Prostate cancer (PC) is the second-most frequent malignancy and the sixth major cause of cancer-related fatalities in men globally (1). The prostate cancer incidence rate has risen by 3% per year from 2014 to 2019 in the USA (2), and it is predicted that there will be approximately 2.3 million new cases and 740,000 deaths from PC globally in 2040 (3). Therefore, it is important to detect suspected at-risk patients to reduce the PC incidence and mortality rates. Acne is a very common chronic inflammatory disease of the skin. According to statistics, acne has become the eighth-most prevalent disease worldwide, affecting approximately 9% of the global population (4). The pathogenesis of acne involves multiple factors such as inflammation caused by Propionbacterium acnes and the hormonal influence (5). P. acnes, as an opportunistic pathogen, plays a likely underestimated role in the development of other human diseases such as degenerative disk, prostate disease, and atherosclerosis (6, 7).
Because acne is a proxy for androgen status (8) and P. acnes is reported to be associated with prostatic inflammation and carcinogenesis (9, 10), there have been studies examining the association between acne and the risk of prostate cancer. However, these previous studies exploring the association between acne and prostate cancer reported inconsistent results. A large prospective population-based cohort (11) indicated that acne was associated with an increased risk for prostate cancer. Another study (12) suggested that individuals with acne in adolescence have a higher risk of adult prostate cancer mortality. One study suggested a lack of acne during adolescence reduce high-grade prostate cancer at adulthood (13). Some other studies (14, 15) showed no associations between acne and the risk of prostate cancer. Additionally, one case–control study (16) reported a negative association between acne‐related facial scarring and later prostate cancer. These observational epidemiological studies are limited in assessing causality due to confounding and reverse causation. Therefore, we hope this question will be resolved with our proposed method.
Mendelian randomization (MR) is a genetics-based instrumental variable approach that relies on the random and fixed assignment of genetic variants at conception to estimate the causal effect size of genetically predicted exposures on an outcome. Compared with traditional observational studies, MR is less susceptible to confounding or reverse causation (17). In addition, a meta-analysis is a statistical method that combines the results of different analyses about the same topic, which can solve conflicts somewhat. It can help to integrate the MR results from different populations, thus drawing a reasonable conclusion. In our study, we use publicly available genome-wide association study (GWAS) data setting genetic variations associated with acne as instrumental variables (IVs), then explored the causal relationship between acne and PC through two-sample MR analyses, followed by a meta-analysis to provide an overall evaluation.
2 Materials and methods2.1 Study design overviewThis study considered acne as the exposure and PC as the outcome. The single nucleotide polymorphisms (SNPs) closely related to acne were regarded as IVs. After the selection of IVs, the causal association between acne and PC was explored by two-sample Mendelian randomization analyses. Then, sensitivity tests were carried out to ensure the results were robust and accurate. Finally a meta-analysis was conducted to integrate MR results from different outcome databases (Figure 1).
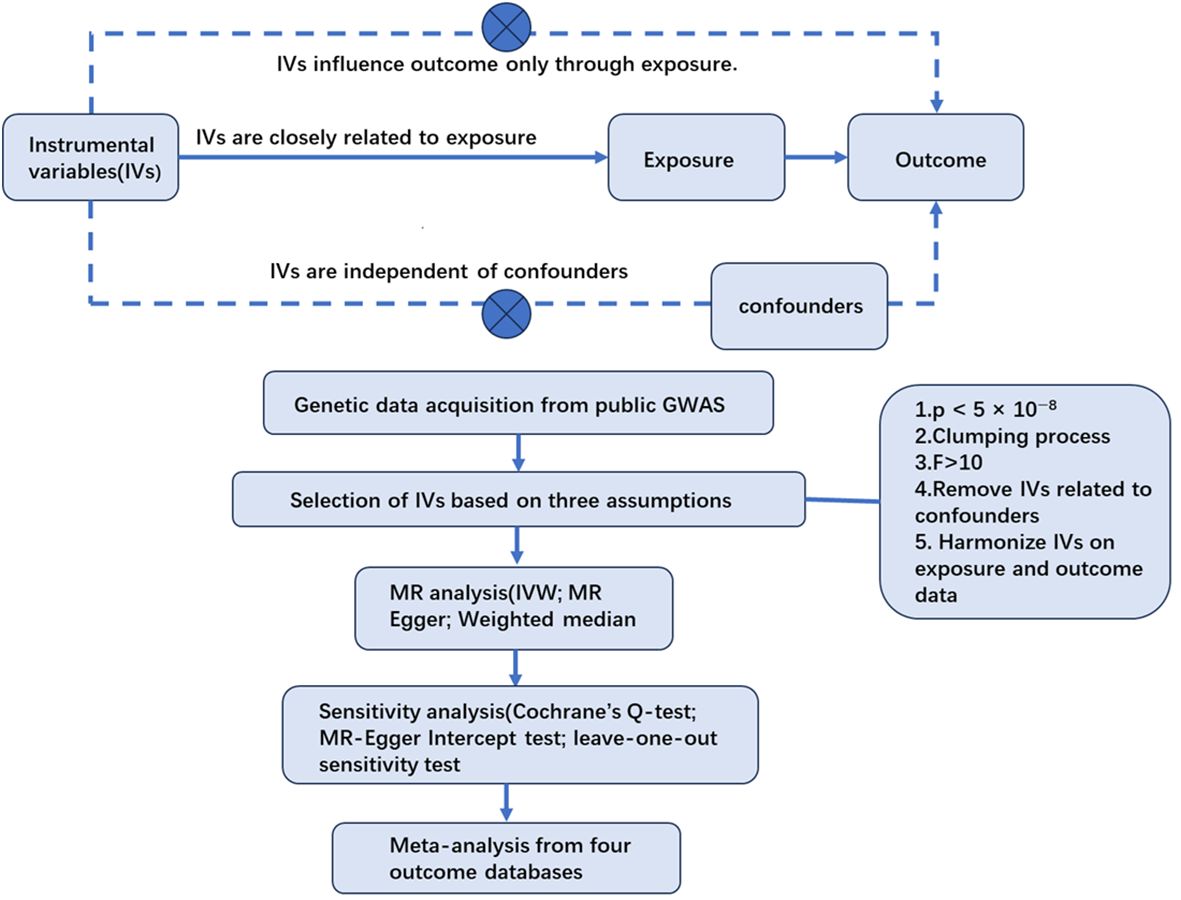
Figure 1. Flowchart of the MR study.
The IVs for MR analysis must meet three basic assumptions (18): (1) the correlation assumption—the IVs are closely related to exposure; (2) the independence assumption —the IVs are independent of confounding factors between exposure and outcome; and (3) the excluding restriction assumption—the IVs influence outcome only through their effects on exposure and not through other casual pathways.
We performed these analyses using the “TwoSampleMR” package and “meta” package in Rstudio (version 4.3.3.).
2.2 Data sourcesThe genetic data for assessing acne were acquired from the largest GWAS meta-analysis of acne by far, which included 20,165 European ancestry cases and 595,231 European ancestry controls (19). The definitions of acne in these cohorts varied across clinical assessments, electronic health records, and self-report questionnaires. The GWAS summary statistics are available in the GWAS catalogue (www.ebi.ac.uk/gwas) (accession number GCST90092000). The genetic data for assessing PC were acquired from four eminent databases: the FinnGen consortium (N=146465), UK Biobank (N=463010), the European Bioinformatics Institute (N=208768), and the IEU OpenGWAS project (N=140254). The detailed information regarding these genetic data are listed in Table 1.
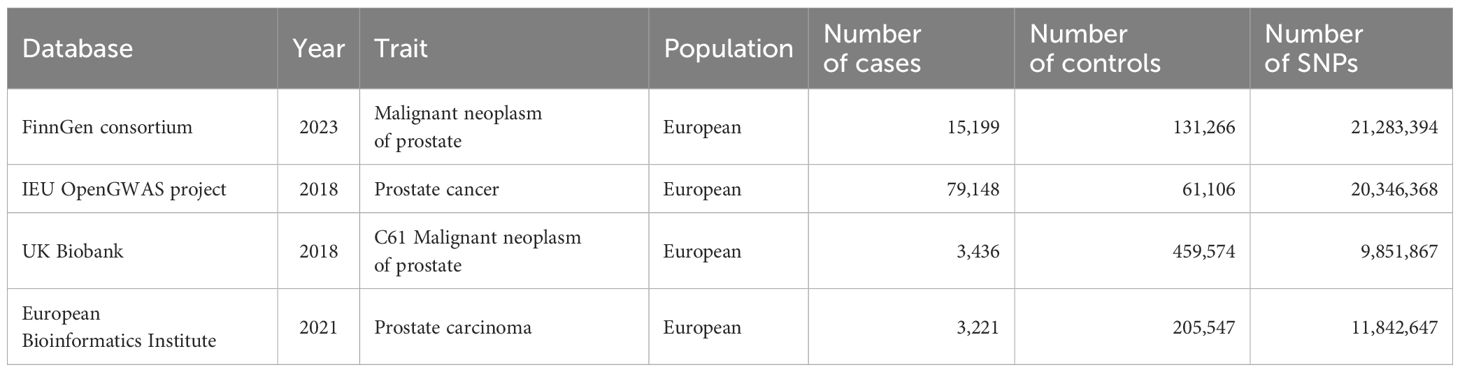
Table 1. Description of the data sources of outcome.
2.3 Selection of IVsTo satisfy the three basic assumptions, we conducted the selection of IVs using the following steps. (1) We used the threshold (p < 5 × 10−8) to select SNPs closely related to acne. (2) We eliminated the linkage disequilibrium (LD) between chosen IVs by controlling parameters of r2 <0.001 and a clumping window of >10,000 kb. LD refers to the phenomenon that genetic variants physically close to each other on the same chromosome are likely to be inherited together. The existence of a LD implies that genetic variants will not be distributed independently and could lead to biased results. (3) We computed F-statistics to assess the strength of IVs to make sure these instruments confidently predicted acne status (20). (4) We examined the chosen SNPs one by one on the phenoscanner database and took away IVs that were highly correlated with prostate diseases. (5) We merged the selected IVs with the genetic data of PC acquired from different outcome databases and removed IVs with palindromic sequences, the orientations of which could not be determined, and incompatible SNPs.
2.4 Statistical analysisFor MR analysis, the inverse variance weighted (IVW) model was adopted as the main causal evaluation method supplemented by weighted median and weighted MR-Egger, as the IVW method assumed that all IVs were valid (21). Additionally, when directional pleiotropy is absent, the IVW method can deliver a relatively stable and accurate causal evaluation by using a meta-analytic approach to combine Wald estimates for each IV (22).
For sensitivity analysis, the heterogeneity between IVs was tested by Cochrane’s Q-statistic. If significant heterogeneity was indicated, a random-effect model would be adopted. Otherwise, a fixed-effect model would be adopted (23). The MR-Egger intercept method was adopted for detecting the horizontal pleiotropy of SNPs. The MR-Egger intercept test is robustly sensitive to directional bias due to pleiotropy, with a p-value of >0.05 indicating the absence of horizontal pleiotropy (24). The leave-one-out sensitivity test was used to judge the stability of the MR results by excluding IVs one by one.
For meta-analysis, the IVW effect estimate as the main evaluation of acne on prostate cancer was acquired from the four outcomes and then combined in a meta-analysis. We adopted a random-effects model to reduce the impact of heterogeneity. Based on the results of the above analyses, we provided an overall evaluation of the genetic causal relationship between acne and prostate cancer.
3 Results3.1 Selected IV dataA cohort of 30 IVs was selected from the FinnGen consortium. A cohort of 27 IVs was selected from the UK Biobank. A cohort of 26 IVs was selected from the IEU OpenGWAS project. A cohort of 30 IVs was selected from the European Bioinformatics Institute. All IVs demonstrated strong validity (an F-statistic greater than 10) (Supplementary Material 1).
3.2 Results of the two-sample MR analysisWe conducted IVW analysis for MR analysis supplemented by MR-Egger and the weighted median. The results of MR analysis from the FinnGen consortium showed a negative causality between acne and PC: IVW [p=0.027; odds ratio (OR)=0.925; 95 confidence interval (CI)% 0.863–0.991], MR-Egger (p=0.018; OR=0.784; 95 CI% 0.648–0.947), weighted median (p=0.114; OR=0.932; 95 CI% 0.857–1.014). The results of MR analysis from the UK Biobank showed no causality between acne and PC: IVW (p=0.466; OR=1; 95 CI% 0.999–1.001), MR-Egger (p=0.573; OR=1.001; 95 CI% 0.998–1.004), weighted median (p=0.394; OR=1; 95 CI% 0.999–1.001). The results of MR analysis from the IEU OpenGWAS project showed no causality between acne and PC: IVW (p=0.388; OR=0.978; 95 CI% 0.929–1.029), MR-Egger (p=0.903; OR=0.991; 95 CI% 0.853–1.151), weighted median (p=0.519; OR=0.981; 95 CI% 0.927–1.039). The results of MR analysis from the European Bioinformatics Institute showed no causality between acne and PC: IVW (p=0.563; OR=1.031; 95 CI% 0.931–1.141), MR-Egger (p=0.921; OR=1.015; 95 CI% 0.753–1.369), weighted median (P=0.877; OR=1.011; 95 CI% 0.881–1.160) (Table 2).
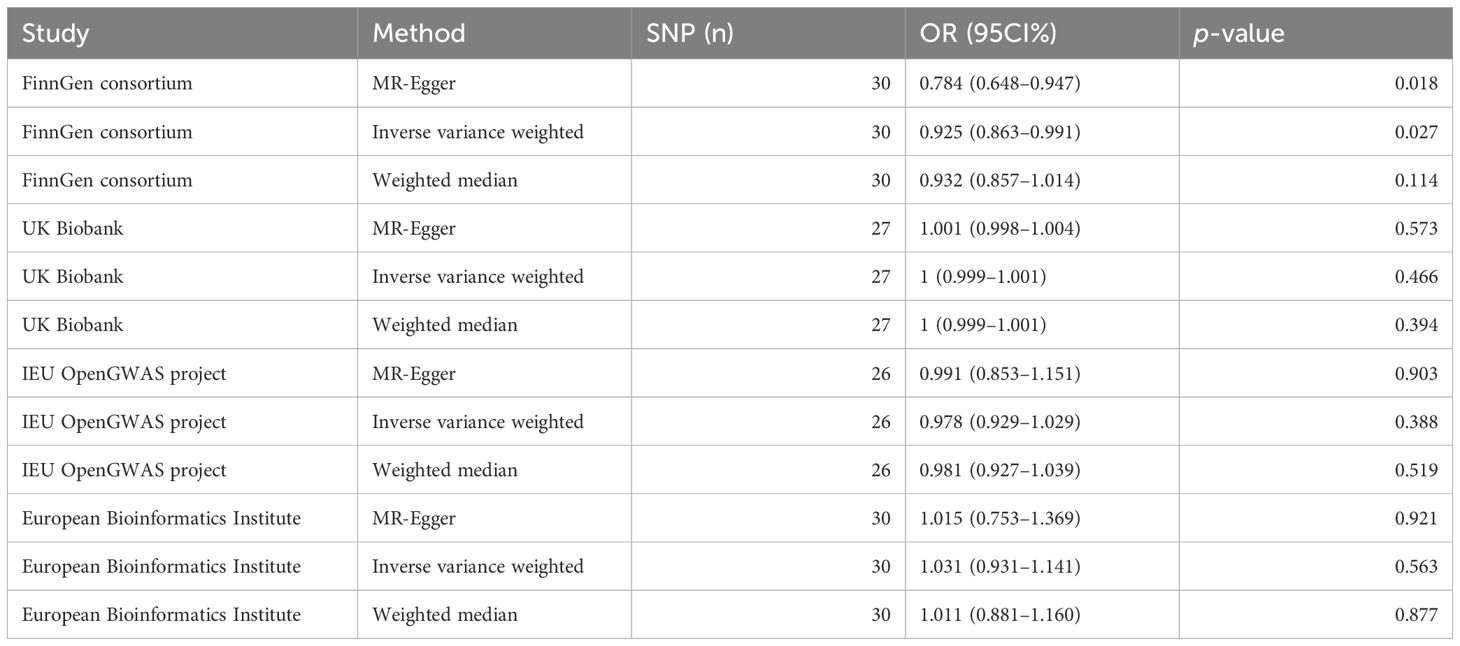
Table 2. Results of MR estimates between acne and PC.
3.3 Results of the sensitivity analysisThe values of intercept were all small, and horizontal pleiotropy was not significant (pFinn=0.078, pUKB=0.718, pIEU=0.855, pEBI=0.918). The results of Cochran’s Q test of MR exploration (pFinn=0.0216, pUKB=0.072, pIEU=0.001, pEBI=0.895) indicated significant heterogeneity. However, it did not affect the accuracy of the result as a multiplicative random-effects model was adopted in this MR analysis. No single SNP was seen in a leave-one-out test that had a disproportionate effect on the overall results (Table 3; Figure 2).

Table 3. Results of the sensitivity analysis.
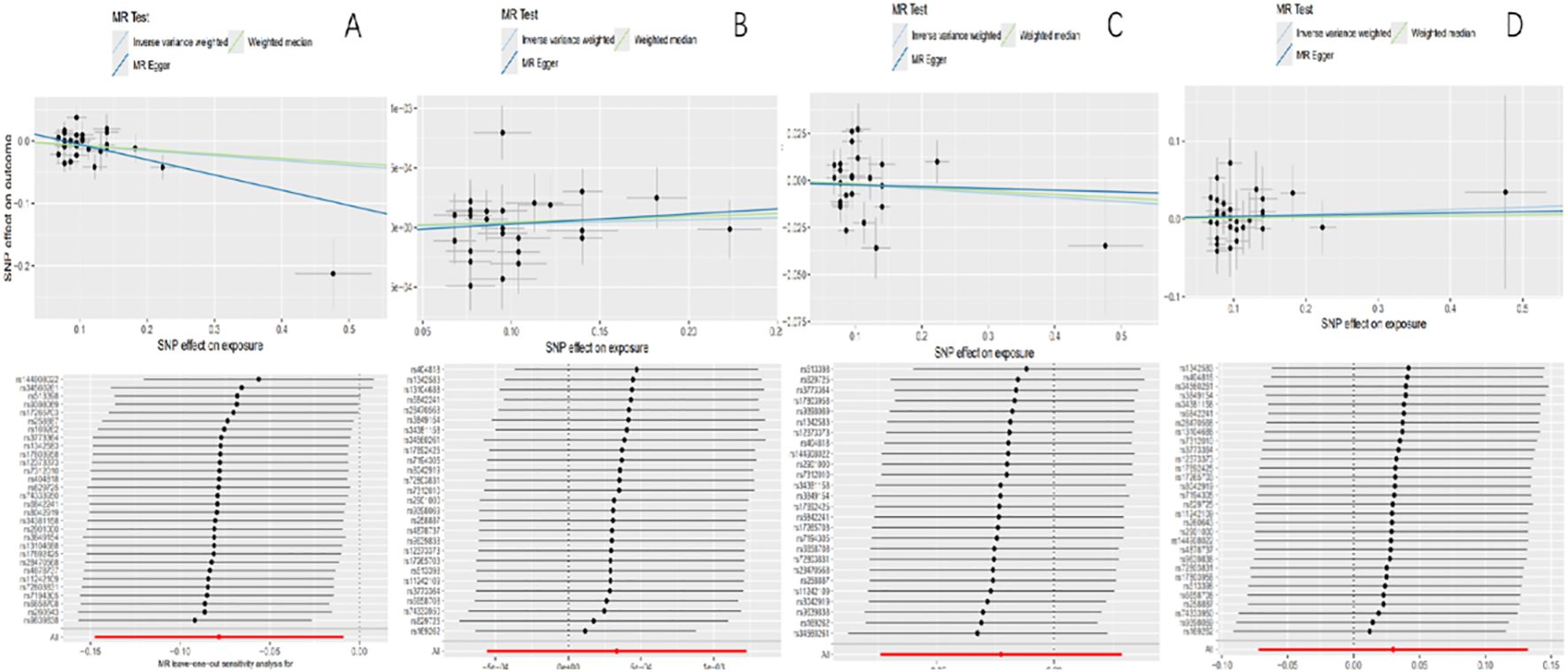
Figure 2. Scatter plots of causality and “leave-one-out” sensitivity tests. (A) The FinnGen consortium. (B) The UK Biobank. (C) The IEU OpenGWAS project. (D) The European Bioinformatics Institute.
3.4 Results of the meta-analysisTaking IVW as the major method, we conducted meta-analysis on the results from different outcome databases. The result did not show significant heterogeneity (I2 = 49.6%; H=1.41; p=0.11) or a genetic causal relationship between acne and PC (p=0.378; OR=0.985; 95% CI 0.954–1.018) (Figure 3).
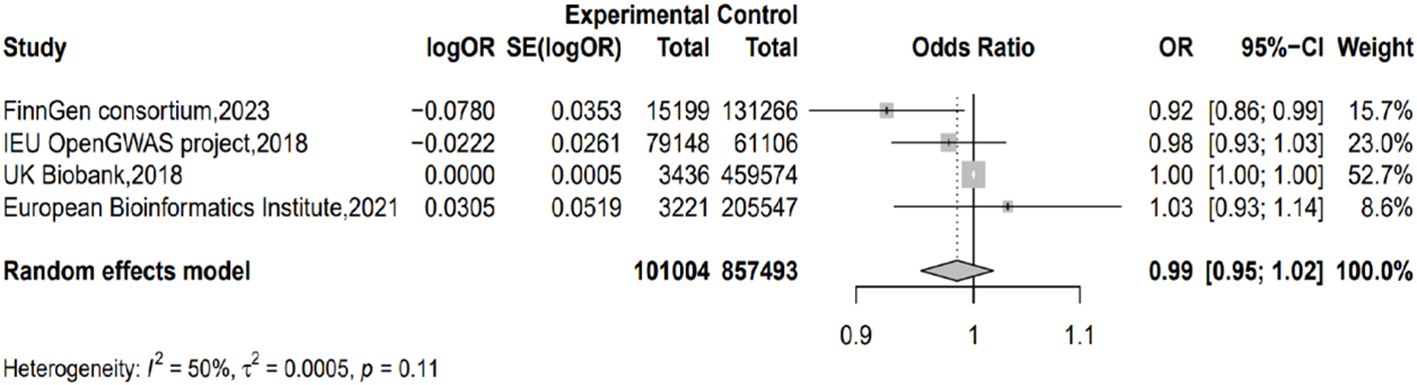
Figure 3. Forest plot of the results from the MR analysis.
4 DiscussionTo the best of our knowledge, this is the first study investigating the association between acne and PC that applied MR analysis. Additionally, we integrate the MR results from different outcome databases to draw a reasonable conclusion. Finally, the result supports there is no genetic causal relationship between acne and PC.
Until now, the relationship between acne and the risk of PC has remained unclarified. Previous studies reported inconsistent results. A large prospective population‐based cohort suggested that a diagnosis of acne classified as severe conferred a sixfold increased risk of PC (11). Another prospective cohort study suggested that acne during young adulthood was associated with an increased risk of PC‐specific death (12). A prospective cohort of American male health professionals showed an increased PC risk for men who reported receiving treatment with tetracycline for more than 4 years, which was regarded as a marker of severe acne (25). Additionally, there were studies that showed no association between acne and the risk of PC (14, 15). There was still a case-control study that reported a negative association between acne‐related facial scarring and later PC (16). However, most of these studies are observational studies. Furthermore, these contentious findings may be influenced by indissoluble or unidentified risk factors and recall bias.
The exact pathogenic mechanism of PC is still largely unknown. There have been two different hypotheses regarding the association between acne and PC so far. The first is that P. acnes-mediated inflammation may contribute to PC. A study (26) found that P. acnes isolated from radical prostatectomy specimens was positively related to the onset and extent of both acute and chronic prostate inflammation. Persistent low-grade P. acnes-mediated inflammation may lead to precursor lesions for PC (25). The second is a strong immune response caused by P. acnes may offer protection from PC. P. acnes can cause inflammatory reactions through complement activation and the induction of proinflammatory cytokines (27) and can also cause a strong cell-mediated T-helper type 1 (Th1) immune response (28). Additionally, Th1 immune responses are detrimental to the establishment and progression of tumors (29). One study (30) reported that high serum titers of antibodies directed against P. acnes were observed to be inversely associated with the risk of PC, and the high serum titers of antibodies directed against P. acnes may be an indirect marker of increased cell-mediated immunity. One of our MR results pointed to a similar conclusion. However, MR analysis of a population only provides a quasi-randomized controlled trial design. The findings require validation in different populations. Additionally, hormonal activity also plays a role in the pathology of acne. In previous studies, the association between P. acnes and PC could not fully represent the association between acne and PC. Our study integrating MR results from different populations suggests acne should not be considered as a morbidity hazard factor for PC. However, the effects of P. acnes on PC still need further research.
There are several strengths in our study. First, to our knowledge, this is the first study using MR analysis to investigate the association between acne and PC. MR studies can effectively avoid confounding bias compared with observational studies. Second, a supplementary meta-analysis and various sensitivity tests were implemented to increase the reliability of the results. Third, the study may help to influence public health policies regarding the early prevention and timely intervention of PC.
There are still some limitations in our study. First, the age discrepancy between acne and PC could introduce bias. Genetic variants for acne may not remain relevant or active during the typical onset age of PC. Age-stratified analyses could mitigate this. However, current GWAS data on acne lack more detailed age information, limiting the possibility of conducting subgroup analysis. Second, the GWAS data came from the European population. Whether our described findings would be consistent in other populations remained to be investigated. Genetic data from more numerous and larger-scale GWASs are needed. Third, the concrete mechanism of PC is still unclear. Interrelationships between P. acnes, other prostate diseases, and PC still need further research.
5 ConclusionOur MR combined with meta-analysis suggests there is no genetic causal relationship between acne and PC. Acne should not be considered as a morbidity hazard factor for PC.
Data availability statementPublicly available datasets were analyzed in this study. These data can be found from FinnGen consortium (https://r10.finngen.fi/), UK Biobank (https://www.ukbiobank.ac.uk/) EuropeanBioinformatics Institute (https://www.ebi.ac.uk/gwas/), and IEUOpenGWAS project (https://gwas.mrcieu.ac.uk/).
Ethics statementEthical approval and consent were not required as this study was based on publicly available data.
Author contributionsMH: Writing – original draft, Data curation, Methodology, Software. XW: Writing – original draft, Supervision. DW: Writing – review & editing, Data curation. YW: Data curation, Writing – review & editing. JQ: Writing – review & editing, Data curation, Supervision. JJ: Writing – review & editing, Data curation. MZ: Writing – review & editing, Data curation. QM: Writing – review & editing, Data curation. BY: Writing – review & editing, Data curation. HG: Writing – review & editing, Data curation. CQ: Writing – review & editing, Funding acquisition, Project administration, Resources, Supervision, Validation.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe extend our gratitude to the researchers behind the GWASs for generously sharing their summary data with the public. Additionally, we thank all the investigators and participants whose valuable contributions were instrumental in these studies.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1460467/full#supplementary-material
References1. Ou J, Zhen K, Wu Y, Xue Z, Fang Y, Zhang Q, et al. Systemic lupus erythematosus and prostate cancer risk: a pool of cohort studies and Mendelian randomization analysis. J Cancer Res Clin Oncol. (2023) 149:9517–28. doi: 10.1007/s00432-023-04853-5
PubMed Abstract | Crossref Full Text | Google Scholar
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
PubMed Abstract | Crossref Full Text | Google Scholar
6. Capoor MN, Birkenmaier C, Wang JC, McDowell A, Ahmed FS, Brüggemann H, et al. A review of microscopy-based evidence for the association of Propionibacterium acnes biofilms in degenerative disc disease and other diseased human tissue. Eur Spine J. (2019) 28:2951–71. doi: 10.1007/s00586-019-06086-y
PubMed Abstract | Crossref Full Text | Google Scholar
8. Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res. (2012) 304:499–510. doi: 10.1007/s00403-012-1265-x
PubMed Abstract | Crossref Full Text | Google Scholar
9. Leheste JR, Ruvolo KE, Chrostowski JE, Rivera K, Husko C, Miceli A, et al. P. acnes-driven disease pathology: current knowledge and future directions. Front Cell Infect Microbiol. (2017) 7:81. doi: 10.3389/fcimb.2017.00081
PubMed Abstract | Crossref Full Text | Google Scholar
10. Davidsson S, Mölling P, Rider JR, Unemo M, Karlsson MG, Carlsson J, et al. Frequency and typing of Propionibacterium acnes in prostate tissue obtained from men with and without prostate cancer. Infect Agent Cancer. (2016) 11:26. doi: 10.1186/s13027-016-0074-9
PubMed Abstract | Crossref Full Text | Google Scholar
11. Ugge H, Udumyan R, Carlsson J, Andrén O, Montgomery S, Davidsson S, et al. Acne in late adolescence and risk of prostate cancer. Int J Cancer. (2018) 142:1580–5. doi: 10.1002/ijc.31192
PubMed Abstract | Crossref Full Text | Google Scholar
12. Galobardes B, Davey Smith G, Jeffreys M, Kinra S, McCarron P. Acne in adolescence and cause-specific mortality: lower coronary heart disease but higher prostate cancer mortality: the glasgow alumni cohort study. Am J Epidemiol. (2005) 161:1094–101. doi: 10.1093/aje/kwi147
PubMed Abstract | Crossref Full Text | Google Scholar
13. Hernández-Pérez JG, López DS, Rodríguez-Valentín R, Vázquez-Salas RA, Sierra-Santoyo A, Torres-Sánchez L. Late puberty onset and lack of acne during adolescence reduce high-grade prostate cancer at adulthood. Prostate. (2023) 83:1342–50. doi: 10.1002/pros.24596
PubMed Abstract | Crossref Full Text | Google Scholar
14. Cremers RG, Aben KK, Vermeulen SH, den Heijer M, van Oort IM, van de Kerkhof PC, et al. Self-reported acne is not associated with prostate cancer. Urol Oncol. (2014) 32:941–5. doi: 10.1016/j.urolonc.2014.02.019
PubMed Abstract | Crossref Full Text | Google Scholar
15. Zhang X, Lin Y, Xie X, Shen M, Huang G, Yang Y. Is acne in adolescence associated with prostate cancer risk? Evidence from a meta-analysis. PloS One. (2018) 13:e0206249. doi: 10.1371/journal.pone.0206249
PubMed Abstract | Crossref Full Text | Google Scholar
16. Giles GG, Severi G, English DR, McCredie MR, MacInnis R, Boyle P, et al. Early growth, adult body size and prostate cancer risk. Int J Cancer. (2003) 103:241–5. doi: 10.1002/ijc.10810
PubMed Abstract | Crossref Full Text | Google Scholar
17. Ebrahim S, Davey Smith G. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology. Hum Genet. (2008) 123:15–33. doi: 10.1007/s00439-007-0448-6
PubMed Abstract | Crossref Full Text | Google Scholar
18. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. (2023) 4:186. doi: 10.12688/wellcomeopenres.15555.3
PubMed Abstract | Crossref Full Text | Google Scholar
19. Mitchell BL, Saklatvala JR, Dand N, Hagenbeek FA, Li X, Min JL, et al. Genome-wide association meta-analysis identifies 29 new acne susceptibility loci. Nat Commun. (2022) 13:702. doi: 10.1038/s41467-022-28252-5
PubMed Abstract | Crossref Full Text | Google Scholar
20. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
PubMed Abstract | Crossref Full Text | Google Scholar
21. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
PubMed Abstract | Crossref Full Text | Google Scholar
22. Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, InterAct Consortium EPIC-. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. (2015) 30:543–52. doi: 10.1007/s10654-015-0011-z
PubMed Abstract | Crossref Full Text | Google Scholar
23. Bowden J, Hemani G, Davey Smith G. Invited commentary: detecting individual and global horizontal pleiotropy in mendelian randomization-A job for the humble heterogeneity statistic? Am J Epidemiol. (2018) 187:2681–5. doi: 10.1093/aje/kwy185
PubMed Abstract | Crossref Full Text | Google Scholar
24. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
PubMed Abstract | Crossref Full Text | Google Scholar
26. Cohen RJ, Shannon BA, McNeal JE, Shannon T, Garrett KL. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol. (2005) 173:1969–74. doi: 10.1097/01.ju.0000158161.15277.78
PubMed Abstract | Crossref Full Text | Google Scholar
28. Mouser PE, Baker BS, Seaton ED, Chu AC. Propionibacterium acnes-reactive T helper-1 cells in the skin of patients with acne vulgaris. J Invest Dermatol. (2003) 121:1226–8. doi: 10.1046/j.1523-1747.2003.12550_6
PubMed Abstract | Crossref Full Text | Google Scholar
29. Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. (2008) 222:145–54. doi: 10.1111/j.1600-065X.2008.00600
PubMed Abstract | Crossref Full Text | Google Scholar
30. Severi G, Shannon BA, Hoang HN, Baglietto L, English DR, Hopper JL, et al. Plasma concentration of Propionibacterium acnes antibodies and prostate cancer risk: results from an Australian population-based case-control study. Br J Cancer. (2010) 103:411–5. doi: 10.1038/sj.bjc.6605757
留言 (0)