zammel I, Rkhami M. A spectacular penetrating craniocerebral trauma from a rake: a case report. Korean J Neurotrauma. (2023) 19(1):109–14. doi: 10.13004/kjnt.2023.19.e8
PubMed Abstract | Crossref Full Text | Google Scholar
31. Rosenfeld JV, Bell RS, Armonda R. Current concepts in penetrating and blast injury to the central nervous system. World J Surg. (2015) 39(6):1352–62. doi: 10.1007/s00268-014-2874-7
PubMed Abstract | Crossref Full Text | Google Scholar
32. Aljuboori Z, McGrath M, Levitt M, Moe K, Chestnut R, Bonow R. A case series of crossbow injury to the head highlighting the importance of an interdisciplinary management approach. Surg Neurol Int. (2022) 13:60. doi: 10.25259/SNI_1166_2021
PubMed Abstract | Crossref Full Text | Google Scholar
33. Joly LM, Oswald AM, Disdet M, Raggueneau JL. Difficult endotracheal intubation as a result of penetrating cranio-facial injury by an arrow. Anesth Analg. (2002) 94(1):231–2, table of contents. doi: 10.1213/00000539-200201000-00045
留言 (0)