Ochrobactrum intermedium (O. intermedium) is a gram-negative, non-fermenting bacterium closely related to Brucella genus (1). O. intermedium resembles a nascent human pathogen that has rarely been detected so far (1–8). To date, there are only 8 case reports of an infection with O. intermedium (1–8). They are found in immunocompromised and immunocompetent patients and the clinical presentation ranges from severe bacteraemia to incidental findings (1–8).
A musculoskeletal infection with O. intermedium has not been reported in the literature. We present the first case of an osteosynthesis-associated infection (OAI) with O. intermedium in a 80-year-old female patient after osteosynthesis of a posterior column acetabular fracture type 62A2.2 according to the AO classification via a Kocher-Langenbeck approach.
Case descriptionThe historical and current information from this episode of care is organized as a timeline in Figure 1. An 80-year-old female was admitted to the emergency department with pain in her left hip. Six months previously she had undergone open reduction and internal plate fixation of the acetabular fracture via a Kocher-Langenbeck approach. At the time of osteosynthesis, there was no indication of immune deficiency. The patient had no history of frequent infections or infections with opportunistic pathogens. The serological infection parameters were normal at the time of osteosynthesis (Leukocytes 10,870/µl, C-Reactive Protein 2.5 mg/L). The postoperative period was without complications and primary wound healing occurred. The patient demonstrated tenderness, redness and swelling at the scar as well as a fistula. The x-ray showed posttraumatic femoral head necrosis with partial protrusion (Figure 2). Two of the screws communicated with the hip joint as confirmed by computed tomography. Laboratory markers were without pathological findings regarding infection (Leukocytes 6,450/µl, C-Reactive Protein 3.6 mg/L). The patient's medical history showed hypertension, hyperlipidaemia and a cholecystectomy. Her Body Mass Index (BMI) was 24 kg/m2. The patient had a history of anterior pelvic ring injury with fracture of the left inferior and superior pubic ramus five years ago, which was treated conservatively.
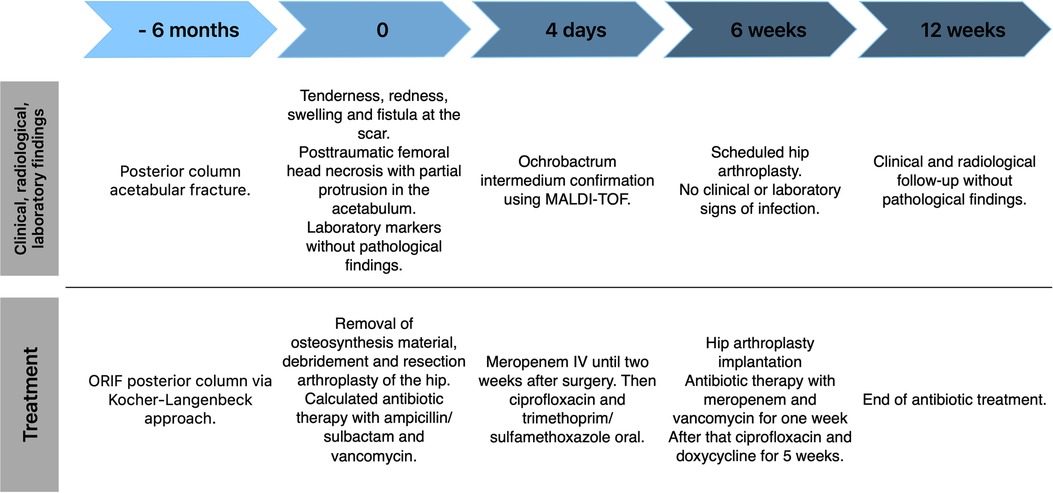
Figure 1. Case report timeline.

Figure 2. (A) AP pelvis x-ray showing partial femoral head necrosis and acetabular protrusion after open reduction and internal plate fixation of a posterior column acetabular fracture via a Kocher-Langenbeck approach. (B) Postoperative anterior posterior (AP) Pelvis 6 weeks after implantation of a hybrid cemented total hip arthroplasty using a cementless revision cup with additional screw fixation and a cemented stem.
Due to the fistula and the clinical presentation, OAI was assumed. Because of the communication of the osteosynthesis material with the hip joint and the femoral head necrosis, an infection of the hip joint was expected as well. A two-stage revision was planned. In the first stage, removal of the osteosynthesis material, debridement and resection arthroplasty of the hip joint with removal of the femoral head (Girdlestone procedure) was performed. Due to the risk of protrusion into the pelvis following the acetabular fracture, we did not use a spacer. Calculated postoperative antibiotic therapy was performed with ampicillin/sulbactam and vancomycin. All of the seven intraoperative microbiological cultures from the site of the osteosynthesis and from the hip joint yielded O. intermedium. The pathogen was determined using the matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) method. The antibiotic susceptibility profile is displayed in Table 1.
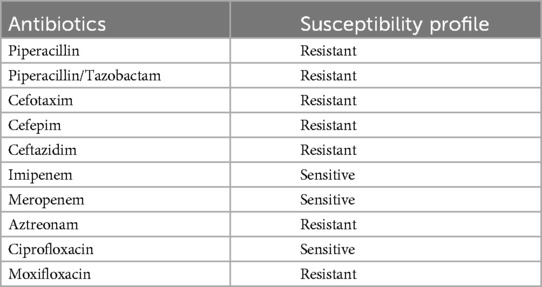
Table 1. Antibiotics susceptibility table for Ochrobactrum intermedium.
After detection of the pathogen, the antibiotic therapy was changed to meropenem 3 × 1 g IV. After IV therapy for two weeks, the therapy was changed to oral antibiotics with ciprofloxacin 2 × 750 mg and cotrimoxazole 3 × 960 mg. The antimicrobial therapy was planned in consultation with an infectious disease specialist according to the Pro-Implant Foundation criteria.
Mobilization was performed with minimal weight bearing on crutches until the final hip arthroplasty implantation. The inpatient stay was 14 days and without complications. Wound healing was normal. The laboratory infection parameters, which increased after the operation and continuously decreased until discharge.
Six weeks after debridement and resection arthroplasty, the scar showed no signs of infection and the inflammatory biomarkers were in the normal range (Leukocytes 6,010/µl, C-Reactive Protein 1.6 mg/L). Implantation of the hip arthroplasty was performed via the same posterolateral approach using a cementless revision cup with screw fixation (Plasmafit® Revision, Aesculap, Tuttlingen, Germany) for the acetabulum and a cemented standard stem (CoreHip®, Aesculap, Tuttlingen, Germany). The postoperative antibiotic therapy consisted of meropenem 3 × 1 g and vancomycin 2 × 1 g IV for one week. After that, an oral antibiotic therapy with ciprofloxacin 2 × 750 mg and doxycycline 2 × 100 mg was started and continued for another 5 weeks. Application of local antibiotics was not performed in this case. After hip arthroplasty the wound healing proceeded normally and the infection parameters decreased until discharge. After implantation of the hip arthroplasty, the patient was allowed full weight bearing.
At the 6-week follow-up the patient showed no pain and no clinical or laboratory signs of infection. Radiological control showed a normal position of the hip arthroplasty without signs of loosening or periprosthetic fracture (Figure 2).
24 Months after implantation of the hip arthroplasty the patient is free of infection and satisfied with the result.
DiscussionO. intermedium is a gram-negative, non-fermenting organism and represents an emerging species that was first separated from O. anthropi in 1998 since standard cultivation and tests did not allow an exact differentiation (9). Until then the non-fermenting bacterium Ochrobactrum anthropi has been the only species of the Ochrobactrum genus (9).
Sharing nearly 99% of its 16S rRNA, O. intermedium has a closer relation to the Brucella genus than other species of Ochrobactrum. Differentiation is only possible with advanced molecular tests like 16S rDNA gene sequencing recA-PCR RFLP (Restriction Fragment Length Polymorphism), and the MALDI-TOF method (9). In our case O. intermedium was specified using the MALDI-TOF method.
So far, the non-fermenting bacterium Ochrobactrum anthropi has been the only species of the genus (9). Ochrobactrum, a common environmental pathogen found in soil and water, is closely related to Brucella spp. due to their similar genetic and physiological characteristics (10).
Ochrobactrum anthropic could be isolated in various clinical specimens and seems to be part of the flora of the large intestine (11). In contrast to O. anthropic, only a few cases of O. intermedium infections have been described in the literature. Möller et al. presented the first case of an infection with O. intermedium in 1999 in a liver transplanted patient with bacteraemia and liver abscesses due to O. intermedium (1). Other case reports include immunocompromised patients with bowel obstruction and bladder cancer as well as a case of endocarditis and bacteraemia after cholangitis (2–4). Other case reports present immunocompetent patients with appendicitis complicated by a perirectal abscess and a catheter-associated infection after a stroke (5, 6). In one case O. intermedium was found incidentally (7). In our case the patient's comorbidities were limited to hypertension, hyperlipidaemia and a cholecystectomy many years ago. There was no history of frequent or opportunistic infections or other chronic diseases. Malnutrition might have played a role in the patient with a BMI of 24 kg/m2, but since it was only slightly below 25 kg/m2, we did not rate this factor to be clinically relevant. We consider the patient to be immunocompetent.
This is the first case to present an implant related infection with O. intermedium.
A limitation of this case report that should be acknowledged is the mid-term follow-up period of 24 months, so far. We will continue to follow the patient after hip arthroplasty implantation.
Identification of the pathogen in an osteosynthesis-associated infection or a periprosthetic joint infection is crucial in order to be able to initiate targeted therapy. Culture negative infection is associated with worse treatment success in periprosthetic joint infections (12). The challenge lies in the identification of O. intermedium and highlights the potential of advanced molecular tests like 16S rDNA gene sequencing recA-PCR RFLP and the MALDI-TOF method.
ConclusionInfection with O. intermedium is rarely identified due to the need of advanced molecular tests for its differenciation. Still O. intermedium has been recognized as pathogen seen in immunodeficient and immunocompetent patients. We described the first case of an osteosynthesis-associated infection with O. intermedium in an 80-year-old patient after osteosynthesis for acetabular fracture. With this case report we would like to increase awareness of possible implant-associated bacterial infections caused by O. intermedium. Two-stage hip arthroplasty with implant removal, debridement and resection arthroplasty in the first stage and hip arthroplasty implantation in the second stage with targeted antibiotic therapy led to a good short-term result in this patient.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementEthical approval was not required for the studies involving humans because this is a case report of a standard clinical treatment. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The patient provided written informed consent for the publication of this case report. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsDK: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. KH: Resources, Supervision, Writing – review & editing, Investigation, Methodology, Validation. AT: Methodology, Supervision, Writing – review & editing. SW: Data curation, Investigation, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Möller LVM, Arends JP, Harmsen HJ, Talens A, Terpstra P, Slooff MJ. Ochrobactrum intermedium infection after liver transplantation. J Clin Microbiol. (1999) 37(1):241–4. doi: 10.1128/JCM.37.1.241-244.1999
Crossref Full Text | Google Scholar
2. Kassab I, Sarsam N, Affas S, Ayas M, Baang JH. A case of Ochrobactrum intermedium bacteremia secondary to cholangitis with a literature review. Cureus. (2021) 13(4):e14648. doi: 10.7759/cureus.14648
PubMed Abstract | Crossref Full Text | Google Scholar
3. Apisarnthanarak A, Kiratisin P, Mundy LM. Evaluation of Ochrobactrum intermedium bacteremia in a patient with bladder cancer. Diagn Microbiol Infect Dis. (2005) 53(2):153–5. doi: 10.1016/j.diagmicrobio.2005.05.014
PubMed Abstract | Crossref Full Text | Google Scholar
4. Bharucha T, Sharma D, Sharma H, Kandil H, Collier S. Ochromobactrum intermedium: an emerging opportunistic pathogen—case of recurrent bacteraemia associated with infective endocarditis in a haemodialysis patient. New Microbes New Infect. (2017) 15:14–5. doi: 10.1016/j.nmni.2016.09.016
PubMed Abstract | Crossref Full Text | Google Scholar
5. Hirai J, Yamagishi Y, Sakanashi D, Koizumi Y, Suematsu H, Mikamo H. A case of bacteremia caused by ochrobacterium Intermedium. Kansenshogaku Zasshi. (2016) 90(2):129–33. doi: 10.11150/kansenshogakuzasshi.90.129
PubMed Abstract | Crossref Full Text | Google Scholar
6. Vaidya SA, Citron DM, Fine MB, Murakami G, Goldstein EJ. Pelvic abscess due to Ochrobactrum intermedium [corrected] in an immunocompetent host: case report and review of the literature. J Clin Microbiol. (2006) 44(3):1184–6. doi: 10.1128/JCM.44.3.1184-1186.2006
PubMed Abstract | Crossref Full Text | Google Scholar
7. Dharne MS, Misra SP, Misra V, Dwivedi M, Patole MS, Shouche YS. Isolation of urease-positive Ochrobactrum intermedium in the stomach of a non-ulcer dyspeptic patient from north India. J Microbiol Immunol Infect. (2008) 41(2):183–6.18473108
PubMed Abstract | Google Scholar
8. Jacobs DJ, Grube TJ, Flynn HW Jr., Greven CM, Pathengay A, Miller D, et al. Intravitreal moxifloxacin in the management of Ochrobactrum intermedium endophthalmitis due to metallic intraocular foreign body. Clinical Ophthalmol. (2013) 7:1727–30. doi: 10.2147/OPTH.S44212
Crossref Full Text | Google Scholar
9. Velasco J, Romero C, Lopez-Goni I, Leiva J, Diaz R, Moriyon I. Evaluation of the relatedness of Brucella spp. And Ochrobactrum Anthropi and description of Ochrobactrum intermedium Sp. Nov., a new Species with a closer relationship to Brucella spp. Int J Syst Bacteriol. (1998) 48(Pt 3):759–68. doi: 10.1099/00207713-48-3-759
PubMed Abstract | Crossref Full Text | Google Scholar
10. Holmes B, Popoff M, Kiredjian M, Kersters K. Ochrobactrum Anthropi gen. Nov., Sp. Nov. From human clinical specimens and previously known as group vd. Int J Syst Evol Microbiol. (1988) 38(4):406–16. doi: 10.1099/00207713-38-4-406
Crossref Full Text | Google Scholar
11. Alnor D, Frimodt-Meller N, Espersen F, Frederiksen W. Infections with the unusual human pathogens Agrobacterium Species and Ochrobactrum Anthropi. Clin Infect Dis. (1994) 18(6):914–20. doi: 10.1093/clinids/18.6.914
PubMed Abstract | Crossref Full Text | Google Scholar
12. Tan TL, Kheir MM, Shohat N, Tan DD, Kheir M, Chen C, et al. Culture-Negative periprosthetic joint infection: an update on what to expect. JB JS Open Access. (2018) 3(3):e0060. doi: 10.2106/JBJS.OA.17.00060
留言 (0)