The guidelines for treating basal cell carcinoma (BCC) suggest standard surgical excision with a 4–5 mm margin for low-risk cases. For high-risk BCCs, Mohs micrographic surgery (MMS) is the preferred method1. MMS involves immediate horizontal section-guided surgical excision, with the standard procedure involving frozen section microscopy of the excised tumour, ideally under direct observation of the operating surgeon. The surgical margins are further excised till the sections return negative for tumour cells, which involves elaborate tissue processing and repeated examination of multiple frozen sections which is time-consuming and delays wound closure. Owing to the above reasons and the high operational costs, MMS is mostly unavailable in developing countries. An alternative variation named “en face” frozen sectioning, has been reported,2,3 but this technique requires larger surgical margins of up to 4 mm and tangential histopathology sections, which may be more difficult to orient as compared to the horizontal sections of MMS.
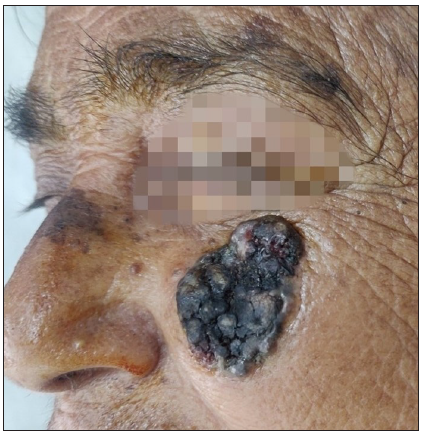
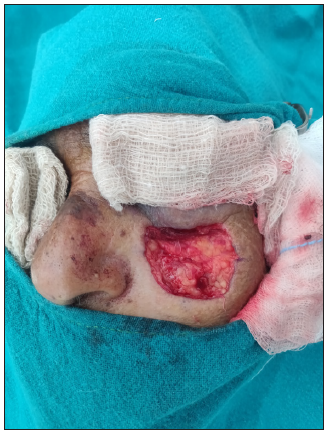
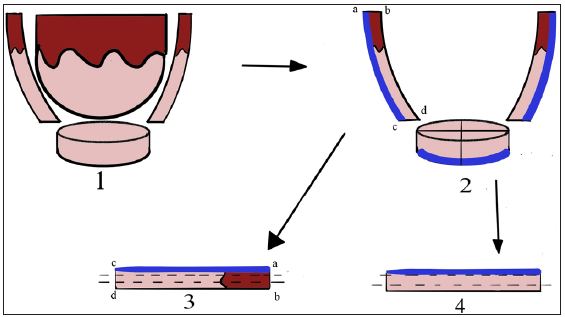
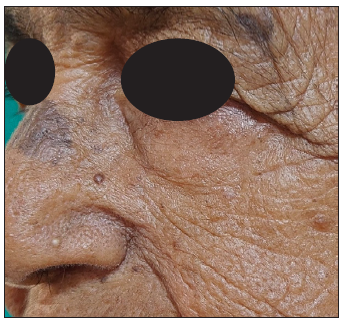
SolutionA pre-operative dermoscopy is performed to mark the boundary of the healthy skin around the pigmented BCCs. The excision is done using an incision line abutting the tumour ensuring complete excision on the surface and the specimen is sent for routine paraffin sectioning. The deeper and side margins of the tumour are removed until the end of the pigmented area, superficial fat lobules or altered consistency, whichever is the deepest. The defect’s edge is then divided into clock-face-like four quadrants, labelled I (12 to 3 o’clock), II (3 to 6 o’clock), III (6 to 9 o’clock), and IV (9 to 12 o’clock). Next, a strip of tissue with 1–2 mm skin width is excised from the edge and a 1–2 mm disc from the floor of the wound is similarly excised and both are divided as above [Figures 1a–c]. The inner edges are inked using a marking pen and the inner part of the deeper disc is labelled with micropore tape, and the tissue bits are transported to pathology on a labelled gauze. After washing, the outer and deeper edges are inked in the pathology department before freezing. The side margins are flipped and positioned horizontally on the slides and thereafter the side and deep margins are processed separately. Both undergo horizontal sectioning at a thickness of 4–5 microns commencing from the outermost/deeper edges [Figure 1c]. If the outermost/deepest sections return tumour negative, then further frozen sectioning is suspended and the wound is closed. If however, any slide shows the tumour, then the corresponding parts of the post-excision wound undergo proportionate re-excision until the margins return tumour negative. In both scenarios, the remaining marginal tissue is further subjected to routine paraffin sectioning and, if needed, immunohistochemistry. The wound healing using the appropriate closure technique is excellent as repeated tissue handling is avoided [Figure 2].
Export to PPT
Export to PPT
Export to PPT
Export to PPT
留言 (0)