Gastric cancer (GC) is responsible for over one million new cases in 2020 and estimated 769,000 deaths, ranking fifth for incidence and fourth for mortality globally (1). In China, approximately 358,700 new cases of GC and 260,400 deaths occurred in 2022, which is the third largest number of cancer deaths (2). Currently, systemic therapy for patients with HER-2 negative, unresectable advanced or recurrent gastric/gastroesophageal junction cancer (GC/GEJC) has been dominated by chemotherapy, and the common first-line agents include platinum, fluorouracil and taxane drugs worldwide (3–5). However, the efficacy of these treatments is not ideal, with the median overall survival (mOS) at approximately only 1 year (6).
Attraction 4, Checkmate 649, and Orient 16 have demonstrated a synergistic effect of immune checkpoint inhibitors (ICIs) in combination with chemotherapy in patients with HER-2 negative, unresectable advanced or recurrent GC/GEJC (7–9). Studies have found that chemotherapy can not only kill tumor cells through cytotoxic effects directly, but also promote anti-tumor immune responses by inducing immunogenic cell death (10–12). As of now, several guidelines, such as the Chinese Society of Clinical Oncology, the European Society for Medical Oncology, and the National Comprehensive Cancer Network, suggest that ICIs together with chemotherapy are used as the first-line treatment for patients with advanced GC especially who exhibit a high combined positive score (CPS) (5, 13, 14).
In China, ICIs are frequently applied to treat unresectable advanced or recurrent GC/GEJC. In order to explore the efficacy and safety of ICIs combined with chemotherapy in these patients, here we examined the short-term and long-term outcomes as well as the adverse events (AEs) of patients who received chemotherapy alone or chemotherapy combined with ICIs.
2 Materials and methods2.1 Study design and participantsThis retrospective study involved patients with HER-2 negative, unresectable advanced or recurrent GC/GEJC. All patients were fully aware of the purpose of this study and expressed informed consent. This study retrospectively analyzed clinical data of patients with advanced GC/GEJC from January 1, 2018 to May 31, 2023 at the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine in China. Survival data were obtained through follow-up.
All patients had histologically or cytologically confirmed HER-2 negative, unresectable advanced or recurrent GC/GEJC; had received at least two cycles of chemotherapy or chemotherapy combined with ICIs; had received at least one efficacy assessment; had baseline Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; and had normal hepatic and renal function. Patients were excluded if they could not tolerate immunotherapy or chemotherapy; or had severe systemic or autoimmune disease; or multiple primary tumors or unknown primary sites; or were HER-2 positive; or had incomplete clinical data.
2.2 Study proceduresAll patients included in the final analysis received chemotherapy (XELOX or FOLFOX) and a subset of patients combined with ICIs (nivolumab, sintilimab, tislelizumab and camrelizumab) on this basis.
The baseline information below of each patient were collected: age, sex, family genetic history, history of smoking, history of drinking, ECOG PS, primary tumor location, surgery or not, metastatic site, organs with metastases, and chemotherapy regimen. The clinical efficacy was assessed by outcomes of CT or MRI, which was based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (15).
2.3 OutcomesThe primary endpoint of this study was progression-free survival (PFS), which was estimated from treatment initiation to progression or death. The secondary endpoints included overall survival (OS), which was defined as the duration from treatment initiation to death due to any reason; objective response rate (ORR), which was defined as the proportion of patients with the best overall response of complete response (CR) or partial response (PR); and disease control rate (DCR), which was defined as the proportion of patients with CR, PR, or stable disease (SD). Safety endpoint included evaluation of AEs. AEs were monitored and classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
2.4 Statistical analysisIn this study, SPSS 27.0 and GraphPad Prism 9.0 were used for statistical analysis and scientific mapping. Descriptive statistics were used for the basic characteristic data. Chi-square test or fisher’s exact test was used to analyze the efficacy and incidence of adverse reactions. PFS and OS were estimated with Kaplan-Meier method, which was expressed with the two-sided 95% confidence intervals (CIs), and the differences between groups were compared by log-rank test, and the two-sided significance level was P=0.05. The ORR and DCR were analyzed with the Chi-square test. Univariate and multivariate analysis were performed using the Cox proportional hazards model, and hazard ratios (HRs) and 95% CIs were calculated. The difference of P<0.05 was statistically significant.
3 Results3.1 Baseline characteristicsAfter screening 1745 patients according to the inclusion and exclusion criteria described above, we excluded 136 HER-2 positive patients, 169 patients who could not tolerate chemotherapy or immunotherapy, 179 patients who had severe systemic or autoimmune disease, 248 patients with multiple primary tumors or with unknown primary tumor sites, 123 patients without complete clinical data, and 118 patients without evaluable lesions for efficacy. A total of 210 patients were included in the final analysis (Figure 1).
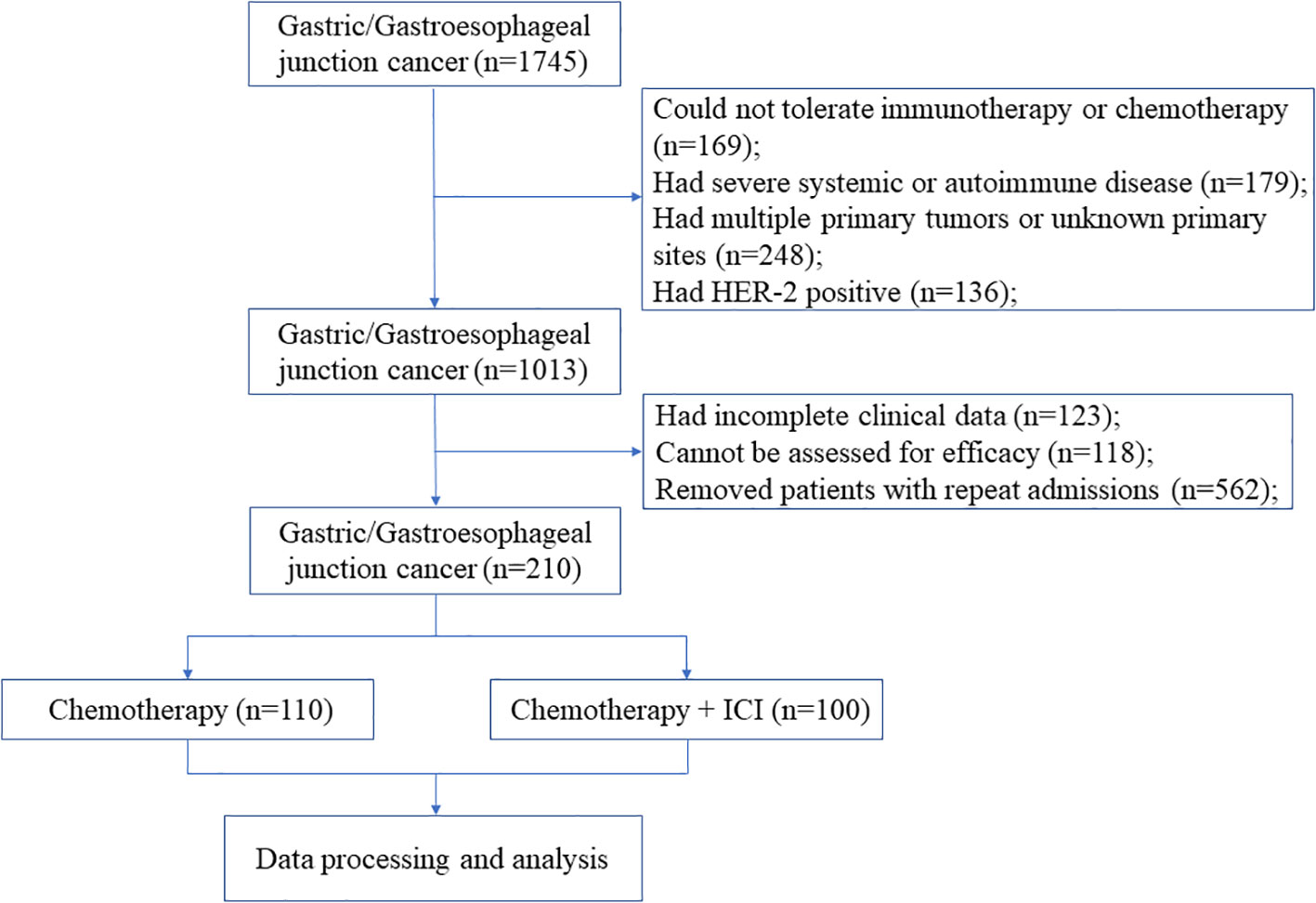
Figure 1. Flow diagram of the study.
The median age of the patients included in the chemotherapy alone group was 64 years old (interquartile range [IQR], 60-70), of which 83 (75.5%) were male, 26 (23.6%) had the family genetic history, 65 (59.1%) had the history of smoking, 52 (47.3%) had the history of alcohol, 101 (91.8%) had a primary tumor site of the stomach, 69 (62.7%) had undergone surgery, half (50%) had two or more sites of tumor metastasis, and the majority of patients (66.4%) received the FOLFOX chemotherapy regimen. The median age of the patients included in the immunotherapy together with chemotherapy group was 66 years old (IQR, 60-73), of whom 66 (66%) were male, 18 (18%) had the family genetic history, 67 (67%) had the history of smoking, 36 (36%) had the history of alcohol, 89 (89%) had a primary tumor site of the stomach, 55 (55%) had undergone surgery, more than half (51%) had two or more sites of metastases, and most (63%) received the FOLFOX chemotherapy regimen. ICIs included nivolumab (6%), sintilimab (70%), tislelizumab (10%), and camrelizumab (14%). All patients had an ECOG performance status of 0-1 (Table 1). Because precise PD-L1 CPS values were not available for more than 80% of enrolled patients (PD-L1 CPS ≤1 6 patients, CPS ≥1 10 patients, CPS ≥5 5 patients, and CPS ≥10 2 patients), this metric was not analyzed. Patients who completed first-line therapy without disease progression and tolerable AEs were eligible for maintenance therapy, which consisted of single-agent chemotherapy (S-1 or Capecitabine) with or without immunotherapy.
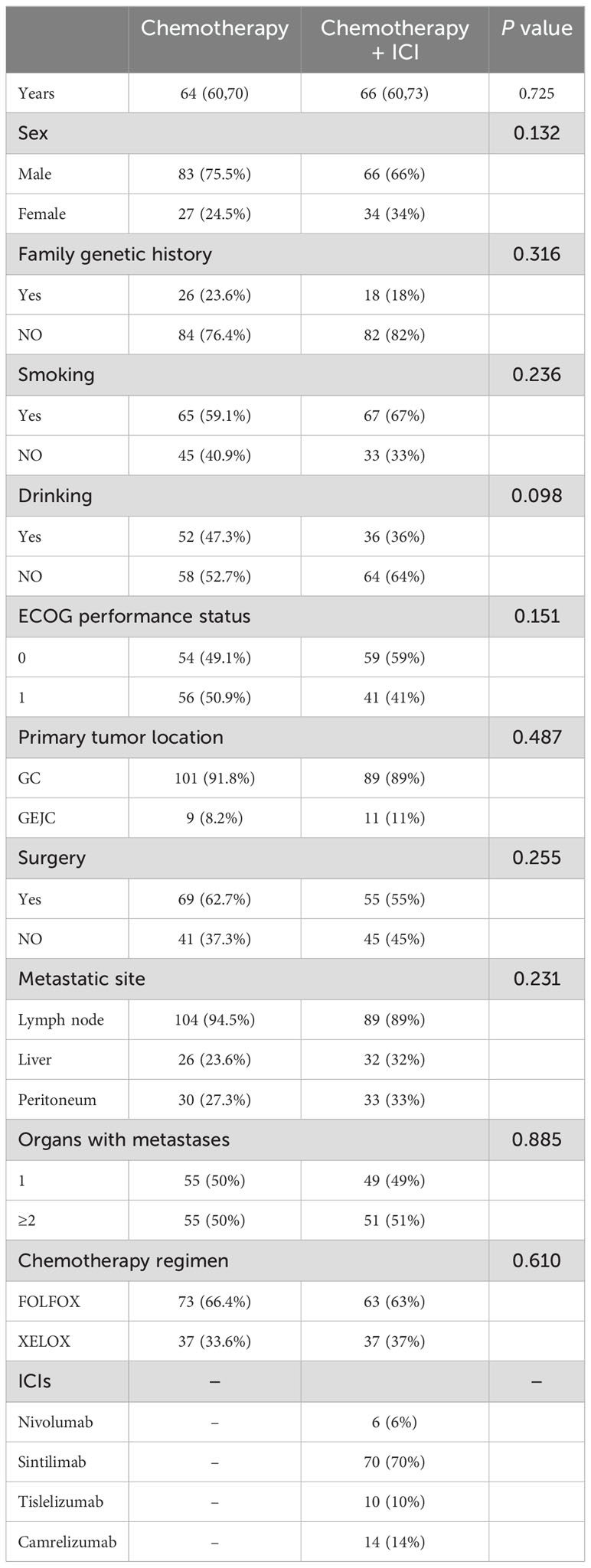
Table 1. Baseline clinical characteristics.
3.2 EfficacyAt the cutoff date, a total of 156 patients out of 210 patients had PD, including 94 in the chemotherapy alone group and 62 in the combination treatment group. Data analysis showed that the median PFS (mPFS) was 270 days (95%CI 177.510-362.490) in the chemotherapy group and 357 days (95%CI 250.103-463.897) in the combination treatment group, and the difference was statistically significant (P<0.05) (Figure 2). A total of 36 patients died in the chemotherapy alone group and 37 patients died in the combination treatment group. The median OS (mOS) was 14.9 months (95%CI 9.831-17.769) in the chemotherapy alone group, and 15 months (95%CI 12.386-17.614) in the combination treatment group, and the difference was not statistically significant (P>0.05) (Figure 3).
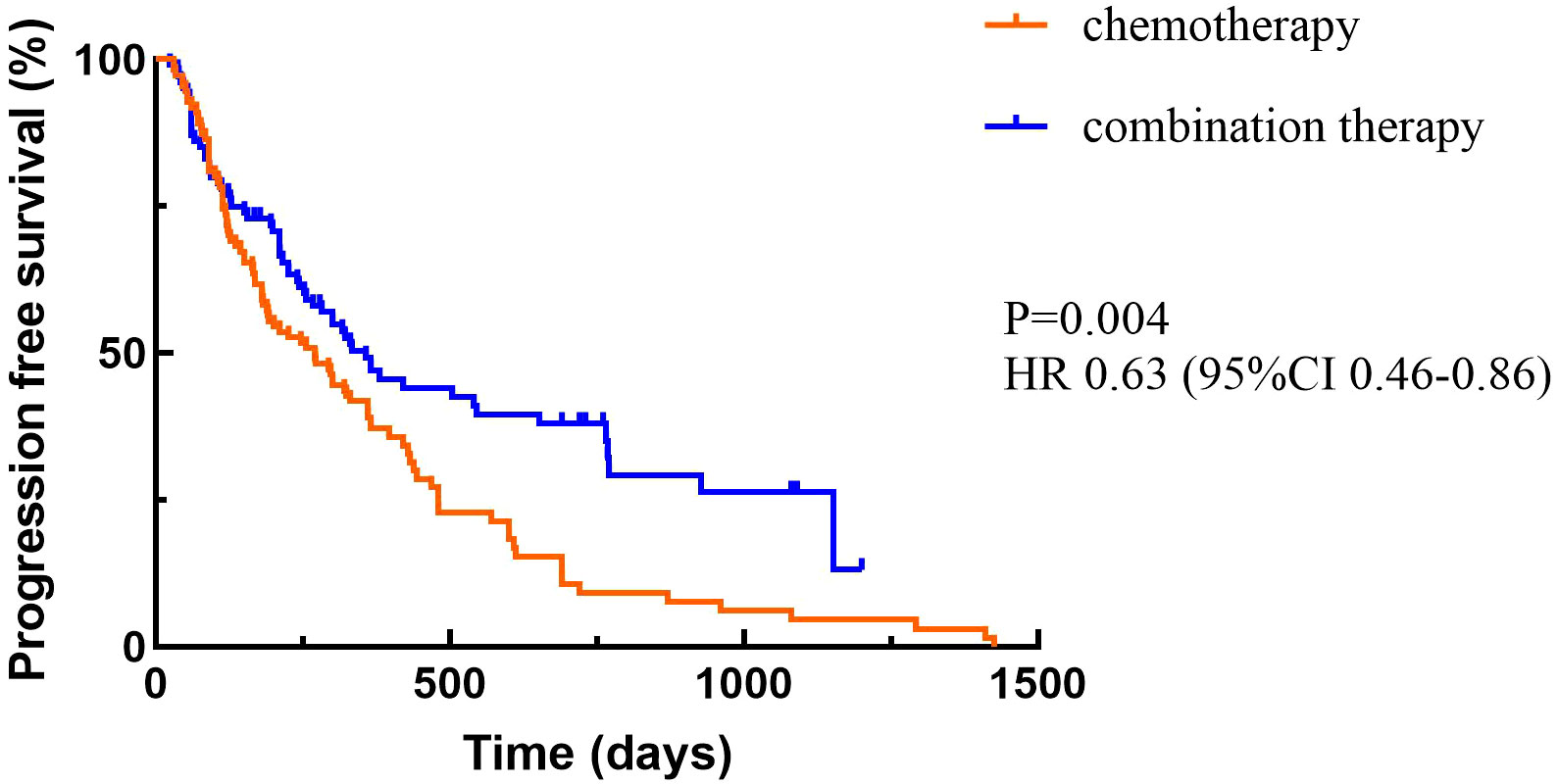
Figure 2. Progression-free survival.
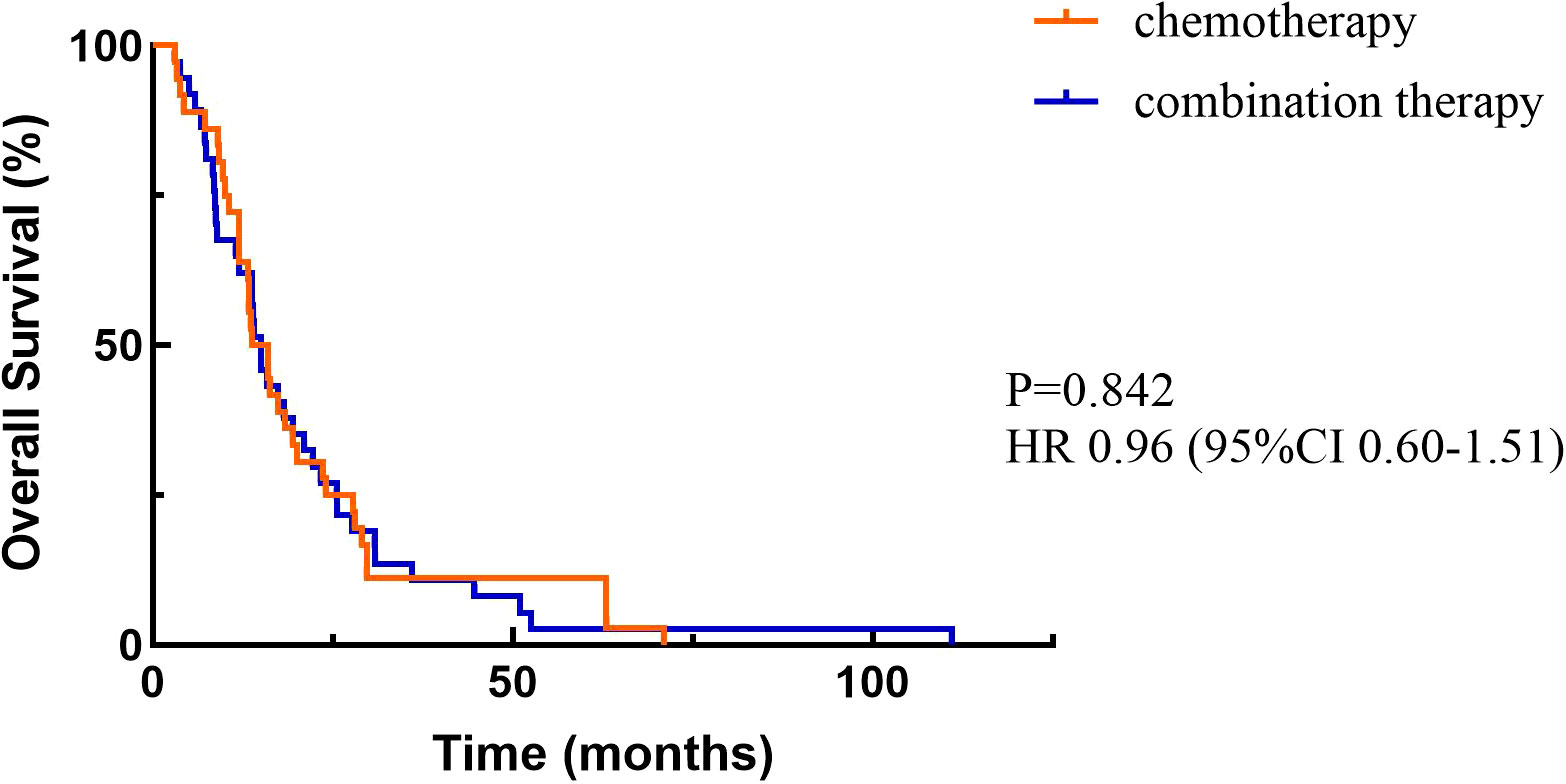
Figure 3. Overall survival.
Based on RECIST1.1 criteria, no patients achieved CR and PR, 16 patients achieved SD in the chemotherapy alone group. In the combination treatment group, no patients achieved CR, 4 patients achieved PR, 34 patients achieved SD, and the ORR was 4%. There was no statistically significant difference in ORR between the two groups (P=0.050). The DCR was 14.5% in the chemotherapy alone group and 38% in the combination treatment group. The difference was statistically significant (P<0.05) (Table 2).
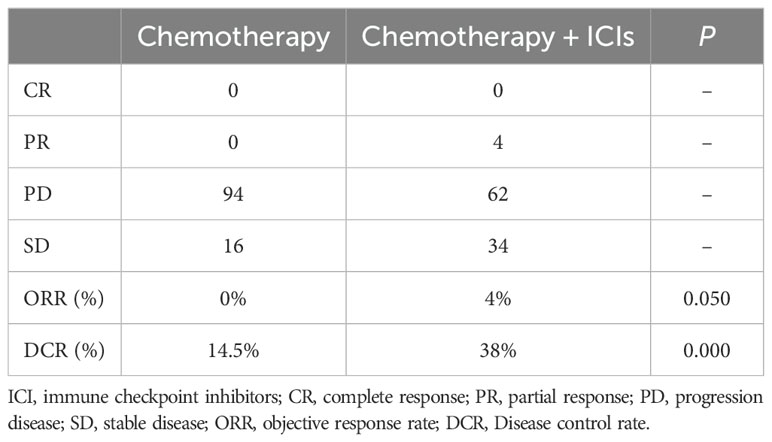
Table 2. ORR and DCR for different treatment regimens.
3.3 Subgroup analysisSubgroup analyses showed that primary tumor location of GEJC, ECOG PS of 1, without liver metastasis, and chemotherapy plus ICIs were associated with PFS benefit. The results of univariate analysis of OS showed that age, family genetic history, surgery or not, and organs with metastases were influencing factors (P<0.05), while in further Cox multivariate analysis showed that only surgery or not was correlated with patients’ prognosis (P<0.05). The results of the subgroup analyses of PFS and OS were shown in Tables 3, 4. In addition, we added the analysis of the effect of different ICIs on the efficacy of combination therapy (Table 5).
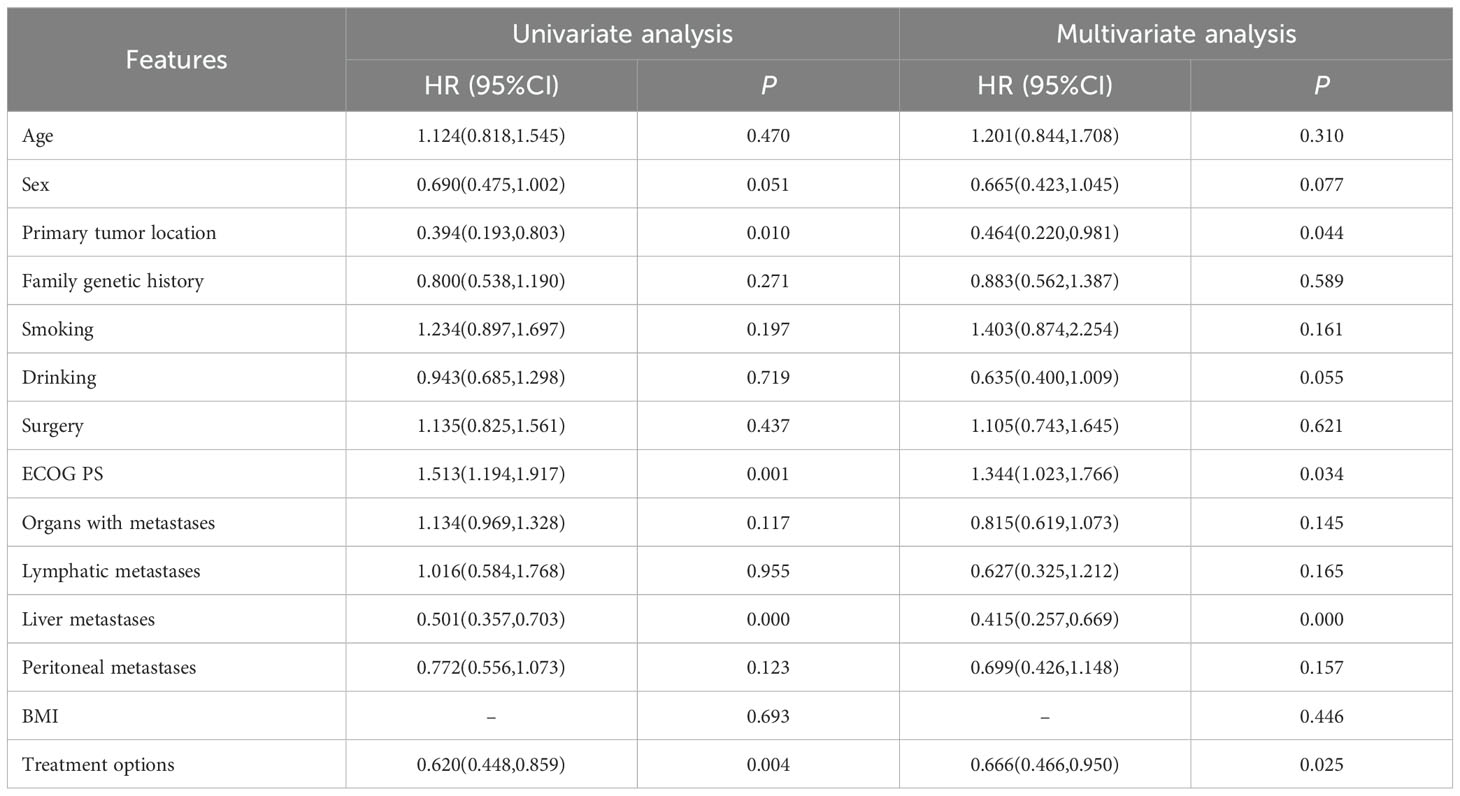
Table 3. Univariate and multivariate analyses of PFS.
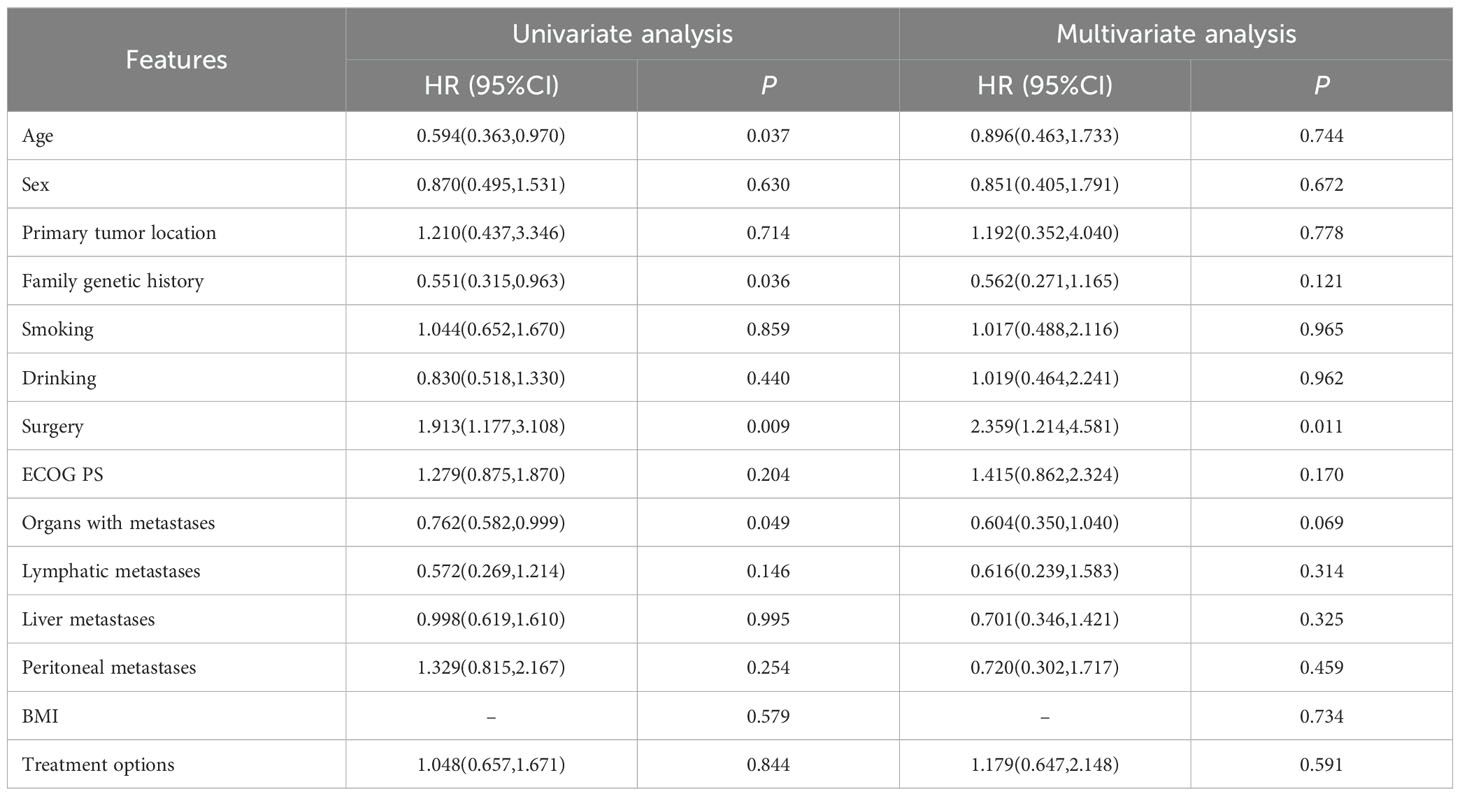
Table 4. Univariate and multivariate analyses of OS.
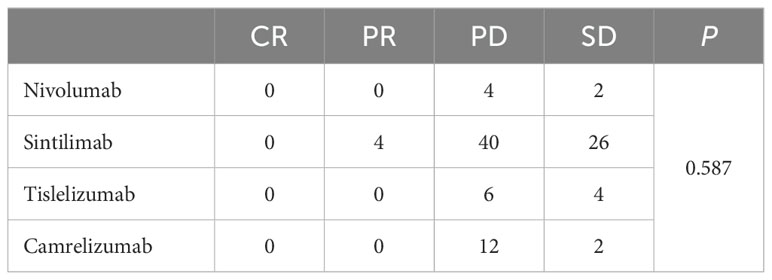
Table 5. Efficacy of different ICIs.
3.4 SafetyDuring the treatment, the most common AEs included: leucopenia, neutropenia, anemia, thrombocytopenia, alanine aminotransferase (ALT) increase, aspartate transaminase (AST) increase, creatinine increase, vomiting, peripheral neuropathy, and diarrhea, most of which were of grade 1-2 and manageable (Table 6). Any grade anemia was more common in the combination treatment group than in the chemotherapy alone group, but there was no significant difference in the incidence of grade 3 and above. Immunotherapy-related AEs included thyroid dysfunction (2 patients), myocarditis (3 patients), and pneumonitis (2 patients).
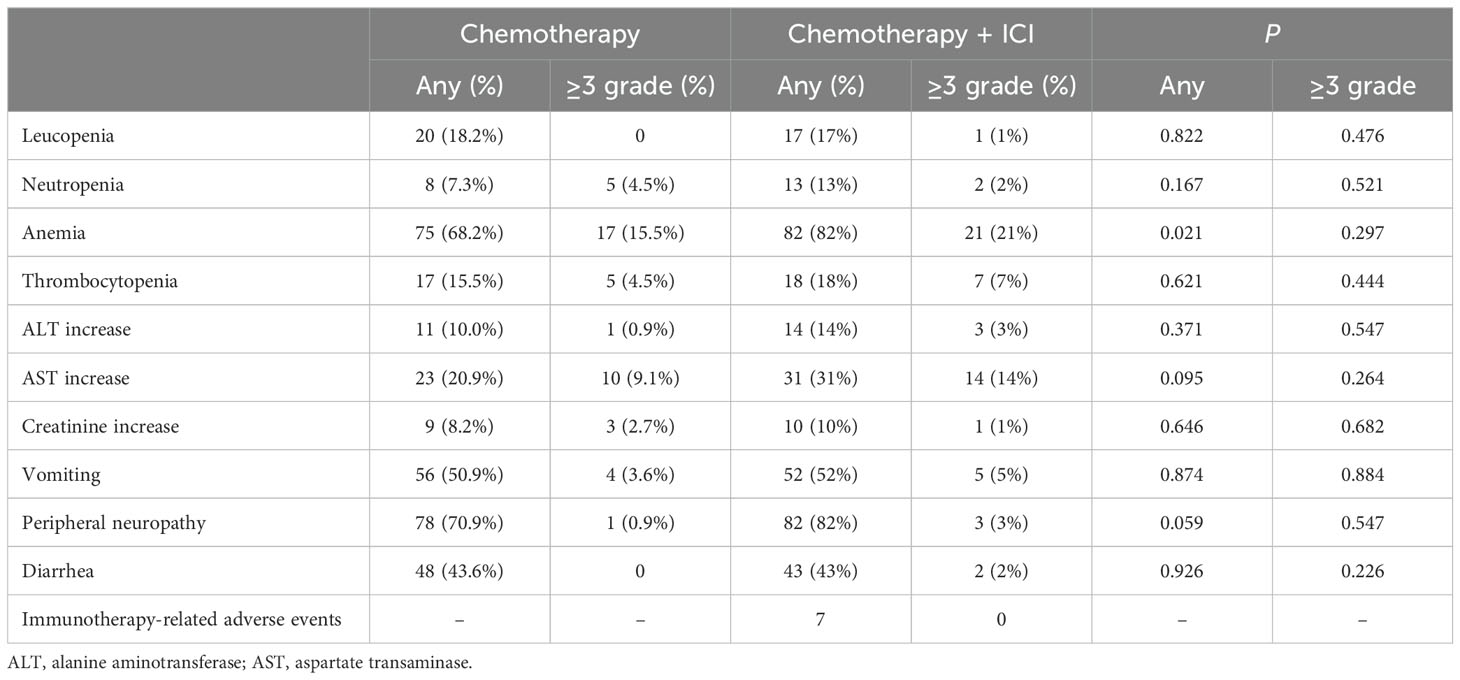
Table 6. Summary of adverse events.
4 DiscussionCheckmate 649 established the importance of immunotherapy in advanced GC. Recent follow-up data showed (16) that nivolumab plus chemotherapy showed benefit in both OS and PFS compared with chemotherapy alone in both patients with PD-L1 CPS≥5 and all randomized patients. In patients with PD-L1 CPS≥5, mPFS was 8.3 versus 6.1 months (HR=0.71, 95%CI 0.61-0.82) and mOS was 14.4 versus 11.1 months (HR=0.70, 95%CI 0.61-0.81). In all randomized patients, mPFS was 7.7 versus 6.9 months (HR=0.80, 95%CI 0.71-0.89) and mOS was 13.7 versus 11.6 months (HR=0.79, 95%CI 0.71-0.88). Orient 16 (17) demonstrated the population-wide benefit of immunotherapy combined with chemotherapy as a first-line treatment for locally advanced/metastatic GC. The final results showed that in patients with PD-L1 CPS≥5 sintilimab combined with chemotherapy could significantly prolong mPFS (7.7 versus 5.8 months, HR=0.628, P=0.0002) and mOS (19.2 versus 12.9 months, HR=0.587, P<0.0001). In the whole population, mOS was 15.2 versus 12.3 months (HR=0.681, P<0.0001) and mPFS was 7.1 versus 5.7 months (HR=0.638, P<0.0001). Results of rationale 305 (18) also showed that immunotherapy combined with chemotherapy as a first-line treatment can significantly prolong survival in patients with locally advanced unresectable or metastatic GC/GEJC.
Notably, keynote 062 (19) and attraction 4 (7) received partially negative results, which were also RCTs comparing the efficacy and safety of immunotherapy plus chemotherapy with chemotherapy alone, and the addition of immunotherapy did not result in a significant final OS benefit. In this retrospective study, we found that chemotherapy combined with ICIs was effective in improving PFS (mPFS 270 versus 357 days, P<0.05), which is consistent with previous studies. However, there was no significant difference in OS (mOS 14.9 versus 15 months, P>0.05), which we considered that it may be related to the level of CPS expression, mismatch repair status, subsequence lines of treatment and the length of follow-up. Although PD-L1 CPS≥5 has been shown to be a good independent prognostic factor for survival (16–18), interestingly a systematic review found that when ICI was combined with chemotherapy, the correlation between PD-L1 expression and ORR was not obvious. The pooled ORR in PD-L1 negative, PD-L1 CPS ≥1, PD-L1 CPS ≥5, and PD-L1 CPS ≥10 population was 57%, 48%, 60%, and 58%, respectively. It seems that the benefit brought about by the rise in PD-L1 expression was not obvious when ICI and chemotherapy were combined (20). This requires further exploration on the effect of CPS on the efficacy of immunotherapy combined with chemotherapy in advanced GC. In addition, OS was closely related to follow-up time. Because the follow-up of this study was only one year, there may be bias, and we will continue to follow up these patients in the future. None achieved CR or PR in the chemotherapy alone group, compared with only 4 patients of PR in the combination group. There was no statistically significant difference in ORR between two groups (P=0.050). The DCR was 14.5% in the chemotherapy alone group and 38% in the combination treatment group (P<0.05). ORR and DCR can be affected by a variety of factors, including the level of immunity, the type of ICIs used, PD-L1 CPS expression level, tumor characteristics, molecular phenotypes, and performance status of patients. The combination of these factors determined the efficacy of patients receiving immunotherapy in combination with chemotherapy. Subgroup analyses showed that primary tumor location of GEJC, ECOG PS of 1, without liver metastasis, and chemotherapy plus ICIs were associated with patient PFS benefit. Cox multivariate analysis showed that only surgery or not was correlated with patients’ prognosis (P<0.05). Although there was no statistically significant difference in the effect of different ICIs on combination therapy in this study, it is still worth further exploring whether this is related to sample size and regional differences. Most AEs were grade 1-2 and manageable. In this study any grade of anemia was more common in the combination treatment group than in the chemotherapy alone group, but there was no significant difference in the incidence of grade 3 and above. Immunotherapy-related AEs included thyroid dysfunction (2 patients), myocarditis (3 patients), and pneumonitis (2 patients). Although the incidence of grade 3 and above AEs in chemotherapy combined with immunotherapy is low, close attention should be paid to prevent the occurrence of severe immune-related AEs and more in-depth analysis of it could follow to provide targeted remissions. At present, large real-world studies comparing the efficacy and safety of immunotherapy combined with chemotherapy are still needed, and there is an urgent need to study the dominant population and dominant stage of immunotherapy.
In this study, patients were collected according to strict inclusion criteria, and also multivariate analysis was used to control for the impact of confounding factors to minimize error. As this was a retrospective real-world study, clinical data collection was based on the extraction of electronic medical records and patient follow-up, and the data for safety analysis were mainly from medical records, laboratory indicators and imaging tests. The potential bias due to the retrospective, non-randomized design remains a limitation of this study. Many pathology centers, including ours, do not perform routine CPS detection, so the records of PD-L1 CPS expression level in patients were incomplete. And this study is only a single-center study, which has the problem of small sample size. In addition, HER-2 positive patients, who account for 20% of all GC patients (21), were not enrolled in the study, and there is also a clinical need to help these patients improve their survival, so we are conducting further prospective studies on different CPS levels, HER-2 expression levels, microsatellite status, and different ICIs.
5 ConclusionIn conclusion, the results of the study showed that in patients with HER-2 negative, unresectable advanced or recurrent GC/GEJC chemotherapy combined with ICIs could greatly prolong PFS, but OS was not significantly improved, and AEs were manageable. The outcomes confirmed the efficacy and safety of immunotherapy in combination with chemotherapy in the real-world setting, which could provide the basis for the standard first-line treatment of these patients.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributionsQX: Conceptualization, Formal analysis, Investigation, Writing – original draft. DY: Conceptualization, Investigation, Writing – original draft. CJ: Investigation, Visualization, Writing – original draft. FK: Writing – review & editing. YJ: Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion Open Funding Project (NCRCOP2023007), First Teaching Hospital of Tianjin University of Traditional Chinese Medicine TuoXin Project (2023008), and Special Fund for Clinical Research of Wu Jieping Medical Foundation (320.6750.2023-10-5).
AcknowledgmentsThanks to all the authors for their contributions to this article, and thanks to the institution for its support.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
2. Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. (2024) 46:221–31. doi: 10.3760/cma.j.cn112152-20240119-00035
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
PubMed Abstract | Crossref Full Text | Google Scholar
4. Muro K, Van Cutsem E, Narita Y, Pentheroudakis G, Baba E, Li J, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. (2019) 30:19–33. doi: 10.1093/annonc/mdy502
PubMed Abstract | Crossref Full Text | Google Scholar
5. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). (2021) 41:747–95. doi: 10.1002/cac2.12193
PubMed Abstract | Crossref Full Text | Google Scholar
6. Zhu Y, Liu K, Zhu H, Wu H. Immune checkpoint inhibitors plus chemotherapy for HER2-negative advanced gastric/gastroesophageal junction cancer: a cost-effectiveness analysis. Therap Adv Gastroenterol. (2023) 16:17562848231207200. doi: 10.1177/17562848231207200
PubMed Abstract | Crossref Full Text | Google Scholar
7. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:234–47. doi: 10.1016/S1470-2045(21)00692-6
PubMed Abstract | Crossref Full Text | Google Scholar
8. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
PubMed Abstract | Crossref Full Text | Google Scholar
9. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
PubMed Abstract | Crossref Full Text | Google Scholar
10. Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. (2014) 20:2831–7. doi: 10.1158/1078-0432.CCR-13-3141
PubMed Abstract | Crossref Full Text | Google Scholar
13. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
PubMed Abstract | Crossref Full Text | Google Scholar
14. Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:844–60. doi: 10.1016/j.annonc.2020.03.304
PubMed Abstract | Crossref Full Text | Google Scholar
15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
PubMed Abstract | Crossref Full Text | Google Scholar
16. Janjigian YY, Ajani JA, Moehler M, Shen L, Garrido M, Gallardo C, et al. First-line nivolumab plus chemotherapy for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma: 3-year follow-up of the phase III checkMate 649 trial. J Clin Oncol. (2024) 42:2012–20. doi: 10.1200/JCO.23.01601
PubMed Abstract | Crossref Full Text | Google Scholar
17. Xu JM, Jiang HP, Pan YY, Gu KS, Cang SD, Han L, et al. Abstract CT078: First-line treatment with sintilimab (sin) vs placebo in combination with chemotherapy (chemo) in patients (pts) with unresectable gastric or gastroesophageal junction (G/GEJ) cancer: Final overall survival (OS) results from the randomized, phase III ORIENT-16 trial. Cancer Res. (2023) 83:CT078. doi: 10.1158/1538-7445.AM2023-CT078
Crossref Full Text | Google Scholar
18. Moehler M, Kato K, Arkenau T, Oh DY, Tabernero J, Correa M, et al. Rationale 305: Phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J Clin Oncol. (2023) 41:abstr 286. doi: 10.1200/JCO.2023.41.4_suppl.286
Crossref Full Text | Google Scholar
19. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:1571–80. doi: 10.1001/jamaoncol.2020.3370
PubMed Abstract | Crossref Full Text | Google Scholar
20. Xie T, Zhang Z, Zhang X, Qi C, Shen L, Peng Z. Appropriate PD-L1 cutoff value for gastric cancer immunotherapy: A systematic review and meta-analysis. Front Oncol. (2021) 11:646355. doi: 10.3389/fonc.2021.646355
PubMed Abstract | Crossref Full Text | Google Scholar
21. Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. (2012) 23:2656–62. doi: 10.1093/annonc/mds104
留言 (0)