As China's economy continues to grow, the pharmaceutical industry has become a vital component of the national economy. However, the Chinese pharmaceutical sector has long been plagued by significant corruption issues, which not only undermine the efficacy and quality of medical services but also severely impact the healthy development of the industry and public trust in the entire medical system (1, 2). Corruption in the pharmaceutical industry includes, but is not limited to, bribery, illegal kickbacks, and the abuse of public office. These practices not only contravene legal provisions but also disrupt the fair allocation of medical resources. Similar challenges are faced globally by the pharmaceutical industry. For example, some major pharmaceutical companies in the United States have been fined heavily for illegal marketing practices, while incidents of bribery and fraud in medical procurement are common in some countries in Africa and South America (3–6). These corruption issues reflect the widespread nature of pharmaceutical industry corruption globally and the complex challenges it poses. Corrupt practices hinder medical innovation, equitable access to medical resources, increase the burden on public health systems, and diminish the effectiveness of treatments and patient satisfaction. For example, the bribery scandal involving GlaxoSmithKline in China in 2013 significantly impacted the company's ability to innovate and maintain market trust (3). Furthermore, a study by Mackey and Cuomo highlights how corruption in drug procurement processes in low- and middle-income countries leads to inequitable access to essential medicines, thereby increasing the public health burden (4).
China places high importance on combating commercial bribery in the pharmaceutical industry, enacting a series of laws and policy measures aimed at regulating the industry's order and promoting healthy development. For instance, the Anti-Unfair Competition Law of the People's Republic of China, enacted in 2018, explicitly prohibits commercial bribery and other unfair competition practices, with Article 8 specifically addressing the prohibition of commercial bribery, including any form of bribe given to a counterparty's staff for the purpose of securing business transactions (PRC Anti-Unfair Competition Law, 2018). The Drug Administration Law of the People's Republic of China, promulgated in 2019, enhanced drug approval and market supervision, with Article 39 requiring comprehensive documentation and transparency throughout the drug production, distribution, and usage processes, and Article 75 imposing stricter penalties for legal violations (PRC Drug Administration Law, 2019). The Anti-Monopoly Law, revised in 2022, strengthened regulation against potential monopolistic behaviors in the pharmaceutical industry, particularly through Article 17 which addresses the abuse of market dominance, promoting fair market competition (PRC Anti-Monopoly Law, 2022). The implementation of these laws and regulations provides legal and institutional support for corruption prevention and rectification in the pharmaceutical field, representing key measures to drive the continuous and healthy development of the industry.
However, relying solely on the formulation of laws and policies is insufficient; effective supervision and anti-corruption mechanisms are equally crucial. In the pharmaceutical industry, the complexity of supervisory entities, the diversity of regulatory tools, and the covert nature of the entities being regulated make supervision challenging. Therefore, understanding the interplay between policy, supervision, and governance is vital for enhancing regulatory standards in the pharmaceutical industry and effectively addressing corruption issues.
This study focuses on the three main stakeholders in China's pharmaceutical industry—pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies—as the core of the study. By analyzing the roles, interrelationships, and strategic interactions of these three parties in addressing corruption, the paper reveals their mechanisms of action and influencing factors in corruption within the pharmaceutical industry. Furthermore, the paper explores how improving the coordination and cooperation among these three parties and strengthening regulatory mechanisms can effectively tackle corruption in the pharmaceutical industry, promote its healthy development, and safeguard public interest. To achieve these objectives, this study adopts a bounded rationality perspective and employs a game-theoretic evolutionary approach. We establish a tripartite evolutionary game model involving pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies. The model analyzes the stable strategies of the parties involved and the sensitivity of key parameters within this tripartite game system.
In summary, this study aims to address the persistent and pervasive corruption issues in China's pharmaceutical industry, which threaten public health and the industry's sustainable development. By employing a game-theoretic evolutionary approach, we seek to explore the strategic interactions among key stakeholders—pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies—and identify stable anti-corruption strategies that can effectively mitigate corruption and promote industry growth. Through an in-depth analysis of the strategic interactions among pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies, this paper aims to provide theoretical insights and policy recommendations for resolving corruption issues in the pharmaceutical sector.
2 Literature review 2.1 Corruption in the pharmaceutical industry: historical context and modern challengesCorruption in the pharmaceutical industry exhibits diversity and persistence, with a complex historical background. From the early days of unregulated competition in the drug market to the regulatory challenges of the modern globalized context, the industry has undergone significant transformations. Initially, the lack of effective regulatory mechanisms led to the proliferation of substandard and counterfeit drugs (7). Since the mid-20th century, as drug approval and market supervision systems were gradually established, the forms of corruption in the pharmaceutical industry became increasingly complex, involving drug price manipulation, falsification of clinical trial data, and inappropriate marketing practices (8). These practices not only endanger public health but also undermine the ethical standards and public trust in the pharmaceutical industry.
Currently, the pharmaceutical industry faces a more diverse and complex set of corruption challenges. Globalization has introduced problems with transnational drug supply chains, the highly competitive market environment, and increasingly complex drug approval processes, all of which provide fertile ground for corrupt practices (9–11). For example, some pharmaceutical companies exploit their market dominance to manipulate prices, or engage in opaque collaborations with doctors and medical institutions, influencing the promotion and use of drugs (3, 5). Additionally, the falsification of clinical trial data has become increasingly scrutinized in recent years, affecting the safety and efficacy of drugs and damaging the scientific credibility of the entire industry (8). Therefore, strengthening regulations, enhancing transparency, and intensifying the punishment of corrupt practices are key challenges currently faced by the pharmaceutical industry.
2.2 Governance of corruption issues in China's pharmaceutical industryThe causes of corruption in the pharmaceutical sector are complex, intertwining historical and contemporary factors, and exhibit both complexity and specificity. Corruption in this field spans various segments including drug distribution, medical examinations and treatments, equipment procurement, supply of consumables, and construction projects. It involves a complex array of personnel and a variety of corrupt practices, making investigations particularly challenging; thus, anti-corruption efforts in healthcare represent a prolonged battle (12, 13). For instance, the Changsheng Biotechnology vaccine scandal in 2018 revealed severe violations in the production of rabies vaccines, including the falsification of production test records and product release data, providing false information during regulatory inspections, and bribing relevant department officials (14, 15). In 2023, Xu Qingfeng, the director of the Guangdong Provincial Administration of Traditional Chinese Medicine, was arrested for accepting substantial bribes. During his tenure, he exploited his position to aid several pharmaceutical companies in matters of drug procurement and project approval, illegally profiting from these transactions (16).
The root causes of corruption in China's pharmaceutical industry primarily stem from an imperfect regulatory system, fierce market competition, and a cultural reliance on networking. These factors collectively create a fertile ground for corruption (17, 18): Regulatory bodies, lacking sufficient human and technical resources, struggle to effectively supervise the broad and complex pharmaceutical industry. The uneven enforcement of laws, combined with local protectionism, further weakens the deterrent power of regulations. Moreover, the high profits at stake drive companies to resort to illegal methods to gain competitive advantages in the fierce market (17, 18). Additionally, the deeply ingrained culture of relationships provides a covert social acceptance for such behaviors, contributing to the longstanding and complex nature of corruption within the industry (17).
In recent years, the pharmaceutical industry in China has made significant progress in anti-corruption efforts, reflecting the government's strong commitment to combating corruption. The government has not only revised laws and regulations such as the Drug Administration Law and the Anti-Unfair Competition Law, thereby strengthening the penalties for illegal activities, but it has also enhanced the regulatory system by increasing industry transparency and promoting public participation, systematically addressing and preventing corrupt practices. Additionally, the use of digital and information technology tools, such as electronic tracking systems and online monitoring platforms, has enhanced the government's ability to monitor the distribution of drugs and medical services in real time. This has increased the likelihood of detecting corrupt activities and raised the cost of non-compliance (19, 20). Furthermore, the rise of social media and online complaint platforms has strengthened public engagement, promoting policy transparency and public oversight of medical practices (21). However, despite notable advancements in anti-corruption within the pharmaceutical sector, challenges remain in ensuring comprehensive transparency and eradicating deep-seated corruption (22). Anti-corruption efforts in the pharmaceutical industry continue to be a crucial focus of the Chinese government's broader campaign against corruption (23).
2.3 Game theory in pharmaceutical research: theory and practiceGame theory, a mathematical framework for analyzing strategic interactions, plays a crucial role in the application within the pharmaceutical industry (24). Historically, the multifaceted stakeholder relationships in the pharmaceutical sector have constituted complex game scenarios, including pharmaceutical companies, regulatory bodies, healthcare providers, and consumers. While each participant pursues their respective interests, their decisions are influenced by the choices of others (25–27). The essence of game theory lies in analyzing the strategic choices and outcomes under these interactions. For example, the decisions of pharmaceutical companies regarding research and development and market strategies depend not only on market demand and competitor behavior but are also constrained by policies, regulations, and public opinion. Concepts such as Nash equilibrium are employed to explain the strategic choices of all parties in a stable state (28–30).
In practice, game theory is extensively used to guide policy-making and managerial decisions in the pharmaceutical industry. For instance, when government agencies formulate regulatory policies for the pharmaceutical sector, they consider the market behaviors and potential reactions of pharmaceutical companies, aiming to achieve optimal social welfare (31, 32). Similarly, pharmaceutical companies facing intense market competition and increasingly stringent regulatory demands use game theory to optimize their business strategies, ensuring sustainable development and compliance (33). The integration of theory and practice not only deepens our understanding of the dynamics within the pharmaceutical industry but also provides a scientific basis for formulating more effective industry regulations (34). This interdisciplinary approach increasingly demonstrates its importance and efficacy in addressing complex issues within the pharmaceutical industry (35).
To enhance the understanding of these dynamics, it is essential to consider the broader applications of game theory in related fields. For example, Hua et al. (36) conducted a game-theoretic analysis of pricing and cooperative advertising in a reverse supply chain for unwanted medications in households, which highlights the strategic interactions and potential cooperative strategies that can be applied to the pharmaceutical industry. Li and Ma (37) explored financial reforms and regional investment conflicts in China through a game-theoretic lens, providing insights into how regulatory changes can influence stakeholder behavior in the pharmaceutical sector (37). Additionally, Tat et al. (38) developed a mathematical model for pharmaceutical supply chain coordination, emphasizing the importance of reselling medicines in an alternative market, which can inform strategies to combat corruption and inefficiencies in the pharmaceutical supply chain. Wen and Zhou (39) examined the impacts of regional governmental incentives on the straw power industry in China, using game theory to understand the implications of policy incentives on industry practices, which can be analogously applied to understand the effects of regulatory incentives in the pharmaceutical industry. Finally, Zhu (40) analyzed the deterrent effects of severe penalties on corruption through a game-theoretic approach, providing valuable insights into the effectiveness of stringent anti-corruption measures in the pharmaceutical industry.
2.4 The role and challenges of third-party auditing organizations in the pharmaceutical industryIn the pharmaceutical industry, Third-Party Auditing Organizations play a pivotal role. These organizations are primarily responsible for conducting independent reviews of the financial statements and business operations of pharmaceutical companies to ensure compliance with relevant laws, regulations, and industry standards. Third-party audits are indispensable for protecting public interests and safeguarding the rights of investors and consumers. Through these audit activities, financial irregularities, governance deficiencies, and potential unethical behaviors within the pharmaceutical industry can be uncovered, assisting regulatory bodies in timely identifying and addressing industry issues (41).
However, Third-Party Auditing Organizations face numerous challenges in fulfilling their duties. Firstly, the complexity of the pharmaceutical industry makes the auditing process more technical and specialized. The intricate financial structures and business processes of pharmaceutical companies demand auditors not only have profound expertise but also require adaptability (42, 43). Secondly, conflicts of interest pose a significant challenge. For instance, some auditing organizations may struggle to maintain complete independence due to economic ties, which can lead to questions about the reliability of audit results. With the rapid transformations in the pharmaceutical sector, including new regulatory policies and technological advancements, auditing agencies are tasked with the critical responsibility of adapting to these changes and effectively carrying out their auditing roles (44, 45).
3 Evolutionary model assumptions and description 3.1 Model descriptionIn this study, we employ an evolutionary game theory model to analyze the interactions and behavioral patterns of pharmaceutical companies, Third-Party Auditing Organizations, and Medical Insurance Regulatory Agency in China's pharmaceutical industry, particularly in addressing corruption issues. The model focuses on the strategic choices of each party under economic incentives and punitive mechanisms, as well as their impact on the safety of health insurance funds and industry governance. Specifically, the model quantifies the decision-making processes of each entity through a set of variables: pharmaceutical companies decide between compliance and non-compliance with health insurance regulations, weighing the benefits and costs associated with each state; Third-Party Auditing Organizations choose between performing their duties and accepting bribes, balancing the costs of audits against potential rewards or penalties; and Medical Insurance Regulatory Agency focus on formulating and enforcing regulatory policies to maximize social welfare and minimize losses to the health insurance fund. These variables interact to create a dynamic game environment where parties adjust their strategies in response to others' actions and changes in the external environment. Through this model, the study aims to reveal the equilibrium states under different strategic combinations and their potential impacts on governance in the pharmaceutical industry, providing theoretical support for the formulation of effective policies and countermeasures. All variables used in the model and their meanings are listed in Table 1.
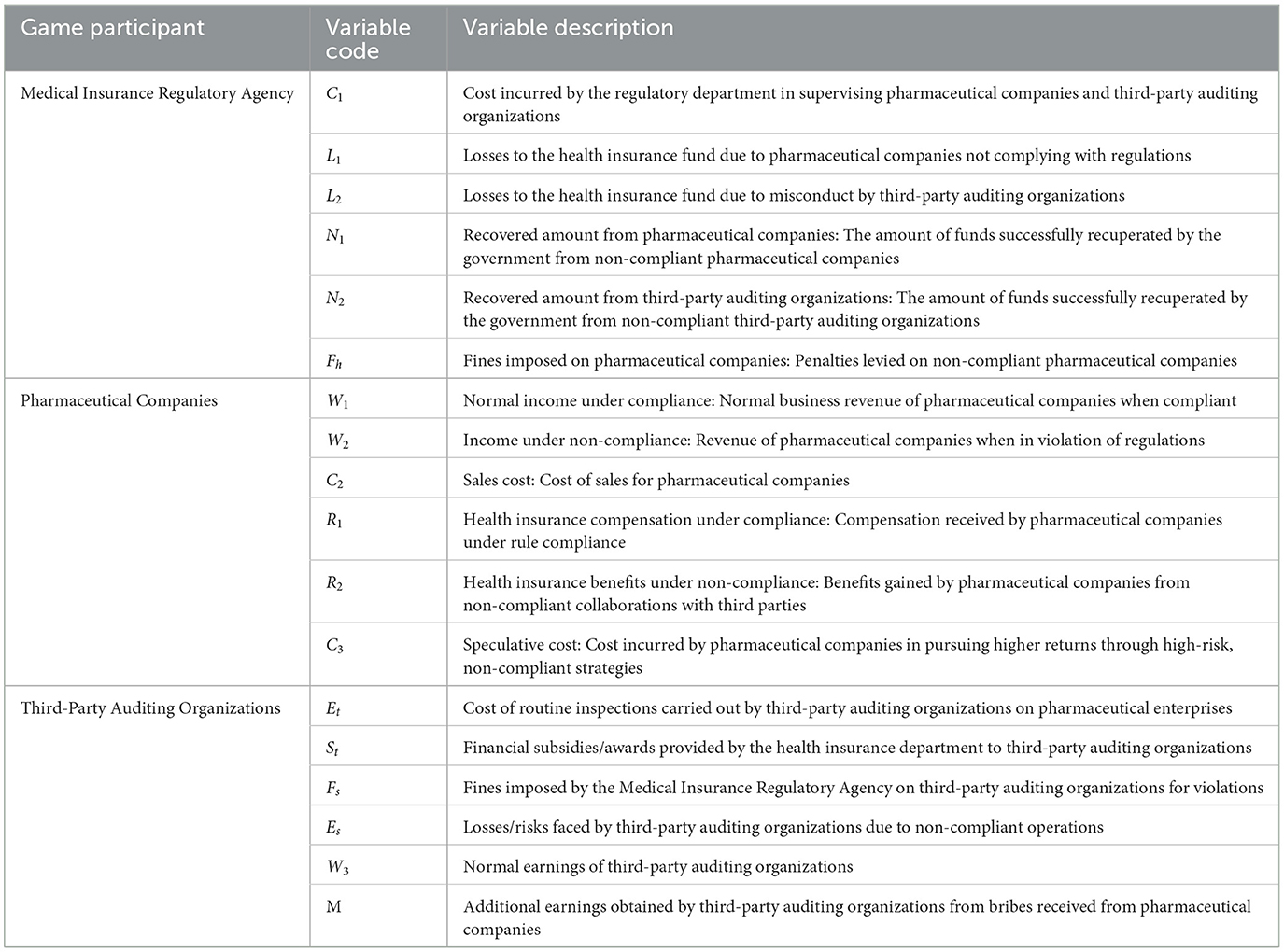
Table 1. Variables and variable description.
In the context of addressing governance and corruption within China's pharmaceutical industry, a complex tripartite game relationship forms between pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies. Each party pursues its own interests and goals while being influenced by the actions of the other two, creating a dynamic decision-making environment. Within this evolutionary game framework, the interactions and feedback among pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies collectively drive the governance process in the pharmaceutical sector. The logical relationships in this tripartite evolutionary game are illustrated in Figure 1.
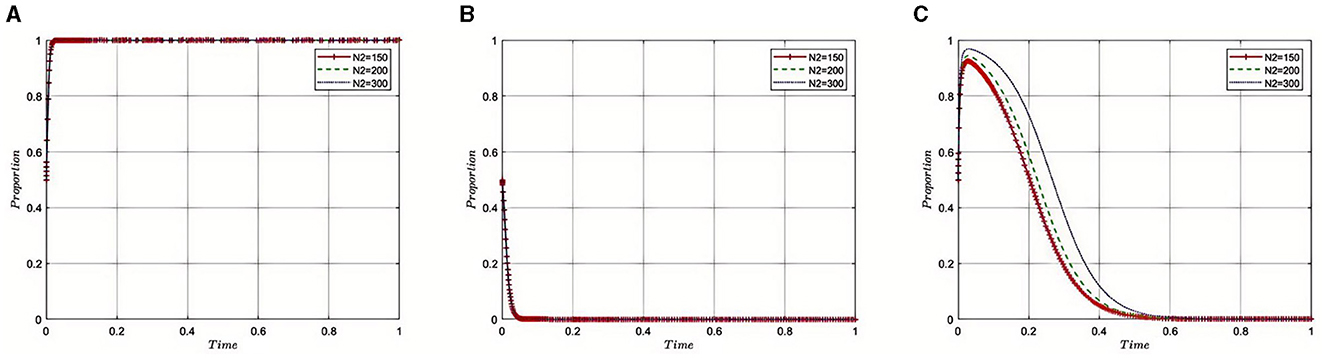
Figure 1. (A–C) Logogram of the evolutionary game between pharmaceutical companies, third-party auditors and health insurance regulators.
3.2 Evolutionary model assumptionsThis study employs the framework of evolutionary game theory to analyze the strategic interactions among pharmaceutical companies, Third-Party Auditing Organizations, and Medical Insurance Regulatory Agency in the Chinese pharmaceutical sector, and their impact on industry governance. Based on this, we propose the following assumptions to construct and analyze the corresponding evolutionary model:
Assumption 1: Key Participants. In the context of corruption in the Chinese pharmaceutical industry, the primary participants include pharmaceutical companies, Third-Party Auditing Organizations, and Medical Insurance Regulatory Agency. This study constructs an evolutionary game model involving these three parties, assuming that each makes strategic choices under bounded rationality to maximize their respective interests.
Assumption 2: Decision-making Assumption for Pharmaceutical Companies. Pharmaceutical companies aim to maximize their total profits in each decision-making process. After considering compliance costs, illicit gains, the risk of penalties, and reputation damage, these companies will decide whether to adhere to health insurance regulations.
Assumption 3: Response Assumption for Third-Party Auditing Organizations. Third-Party Auditing Organizations base their actions on audit efficiency, potential rewards/subsidies, and the risk of fines. After evaluating their economic benefits and professional reputation, these agencies will decide whether to accept bribes from pharmaceutical companies.
Assumption 4: Behavioral Assumption for Medical Insurance Regulatory Agency. Medical Insurance Regulatory Agency adjust their regulatory strategies and costs based on the behaviors of pharmaceutical companies and Third-Party Auditing Organizations. Their goal is to maximize social welfare, ensure the safety of the health insurance fund, and promote compliant operations within the industry.
Assumption 5: Strategic Adjustment. During the game, each party adjusts its strategies based on the outcomes of the game and feedback received. For instance, if pharmaceutical companies frequently incur penalties for non-compliance, they may increase their compliance efforts and reduce violations. Similarly, if auditing agencies face severe penalties for misconduct, they may implement audit standards more rigorously.
4 Evolutionary model construction and analysis 4.1 Model constructionSet up the game model where pharmaceutical companies complying with health insurance regulations is denoted by x, non-compliance by 1-x, Third-Party Auditing Organizations accepting bribes by y, refusing bribes by 1-y, health insurance regulatory agencies enforcing strict regulation by z, and lenient regulation by 1-z, with the assumption that x, y, z∈(0, 1). Based on these assumptions and variable definitions, construct a mixed-strategy game payoff matrix involving pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies as shown in Table 2.
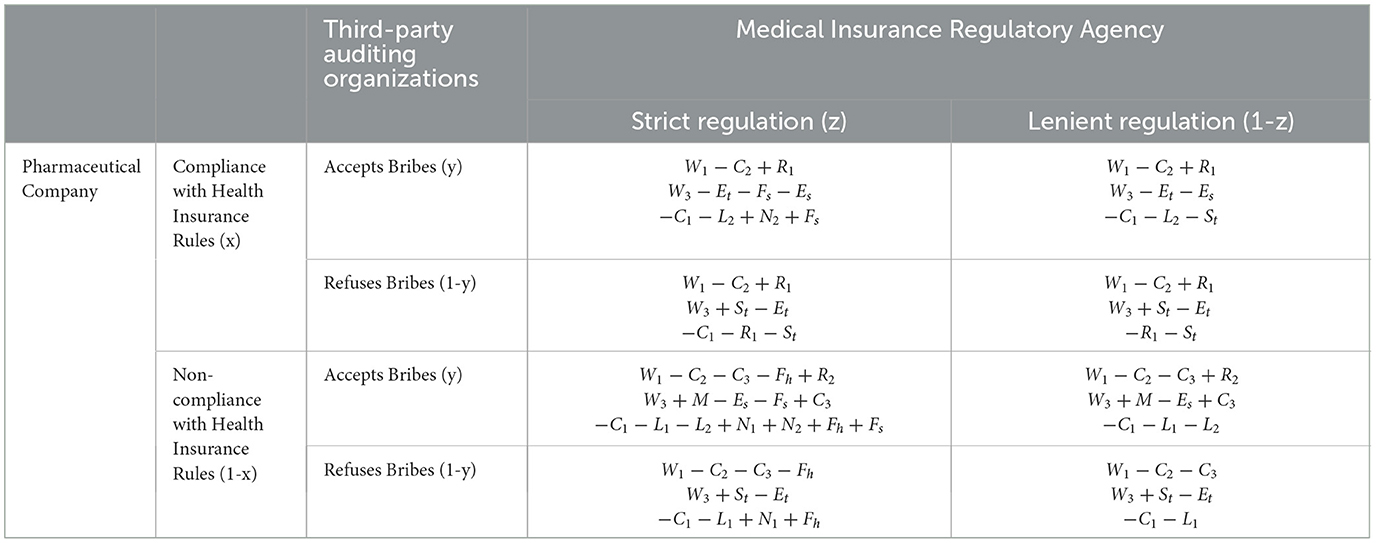
Table 2. Mixed-strategy game payoff matrix for pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies.
4.2 Game model analysis 4.2.1 The gains of pharmaceutical companies complying with the health care rules, not complying with the health care rules, and the average gains, respectivelyEX=(W1-C2+R1)*y*z+(W1-C2+R1)*y*(1-z)+(W1-C2+R1)*(1-y)*z+(W1-C2+R1)*(1-y)*(1-z)
E1-X=(W1-C2-C3-Fh+R2)*y*z+(W1-C2-C3+R2)*y*(1-z)+(W1-C2-C3-Fh)*(1-y)*z+(W1-C2-C3)*(1-y)*(1-z)
E¯=xEx+(1-x)E1-x
4.2.2 The gains from accepting bribes, refusing bribes, and the average gain for third-party auditors are, respectivelyEy=(W3-Et-Fs-Es)*x*z+(W3-Et-Es)*x*(1-z)+(1-x)*z*(W3+M-Es-Fs+C3)+(W3+M-Es+C3)*(1-x)*(1-z)
E1-y=(W3+St-Et)*x*z+(W3+St-Et)*x*(1-z)+(1-x)*z*(W3+St-Et)+(W3+St-Et)*(1-x)*(1-z)
E¯=yEy+(1-y)E1-y
4.2.3 The gains from strict and lax regulation by the health care regulator, as well as the average gainsEz=(-C1-L2+N2+Fs)*x*y+(-C1-L2-St)*x*(1-y)+(-C1-L1-L2+N1+N2+Fh+Fs)*(1-x)*y+(-C1-L1+N1+Fh)*(1-x)*(1-y)
Ez=(-C1-L2+N2+Fs)*x*y+(-C1-L2-St)*x*(1-y)+(-C1-L1-L2+N1+N2+Fh+Fs)*(1-x)*y+(-C1-L1+N1+Fh)*(1-x)*(1-y)
E1-z=(-C1-L2-St)*x*y+(-R1-St)*x*(1-y)+(-C1-L1-L2)*(1-x)*y+(-C1-L1)*(1-x)*(1- y)
E¯=zEz+(1-z)E1-z
4.2.4 The replicated dynamic equations for pharmaceutical companies, third-party auditors, and health insurance regulatorsFx=-x*(x-1)*(C3+R1+Fh*z-R2*y)
Fy=y*(y-1)*(Es-C3-Et-M+St+C3*x+Et*x+Fs*z+M*x)
Fz=-z*(z-1)*(Fh+N1-C1*x-Fh*x+Fs*y-N1*x+N2*y+C1*x*y+St*x*y)
4.3 Analysis of evolutionary system equilibriumThe strategic interactions among pharmaceutical companies, third-party auditing organizations, and health insurance regulatory agencies are continuously evolving. Therefore, by establishing a tripartite game model and deriving the replicator dynamic equations, the equilibrium points of this game can be calculated. The evolutionary process of the game involving the three parties is dynamic, with the probability of choosing any strategy being time-dependent. According to the stability principles of differential equations, when all dynamic equations reach zero, it signifies that the entire dynamic system will tend toward stability. Thus, by constructing the replicator dynamic equations for the tripartite game model and setting Fx = 0,Fy = 0,Fz = 0the evolutionary equilibrium points of the game can be determined. Specifically, let:
Fx=-x*(x-1)*(C3+R1+Fh*z-R2*y)=0
Fy=y*(y-1)*(Es-C3-Et-M+St+C3*x+Et*x+Fs*z+M*x)= 0
Fz=-z*(z-1)*(Fh+N1-C1*x-Fh*x+Fs*y-N1*x+N2*y+C1*x*y+St*x*y)= 0
Based on Selten's research findings in non-cooperative game theory, under conditions of asymmetric information, evolutionarily stable strategies are pure strategies. Therefore, it is necessary only to discuss the asymptotic stability of the eight local equilibrium points E1(0, 0, 0), E2(1, 0, 0), E3(0, 1, 0), E4(0, 0, 1), E5(1, 1, 0), E6(1, 1, 0), E7(0, 1, 1), E8(1, 1, 1) that satisfy Fx = 0, Fy = 0, Fz = 0.Using the replicator dynamics equations of the three parties, the Jacobian matrix of the evolutionary game system can be obtained, which facilitates the analysis of the stability of the equilibrium points:
J=[∂Fx∂x∂Fx∂y∂Fx∂z∂Fy∂x∂Fy∂y∂Fy∂z∂Fz∂x∂Fz∂y∂Fz∂z]=[J11J12J13J21J22J23J31J32J33]
J11=-x*(C3+ R1+ Fh*z-R2*y) - (x - 1)*(C3+ R1+ Fh*z-R2*y)
J12=R2*x*(x- 1)
J13= -Fh*x*(x -1)
J21=y*(y - 1)*(C3 + Et + M)
J22=y*(Es - C3 - Et - M + St + C3*x + Et*x + Fs*z + M*x) + (y - 1)*(Es- C3 - Et - M + St+C3*x + Et*x + Fs*z + M*x)
J23= Fs*y*(y-1)
J31=z*(z-1)*(C1+Fh+N1-C1*y-St*y)
J32=-z*(z-1)*(Fs+N2+C1*x+St*x)
J33=-(z-1)*(Fh
留言 (0)