In recent years, the incidence of renal cancer diagnoses has been steadily increasing. According to the American Cancer Society, there were 73,750 new cases of renal cancer diagnosed in 2020 (1, 2). Currently, the surgical treatment for renal cancer is primarily divided into partial nephrectomy and radical nephrectomy. When technically feasible, nephron-sparing surgery is recommended as the preferred surgical treatment for renal tumors. As a result, partial nephrectomy has become the gold standard for surgical treatment of T1 renal cell carcinoma (3–6).
Long periods of preoperative fasting can lead to dehydration, electrolyte imbalance, and reduced blood volume, which in turn can weaken the immune system and increase the risk of postoperative infections (7–9). It may also result in gastrointestinal discomfort during postoperative recovery and adversely affect the recovery process. This study aims to investigate the impact of preoperative fasting time on patients undergoing partial nephrectomy.
Hemorrhage is one of the common complications following partial nephrectomy. It can not only affect the recovery of patients but, in severe cases, can be life-threatening (10). Although hemostatic drugs can control bleeding to some extent, some severe cases require vascular embolization or even reoperation (11, 12). Therefore, the factors contributing to postoperative hemorrhage following partial nephrectomy warrant attention. This study analyzes the risk factors for hemorrhage following partial nephrectomy to provide clinical reference for physicians in their treatment approaches.
2 MethodsA total of 74 renal cancer patients underwent partial nephrectomy at Peking University First Hospital-Miyun hospital between January 2022 and March 2024. Among these patients, 26 had a short-term fasting time, while 48 had a long-term preoperative fasting time. Baseline and perioperative data were collected for all patients. Baseline data included gender, age, body mass index (BMI), presence of hypertension, presence of diabetes, and presence of coronary artery disease (CHD). Perioperative data included tumor size, type of surgery, heart rate at admission, systolic blood pressure at admission, diastolic blood pressure at admission, preoperative creatinine, preoperative blood sodium, preoperative blood potassium, preoperative blood magnesium, preoperative blood phosphorus, preoperative blood calcium, preoperative hemoglobin, intraoperative heart rate, intraoperative systolic blood pressure, intraoperative diastolic blood pressure, postoperative heart rate, postoperative systolic blood pressure, postoperative diastolic blood pressure, surgery duration, intraoperative blood loss, postoperative creatinine, postoperative blood sodium, postoperative blood potassium, postoperative blood magnesium, postoperative blood phosphorus, postoperative blood calcium, and postoperative hemoglobin.
In this study, short-term preoperative fasting was defined as a fasting time of 12 h or less, while long-term preoperative fasting was defined as more than 12 h. The primary endpoint for the analysis of risk factors was postoperative hemorrhage. Postoperative hemorrhage was defined as a decrease in hemoglobin of 20 g/L or more between preoperative levels and the first postoperative day. A decrease of less than 20 g/L was not considered a postoperative hemorrhage complication. The decrease in hemoglobin (g/L) was calculated as preoperative hemoglobin level minus the hemoglobin level on the first postoperative day.
Inclusion criteria: a. Patients aged 18–75 years with T1 tumors and a R.E.N.A.L. nephrometry score of ≤9; b. Complete baseline and perioperative data.
Exclusion criteria: a. Patients with uncontrolled severe disease or acute infection; b. Pregnant or lactating women.
This study was conducted in accordance with the principles of the Declaration of Helsinki (2013 revision) and was approved by the Ethics Committee of Peking University First Hospital-Miyun hospital. The retrospective analysis of this study was exempted from individual consent.
2.1 Surgical techniqueThe patient is placed in a supine position, and standard disinfection and draping are performed. After establishing pneumoperitoneum, trocars are inserted. Using a laparoscope and electrocautery or scissors, the surrounding fat and Gerota's fascia are dissected to expose the kidney and tumor. The renal artery is temporarily clamped using vascular clamps or other devices to reduce intraoperative bleeding. This step must be performed quickly and accurately to minimize renal ischemia time. The tumor and a small margin of surrounding healthy renal tissue are excised using laparoscopic surgical instruments, ensuring negative margins. The renal incision is repaired using absorbable sutures or Hem-o-lok clips to restore the integrity of the kidney. Hemostatic agents or fibrin glue may be used to assist in hemostasis. The renal artery clamp is then removed to restore renal blood flow. The surgical field is checked for bleeding or other complications, and once confirmed to be clear, the laparoscopic instruments are removed and the small abdominal incisions are closed.
3 Statistical analysisStatistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Quantitative variables included age, BMI, tumor size, heart rate at admission, systolic blood pressure at admission, diastolic blood pressure at admission, preoperative creatinine, preoperative blood sodium, preoperative blood potassium, preoperative blood magnesium, preoperative blood phosphorus, preoperative blood calcium, preoperative hemoglobin, intraoperative heart rate, intraoperative systolic blood pressure, intraoperative diastolic blood pressure, postoperative heart rate, postoperative systolic blood pressure, postoperative diastolic blood pressure, surgery duration, intraoperative blood loss, postoperative creatinine, postoperative blood sodium, postoperative blood potassium, postoperative blood magnesium, postoperative blood phosphorus, postoperative blood calcium, and postoperative hemoglobin. Qualitative variables included gender, presence of hypertension, presence of diabetes, presence of CHD, and type of surgery. Normally distributed quantitative data were expressed as mean ± standard deviation, while non-normally distributed data were described using median (range). For continuous variables, normally distributed variables were analyzed using the t-test, while the Mann-Whitney U-test was used for non-normally distributed variables. Categorical variables were analyzed using Fisher's exact test. Univariate and multivariate regression analyses were conducted to determine independent risk factors for postoperative hemorrhage following partial nephrectomy. A p-value of <0.05 was considered statistically significant.
4 Results 4.1 Patient baseline dataThe baseline data of the patients are shown in Table 1. A total of 74 patients who underwent partial nephrectomy were included in this study, with an average age of 52.45 ± 12.75 years. The average tumor size was 3.42 ± 1.70 cm. There were 26 (35.14%) patients in the short-term preoperative fasting group and 48 (64.86%) patients in the long-term preoperative fasting group. Among them, 45 patients experienced postoperative complications, with 12 patients having two complications. No postoperative complications of grade III or higher were observed. The incidence of grade I complications was 39.19% (29/74), and the incidence of grade II complications was 36.49% (27/74).
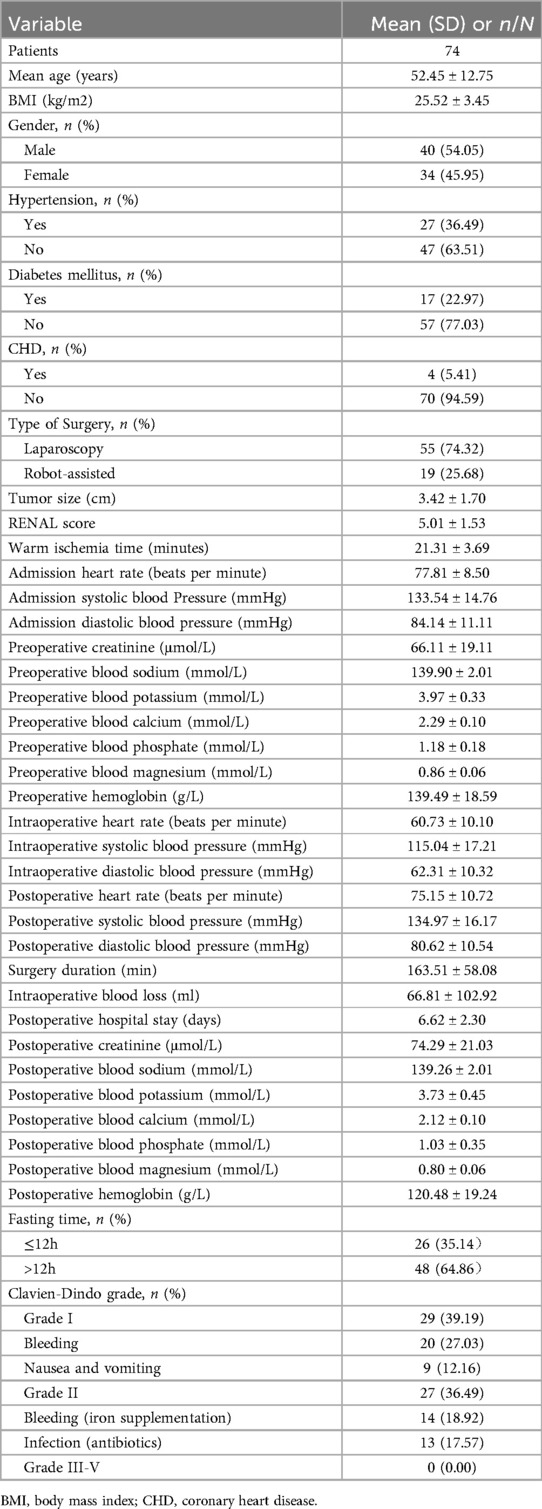
Table 1. Basic characteristics of the patients.
4.2 Comparison between short-term and long-term preoperative fasting in partial nephrectomyThe clinical data of patients in the short-term and long-term preoperative fasting groups are shown in Table 2. The hemoglobin difference in the short-term preoperative fasting group was 21.08 ± 12.44 ml, while in the long-term preoperative fasting group it was 13.65 ± 11.69 ml, showing a statistically significant difference between the two groups (p = 0.020). The calcium difference in the short-term preoperative fasting group was 0.96 ± 1.01 mmol/L, compared to 0.08 ± 0.11 mmol/L in the long-term preoperative fasting group, also showing a statistically significant difference (p = 0.003). The phosphorus difference was 0.54 ± 0.52 mmol/L in the short-term preoperative fasting group and 0.09 ± 0.33 mmol/L in the long-term preoperative fasting group, with a statistically significant difference (p = 0.001). The magnesium difference was 0.35 ± 0.40 mmol/L in the short-term preoperative fasting group and 0.02 ± 0.05 mmol/L in the long-term preoperative fasting group, again showing a statistically significant difference (p = 0.031).
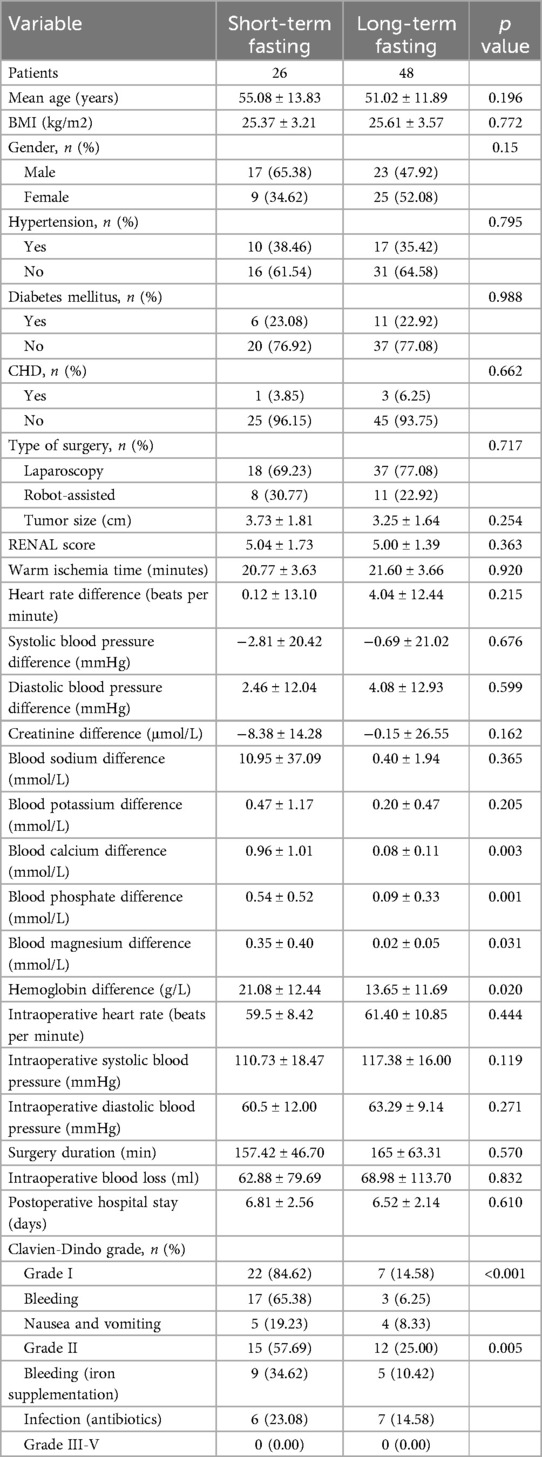
Table 2. Comparing the difference in prognosis between short-term fasting group and long-term fasting group.
4.3 Analysis of risk factors for hemorrhage after partial nephrectomyThe clinical data of the control group and the hemorrhage group are shown in Table 3. Postoperatively, there were 40 (54.05%) patients in the control group and 34 (45.95%) patients in the hemorrhage group. In the control group, 34 (85.00%) patients underwent laparoscopic partial nephrectomy and 6 (15.00%) underwent robot-assisted partial nephrectomy. In the hemorrhage group, 21 (61.76%) patients underwent laparoscopic partial nephrectomy and 13 (38.24%) underwent robot-assisted partial nephrectomy, showing a statistically significant difference between the two groups (p = 0.023). In the control group, 2 (5.00%) patients were in the short-term preoperative fasting group, and 38 (95.00%) were in the long-term preoperative fasting group. In the hemorrhage group, 24 (70.59%) patients were in the short-term preoperative fasting group, and 10 (29.41%) were in the long-term preoperative fasting group, also showing a statistically significant difference (p < 0.001).
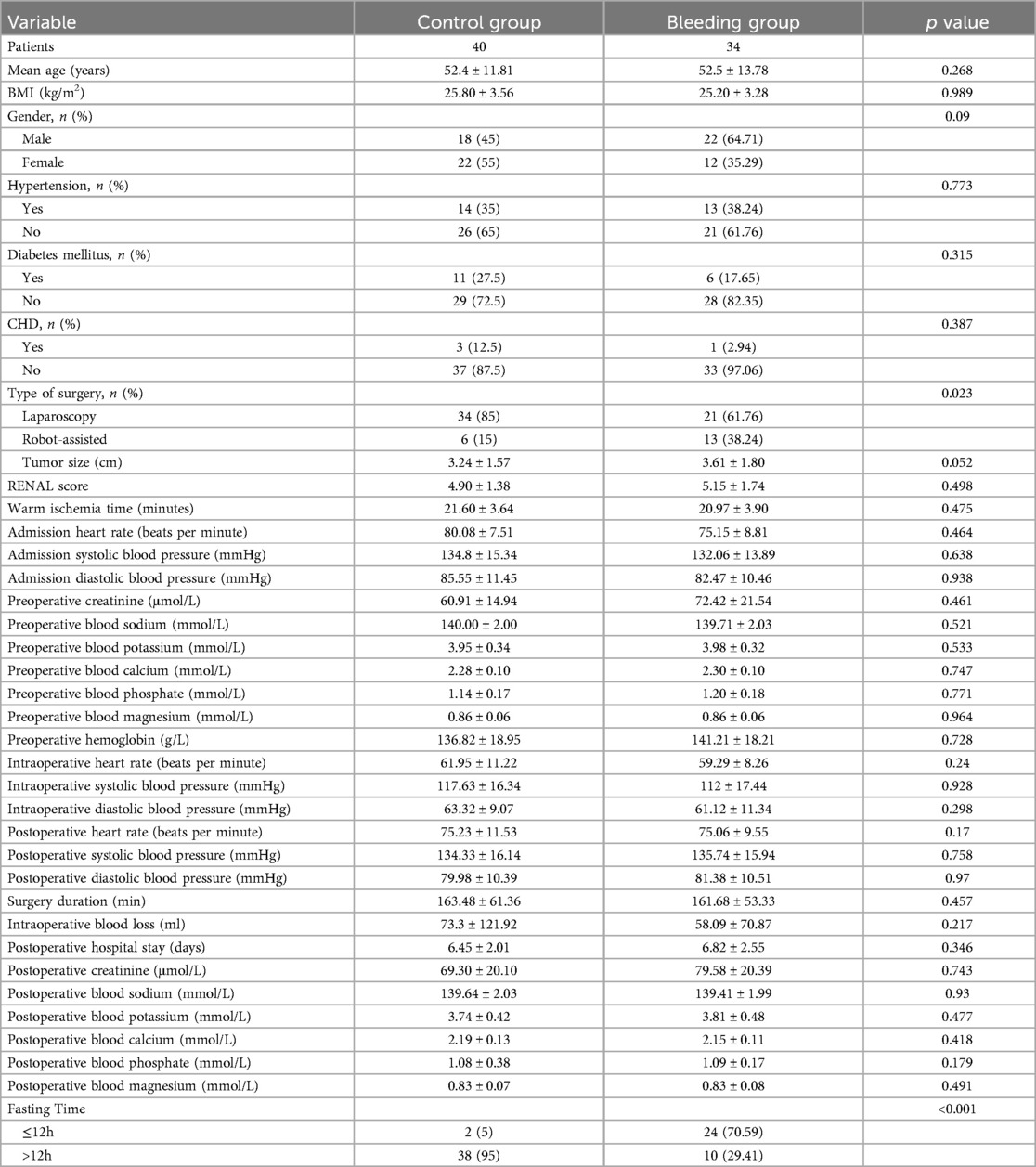
Table 3. Comparing the difference in prognosis between control group and bleeding group.
Univariate and multivariate regression analyses identified the type of surgery (p = 0.014) and preoperative fasting time (p < 0.001) as risk factors for postoperative hemorrhage. Both the type of surgery (p = 0.050) and preoperative fasting time (p < 0.001) were also found to be independent risk factors for postoperative hemorrhage in partial nephrectomy (Table 4).
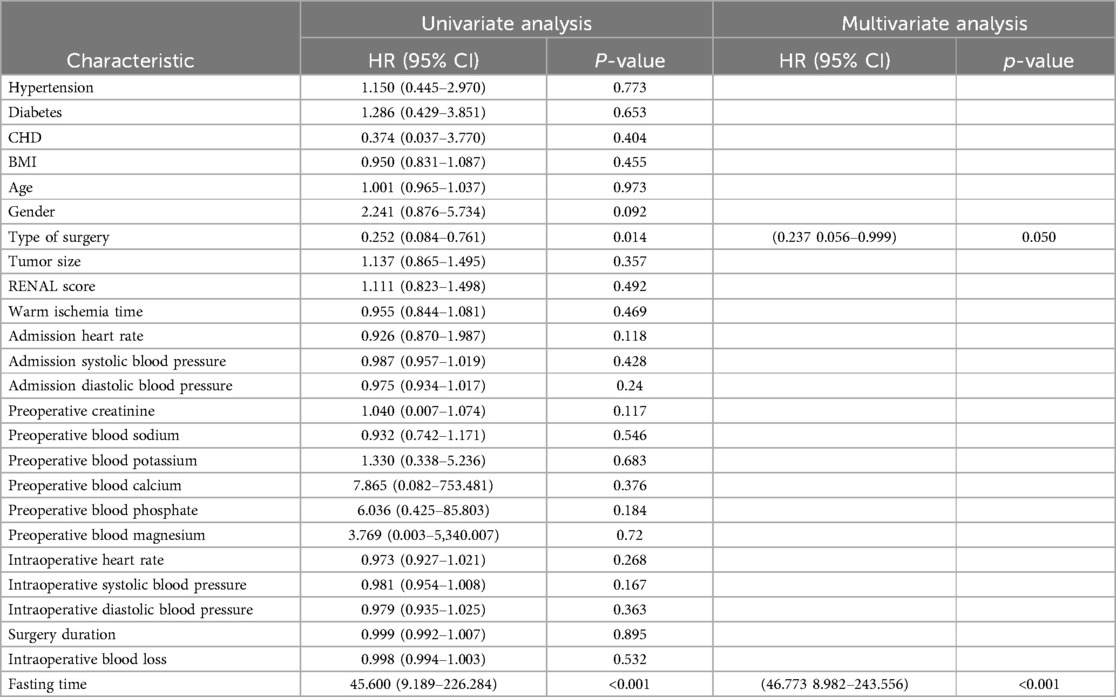
Table 4. Univariate analysis and multivariate analysis of bleeding in patients with partial nephrectomy.
5 DiscussionRenal cancer accounts for more than 3% of all new cancer cases worldwide, and its incidence is rising annually (13, 14). Numerous studies have shown a threefold increase in the number of partial nephrectomies performed annually, while cases of radical nephrectomy have decreased by 40% in recent years. We found that radical nephrectomy increases the risk of surgical wound infection by 195%, sepsis by 160%, pancreatitis by 110%, blood transfusion by 100%, acute thromboembolism by 90%, acute respiratory failure and acute kidney disease by 60%, intestinal obstruction by 40%, and intensive care unit admission by 20% (15). Consequently, partial nephrectomy is becoming increasingly common in clinical practice. This study compares the effects of short-term and long-term preoperative fasting in patients undergoing partial nephrectomy and analyzes the risk factors for postoperative hemorrhage to provide clinical reference for physicians.
Previous studies have generally considered long-term preoperative fasting to be detrimental (9, 16–19). This issue is also present in partial nephrectomy, where some patients may need to fast for extended periods due to surgical scheduling, potentially causing adverse effects. Long-term preoperative fasting can lead to dehydration, electrolyte imbalance, and impaired cardiac and renal function. It also increases the body's stress response, potentially negatively affecting surgical tolerance and postoperative recovery. However, in this study, it was found that long-term preoperative fasting resulted in less loss of blood calcium, magnesium, phosphorus, and hemoglobin, differing from previous studies. This might be because long-term fasting leads to blood concentration and reduced plasma volume, thereby decreasing intraoperative blood loss due to lower intravascular pressure. Additionally, preoperative dehydration might reduce electrolyte loss. However, this study did not account for postoperative hospital stay and adverse outcomes. Gisele et al. (20) found that shortening preoperative fasting time can reduce the occurrence of postoperative discomfort and complications. Therefore, while long-term fasting may reduce the loss of ions and hemoglobin, it could adversely affect postoperative prognosis.
There are relatively few reports on prolonged fasting and water restriction before surgery, but multiple studies suggest that extended fasting may have certain benefits for lowering blood pressure, reducing cardiovascular risk, and extending lifespan (21–23). Ezpeleta et al. (21) explored the effects of prolonged fasting, noting significant benefits in weight loss and blood pressure reduction, though the long-term benefits require further study. Wilhelmi et al. (22) investigated the effects of fasting on healthy lifespan and cognitive function, highlighting that extended fasting can enhance intrinsic defense mechanisms and reduce cardiovascular risk. Sang et al. (24) studied the impact of prolonged fasting on the gut microbiota of obese individuals, finding that fasting significantly altered the composition and metabolic capacity of the gut microbiome. Reasonably timed fasting and water restriction may offer certain health benefits. During fasting, the body activates the process of autophagy, which breaks down and recycles damaged or aging cell components, helping to remove harmful substances from the body and promoting cellular renewal. Fasting also lowers blood glucose and insulin levels, which may help improve insulin sensitivity and regulate blood sugar fluctuations. However, excessive fasting and water restriction can lead to potential risks such as dehydration, electrolyte imbalances, and nutrient deficiencies. Therefore, the duration of fasting and water restriction should be managed carefully.
Hemorrhage is one of the common complications following partial nephrectomy (10, 25, 26). By comparing the control group and the hemorrhage group after partial nephrectomy, it was found that the type of surgery significantly impacts hemorrhage (p = 0.023). Robot-assisted partial nephrectomy had a greater impact on postoperative hemorrhage compared to laparoscopic partial nephrectomy. This finding differs from previous studies and may be related to the shorter implementation period and less mature technique of robot-assisted partial nephrectomy at our center. The comparison between the control group and the hemorrhage group also showed that preoperative fasting time significantly impacts hemorrhage (p < 0.001), with short-term fasting having a greater impact on hemorrhage. This could be related to blood concentration after fasting.
Analysis of risk factors for postoperative hemorrhage after partial nephrectomy identified surgical method (p = 0.014) and preoperative fasting time (p < 0.001) as significant risk factors. Both surgical method (p = 0.050) and preoperative fasting time (p < 0.001) were also found to be independent risk factors for postoperative hemorrhage. Tsai et al. (26) similarly identified preoperative fasting time and surgical duration as risk factors for postoperative hemorrhage after partial nephrectomy. Bai et al. (27) also found preoperative fasting time and surgical duration to be significant risk factors in their study. Lorenzo et al. (28) indicated that robot-assisted partial nephrectomy is superior to laparoscopic and open surgeries in reducing transfusions and overall complications, which contrasts with our findings. This discrepancy may be related to the surgeon's experience. The robotic system provides a three-dimensional high-definition view and seven degrees of freedom in surgical instruments, allowing more precise control and reducing damage to surrounding blood vessels and tissues. Despite the enhanced precision offered by robotic systems, the surgeon must still possess adequate robotic surgical experience. In our study, the higher incidence of postoperative hemorrhage in robot-assisted partial nephrectomy may reflect the single-center experience and the relatively short period since the introduction of robotic surgery at our institution.
Long-term preoperative fasting should be a greater focus for clinicians and nurses. Although our study found short-term fasting to be a risk factor for postoperative hemorrhage, most previous studies suggest that long-term fasting adversely affects patient prognosis. Therefore, the duration of preoperative fasting should be reasonably adjusted based on the patient's condition.
This study has some limitations. Firstly, the sample size is relatively small, which may introduce bias in the analysis. Additionally, as the study was conducted in a single center, the lack of multicenter data may limit the generalizability of the findings.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statementThe studies involving humans were approved by Beijing University first hospital Miyun hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because we retrospectively collected some clinical data of patients without interfering with patients. Meanwhile, it is difficult for these patients to contact and sign the consent form again, which our ethics committee believes is visa-free.
Author contributionsCW: Writing – original draft, Writing – review & editing. JC: Writing – original draft, Writing – review & editing. ZG: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsBMI, body mass index; CHD, coronary artery disease.
References2. Cooperberg MR, Mallin K, Ritchey J, Villalta JD, Carroll PR, Kane CJ. Decreasing size at diagnosis of stage 1 renal cell carcinoma: analysis from the national cancer data base, 1993 to 2004. J Urol. (2008) 179(6):2131–5. doi: 10.1016/j.juro.2008.01.097
PubMed Abstract | Crossref Full Text | Google Scholar
3. Li M, Cheng L, Zhang H, Ma L, Wang Y, Niu W, et al. Laparoscopic and robotic-assisted partial nephrectomy: an overview of hot issues. Urol Int. (2020) 104(9–10):669–77. doi: 10.1159/000508519
PubMed Abstract | Crossref Full Text | Google Scholar
4. Hevia V, Boissier R, Rodríguez-Faba Ó, Fraser-Taylor C, Hassan-Zakri R, Lledo E, et al. Management of localised prostate cancer in kidney transplant patients: a systematic review from the EAU guidelines on renal transplantation panel. Eur Urol Focus. (2018) 4(2):153–62. doi: 10.1016/j.euf.2018.05.010
PubMed Abstract | Crossref Full Text | Google Scholar
5. Grivas N, Kalampokis N, Larcher A, Tyritzis S, Rha KH, Ficarra V, et al. Robot-assisted versus open partial nephrectomy: comparison of outcomes. A systematic review. Minerva Urol Nefrol. (2019) 71(2):113–20. doi: 10.23736/S0393-2249.19.03391-5
PubMed Abstract | Crossref Full Text | Google Scholar
6. Antonelli A, Minervini A, Mari A, Bertolo R, Bianchi G, Lapini A, et al. Trimatch comparison of the efficacy of FloSeal versus TachoSil versus no hemostatic agents for partial nephrectomy: results from a large multicenter dataset. Int J Urol. (2015) 22(1):47–52. doi: 10.1111/iju.12603
PubMed Abstract | Crossref Full Text | Google Scholar
7. de Assis MCS, de Moraes Silveira CR, Beghetto MG, De Mello ED. Is duration of postoperative fasting associated with infection and prolonged length of stay in surgical patients? Nutr Hosp. (2014) 30(4):919–26. doi: 10.3305/nh.2014.30.4.7528
PubMed Abstract | Crossref Full Text | Google Scholar
8. Falconer R, Skouras C, Carter T, Greenway L, Paisley AM. Preoperative fasting: current practice and areas for improvement. Updates Surg. (2014) 66(1):31–9. doi: 10.1007/s13304-013-0242-z
PubMed Abstract | Crossref Full Text | Google Scholar
10. Styopushkin S, Chaikovskyi V, Chernylovskyi V, Sokolenkо R, Bondarenko D. Postoperative hemorrhage as a complication of a partial nephrectomy: frequency, features and management. Georgian Med News. (2021) 313:12–20.
11. Bigot P, Bouvier A, Panayotopoulos P, Aubé C, Azzouzi AR. Partial nephrectomy after selective embolization of tumor vessels in a hybrid operating room: a new approach of zero ischemia in renal surgery. J Surg Oncol. (2016) 113(2):135–7. doi: 10.1002/jso.24120
PubMed Abstract | Crossref Full Text | Google Scholar
12. Yucel MO, Polat H, Bagcioglu M, Karakan T, Benlioglu C, Cift A, et al. Comparison of the efficacy and histopathological effects of three hemostatic agents in a partial nephrectomy rat model. Int Urol Nephrol. (2016) 48(1):65–71. doi: 10.1007/s11255-015-1129-3
PubMed Abstract | Crossref Full Text | Google Scholar
13. Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. (2022) 82(5):529–42. doi: 10.1016/j.eururo.2022.08.019
PubMed Abstract | Crossref Full Text | Google Scholar
14. Huang J, Leung DK-W, Chan EO-T, Lok V, Leung S, Wong I, et al. A global trend analysis of kidney cancer incidence and mortality and their associations with smoking, alcohol consumption, and metabolic syndrome. Eur Urol Focus. (2022) 8(1):200–9. doi: 10.1016/j.euf.2020.12.020
PubMed Abstract | Crossref Full Text | Google Scholar
15. Pyrgidis N, Schulz GB, Stief C, Blajan I, Ivanova T, Graser A, et al. Surgical trends and complications in partial and radical nephrectomy: results from the GRAND study. Cancers (Basel). (2023) 16(1):97. doi: 10.3390/cancers16010097
PubMed Abstract | Crossref Full Text | Google Scholar
16. Cheng P-L, Loh E-W, Chen J-T, Tam K-W. Effects of preoperative oral carbohydrate on postoperative discomfort in patients undergoing elective surgery: a meta-analysis of randomized controlled trials. Langenbecks Arch Surg. (2021) 406(4):993–1005. doi: 10.1007/s00423-021-02110-2
PubMed Abstract | Crossref Full Text | Google Scholar
17. Rizvanović N, Nesek Adam V, Čaušević S, Dervišević S, Delibegović S. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int J Colorectal Dis. (2019) 34(9):1551–61. doi: 10.1007/s00384-019-03349-4
PubMed Abstract | Crossref Full Text | Google Scholar
18. Lin M-W, Chen C-I, Cheng T-T, Huang C-C, Tsai J-W, Feng G-M, et al. Prolonged preoperative fasting induces postoperative insulin resistance by ER-stress mediated Glut4 down-regulation in skeletal muscles. Int J Med Sci. (2021) 18(5):1189–97. doi: 10.7150/ijms.52701
PubMed Abstract | Crossref Full Text | Google Scholar
19. Zhou G, Zhu F, An Y, Qin L, Lv J, Zhao X, et al. Prolonged preoperative fasting and prognosis in critically ill gastrointestinal surgery patients. Asia Pac J Clin Nutr. (2020) 29(1):41–7. doi: 10.6133/apjcn.202003_29(1).0006
PubMed Abstract | Crossref Full Text | Google Scholar
20. Marquini GV, Pinheiro FES, Vieira AUC, Pinto RMC, Uyeda MGBK, Girão MJBC, et al. Effects of preoperative fasting abbreviation with carbohydrate and protein solution on postoperative symptoms of gynecological surgeries: double-blind randomized controlled clinical trial. Rev Col Bras Cir. (2020) 46(5):e20192295. doi: 10.1590/0100-6991e-20192295
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ezpeleta M, Cienfuegos S, Lin S, Pavlou V, Gabel K, Varady KA. Efficacy and safety of prolonged water fasting: a narrative review of human trials. Nutr Rev. (2024) 82(5):664–75. doi: 10.1093/nutrit/nuad081
PubMed Abstract | Crossref Full Text | Google Scholar
22. de Toledo F W, Grundler F, Sirtori CR, Ruscica M. Unravelling the health effects of fasting: a long road from obesity treatment to healthy life span increase and improved cognition. Ann Med. (2020) 52(5):147–61. doi: 10.1080/07853890.2020.1770849
PubMed Abstract | Crossref Full Text | Google Scholar
23. Nugraha B, Riat A, Ghashang SK, Eljurnazi L, Gutenbrunner C. A prospective clinical trial of prolonged fasting in healthy young males and females-effect on fatigue, sleepiness, mood and body composition. Nutrients. (2020) 12(8):2281. doi: 10.3390/nu12082281
PubMed Abstract | Crossref Full Text | Google Scholar
24. Sang X, Li S, Guo R, Yan Q, Liu C, Zhang Y, et al. Dynamics and ecological reassembly of the human gut microbiome and the host metabolome in response to prolonged fasting. Front Microbiol. (2023) 14:1265425 doi: 10.3389/fmicb.2023.1265425
PubMed Abstract | Crossref Full Text | Google Scholar
25. Gomella PT, Solomon J, Ahdoot M, Gurram S, Lebastchi AH, Levy E, et al. Timing, incidence and management of delayed bleeding after partial nephrectomy in patients at risk for recurrent, bilateral, multifocal renal tumors. Urol Oncol. (2024) 42(7):222.e221–222.e227. doi: 10.1016/j.urolonc.2024.03.004
PubMed Abstract | Crossref Full Text | Google Scholar
26. Tsai C-H, Chung H-J, Huang EYH, Lin T-P, Huang T-H, Huang WJ. Risk factors for hemorrhagic complications following robotic-assisted partial nephrectomy. J Chin Med Assoc. (2023) 86(3):295–9. doi: 10.1097/JCMA.0000000000000857
PubMed Abstract | Crossref Full Text | Google Scholar
27. Bai N, Qi M, Shan D, Liu S, Na T, Chen L. Trifecta achievement in patients undergoing partial nephrectomy: a systematic review and meta-analysis of predictive factors. Int Braz J Urol. (2022) 48(4):625–36. doi: 10.1590/s1677-5538.ibju.2021.0095
PubMed Abstract | Crossref Full Text | Google Scholar
28. Luciani LG, Chiodini S, Mattevi D, Cai T, Puglisi M, Mantovani W, et al. Robotic-assisted partial nephrectomy provides better operative outcomes as compared to the laparoscopic and open approaches: results from a prospective cohort study. J Robot Surg. (2017) 11(3):333–9. doi: 10.1007/s11701-016-0660-2
留言 (0)