First discovered in 1969 by Cashel and Gallant as spots appearing on autoradiograms from amino acid-starved Escherichia coli cells 1, the universal stringent response alarmone guanosine-3ʹ,5ʹ-bisdiphosphate (ppGpp) and guanosine-5ʹ-triphosphate-3ʹ-diphosphate (pppGpp) (collectively as (p)ppGpp) is well known for its importance in bacterial virulence, and tolerance and persistence to antibiotics 2, 3, 4, 5. Various stress conditions are now known to induce the production of (p)ppGpp, which affect bacterial global transcription, translation, and metabolism via its numerous target proteins 6, 7 to redistribute resources and, thus, survive the fluctuating environments 8, 9. Elevated levels of (p)ppGpp are associated with antibiotic persistence, where bacteria enter a dormant state, leading to recurring and recalcitrant infections, and thereby occurrence of antibiotic resistance mutations 5, 10, 11. Thus, targeting the stringent response is a promising strategy to combat bacterial antibiotic persistence and resistance 5.
The (p)ppGpp levels are regulated by the RelA-SpoT homologue (RSH) family proteins 12. RelA in E. coli has only the synthetase (SYN) activity, producing ppGpp and pppGpp by transferring the pyrophosphate (PPi) of ATP to GDP and GTP, respectively 1, 13. Whereas, SpoT has a weak SYN activity but a strong hydrolase (HYD) activity, degrading ppGpp and pppGpp in the presence of the co-factor Mn2+ 14, 15, 16. Given the prominent role of RelA in producing (p)ppGpp, previous attempts have focused on inhibiting the SYN activity of RelA, such as the well-known example of relacin, which inhibits the SYN activity of RelA 17, 18. Other similar molecules 17, 19 include the peptide 1018 that disperse biofilm by preventing the accumulation of (p)ppGpp 19 and a lead compound X9 targeting Mycobacterium tuberculosis Relmtb 20.
Despite the strong SYN activity of RelA, in many bacteria SpoT, instead of RelA, is more important for persistence 10, 21 and virulence 2, 22. The HYD activity of SpoT is also essential to dynamically regulate intracellular levels of (p)ppGpp, as the key modulator of bacterial physiological changes in response to fluctuating environments. Consistently, deletion of spoT gene is impossible while relA is present 6, likely due to the uncontrolled production of (p)ppGpp, resulting in cell toxicity. Targeting the SpoT HYD activity is thus also promising.
Nevertheless, there are no reports about SpoT inhibitors. The lack of such reports may be due to the difficulty in purifying a stable full-length recombinant SpoT protein 4. Another difficulty is the lack of a simple, accessible detection method of (p)ppGpp. The current methods used for detecting (p)ppGpp include the thin layer chromatography 1, radioimmunoassay 23, enzyme coupled reactions 24 or HPLC 25, which required three days to analyse 50 samples, being very time-consuming. Further, a fluorescent detection method for ppGpp, via the use of Eu3+ ion-functionalised fluorescent molybdenum sulfide quantum dots test paper, requires specific synthesis method, instrument and expertise 26. A recent colourimetric method requires less preparation time as it utilises the redox reaction occurring between a Fenton-like reagent and 2,20-azino-bis-(3-ethylbenzthiazoline-6-sulfonic) acid 27; however, PPi, which is the product of the SpoT HYD reaction, is also detected by this method.
In this study, we demonstrate the surprising discovery of a commercially available, low-cost malachite green (MG) detection kit, originally designed for orthophosphate (Pi) detection 28, for detecting (p)ppGpp and its analogues, especially pGpp 29. We further developed a simple method utilizing MG to analyze the HYD activity of E. coli SpoT and show that SpoT can hydrolyze pGpp, pGp, and ppGp besides (p)ppGpp. Additionally, we screened both an in-house and commercial library of chemicals and identified the antibiotic thermorubin and two (p)ppGpp analogues DR-5839A and DR-6459 as novel inhibitors of the SpoT HYD activity. This study thus provides a very useful tool for the study of (p)ppGpp, pGpp and potential antimicrobial research targeting the RSH proteins.
RESULTSMalachite green directly detects pppGpp, ppGpp and their analogs
To find a simple and suitable method for quantifying the SpoT HYD reaction, we suspected that both PPi and Pi may be produced by SpoT. The orthophosphate can be readily detected by the commercial MG (SIGMA-Aldrich, MAK307). To test, we purified a SpoT protein with a N-terminal Histidine-MBP-SUMO tag and a E319Q mutation. The SpoT E319Q mutation is expected to inactivate the SYN activity of SpoT (hereafter SYNoff) 30, avoiding its confounding effect on the HYD reaction. We then started two parallel SpoT HYD reactions with ppGpp, one with an active SpoT protein and the other with a heat inactivated SpoT as the negative control (see Material and Method for details). After 90 minutes’ reaction, we added the MG detection mix to stop the reaction and detect the produced orthophosphates by spectrophotometric reading at 620 nm. Surprisingly, instead of an expected higher A620nm value of the experimental group versus the negative control, the reverse was observed (Figure 1A). We thus suspected that either no Pi was produced and/or that ppGpp was detected by MG directly.
–
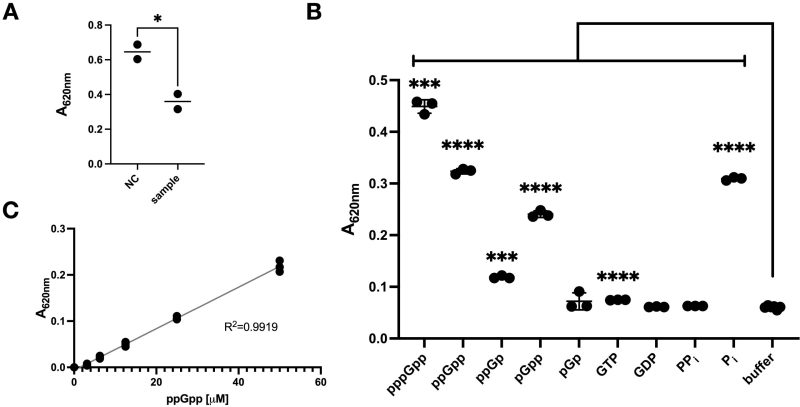
FIGURE 1: Malachite green (MG) directly detects (p)ppGpp and its analogues. (A) Detection of potential SpoT HYD reaction products with MG. ppGpp was incubated (90 min, room temperature) with purified SpoT protein with only the HYD activity, i.e., SYNoff . Afterwards, MG reagent was added to the reaction mixes and read at 620 nm (A620nm). NC, sample: the two reactions with either heat inactivated or native SpoT SYNoff proteins, respectively. (B) Direct detection of the indicated chemicals by MG. The concentration of each compound was 35.6 µM (total volume 45 µl) before adding the MG detection mix. After 30 min incubation time, the absorbance at 620 nm (A620nm) was assessed. (C) Standard curve of detecting ppGpp via the MG mix. The data shown are the mean ± SD of three technical replicates except for (A), which is of two replicates. The significance of unpaired t-test is indicated by the symbols * (p-value < 0.05), *** (p-value < 0.001), and **** (p-value < 0.0001).
To discriminate, we mixed equal amount (35.6 µM) of pppGpp, ppGpp and their analogues, plus other relevant nucleotides directly with MG (Figure 1B). We found that, the highest A620nm read was generated by pppGpp, followed by ppGpp, which is slightly higher than Pi. As stated in the manufacturer’s manual, GTP had a slightly higher read compared to the buffer control. Interestingly, the (p)ppGpp analogues, pGpp, ppGp and pGp, were also detected, and pGpp gave a reading slightly lower than ppGpp/Pi but much higher than ppGp. pGp gave similar low readings as GTP, and GDP and PPi were not significantly detected. These data demonstrate that the (p)ppGpp, pGpp, and ppGp can be detected by MG directly. Furthermore, the higher reading of pppGpp compared to ppGpp and higher reading of pGpp compared to ppGp, indicate that both the total number of Pi groups and the presence of an extra 5’- or 3′-phosphate are important for binding to MG. We further determined the detection limit of ppGpp to be about 2.94 µM (Figure 1C).
The malachite green kit can be used to assess the SpoT HYD activity
With the confirmed capacity of MG in detecting (p)ppGpp directly, we tested if the SpoT HYD activity can be assessed with MG. To this, we firstly performed the ppGpp hydrolysis reactions by using three different concentrations of SpoT SYNoff (50 nM, 100 nM, 200 nM). As a negative control (control-1, Figure 2A), heat inactivated SpoT SYNoff was used. Additionally, another control (control-2, Figure 2A) was included, which contained the reaction mix and the SpoT SYNoff protein but without ppGpp. As the anticipated products of the HYD reaction, GDP and PPi, were shown not to give a significant signal (Figure 1C), a drop in A620nm was expected. Indeed, this drop was observed (Figure 2A, 2B), for the samples containing active SpoT SYNoff, and that the greater the amount of SpoT used, the more pronounced the decrease in A620nm became. On the other hand, the negative control-1 with heat inactivated SpoT gave highest A620nm due to the non-hydrolyzed ppGpp and the control-2 gave lowest A620nm due to the absence of ppGpp. These results confirmed that the reduction of A620nm depends on the active SpoT SYNoff protein and its concentration, showing that ppGpp was detected by MG and that it can be used to assess the SpoT HYD activity in vitro.
–
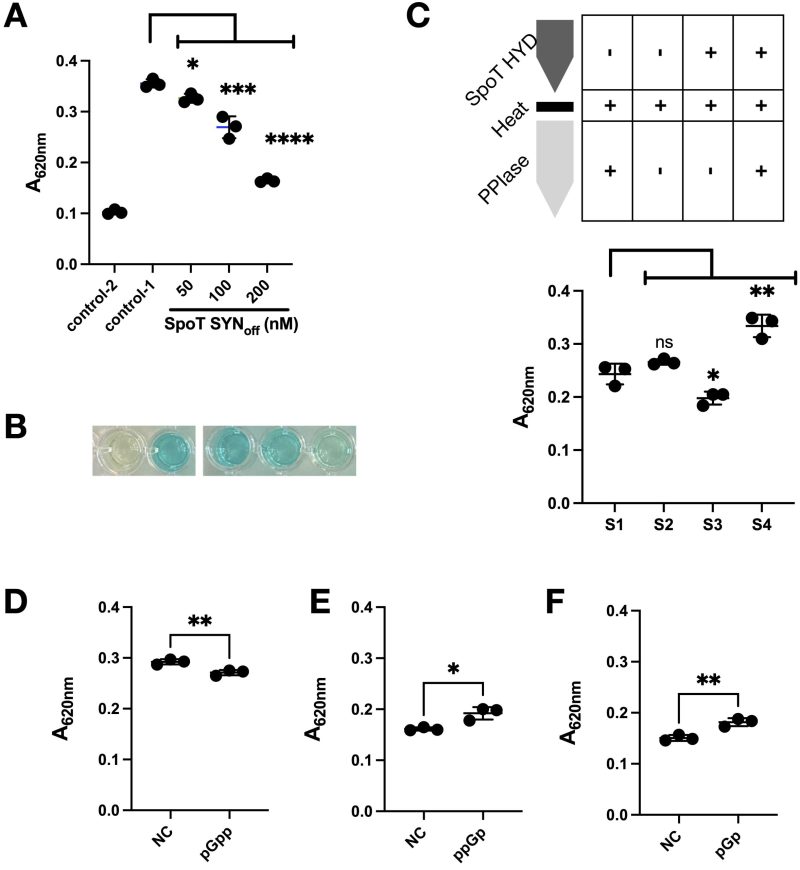
FIGURE 2: Assessing the hydrolase activity of SpoT by using MG. (A) SpoT concentration dependent reduction of MG detection signal (A620nm). The SpoT reaction was given 90 minutes to occur before the MG detection mix was added. After 30 min incubation time, the absorbance (A620nm) was read via a plate reader. Control-2 contained the reaction components without ppGpp but with SpoT SYNoff protein. Control-1 is identical with the SpoT SYNoff samples except that SpoT SYNoff was added just before the reaction was stopped with MG. (B) A representative picture of the sample wells of (A). (C) (top) The scheme of the reactions that were performed. PPiase = pyrophosphatase. (bottom) Absorbance at 620 nm for the reactions as indicated above. (D, E, F) Hydrolysis of (p)ppGpp analogues, pGpp, ppGp, and pGp (each at 35.6 µM) by SpoT SYNoff(100 nM, 90 min) and their detection by MG. NC, the SpoT HYD reactions were similarly set up, but MG working reagent was added immediately to stop the HYD reaction. The mean ± SD of three technical replicates were plotted. Unpaired t-test was performed and the significance is displayed by the symbols * (p-value < 0.05), ** (p-value < 0.005).
PPi, instead of Pi, is the product of the SpoT hydrolase reaction
To have additional confirmation of the surprising ability of MG to detect (p)ppGpp, we tested if converting the product PPi to Pi will increase A620nm (Figure 2C). For this, we performed two parallel SpoT HYD reactions (S3, S4) for 60 min. After this, both reactions were stopped by heat inactivation, cooled down, and one reaction (S4) was added with pyrophosphatase (PPiase) for another 15 min before terminated and detected with MG reagent. The other reaction (S3) was added with both the PPiase and MG simultaneously. As controls, two other reactions (S1, S2) were performed. Both reactions contain the same setup as S3 and S4 but they contain heat inactivated SpoT SYNoff instead. Further, S1 was supplied with PPiase while S2 was not. Comparing the A620nm readings, the similar readings of S1 and S2 indicate no residual PPi in the reaction mix. The lower A620nm of S3 compared to S2 demonstrate the HYD activity of SpoT, while the much higher A620nm of S4 compared to S3 demonstrates the further cleavage of PPi to Pi, which boosted the A620nm significantly. Altogether, these tests show that PPi, instead of Pi, is the (main) product of SpoT hydrolytic reaction of ppGpp.
SpoT hydrolyses the 3’-phosphates of the (p)ppGpp analogues
Given the above result (Figure 2C) and the observations in Figure 1B, we were intrigued to test the substrate range of SpoT HYD and the detecting capacity of MG. For this, we performed the SpoT HYD assays towards pGpp, ppGp, and pGp (Figure 2D-F). As expected, SpoT SYNoff cleaves off the 3’-PPi of pGpp, resulting in GMP and thus a decreased reading (Figure 2D). The cleavage of 3’-Pi of both ppGp and pGp result in GDP/GMP and Pi, increasing the A620nm, as seen in Figure 2E, 2F. Besides confirming the 3’-phosphate(s) HYD activity of SpoT, these results further validate that MG can detect (pp)pGp(p), despite with differential sensitivities.
Two ppGpp analogs, DR-5839A and DR-6459, inhibit SpoT HYD activity
The newly discovered detection ability of MG allowed us to design a facile screening method for chemicals that inhibit or accelerate the SpoT HYD activity. For this, we note that previously, (p)ppGpp analogues were found to inhibit the SYN activity of the SpoT homologue RelA, such as Relacin 18 or DR-4250 and DR-M014 17. We thus firstly tested the robustness of the MG method for screening in a 96-well plate, and the z’ value 31 was high at 0.644 (Supplementary Figure S1). Thus, we decided to screen a small number of (p)ppGpp analogues for potential effect on SpoT HYD activity. Out of the 30 ppGpp analogues (Supplementary Table S1), two (DR-5839A, DR-6459) displayed a clear inhibition of SpoT HYD activity (Figure 3A). Consistently, their chemical structures are highly similar (Figure 3B, 3C), with DR-5839A more similar as ppGpp, which is in line with its more pronounced inhibitory effect. However, none of them were significantly detected by MG directly (compare the two N.C. in Figure 3D, 3E and ppGpp in Figure 1B), possibly due to their chemical differences with ppGpp (see discussion below). The further incubation of SpoT SYNoff with either chemical alone did not generate a different reading of A620nm (Figure 3D, 3E), suggesting that they cannot be hydrolysed by SpoT.
–
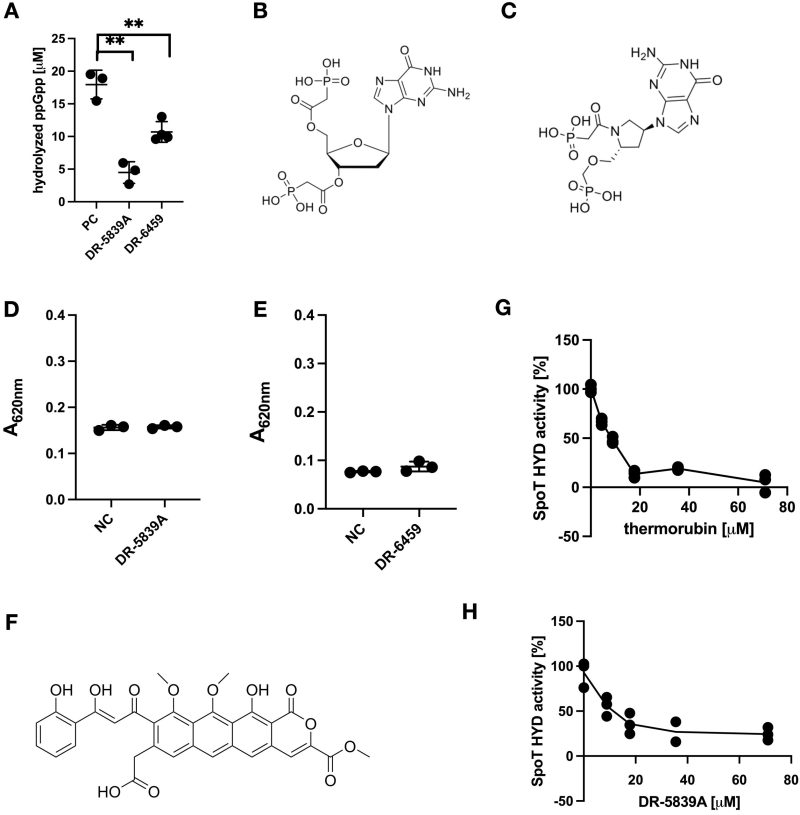
FIGURE 3: Identification of novel chemical inhibitors of SpoT HYD activity. (A) Hydrolysis of ppGpp (in µM) by SpoT SYNoff in the absence (PC) or presence of two ppGpp chemical analogues (DR-5839A, DR-6459). Unpaired t-test significance was displayed (** p-value < 0.005). (B, C) Chemical structures of (B) DR-5839A and (C) DR-6459. (D, E) Test if DR-5839A and DR-6459 can be a substrate of SpoT HYD activity using MG. NC, the SpoT HYD reactions were similarly set up, but MG working reagent was added immediately to stop the HYD reaction. (F) Chemical structure of thermorubin. (G, H) IC50 measurement of both thermorubin (G) and DR-5839A (H). The activities shown in percentage were normalised to the sample without the respective chemical. The mean ± SD of three technical replicates were plotted.
Thermorubin inhibits the SpoT HYD activity
Encouraged by the above small-scale screening, we decided to screen natural products affecting the SpoT HYD activity. To this end, we screened a collection of 60 known metabolites/antibiotics produced by actinomycetes (Supplementary Table S2). Remarkably, out of this screening, we identified thermorubin (Figure 3F) as a strong inhibitor of SpoT HYD activity. Thermorubin was discovered in 1964 as a natural product of Thermoactinomyces antibioticus 32 with antibacterial activities against Gram-negative and Gram-positive bacteria by inhibiting translation process 33, 34, 35, 36. Notably, the structurally similar tetracycline showed no effect on SpoT HYD activity (Supplementary Figure S2). Both tetracycline and thermorubin were dissolved in 10% DMSO. Together, these data suggest the specific binding and inhibition of SpoT by thermorubin. To compare the potency of thermorubin and DR-5839A, both the half-maximal inhibitory concentrations (IC50) were determined. Thermorubin showed an IC50 of 7.43 ± 1.32 µM, while DR-5839A showed an approximately two-times higher IC50 of 13.47 ± 3.73 µM (Figure 3G, 3H). Hence, thermorubin is a more potent inhibitor of SpoT HYD activity.
Given the higher in vitro inhibitory efficacy of thermorubin, we tested if thermorubin affects E. coli physiology. For this, we used the minimal medium (M9) supplemented with three amino acids, serine, methionine, and glycine (SMG) 37. The presence of SMG applies a starvation condition that a high level of (p)ppGpp produced by RelA is required to support E. coli growth. Since thermorubin inhibits the HYD activity of SpoT, we suspect that it may allow the accumulation of ppGpp produced by SpoT, thus supporting cell growth of the ΔrelA spoTwt strain in M9-SMG medium. As expected, ΔrelA spoTwt is defective (Figure S3B) in growing in M9-SMG as compared to wild type (wt) E. coli (Figure S3A) and the double deletion strain ΔrelA ΔspoT 38 cannot grow at all (Figure S3C). Further, addition of thermorubin did not enhance, but instead inhibited, the growth of ΔrelAspoTwt in M9-SMG. Such an inhibitory effect was also observed for wt E. coli (Figure S3A). This could be due to the fact that thermorubin itself as an antibiotic directly binds to ribosome and inhibits translation process 33, 34, 35, thereby cell growth. Consistently, in a dose dependent manner thermorubin inhibits cell growth of all three E. coli strains, i.e., wt, ΔrelA spoTwt, ΔrelA ΔspoT, in the rich LB broth (Figure S4), wherein (p)ppGpp is dispensable for growth. In conclusion, the inhibitory effect of thermorubin on translation may have outweighed its ability to boost (p)ppGpp levels by inhibiting the SpoT HYD activity.
DISCUSSIONSimple and cost-effective (pp)pGpp detection method using MG
This study serendipitously discovered that MG (out of the MAK307 kit, Sigma-Aldrich) can directly detect (p)ppGpp in vitro. Several lines of evidence support this. 1) Heat inactivated SpoT produced higher A620nm than active SpoT SYNoff protein (Figure 1A, 2C); and the more SpoT SYNoff proteins used, the lower the A620nm became (Figure 2A). 2) (p)ppGpp and its analogues produced significant A620nm readings when mixed up directly with MG, while GDP and PPi did not (Figure 1B). 3) Addition of PPiase restored the decreased A620nm of the SpoT HYD reaction (Figure 2C), demonstrating that SpoT cleaves ppGpp to release PPi, which was hydrolyzed by PPiase, further increasing the A620nm readings. 4) Cleavage of pGpp decreased the A620nm, while cleavage of ppGp and pGp increased the A620nm, demonstrating that SpoT cleaves the 3’ C-O bond, releasing either PPi or Pi depending on the substrates. Altogether, these data showed that MG directly detects (p)ppGpp, besides confirming the cleavage position of SpoT HYD activity on (p)ppGpp and its analogues pGpp, ppGp, and pGp.
The advantages of this MG method are clear to other methods as described above. MG is a well-developed chemical that is readily available, cheap and the detection reaction is fast (30 min). Therefore, MG could be used in large-scale high-throughput manner to screen for chemicals and proteins influencing the HYD (and SYN) activities of RSH proteins, as demonstrated in this study. However, limitations are also notable. Since MG does not discriminate Pi and (pp)pGpp, and the fact that biological samples without pre-clean up contain Pi and other potentially interfering molecules, one can only obtain at most the sum of all four molecules (i.e. Pi and (pp)pGpp) with MG, limiting its usage. Additionally, the ppGpp detection limit of MG is relatively high, despite an anticipated lower limit for pppGpp (Figure 1B). Nevertheless, we showed that MG could still be used for studying the HYD activity of SpoT, underscoring its usefulness in in vitro systematic studies. Lastly, MG could be used to study pGpp as well, a third stringent alarmone confirmed recently 29.
The (p)ppGpp detection mechanism by MG
Previously, MG was known to detect Pi by forming a complex with MG and molybdate, resulting in a shift in absorbance at 620 nm 28, 39; however, the detection mechanism remains elusive. How does MG detect (p)ppGpp? Several features can be deduced from our data. pppGpp gave higher A620nm than ppGpp, which is similar as Pi; however, ppGp gave much lower A620nm than pGpp (Figure 1B). These data suggest the more of the total 3’ and 5’ Pi and particularly those at the 3’ position give higher A620nm and thus detection by MG. However, PPi is not detected by MG, suggesting two different manners that how Pi and (p)ppGpp are detected by MG. Furthermore, both DR-5839A and DR-6459 gave lower A620nm readings than ppGpp (Figure 3B-3E, 1B), suggesting that the 5’and 3’ Pi groups and their three-dimensional configurations also affect their detection by MG.
We note that, previously, the guanine-rich quadruplex could be detected by MG 40. The potential mechanism involves pi-stacking between guanine and the triple phenyl ring of MG and maybe also electrostatic interactions. Therefore, the guanine ring of (p)ppGpp may pi-stack with MG, facilitating their interactions. Then, the 5’and 3’phosphates of (p)ppGpp may assume the similar position of free Pi, to be detected by MG. In support of this, both the 5’ and 3’ phosphates of (p)ppGpp are known to assume very flexible conformations 41, that may allow their detection of MG. Consistently, the one more 5’-gamma Pi of pppGpp produces higher A620nm than ppGpp. We further suspect that MG may also detect other well-known nucleotide messengers such as AppppA 42 and ppApp 43, 44.
Novel inhibitors of SpoT HYD activity
Regardless the detection mechanism, MG provides a low-cost, fast, and simple spectrophotometric method for (p)ppGpp detection. MG can be used to study the HYD activity of SpoT in vitro as shown in this study (Figure 3). Via two screenings, we identified three inhibitors of the SpoT HYD activity, namely two (p)ppGpp analogues (DR-5839A and DR-6459) and the antibiotic thermorubin. Interestingly, tetracycline did not inhibit SpoT HYD despite its chemical similarity to thermorubin (Figure S2), confirming the specificity of thermorubin. Although the inhibitory manner of thermorubin remains elusive, both DR-5839A and DR-6459 potentially bind to the SpoT HYD active site, competitively inhibiting the HYD activity given the similar chemical structures to (p)ppGpp.
MATERIAL AND METHODSStrains
Strains used in this study are listed in Table 1.
Table 1. Strains used in this study.
YZ37
MG1655 Wild type
Laboratory stock
YZ38
MG1655 ΔrelA
Laboratory stock
YZ841
MG1655 ΔrelA ΔspoT
Substrates and reagents
ppGpp and pppGpp were purchased from Jena Bioscience. pGpp was synthesized according to 45. The other ppGpp analogues except for DR-6459 (synthesis described in the Supplementary Document S2) were synthesized as described in 17 (see detailed information in Supplementary document S1) and dissolved in 50 mM HEPES pH 8.0 with 150 mM NaCl. The PPiase and the PPi standard were taken from the EnzChekTM PPiAssay kit (ThermoFisher Scientific). The malachite green kit (MAK307) was purchased from Sigma-Aldrich, as well as all other remaining reagents. Tetracycline hydrochloride was purchased from Sigma-Aldrich. Thermorubin was provided by NAICONS Srl, purified from Thermoactinomyces antibioticus as previously described 32. Both tetracycline and thermorubin were dissolved in 10% DMSO.
MG dye preparations
The MG detection mix was prepared as described in the manufacturer’s manual (SIGMA-Aldrich, MAK307) by adding 1 volume of component B to 100 volumes of component A 30 min prior to use at room temperature (RT). For 45 µl reaction volume, 12 µl detection mix was added, and after 30 min incubation at RT, absorbance was measured at 620 nm.
SpoT SYNoff protein purification
E. coli RosettaTM (DE3) (Novagen) was used in this study to express the SpoT SYNoff protein with a N-terminal Histidine-MBP-SUMO tag. Expression of spoT was induced at an optical density at 600 nm (OD600nm) = 0.6 – 0.7 via the addition of 0.1 mM IPTG and subsequently shaking overnight at 20°C. The bacterial cells were collected and resuspended in 50 mM HEPES pH 8.0, 1 M NaCl, 10 mM imidazole, 5 mM beta-mercaptoethanol (BME) together with protease inhibitor (cOmpleteTM, Mini, EDTA-free Protease inhibitor cocktail). The cells were lysed by sonication (Amplitude 20%) and incubated with equilibrated Ni-NTA Agarose (Qiagen) and washed (50 mM HEPES pH 8.0, 1 M NaCl, 20 mM imidazole, 5 mM BME) before eluted (50 mM HEPES pH 8.0, 1 M NaCl, 500 mM imidazole, 5 mM BME) using a polypropylene chromatography column. The eluted protein was loaded on a size exclusion column Superdex 200 10/300 GL (GE Healthcare) equilibrated with 50 mM HEPES pH 8.0, 1 M NaCl, and 5% glycerol. Protein concentrations were assessed using a Bradford assay (Bio-Rad). The purified protein was frozen in liquid nitrogen and stored at -80°C.
SpoT HYD activity assay
All SpoT HYD reactions were carried out with a total reaction volume of 45 µl and a total reaction time of 90 min. The hydrolase activity reaction was performed at RT with 100 nM (or 200 nM and 50 nM when specified) SpoT SYNoff as the standard protein concentration. The reaction mix contained 50 mM HEPES pH 8.0, 150 mM NaCl, 2 mM BME, 0.2 mg/ml BSA, and 5 mM MnCl2 and 35.6 µM ppGpp for the standard reaction. The reaction was started upon the addition of SpoT SYNoff. After 90 min, the reaction was stopped via the MG detection mix and the samples were assessed by absorbance at 620 nm.
Pyrophosphatase assay
After the SpoT HYD reaction is done and heat inactivated, 0.349 U PyrophosphataseTM was added to the samples for 15 min incubation at RT, before terminated by adding the MG detection mix.
IC50 determination
The SpoT HYD activity assay was performed as described above, without or with the presence of respective concentrations of DR-5839A or thermorubin. The relative SpoT HYD activities were obtained by normalizing to that performed without either compound. The IC50 was calculated according to the 4-parameter logistic-model formula as described in 46.
Variation test of microtitre plate screening
The variation of the SpoT HYD assay was tested as described in the “SpoT HYD activity assay” except that the SpoT reaction time was 120 min and 84 positive controls (with reaction time 120 min) and 12 negative controls (with reaction time 0 min) were tested.
E. coli growth tests in M9-SMG medium
Overnight precultures were grown in Luria Bertani (LB) for 18 hours at 37°C, and cells were washed twice in 1x Phosphate Buffered Saline (PBS) and normalised in PBS before inoculated in M9-SMG medium to OD600nm = 0.005. The M9-SMG medium contains M9Glc minimal media (1x M9 salt, 1 mM MgSO4, 0.2 % glucose, 0.1 mM CaCl2, Thiamine (0.001 mg/1 ml)) and 100 µg/ml each of serine, glycine, and methionine 37. In addition, the indicated concentration of thermorubin was added to the medium, and the growth was measured every 15 min for 24 hours in a plate reader (Biotek) at 37°C with double orbital agitation with a frequency of 548 cpm (2 mm).
Data analysis
Figures were generated via the software GraphPad Prism version 9.5.0, which was also used to perform unpaired t-test when indicated. At least three technical replicates were used for all reactions.
留言 (0)