This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Andrew C. Hean, PharmD; Andres R. Schneeberger, MD; and Kelly C. Lee, PharmD, MAS
Published: August 29, 2024
Lurasidone is a second-generation antipsychotic with US Food and Drug Administration–approved indications for schizophrenia and bipolar I depression.1 Glucagon-like peptide-1 receptor agonists (GLP 1RAs) are being increasingly prescribed for weight loss, with nausea and vomiting being common limiting side effects.2 Compared to other weight-loss medications, GLP-1RAs show the most evidence in treating antipsychotic-induced weight gain.3 In this case report, we describe a potential drug interaction between lurasidone and semaglutide causing new intolerable nausea following lurasidone administration.
Case ReportA 35-year-old woman with a history of bipolar II disorder, posttraumatic stress disorder, atrial fibrillation, asthma, prediabetes, and hypertension was prescribed lamotrigine 400 mg daily, gabapentin 1,200 mg daily, and quetiapine 300 mg nightly. Upon initiating quetiapine, she noticed increased impulsive eating and weight gain. She was switched to lurasidone 40 mg daily with food and noticed improvement in mood with good tolerability. She was then titrated to 80 mg daily over 3 months with no adverse effects. Two days prior to titration to 80 mg, she was prescribed semaglutide 0.25 mg subcutaneously weekly for weight loss by another provider.
During month 2 of concurrent semaglutide and lurasidone, lurasidone was titrated to 120 mg daily, while semaglutide was dosed at 1 mg weekly. She endorsed mild nausea thought to be due to semaglutide. During month 3 of semaglutide and lurasidone treatment, she continued to endorse nausea but to a lesser extent; she continued to have mood symptoms, and lurasidone was increased to 160 mg daily. After 13 days, she reported intolerable nausea 1 hour after taking lurasidone 160 mg while on semaglutide 1.7 mg weekly. The psychiatry provider recommended splitting lurasidone dosing to 80 mg twice daily. This did not alleviate nausea, and lurasidone was decreased to 120 mg daily; semaglutide was given at 2.4 mg weekly. During month 5 of semaglutide and lurasidone treatment, she endorsed continued nausea 1 hour after taking lurasidone, now at 80 mg every other day. Nausea did not occur on days she did not take lurasidone. She endorsed the same quantity and quality of food regardless of taking lurasidone. Due to intolerable nausea, she was switched back to quetiapine 200 mg. She endorsed good efficacy from quetiapine with remission of nausea after stopping lurasidone and continued semaglutide 2.4 mg weekly. Figure 1 provides the timeline of medication changes, adverse effects, and monitoring parameters.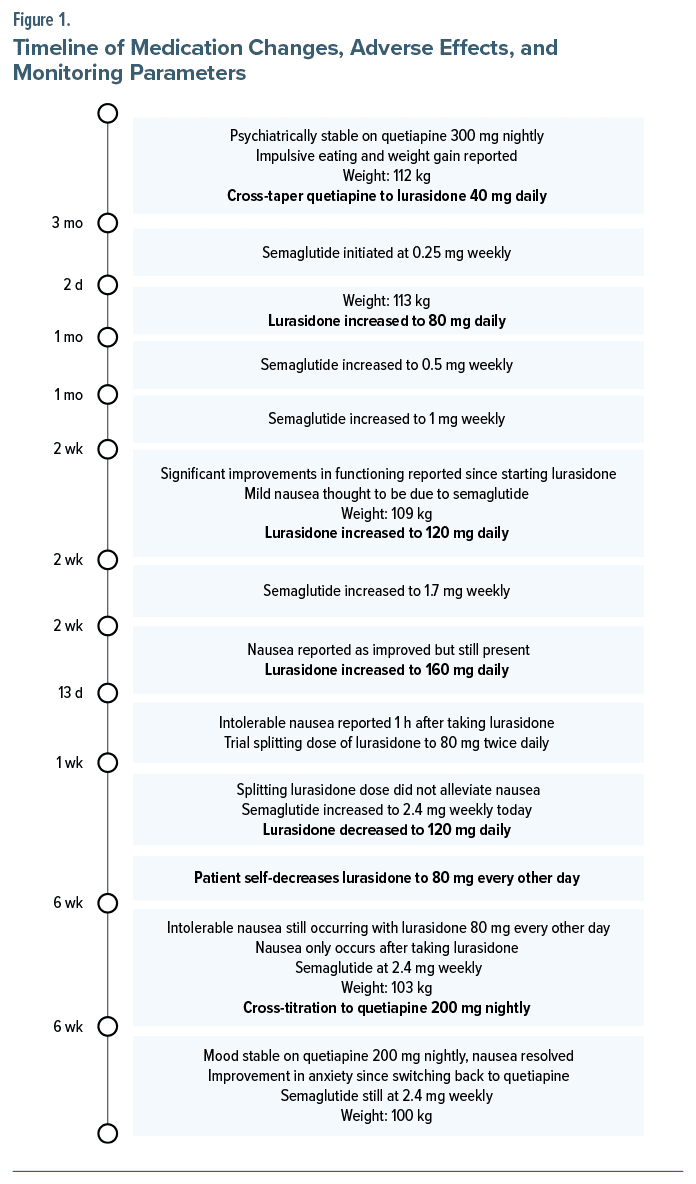

From our literature search, this is the first report describing new-onset intolerable nausea from lurasidone in the setting of semaglutide initiation and may suggest a drug interaction between the 2 medications that has yet been documented in the literature. The patient had been stable on lurasidone without side effects, but it was not until the initiation of semaglutide that she began to develop intolerable nausea, even at doses of lurasidone previously tolerated.
Both medications have shown higher incidences of nausea compared to placebo in clinical trials. The incidence of nausea for lurasidone in bipolar disorder trials was pooled at 14% for 20–120 mg daily compared to 8% in placebo.1 The incidence of nausea in weight loss trials for semaglutide 2.4 mg weekly was 44% versus 16% for placebo.4
Studies in mice receiving semaglutide show increased dopamine signaling in the ventral tegmental area.5 Lurasidone is theorized to cause nausea due to partial—rather than full—agonism of 5-HT1a receptors with decreased D2 receptor antagonism at doses lower than 160 mg daily.6 We theorize that increased dopamine release from semaglutide, with lurasidone’s reduced partial agonist activity at 5-HT1a receptors, may have contributed to the unremitting nausea.
ConclusionDue to the rise in GLP-1RA prescribing for weight loss, we recommend clinicians be vigilant regarding unremitting nausea/vomiting that occurs with lurasidone and possibly other second-generation antipsychotics upon initiation of GLP-1RAs.
Published Online: August 29, 2024. https://doi.org/10.4088/PCC.24cr03736
© 2024 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2024;26(4):24cr03736
Submitted: February 17, 2024; accepted May 2, 2024.
To Cite: Hean AC, Schneeberger AR, Lee KC. Unremitting nausea due to lurasidone after semaglutide initiation. Prim Care Companion CNS Disord. 2024;26(4):24cr03736.
Author Affiliations: Department of Pharmacy, University of California, San Diego Health, San Diego, California (Hean); Department of Psychiatry, University of California, San Diego, California (Schneeberger); Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, California (Lee).
Corresponding Author: Kelly C. Lee, PharmD, MAS, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, 9500 Gilman Dr, Mail Code 0719, Pharmaceutical Sciences Bldg, La Jolla, CA 92093 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was received from the patient to publish this case report. Details have been de-identified to protect the patient’s anonymity.
ORCID: Andrew C. Hean: https://orcid.org/0000-0002-8340-2296; Andres R. Schneeberger: https://orcid.org/0000-0001-8176-9126; Kelly C. Lee: https://orcid.org/0000-0002-1674-4210
留言 (0)