Hidradenitis suppurativa (HS) is a chronic inflammatory process of the skin resulting in high morbidity (1) and profound patient distress (2). It has a prevalence of 1–4% and more commonly occurs in women aged 20–40 years old (2). Approximately 13% of these patients eventually develop severe, Hurley stage III disease, often experiencing rapid disease progression (3). The exact etiology and pathogenesis remain unclear. However, the disease is characterized by follicular occlusion and rupture, followed by a consequent inflammatory response that drives the immune activation necessary for clinical disease progression (4). HS has been associated with various laboratory anomalies reflecting an underlying chronic inflammatory state, including anemia, thrombocytopenia, and leukocytosis. These abnormalities are common in those with severe HS but can normalize with the initiation of immunomodulatory treatment (5). Recently, the immune response in HS has been of great interest to researchers for purposes of understanding disease progression and identifying immune targets for therapy. Increasingly, reports have described elevated immunoglobulins such as IgG in the HS disease process, hinting toward an exaggerated immune response taking place (6–9). Separately, IgG4 has been a recent topic of intense immunologic investigation for its role as a mediator of certain rheumatologic diseases, including IgG4-RD, rheumatoid arthritis (RA), Sjogren’s syndrome, and systemic lupus erythematosus, for instance (10). Herein, we describe the first report of IgG4 elevation in a patient with HS. A review of the literature, including the causes of IgG4 elevation and the role of serum immunoglobulins in HS, is discussed.
Case presentationA 23-year-old woman with polycystic ovary syndrome and a 10-year history of Hurley stage III HS was treated for several years with a combination of clindamycin, rifampin, and doxycycline. She was then started on adalimumab for uncontrolled HS. One year later, she presented to her primary care provider with a 1-month history of progressive weight loss, nausea, malaise, and persistent fevers. She was treated for a presumed urinary tract infection with cefdinir.
After a lack of improvement, she was then evaluated with a computed tomography (CT), which found heterogeneous enhancement of the kidneys bilaterally, without stranding or abscess. She was subsequently admitted for antibiotic management for treatment of presumed pyelonephritis. However, despite treatment with ceftriaxone and later cefepime, her symptoms did not improve. A repeat CT was unchanged, arguing against pyelonephritis as the source of her fever, fatigue, and other symptoms. In addition to persistent fevers and tachycardia despite antibiotic treatment, she noted jaw swelling on admission, which progressively worsened throughout her hospital stay (Figure 1).

Figure 1. Patient timeline summarizing notable treatment changes and clinical events.
Infectious workup was notable for positive monospot, negative Epstein–Barr virus IgM and polymerase chain reaction (PCR), and positive viral capsid IgGS but was otherwise negative. Rheumatologic workup revealed elevated total IgG and IgG4 serologies (Table 1), along with elevated lysozyme and hypercalcemia. Neck CT imaging was then performed and revealed sialadenitis of the parotids and submandibular glands. Positron emission tomography/computed tomography revealed no evidence of malignant or clonal disease. Instead, it identified hypermetabolic cutaneous thickening of the axillae, inguinal folds, and upper thighs, which was consistent with an infectious or inflammatory process.
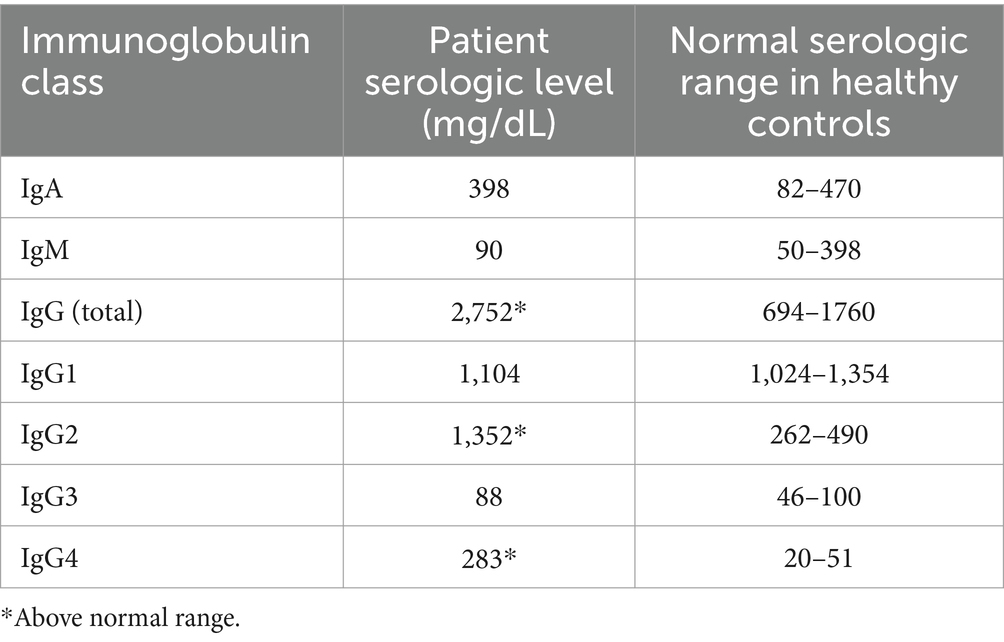
Table 1. Summary of serum IgA, IgM, IgG, and respective subclass levels in our patient compared to healthy controls (20, 21).
Due to the parotitis and concern for IgG4-RD, cervical lymph node excision and parotid gland biopsies were conducted. Biopsy showed focal chronic sialadenitis and focal fibrosis, and IgG4 immunohistochemical stain of the biopsies revealed that less than 5% of IgG-positive plasma cells were IgG4-positive. Her persistent fevers and jaw swelling resolved by the day of discharge, with no clear underlying cause identified in addition to a positive monospot test. Her adalimumab was held upon admission due to infection concerns, and she was discharged on triple antibiotic therapy with metronidazole, moxifloxacin, and rifampin.
At 1-month follow-up with dermatology, the patient was started on spironolactone, had her antibiotics switched to doxycycline, and received intralesional triamcinolone injections. At a later follow-up with rheumatology, the patient was found to have persistently high IgG4 levels along with elevated lysozyme. Despite a lack of symptoms and non-diagnostic biopsy results for either pathology, the patient was started on azathioprine for suspicion of IgG4-RD vs. sarcoidosis. Six months later, she presented to dermatology in an active HS flare with many draining inflamed nodules and interconnected sinus tracts in axillae, inguinal folds, and mons pubis. Her laboratory results were notable for absolute neutrophilia and a white blood cell count of 18 × 109/L, slightly elevated compared to her hospital admission at 16.7 × 109/L. She received seven injections of intralesional triamcinolone to the nodules, and her antibiotics were switched to levofloxacin for bacterial coverage. She was referred to plastic surgery for urgent debridement of the right axillary skin with a split-thickness skin graft, which was resolved with vacuum-assisted closure and physical therapy. After surgery, secukinumab was started, and spironolactone, rifampin, and clindamycin were continued. At a 3-month follow-up, she presented with a well-healed right axilla and an active flare in the left axilla.
DiscussionThe role of IgG4 across varying disease states is nuanced and a topic of continued investigation. IgG4 has the potential to activate or inhibit inflammation depending on the context of the specific disease state (10). Elevated serum IgG4 has thus been described in a wide range of conditions (11) including autoimmune diseases, cancer, and infections. Discussion of the source of IgG4 elevation is of great importance because management differs depending on the underlying pathology. One report has demonstrated that cancer cells can utilize the inhibitory effect of IgG4 to achieve host immune evasion (12). IgG4 elevation has also been reported in chronic inflammatory states such as RA. Patients with RA and elevated IgG4 out of proportion to total IgG were found to have higher levels of total antibodies, decreased responsiveness to standard RA treatments, and higher disease activity (13). Another report described the association of elevated IgG4 levels with IgG4-RD. This complex clinical condition is characterized by elevated IgG4 levels and infiltration of IgG4-positive plasma cells into organs which drives an inflammatory response. This can present as the enlargement of one or more organs, including the salivary glands, pancreas, and kidney most commonly. In this case, a patient with IgG4 elevation in serum and salivary gland swelling was concerned for the complex IgG4-RD leading to invasive testing for malignancy and heavy immunosuppression. Treatment includes glucocorticoids and adjuvant immunosuppressants, depending on the context (14). Diagnosis primarily relies on tissue biopsy demonstrating typical histopathologic findings combined with an increased tissue presence of IgG4-positive plasma cells. The suggestive morphologic findings on tissue biopsy include storiform fibrosis, dense lymphoplasmacytic infiltrate, and obliterative phlebitis. Elevated IgG4 serologies or IgG4 cell count are both non-specific findings (15). Therefore, excluding other causes of IgG4 elevation is a critically important step in the diagnosis and workup of IgG4-RD.
Although the exact role of IgG in the etiopathogenesis of HS is still being investigated, current research supports that total immunoglobulins are elevated in severe disease (6, 7). The presence of IgG antibodies has been established in both the serum and skin of patients with HS (8, 16). Correlating skin and serum levels of IgG can serve as an accurate predictor of HS disease severity (7, 16) with a threshold of approximately 1,300 mg/mL being predictive of Hurley Stage III disease (7). Our patient had a serum IgG of 2,700 mg/mL that was markedly above the proposed threshold (Table 1). These serologic findings correlated with our clinical observations of her severe Hurley stage III disease. Laboratory analysis demonstrated other known findings of longstanding inflammation in the setting of severe HS, including leukocytosis. In part with the total elevation of the IgG subclass, some degree of IgG4 elevation could thus be expected in the setting of severe HS. Notably, tumor necrosis factor-alpha inhibitors have a variable impact on IgG4 levels (17–19). Long-term treatment with adalimumab and infliximab may result in the development of anti-drug IgG and IgG4 antibodies, and reports have shown that anti-drug IgG4 antibodies are present in 27 and 29% of patients receiving infliximab (17) and adalimumab (18), respectively. Within the context of RA, reports have demonstrated that sustained treatment with adalimumab generally decreased total IgG4 levels (19), so serologic elevation may reflect underlying disease severity. While our patient may have developed anti-adalimumab IgG4 antibodies, it is unclear to what extent these would contribute to her total IgG4 serologic elevation more broadly. Considering the extensive infectious, rheumatologic, and oncologic workups that were negative in our patient, severe HS disease is a reasonable explanation for IgG and IgG4 elevation.
Herein, we describe the first case in the literature of HS associated with elevated IgG4 with no other contributing etiology for the elevated IgG4. Long-term follow-up is needed to monitor how IgG4 levels change over time in our patient on biologic therapy to correlate any decrease or normalization of total IgG and IgG4 with clinical improvement in HS.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsAG: Conceptualization, Investigation, Writing – original draft. MF: Supervision, Writing – review & editing. CB: Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. von der Werth, JM, and Jemec, GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. (2001) 144:809–13. doi: 10.1046/j.1365-2133.2001.04137.x
Crossref Full Text | Google Scholar
2. Seyed Jafari, SM, Hunger, RE, and Schlapbach, C. Hidradenitis suppurativa: current understanding of pathogenic mechanisms and suggestion for treatment algorithm. Front Med. (2020) 7:68. doi: 10.3389/fmed.2020.00068
PubMed Abstract | Crossref Full Text | Google Scholar
3. Vanlaerhoven, AMJD, Ardon, CB, van Straalen, KR, Vossen, ARJV, Prens, EP, and van der Zee, HH. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. (2018) 234:232–3. doi: 10.1159/000491547
PubMed Abstract | Crossref Full Text | Google Scholar
4. Napolitano, M, Megna, M, Timoshchuk, EA, Patruno, C, Balato, N, Fabbrocini, G, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. (2017) 10:105–15. doi: 10.2147/CCID.S111019
Crossref Full Text | Google Scholar
5. Morss-Walton, PC, Greif, C, Holcomb, ZE, Salian, P, Yankama, T, Kim, D, et al. Treatment of hidradenitis suppurativa resolves associated hematologic abnormalities. Int J Womens Dermatol. (2022) 8:e011. doi: 10.1097/JW9.0000000000000011
PubMed Abstract | Crossref Full Text | Google Scholar
6. Byrd, AS, Carmona-Rivera, C, O'Neil, LJ, Carlucci, PM, Cisar, C, Rosenberg, AZ, et al. Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci Transl Med. (2019) 11:1–12. doi: 10.1126/scitranslmed.aav5908
PubMed Abstract | Crossref Full Text | Google Scholar
8. Hoffman, LK, Tomalin, LE, Schultz, G, Howell, MD, Anandasabapathy, N, Alavi, A, et al. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS One. (2018) 13:e0203672. doi: 10.1371/journal.pone.0203672
Crossref Full Text | Google Scholar
9. Frew, JW, Grand, D, Navrazhina, K, and Krueger, JG. Beyond antibodies: B cells in hidradenitis suppurativa: bystanders, contributors or therapeutic targets? Exp Dermatol. (2020) 29:509–15. doi: 10.1111/exd.14092
Crossref Full Text | Google Scholar
11. Ebbo, M, Grados, A, Bernit, E, Vély, F, Boucraut, J, Harlé, JR, et al. Pathologies associated with serum IgG4 elevation. Int J Rheumatol. (2012) 2012:602809. doi: 10.1155/2012/602809
Crossref Full Text | Google Scholar
12. Wang, H, Xu, Q, Zhao, C, Zhu, Z, Zhu, X, Zhou, J, et al. An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. J Immunother Cancer. (2020) 8:e000661. doi: 10.1136/jitc-2020-000661
PubMed Abstract | Crossref Full Text | Google Scholar
13. Chen, LF, Mo, YQ, Ma, JD, Luo, L, Zheng, DH, and Dai, L. Elevated serum IgG4 defines specific clinical phenotype of rheumatoid arthritis. Mediat Inflamm. (2014) 2014:635293. doi: 10.1155/2014/635293
PubMed Abstract | Crossref Full Text | Google Scholar
15. Deshpande, V, Zen, Y, Chan, JK, Yi, EE, Sato, Y, Yoshino, T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. (2012) 25:1181–92. doi: 10.1038/modpathol.2012.72
PubMed Abstract | Crossref Full Text | Google Scholar
16. Carmona-Rivera, C, O'Neil, LJ, Patino-Martinez, E, Shipman, WD, Zhu, C, Li, QZ, et al. Autoantibodies present in hidradenitis suppurativa correlate with disease severity and promote the release of Proinflammatory cytokines in macrophages. J Invest Dermatol. (2022) 142:924–35. doi: 10.1016/j.jid.2021.07.187
PubMed Abstract | Crossref Full Text | Google Scholar
17. Vultaggio, A, Nencini, F, Carraresi, A, Pratesi, S, Movérare, R, Eriksson, C, et al. IgG4 anti-infliximab in treated patients: clinical impact and temporal evolution. Allergy. (2018) 73:2172–81. doi: 10.1111/all.13471
PubMed Abstract | Crossref Full Text | Google Scholar
18. van Schouwenburg, PA, Krieckaert, CL, Nurmohamed, M, Hart, M, Rispens, T, Aarden, L, et al. IgG4 production against adalimumab during long term treatment of RA patients. J Clin Immunol. (2012) 32:1000–6. doi: 10.1007/s10875-012-9705-0
PubMed Abstract | Crossref Full Text | Google Scholar
19. Sakthiswary, R, Shaharir, SS, and Wahab, AA. Frequency and clinical significance of elevated IgG4 in rheumatoid arthritis: a systematic review. Biomedicine. (2022) 10:558. doi: 10.3390/biomedicines10030558
Crossref Full Text | Google Scholar
20. Pellicano, C, Colalillo, A, Cusano, G, Palladino, A, Pellegrini, M, Callà, CAM, et al. Serum immunoglobulin G (IgG) subclasses in a cohort of systemic sclerosis patients. J Pers Med. (2023) 13:309. doi: 10.3390/jpm13020309
PubMed Abstract | Crossref Full Text | Google Scholar
21. Gonzalez-Quintela, A, Alende, R, Gude, F, Campos, J, Rey, J, Meijide, LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. (2008) 151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x
留言 (0)