Hypertension is a common chronic disease causing cardiac and cerebrovascular complications, increasing deaths annually (1). Despite the availability of a wide range of antihypertensive medications, 71% of patients fail to control their blood pressure below 130/80 mmHg (2) (2023 ESH Arterial Hypertension Management Guidelines European Society of Hypertension Arterial Hypertension Management Working Group: Recognized by the International Society of Hypertension (ISH) and the European Kidney Association (ERA)—doi: 10.1097/HJH0000000003480). At present, the mechanisms underlying the development of hypertension are still not fully understood clinically. Although there are a large number of antihypertensive drugs that can act on this type of disease, the majority of patients are still not effectively controlled (3). This highlights the urgent need for further improvement in risk assessment. Despite recent significant progress, identifying and managing primary and secondary hypertension still faces significant challenges.Therefore, there is an urgent clinical need for treatments and drugs with better efficacy.
Hypertension is the result of a combination of environmental and genetic factors, an important one being low-grade inflammation (4). Hypertension is caused by the activation of complement (inflammasome) and changes in the phenotype of circulating immune cells, especially bone marrow cells. These inflammatory events are interdependent and ultimately participate in the progression of hypertension through mechanisms involving oxidative stress, endogenous protein modifications, and alterations in antigen processing and presentation (5). Microvascular remodeling, autonomic nervous system (ANS) imbalance, and RAAS activation are the main pathological mechanisms of hypertension (6). Inflammation has been found to be associated with endothelial cell activation and dysfunction in hypertension, and the two pathologic processes are interrelated. Clinical observation evidence and animal experiments both indicate that inflammation plays an important role in the development of hypertension (7). The biomarkers of inflammation, including high sensitivity C-reactive protein, various cytokines, and products of complement pathways, are elevated in hypertensive patients (8). The inflammation-mediated endothelial cell activation is an important step in triggering hypertension. Both innate immunity and adaptive immunity can promote blood pressure elevation by triggering vascular inflammation and microvascular remodeling. For example, classically activated macrophages (M1), neutrophils, and dendritic cells cause hypertension by secreting inflammatory cytokines. Interferon gamma (IFN - γ) and interleukin-17 (IL-17) induce oxidative stress damage and endothelial dysfunction, leading to hypertension (6). Long term inflammatory processes can increase the production of ROS, leading to oxidative stress and endothelial dysfunction, regulating the tension and structure of blood vessels (9). It can provide a substrate for the inflammation of primary hypertension (2). While, hypertension also deteriorates the endothelium, which leads to further inflammation, deteriorating blood pressure in a vicious cycle. Hypertension is proved accompanied by the activation of inflammasome complement and circulating immune cells (8). It is suggested that inflammatory factors are involved in HP progression or are associated with patient mortality, but the exact correlation is unknown.
Mendelian randomization (MR) is an analytical method that uses genetic variation in non-experimental data to infer causal effects of exposure on outcomes (10). Because alleles are randomly assigned during meiosis, MR reduces traditional confounding variables and reverse causation, providing better evidence for causal inference (11). MR analysis of two samples allows researchers to assess instrument-exposure and instrument-outcome associations in two independent population samples, thereby enhancing the applicability and validity of the test (12). Therefore, we aimed to utilize serum data from Finnish patients with hypertension and the International Consortium on Blood Pressure (ICBP) Genome-Wide Associated Study (GWAS) population to perform Mendelian randomization (MR) analyses, in order to further explore the correlation between specific 43 inflammatory cytokines and hypertension progression, and then to further explore risk factors for hypertension stratification.
Material and methods Study designA two-sample Mendelian randomization (MR) study was conducted to assess the causal relationship between systemic inflammatory regulators and hypertension (primary or secondary types). All data used in this study were obtained from previously published research, with informed consent already acquired in the original studies. We first employed genetic variants associated with inflammatory regulators to determine their causal relationship with hypertension. In our study, the chosen valid instrumental variables (SNPs) strictly adhered to the three assumptions of Mendelian randomization analysis.
Data source for systemic inflammatory regulatorsIn this study, we utilized a large-scale, published genome-wide association study (GWAS) meta-analysis encompassing 41 cytokines, representing the most comprehensive data set available to date (PMID: 27989323). The study involved 8,293 Finnish participants from three independent population cohorts: the Cardiovascular Risk in Young Finns Study (YFS), FINRISK1997, and FINRISK2002. Cytokines were measured in EDTA plasma from FINRISK1997, heparin plasma from FINRISK2002, and serum from YFS. Subjects with cytokine concentrations below or above the detection limits of laboratory analysis were omitted from the analyses. Additionally, cytokines with more than 90% missing values (7 out of 48) were excluded from the study. Generally, the YFS participants are generally younger than those in the FINRISK1997 and FINRISK2002 studies, with an average age of 37 compared to 60 years. More detailed characteristics of above three study cohorts are reported in Supplementary Table S1.
Data sources for hypertensionGWAS summary data for primary or secondary hypertension were both obtained from FinnGen consortium R9 release data. The FinnGen study aims to produce genomic data with linkage to national health register data of 500,000 Finnish individuals, which is enriched for disease end points (PMID: 36653562). The end point “Hypertension, essential” and “Secondary hypertension” were used in this study. These two GWAS consisted of 92,462 cases and 265,626 controls and 2,597 cases and 191,924 controls, respectively, and the median age at first event was 65 years for “Hypertension, essential” and 57 years for “Secondary hypertension”. The following variables were adjusted during the analysis: gender, age, first 10 main components, and genotyping batch. More details about the endpoint were provided at https://risteys.finregistry.fi/.
Selection of instrumental variablesGiven a p-value threshold of <5 × 10−8 for SNP selection did not yield three or more significant SNPs for all 41 systemic inflammatory regulators, to obtain a more comprehensive set of SNPs for inflammatory regulators, we adjusted the significance threshold to P < 5 × 10−6. We employed a stringent clumping procedure to ensure that independent SNPs met the linkage disequilibrium (LD) coefficient criteria (r2 < 0.001, window size = 10,000 kb) and removed palindromic SNPs. To mitigate the impact of weak instrumental variables on the MR model's performance, we used the F statistic of SNPs to evaluate the strength of instrumental variables. Typically, an F value >10 indicates no apparent bias caused by weak IVs.
Statistical analysisGiven it provides the highest statistical power, the inverse-variance weighted (IVW) approach was used as our primary analysis (PMID: 26661904). When these conditions are not met, the results may be biased. Therefore, a variety of analyses including MR-Egger, weighted median, simple mode and weighted mode were used as sensitive analyses, to corroborate the causal relationship between inflammatory regulators and hypertension (PMID: 29040600). Additionally, we used MR-Egger intercept test and Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO global test) to further evaluate the presence of horizontal pleiotropy (PMID: 29686387, PMID: 26050253). An MR-Egger regression intercept not equal to zero and P < 0.05 indicates that the inflammatory factors may not be the sole pathway through which IVs influence hypertension. The MR-PRESSO global test can further correct for potential outlier pleiotropy by removing outlier SNPs when necessary. We also performed heterogeneity tests on IVW and MR-Egger results using the Cochrane Q test; when P < 0.05, heterogeneity is present, and we need to evaluate outliers in conjunction with other results. It is generally believed that the heterogeneity of a single result will not significantly impact the prediction of causal relationships. The leave-one-out sensitivity analysis, which involves the sequential exclusion of SNPs, is commonly used to find and eliminate SNPs that have a significant impact on the results, ensuring the reliability of the outcomes (PMID: 27749700).
We consider a significant causal relationship to exist between inflammatory factors and hypertension when the following three conditions are met: (1) A significant difference exists in the primary analysis method IVW results (P < 0.05); (2) Other sensitive methods are consistent with the IVW estimate direction; (3) No pleiotropy was found in MR-Egger intercept test or MR-PRESSO global test results (P < 0.05).
Results Selection of instrumental variables (IVs)A total of 840 SNPs were screened as IVs for 41 systemic inflammatory regulators. These IVs explained 2.3%–24.7% of the variance and the F-statistics of them were all greater than 10 (ranged 20.1–128.3), signifying the robustness and strength of the IVs employed in this study. This indicates the absence of any significant weak instrument bias in the results, making the findings reliable and acceptable (Supplementary Table S2).
Two-sample MR analysisAmong all 41 inflammatory regulators analyzed in our study, 3 inflammatory regulators were found related to secondary hypertension according to the criteria mentioned above in which the IVW method demonstrated a significant difference (P < 0.05), and the other methods indicated consistent directions. In the IVW analysis, genetically predicted TNFb was negatively associated with risk of secondary hypertension, with a OR of one SD increase in genetically predicted TNFb causing 16.6% (95% CI: 4.4%–27.1%) lower risk of secondary hypertension. Similar trend was also found in MIP1b (OR = 0.91; 95% CI 0.84–0.99, p = 0.024) and MIG (OR = 0.88; 95% CI 0.78–0.99, p = 0.040) (Table 1, Supplementary Table S3). Additionally, there was not any evidence of 41 inflammatory regulators associated with primary hypertension (Supplementary Table S3).
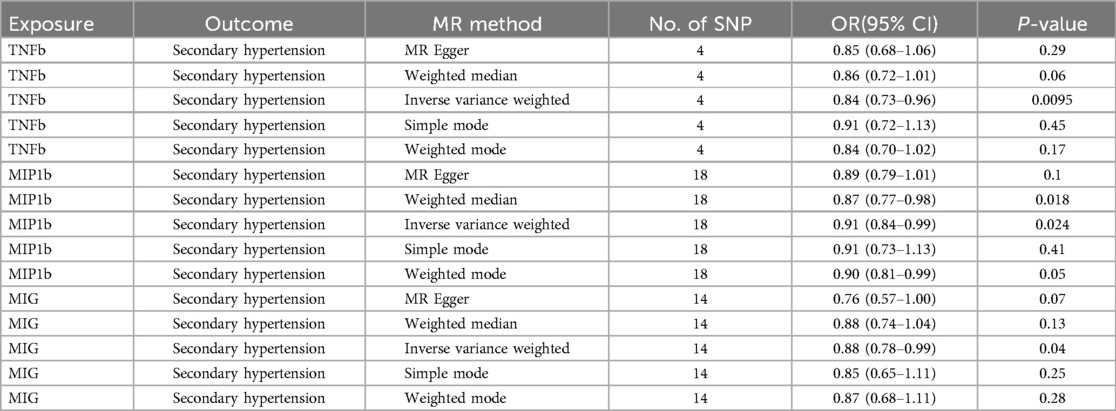
Table 1. Result of MR estimates for three systemic inflammatory regulators.
Sensitivity analyses and leave-one-out analysisFor these three inflammatory regulators, there was not any heterogeneity found in the IVW method or the MR-Egger regression, the P-value for Cochran's Q test were all >0.05 (Supplementary Table S4). MR Egger regression intercepts were near zero and revealed little evidence of horizontal pleiotropy in the IVs (Supplementary Table S5). Using the MR-PRESSO global outlier test, no evidence for outliers was observed (Supplementary Table S5). The scatter plots did not demonstrate any potential outlier in the IVs for these three inflammatory regulators (Figure 1). In the leave-one SNP-out analyses, the risk estimates of all inflammatory regulators generally remained consistent after eliminating each single SNP at a time, that it is, not any leverage point with high influence was identified (Figure 2). The full results of MR estimate for the 41 inflammatory regulators on hypertension are presented in Supplementary Tables S3–S6.
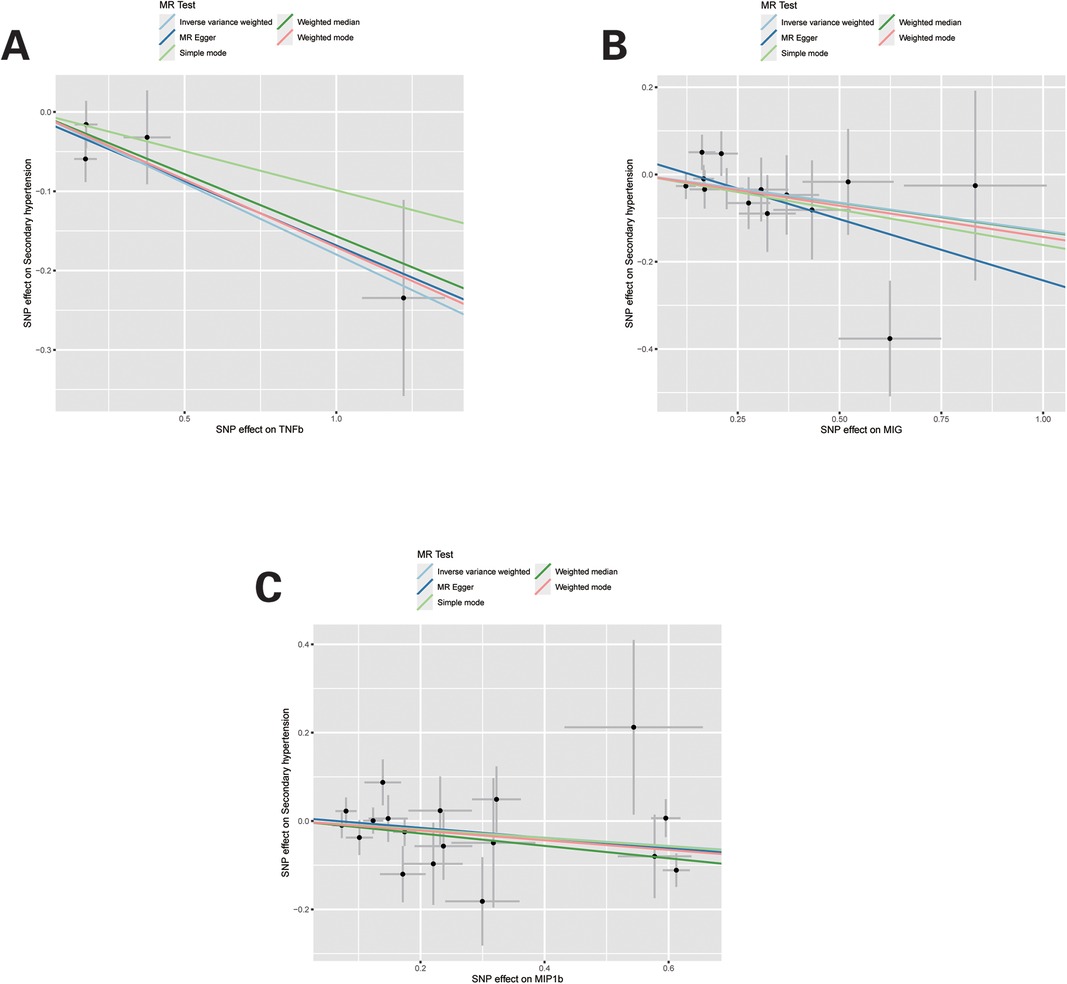
Figure 1. Scatter plots of Mendelian randomization (MR) analyses for TNFb, MIG and MIP1b in secondary hypertension. Individual inverse variance (IV) associations with systemic inflammatory regulators risk are displayed vs. individual IV associations with Secondary hypertension in black dots. The 95% CI of odd ratio for each IV is shown by vertical and horizontal lines. The slope of the lines represents the estimated causal effect of the MR methods.
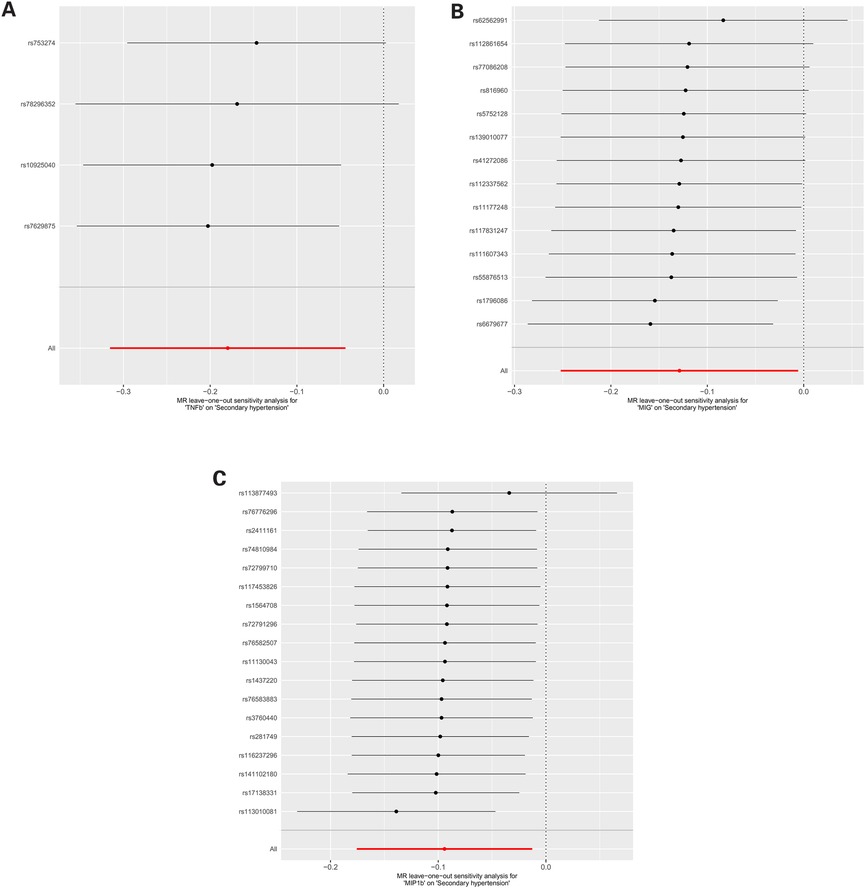
Figure 2. (A) MR leave-one-out sensitivity analysis for “TNFb” on “Secondary hypertension”. (B) MR leave-one-out sensitivity analysis for “MIG” on “Secondary hypertension”. (C) MR leave-one-out sensitivity analysis for “MIP1b” on “Secondary hypertension”.
DiscussionNumerous studies based on the inflammatory response in hypertension have made breakthroughs and confirmed the efficacy of many drugs on the inflammatory response in Primary and secondary hypertension (13). 5%–10% of hypertensive patients suffer from secondary hypertension. The main causes of secondary hypertension are substantial kidney disease, primary aldosteronism, and renal vascular hypertension. Identifying these patients can enable etiological management of potential diseases (14). Many inflammatory markers have been shown to be elevated in hypertensive patients, such as CRP, the most widely studied inflammatory marker, which has been shown to have elevated levels in the blood of hypertensive patients and has been associated with prognosis. Elevated levels of other markers have also been observed in hypertensive patients, such as IL-1β, IL-6, TNF-α, and leukocytes (15). However, there are currently no reports on distinguishing inflammatory factors from primary and secondary hypertension. In this study we utilized a large-scale, published genome-wide association study (GWAS) meta-analysis encompassing 41 cytokines (involved 8,293 Finnish participants from three independent population cohorts: the Cardiovascular Risk in Young Finns Study (YFS), FINRISK1997, and FINRISK2002.), to corroborate the causal relationship between inflammatory regulators and hypertension. Finally, we found 3 inflammatory regulators were found related to secondary hypertension according to the criteria mentioned above in which the IVW method demonstrated a significant difference (P < 0.05). TNFb was negatively associated with risk of secondary hypertension Similar trend was also found in MIP1b and MIG. Additionally, there was not any evidence of 41 inflammatory regulators associated with primary hypertension.
The TNF genes are located in the HLA complex on chromosome 6p21.3 and include TNF-α and TNFb, which play important roles in cell growth, differentiation, proliferation and immunity, apoptosis, and are involved in a wide range of disease progression. Tumor necrosis factor B (TNFB) is a cytokine produced by activated macrophages and is closely related to the TNFA gene (16). TNFB is a Th1 cytokine produced mainly by activated T and B lymphocytes. It is a potent mediator of inflammation and immune suppression (17). TNF-B was associated with serum triglyceride and very low density lipoprotein (VLDL) levels in interaction with TNF-B (18). The interaction between long-chain polyunsaturated fatty acids and nitric oxide, superoxide anions, and transforming growth factor beta can effectively prevent hypertension (19). Recently, it was found that inflammatory biomarkers were significantly elevated in a cohort of patients with idiopathic hypertension, with TNF-b and NT-proBNP being significantly correlated with 5-year survival (20). TNFB can induces an inflammatory response through activation of NF-kB nuclear proteins (16). TNFB promotes the progression of intestinal dermatitis in a YAP dependent manner (21). TGF β subtypes induce EndMT in human microvascular endothelial cells (22), indicating that TNF β is positively correlated with the progression of primary hypertension. However, in this Mendelian analysis, we found a negative correlation between TNF β and secondary hypertension. Previous studies have showed the cytokine TGF - β (transforming growth factor - β) is independent of the production of inflammatory cytokines and can increase the expression and activity of glycolytic enzyme PFKL (phosphofructokinase-1 liver type), promote glycolysis, but inhibit the production of pro-inflammatory cytokines (23). Dysregulation of BMP/TGF - β signaling is associated with disease progression in human PAH and rodent model PH (24). The integrated changes of TGF - β superfamily growth signals may lead to the pathogenesis of secondary hypertension (25). TGF - β (1) mRNA may be a useful gene therapy agent for treating hypertensive vascular diseases (26). Consistent with the findings of the present study, and TNF β may be a risk factors for primary hypertension, and a protect factor for secondary hypertension.
The macrophage inflammatory protein-1 (MIP-1) protein is structurally related to proinflammatory chemokine of the CC subfamily, one of the four chemokine groups defined by their primary structure (i.e., CXC, C, CC, and CX3C) (27). Macrophage Inflammatory Protein (MIP) 1b is a member of the inflammatory cytokine gene family whose expression is induced by pro-inflammatory and pro-mitotic stimuli (28).The gene is rapidly induced by LPS or IL-7 in human peripheral blood mononuclear cells, Elevated MIP1b gene is associated with an increased risk of total breast cancer and increased number of ER-positive BC cases (29). MIP-1β levels are elevated in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis (30). Additionally, research has confirmed that MIP1b[ORIVW:0.92; 95% confidence interval: 0.85–0.98; P = 0.022% was negatively correlated with the risk of diabetes (31). Inhibition of macrophage factor MIP1b can mediate hypoxic pulmonary hypertension (32). No studies have reported a correlation between MIP1b and mortality or disease progression in patients with secondary hypertension, and the present study is the first to demonstrate a positive correlation between MIP1b and the progression of secondary hypertension.
Monokine induced by gamma interferon (MIG) induces adhesion of interleukin (IL)- 2 rapidly transiently processed T lymphocytes to immobilized integrin receptors through activation of the receptor CXCR3 on T cells (33). Previous studies have shown that the inflammatory chemokine MIG is increased in serum species of patients with left ventricular dysfunction (34). In serum of patients with idiopathic pulmonary arterial hypertension (IPAH) only IP-10 and MIG were significantly elevated (35). A model of 3 cytokines (IL-7, MIG, and SCF) was effective in predicting the multifactorial combination of PIH in ART pregnancies (36). MIG is an effective measure to improve compliance in hypertensive patients (15). MIG-7 and other MIG family molecules are positively correlated with the progression of hypertension (37, 38). While Mig-6 is necessary for proper lung development and adult lung homeostasis. MIG-6 negatively regulates STAT3 phosphorylation in uterine epithelial cells and inhibits inflammation progression (39). Consistent with the relationship between MIG and secondary hypertension obtained from the analysis of this study. Perhaps MIG-6 is negatively correlated with secondary hypertension.
The present study is the first to use MR in two samples to infer a causal relationship between inflammatory factors and blood pressure indices. Although recently published GWAS studies have helped to identify possible genetic causes of HP (40), there is the limitation of a small number of instrumental variables available. Estimates of the heritability of inflammation are variable depending on the method of calculation (41). However, it did succeed in identifying correlations between inflammation-related SNPs and systolic and diastolic blood pressure.
In conclusion, this study supported a negative relationship between TNFb, MIP1b 和 MIG6 and secondary hypertension. While the mechanism of this relationship warrants further study, we need to be confirmed in a large number of hypertensive patients. Also, the absence of a causal relationship found in the study between inflammation and primary hypertension requires future investigation (this could be due to the kind of inflammatory markers explored belong to CC chemokine family and had limited variety). This study suggested that hypertension can occur without traditional risk factors, so a shift in prevention methods is needed. Targeting secondary hypertension rather than just traditional risk factors is crucial for preventing adverse events. At the same time, personalized medicine and biomarker research can be combined to provide new ways to improve risk stratification, and integrate genetic and biomarker data, as well as artificial intelligence tools, which is expected to optimize hypertension risk management.
Data availability statementPublicly available datasets were analyzed in this study. This data can be found here: https://r9.finngen.fi/pheno/I9_HYPTENSESS, https://r9.finngen.fi/pheno/I9_HYPTENSEC
Ethics statementEthical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the [patients/ participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributionsXJ: Writing – review & editing, Writing – original draft. JR: Writing – original draft, Formal Analysis, Data curation. FD: Writing – review & editing. WP: Writing – review & editing, Project administration, Methodology, Data curation.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors thank the participants of all GWAS cohorts included in the present work.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1335395/full#supplementary-material
References1. Barrows I, Ramezani A, Raj D. Inflammation, immunity, and oxidative stress in hypertension-partners in crime? Adv Chronic Kidney Dis. (2019) 26(2):122–30. doi: 10.1053/j.ackd.2019.03.001
PubMed Abstract | Crossref Full Text | Google Scholar
2. Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, Nosalski R, Pelka P, Nowakowski D, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. (2019) 40(42):3459–70. doi: 10.1093/eurheartj/ehz646
PubMed Abstract | Crossref Full Text | Google Scholar
3. Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, et al. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. (2020) 324(12):1190–200. doi: 10.1001/jama.2020.14545
PubMed Abstract | Crossref Full Text | Google Scholar
6. Zhang Z, Zhao L, Zhou X, Meng X, Zhou X. Role of inflammation, immunity, and oxidative stress in hypertension: new insights and potential therapeutic targets. Front Immunol. (2022) 13:1098725. doi: 10.3389/fimmu.2022.1098725
PubMed Abstract | Crossref Full Text | Google Scholar
9. Agita A, Alsagaff M. Inflammation, immunity, and hypertension. Acta Med Indones. (2017) 49(2):158–65.28790231
PubMed Abstract | Google Scholar
10. Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GM, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med. (2020) 50(14):2435–43. doi: 10.1017/S0033291719002678
PubMed Abstract | Crossref Full Text | Google Scholar
11. Burgess S, Scott RA, Timpson NJ, Smith GD, Thompson SG, Consortium E. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. (2015) 30(7):543–52. doi: 10.1007/s10654-015-0011-z
PubMed Abstract | Crossref Full Text | Google Scholar
12. Hartwig FP, Davies NM, Hemani G, Smith GD. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. (2016) 45(6):1717–26. doi: 10.1093/ije/dyx028
PubMed Abstract | Crossref Full Text | Google Scholar
13. Muñoz Aguilera E, Leira Y, Miró Q, Orlandi M, Czesnikiewicz-Guzik M, Guzik TJ, et al. Is systemic inflammation a missing link between periodontitis and hypertension? Results from two large population-based surveys. J Intern Med. (2021) 289(4):532–46. doi: 10.1111/joim.13180
PubMed Abstract | Crossref Full Text | Google Scholar
15. Contreras EM, García OV, Claros NM, Guillén VG, Wichmann M, Martínez JC, et al. Efficacy of telephone and mail intervention in patient compliance with antihypertensive drugs in hypertension. ETECUM-HTA study. Blood Press. (2005) 14(3):151–8. doi: 10.1080/08037050510008977
PubMed Abstract | Crossref Full Text | Google Scholar
16. Laddha NC, Dwivedi M, Gani AR, Mansuri MS, Begum R. Tumor necrosis factor B (TNFB) genetic variants and its increased expression are associated with vitiligo susceptibility. PLoS One. (2013) 8(11):e81736. doi: 10.1371/journal.pone.0081736
PubMed Abstract | Crossref Full Text | Google Scholar
17. Segaert S, Hermans C. Clinical signs, pathophysiology and management of cutaneous side effects of anti-tumor necrosis factor agents. Am J Clin Dermatol. (2017) 18(6):771–87. doi: 10.1007/s40257-017-0296-7
PubMed Abstract | Crossref Full Text | Google Scholar
18. Garg PR, Saraswathy KN, Kalla AK, Sinha E, Gho PK. Pro-inflammatory cytokine gene polymorphisms and threat for coronary heart disease in a North Indian Agrawal population. Gene. (2013) 514(1):69–74. doi: 10.1016/j.gene.2012.10.011
PubMed Abstract | Crossref Full Text | Google Scholar
19. Das U. Long-chain polyunsaturated fatty acids interact with nitric oxide, superoxide anion, and transforming growth factor-beta to prevent human essential hypertension. Eur J Clin Nutr. (2004) 58(2):195–203. doi: 10.1038/sj.ejcn.1601766
PubMed Abstract | Crossref Full Text | Google Scholar
20. Hirsch K, Nolley S, Ralph DD, Zheng Y, Altemeier WA, Rhodes CJ, et al. Circulating markers of inflammation and angiogenesis and clinical outcomes across subtypes of pulmonary arterial hypertension. J Heart Lung Transplant. (2023) 42(2):173–82. doi: 10.1016/j.healun.2022.10.026
PubMed Abstract | Crossref Full Text | Google Scholar
21. Liu L, Wang Y, Yu S, Liu H, Li Y, Hu S, et al. Transforming growth factor beta promotes inflammation and tumorigenesis in smad4-deficient intestinal epithelium in a YAP-dependent manner. Adv Sci. (2023) 10(23):e2300708. doi: 10.1002/advs.202300708
Crossref Full Text | Google Scholar
22. Sabbineni H, Verma A, Somanath P. Isoform-specific effects of transforming growth factor β on endothelial-to-mesenchymal transition. J Cell Physiol. (2018) 233(11):8418–28. doi: 10.1002/jcp.26801
PubMed Abstract | Crossref Full Text | Google Scholar
23. Gauthier T, Yao C, Dowdy T, Jin W, Lim YJ, Patiño LC, et al. TGF-β uncouples glycolysis and inflammation in macrophages and controls survival during sepsis. Sci Signal. (2023) 16(797):eade0385. doi: 10.1126/scisignal.ade0385
PubMed Abstract | Crossref Full Text | Google Scholar
24. Wang C, Xing Y, Zhang J, He M, Dong J, Chen S. MED1 Regulates BMP/TGF-β in endothelium: implication for pulmonary hypertension. Circ Res. (2022) 131(10):828–41. doi: 10.1161/CIRCRESAHA.122.321532
PubMed Abstract | Crossref Full Text | Google Scholar
25. Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. (2001) 104(7):790–5. doi: 10.1161/hc3201.094152
PubMed Abstract | Crossref Full Text | Google Scholar
26. Teng J, Fukuda N, Hu WY, Nakayama M, Kishioka H, Kanmatsuse K. DNA-RNA chimeric hammerhead ribozyme to transforming growth factor-beta(1) mRNA inhibits the exaggerated growth of vascular smooth muscle cells from spontaneously hypertensive rats. Cardiovasc Res. (2000) 48(1):138–47. doi: 10.1016/S0008-6363(00)00157-7
PubMed Abstract | Crossref Full Text | Google Scholar
28. Ziegler SF, Tough TW, Franklin TL, Armitage RJ, Alderson MR. Induction of macrophage inflammatory protein-1 beta gene expression in human monocytes by lipopolysaccharide and IL-7. J Immunol. (1991) 147(7):2234–9. doi: 10.4049/jimmunol.147.7.2234
PubMed Abstract | Crossref Full Text | Google Scholar
29. Li S, Li L, Shu XO, Xiang YB, Luo H, Chen W, et al. Mendelian Randomization analyses of genetically predicted circulating levels of cytokines with risk of breast cancer. NPJ Precis Oncol. (2020) 4:25. doi: 10.1038/s41698-020-00131-6
PubMed Abstract | Crossref Full Text | Google Scholar
30. Martínez HR, Escamilla-Ocañas CE, Camara-Lemarroy CR, González-Garza MT, Moreno-Cuevas J, García Sarreón MA. Increased cerebrospinal fluid levels of cytokines monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1β (MIP-1β) in patients with amyotrophic lateral sclerosis. Neurologia. (2020) 35(3):165–9. doi: 10.1016/j.nrl.2017.07.020
PubMed Abstract | Crossref Full Text | Google Scholar
31. Li A, Ren X, Pan Y, Gao W, Ren L, Wang J, et al. IFN-γ, SCF, MIP1b and IL-16 were associated with risk of diabetic nephropathy: a Mendelian randomization study. Diabetes Metab Syndr Obes. (2024) 17:851–6. doi: 10.2147/DMSO.S452227
PubMed Abstract | Crossref Full Text | Google Scholar
32. Zhang Y, Talwar A, Tsang D, Bruchfeld A, Sadoughi A, Hu M, et al. Macrophage migration inhibitory factor mediates hypoxia-induced pulmonary hypertension. Mol Med. (2012) 18(1):215–23. doi: 10.2119/molmed.2011.00094
PubMed Abstract | Crossref Full Text | Google Scholar
33. Yu HT, Oh J, Chang HJ, Lee SH, Shin EC, Park S. Serum monokine induced by gamma interferon as a novel biomarker for coronary artery calcification in humans. Coron Artery Dis. (2015) 26(4):317–21. doi: 10.1097/MCA.0000000000000236
PubMed Abstract | Crossref Full Text | Google Scholar
34. Altara R, Scuteri P, Berardi V, Volpe F, Pilotto L, Galanti M, et al. Left ventricular dysfunction and CXCR3 ligands in hypertension: from animal experiments to a population-based pilot study. PLoS One. (2015) 10(10):e0141394. doi: 10.1371/journal.pone.0141394
PubMed Abstract | Crossref Full Text | Google Scholar
35. Zabini D, Heinemann A, Foris V, Nagaraj C, Nierlich P, Bálint Z, et al. Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur Respir J. (2014) 44(4):951–62. doi: 10.1183/09031936.00145013
PubMed Abstract | Crossref Full Text | Google Scholar
36. Lan X, Guo L, Zhu S, Cao Y, Niu Y, Han S, et al. First-trimester serum cytokine profile in pregnancies conceived after assisted reproductive technology (ART) with subsequent pregnancy-induced hypertension. Front Immunol. (2022) 13:930582. doi: 10.3389/fimmu.2022.930582
PubMed Abstract | Crossref Full Text | Google Scholar
37. Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. (2003) 197(11):1537–49. doi: 10.1084/jem.20021897
PubMed Abstract | Crossref Full Text | Google Scholar
38. Huang B, Yin M, Li X, Cao G, Qi J, Lou G, et al. Migration-inducing gene 7 promotes tumorigenesis and angiogenesis and independently predicts poor prognosis of epithelial ovarian cancer. Oncotarget. (2016) 7(19):27552–66. doi: 10.18632/oncotarget.8487
PubMed Abstract | Crossref Full Text | Google Scholar
39. Yoo JY, Yang WS, Lee JH, Kim BG, Broaddus RR, Lim JM, et al. MIG-6 negatively regulates STAT3 phosphorylation in uterine epithelial cells. Oncogene. (2018) 37(2):255–62. doi: 10.1038/onc.2017.335
PubMed Abstract | Crossref Full Text | Google Scholar
40. Munz M, Richter GM, Loos BG, Jepsen S, Divaris K, Offenbacher S, et al. Meta-analysis of genome-wide association studies of aggressive and chronic periodontitis identifies two novel risk loci. Eur J Hum Genet. (2019) 27(1):102–13. doi: 10.1038/s41431-018-0265-5
PubMed Abstract | Crossref Full Text | Google Scholar
41. Nibali L, Bayliss-Chapman J, Almofareh SA, Zhou Y, Divaris K, Vieira AR. What is the heritability of periodontitis? A systematic review. J Dent Res. (2019) 98(6):632–41. doi: 10.1177/0022034519842510
留言 (0)