Chagas disease (ChD) is a neglected tropical disease caused by the flagellate protozoan Trypanosoma cruzi (T. cruzi) that is endemic in 21 countries in Americas, affecting about 6 to 8 million people worldwide (1). About 70 million people in the Americas are still at a high risk of ChD since they are living in areas of active transmission (2). Moreover, due to migratory movements around the world, ChD has exceeded international borders and can be considered a current global epidemic (1).
In 2020, a new viral infectious disease called Coronavirus Disease 2019 (COVID-19) emerged worldwide, caused by the severe acute respiratory syndrome (SARS-CoV-2 virus) (3, 4). The COVID-19 virus rapidly spread around the world, leading the World Health Organization (WHO) to declare a pandemic state only a few months after the first described cases. COVID-19 is a highly contagious disease that accounted to a high number of infected cases and deaths (5, 6). To mitigate the dissemination of new cases of COVID-19, preventive strategies including social isolation were advocated by many scientific societies around the world (7). While these strategies were fundamental for public health, the inevitable negative consequences of social isolation included the disruption of daily routines, reduced social interaction, mental health challenges, changes in eating patterns, and decreased levels of physical activity (PA) (8–10).
Studies conducted during the COVID-19 pandemic period indicate a decrease in overall PA levels, potentially associated with health impairments (11). In this setting, a better understanding of the consequences of the COVID-19 pandemic on patients with ChD is of paramount importance to facilitate the implementation of intervention strategies to improve health of this specific population. To the best of our knowledge, no previous study has examined the PA levels of patients with ChD during the COVID-19 pandemic period. Therefore, the present study aimed to evaluate the level of PA in patients with ChD during the COVID-19 pandemic and its main associated factors.
2 Methods 2.1 Study design and populationThis is a cross-sectional observational study, carried out from November 2020 to June 2021, including ChD patients confirmed by two simultaneously positive serological tests at the time of study entrance (immunosorbent linked to enzyme assay and indirect immunofluorescence), both sexes, aged ≥18 years. All patients were under regular clinical follow-up at Evandro Chagas National Institute of Infectious Diseases/Oswaldo Cruz Foundation (INI/Fiocruz), a national reference center for treatment and research in tropical and infectious diseases located in Rio de Janeiro, Brazil. At the time of entrance, participants had already been under regular follow-up at INI/Fiocruz for at least 6 months (mean of 16 years). Participants were excluded if they had other infectious diseases at the time of data collection, if they were immunocompromised, or if they had non-Chagasic heart disease.
2.2 Sample sizeThe sample size calculation was based on the study published by Puccinelli et al. (12), which estimated a prevalence of physically active individuals of 76.5% in the Brazilian population during the COVID-19 pandemic. Considering the population of patients with ChD regularly followed at INI/Fiocruz in 2020 (around 900 patients), a 95% confidence interval, 6% accuracy estimate, and increasing the sample size by 10% due to losses and refusals, 175 patients were necessary for the present study.
2.3 Ethical considerationsAll participants received information about the goals and procedures of the study and agreed to participate by signing an informed consent form. The study was approved on 18/09/2020 by the Research Ethics Committee of the Evandro Chagas National Institute of Infectious Diseases in accordance with resolution 466/2012 of the National Health Council (CAAE: 37026320.2.0000.5262).
2.4 Study proceduresPatients were invited to participate during their regular outpatient visits and were submitted to study procedures in two moments. The first moment was performed during patient’s regular medical appointment, in which patients signed the informed consent and performed anthropometric measurements and blood collection. The second moment comprised the application of socioeconomic and clinical questionnaires by telephone, within a period of no more than one month after visit one, and the obtention of clinical information from patient’s medical records. The interviews and questionnaires were conducted in Portuguese, the native language of the study participants. The form is available at: https://osf.io/p9qc8/.
2.5 Physical activityThe level of PA was determined by the International Physical Activity Questionnaire (IPAQ) short version, previously adapted and validated for use in the Brazilian population (13). The IPAQ-short consists of 6 questions about the duration and frequency of participation in vigorous, moderate, and walking activities over the previous seven days (14). Energy expenditure is expressed in metabolic equivalent of task (METs) using the compendium of PA (15). The total volume of PA was quantified in MET-minutes per week and was calculated by summing up the total time per week spent in light (3.3 METs), moderate (4.0 METs), and vigorous (8.0 METs) activities (16).
Moreover, individuals were classified as meeting the PA recommendations per week using the criteria determined by the 2020 WHO guideline for PA and sedentary behavior as follows: >150 min of moderate-intensity PA, >75 min of vigorous-intensity PA, or > 150 min of a combination of moderate and vigorous PA (17).
2.6 Clinical form of ChDThe classification of the clinical presentation of ChD (indeterminate, cardiac, and digestive) and cardiac stages was performed using information from medical records based on clinical, electrocardiographic, echocardiographic, and digestive examination following the criteria of the 2nd Brazilian Consensus of Chagas Disease (18). To facilitate data analysis and following clinical reasoning, patients with cardiac form of ChD were recategorized as cardiac without heart failure and with cardiac failure.
2.7 ComorbiditiesInformation on comorbidities (systemic arterial hypertension, diabetes mellitus, dyslipidemia, obesity, respiratory diseases, cancer, kidney disease, hepatic and other chronic diseases) was obtained using medical records. Anthropometric measures were obtained during the clinical evaluation. Obesity was determined if body mass index [BMI = weight (kg)/squared height (m2)] was ≥30 kg/m2. Self-reported data on anxiety, depression, fear, and sadness were collected through direct questions to patients (“During the quarantine you presented anxiety? Did you have depression during quarantine? During the quarantine were you afraid? During quarantine did you feel sad?”).
2.8 Socioeconomic dataInformation on age, sex, race, marital status, educational level, income, number of rooms per domicile, and number of residents per domicile were collected. Race was self-reported as white, black, mulatto, yellow and indigenous. For data analysis, race was recategorized into white and non-white (black, mixed, yellow, and indigenous). The marital status was self-declared by the participant among the options single, married/stable, divorced, and widowed. Schooling was categorized based on the formal years of study into <9 years, ≥9 to 12 years and ≥ 12 years. Family income was determined by the sum of incomes of all individual residents at home, including wages, pensions, and any other income at the time of the collection. The number of rooms and the number of residents per domicile were collected using a direct question.
2.9 COVID-19 antibodiesImmunoglobulin M (IgM) and Immunoglobulin G (IgG) analysis for SARS CoV-2 were performed through the chemiluminescence test (Abott) in serum samples of the participants, following the manufacturer’s protocol.
2.10 Data analysisExploratory data analysis was performed by calculating means (standard deviations) and frequency (percentages) of the variables of interest (overall and stratified by tertiles of total PA levels). The variables associated with total PA levels (as a continuous variable) were identified using exploratory generalized linear regression models with a logarithmic link and gamma distribution due to the asymmetric and heteroscedastic nature of the residuals. The coefficients (beta) were exponentiated (Exp β) to facilitate the interpretation of the results. Only variables with p ≤ 0.10 in the univariate analysis were included in the multivariate model. A sensitivity analysis including only the variables with p ≤ 0.05 in the univariate analysis in the full multivariate model was also performed. The backwards method was used to sequentially remove variables with p > 0.05 values in multivariate analysis, until the final model maintained only those with p ≤ 0.05.
The Research Electronic Data Capture (REDCap) web application was used for data management and the data analysis was conducted using Stata 13.0 software. Statistical significance was set at p ≤ 0.05 for all analyses.
3 ResultsOf 350 invited patients, 187 agreed to participate in the study and were included in the analysis. The percentage of individuals who met the 2020 WHO PA recommendation was 52.0%. The general characteristics of the patients included in the study are presented in Table 1 (overall and stratified by tertiles of PA). The mean age of individuals included in the study was 61.1 years, with 62.0% of women. The mixed race was predominant (50.8%), with most patients having <9 years of schooling (69.5%). Overall, those in the highest tertile of PA were younger, less likely to present comorbidities (arterial hypertension, diabetes mellitus, and dyslipidemia), and with a lower percentage of severe cardiac forms of ChD.
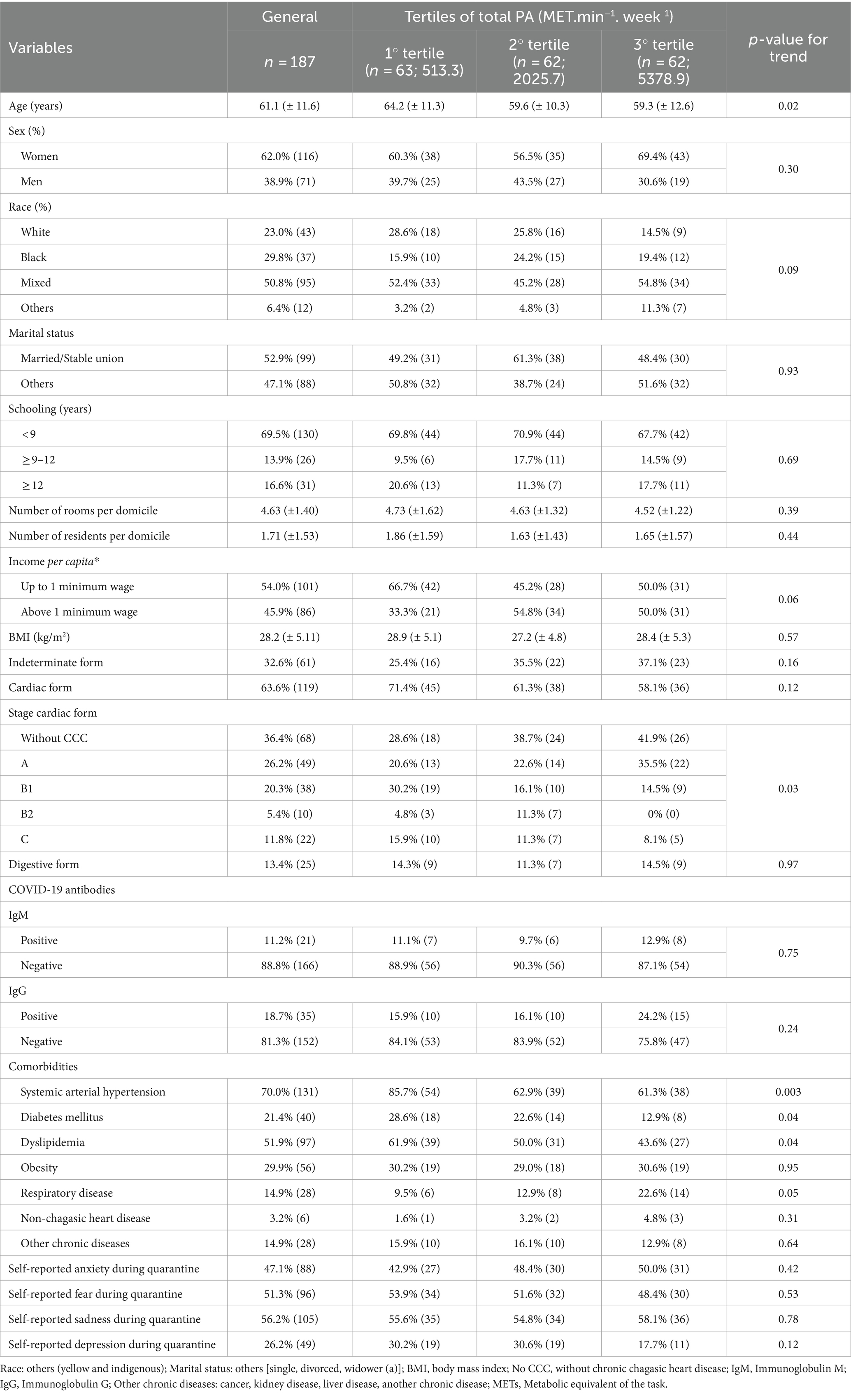
Table 1. Characteristics of participants included in the study (n = 187).
Table 2 depicts the univariate association between investigated exposure variables and total PA levels. The variables that were statistically significant associated with total PA levels were age (Exp β = 0.99; 95% CI 0.97 to 0.99), non-white race (Exp β = 1.37; 95% CI 0.99 to 1.89), education levels ≥12 years (Exp β = 1.41; 95% CI 1.00 to 1.99), income per capita above 1 minimum wage (Exp β = 1.31; 95% CI 1.00 to 1.70), diabetes (Exp β = 1.53; 95% CI 1.12 to 2.11), dyslipidemia (Exp β = 0.69; 95% CI 0.53 to 0.89) and self-reported depression during quarantine (Exp β = 0.71; 95% CI 0.53 to 0.96). In the multivariate model, the variables that were independently associated with total PA levels were non-white race (Exp β = 1.39; 95% CI 1.02 to 1.90), dyslipidemia (Exp β = 0.73; 95% CI 0.56 to 0.95) and self-reported depression during quarantine (Exp β = 0.71; 95% CI 0.52 to 0.96) (Table 3). These variables remained consistent in the sensitivity analysis, which included only the variables with p ≤ 0.05 in the univariate analysis in the full multivariate model. Analysis of deviance residuals and examination of the correlation between explanatory variables did not demonstrate deviations from GLM assumptions (Figure 1).
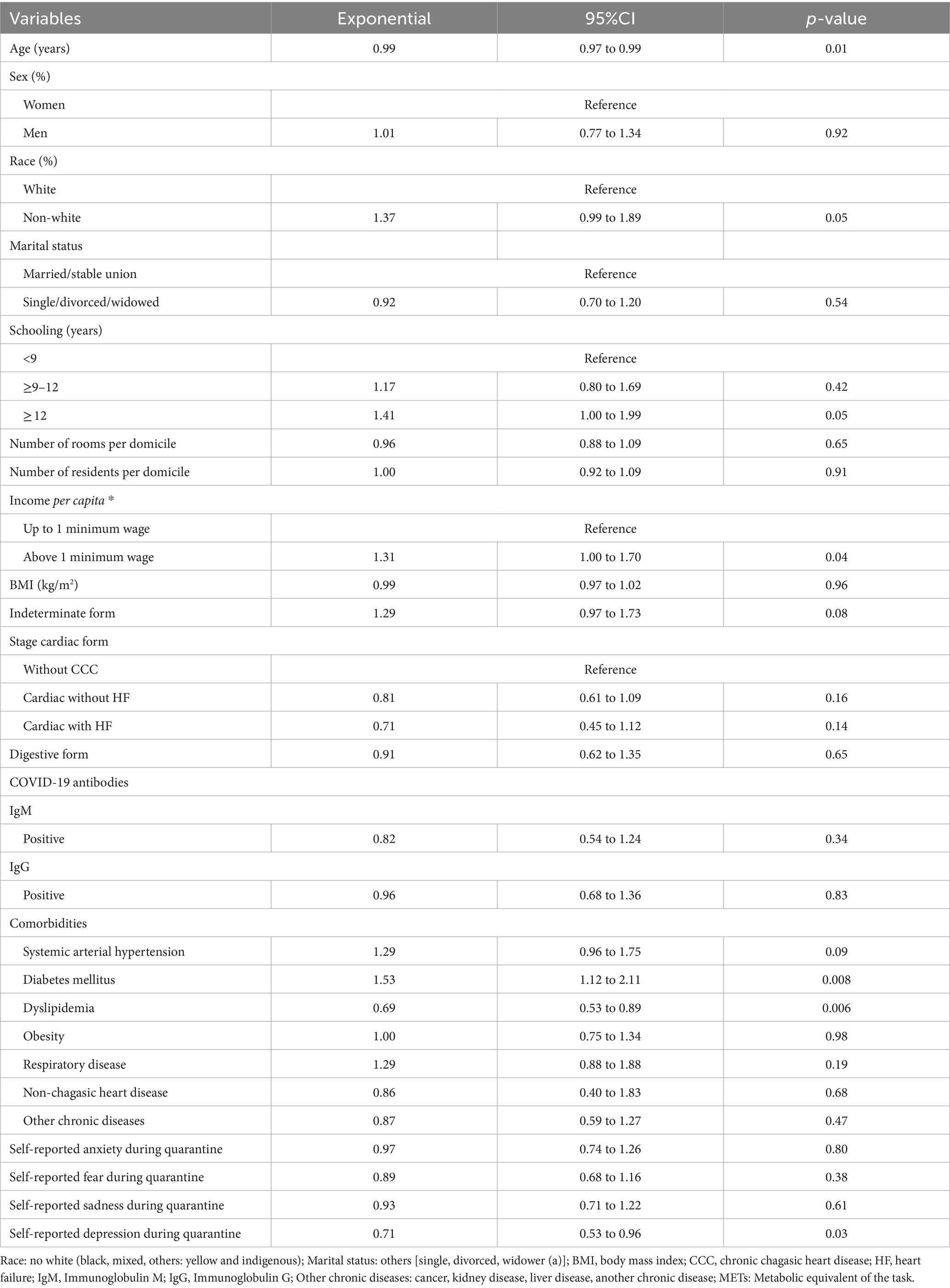
Table 2. Univariate linear regression for association between exposure variables and total PA (METs) in patients with Chagas disease (n = 187).
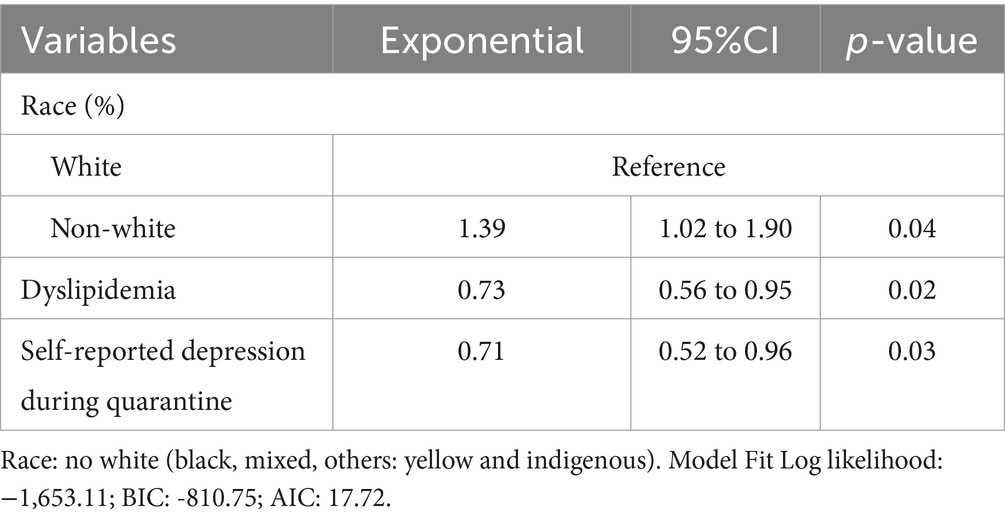
Table 3. Multivariate linear regression for association between exposure variables and total PA (METs) in patients with Chagas disease (n = 187).
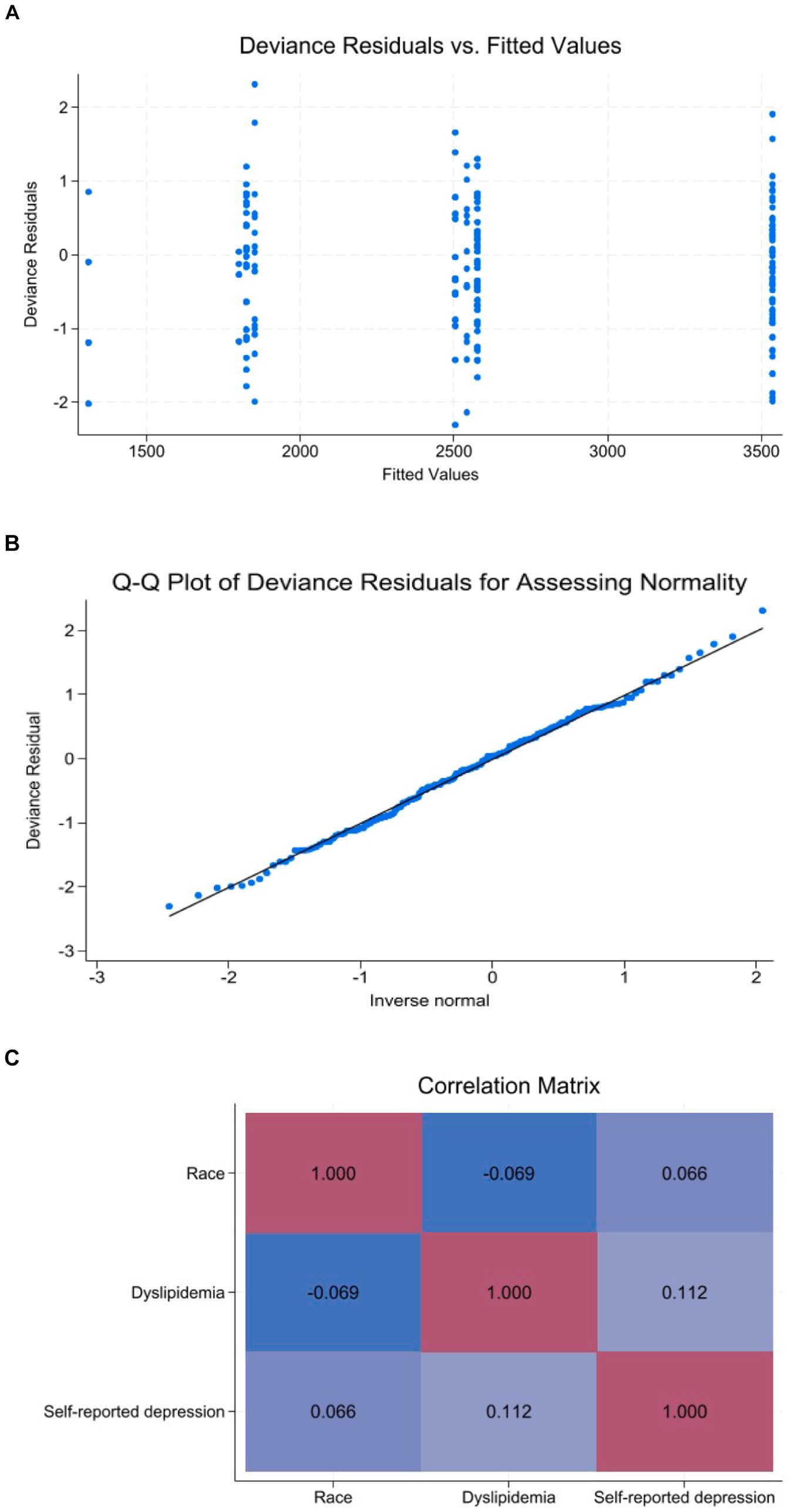
Figure 1. Diagnostic plots for demonstration of GLM assumptions. (A) Deviance residuals vs. fitted values. (B) Q-Q plot of deviance residuals. (C) Correlation matrix map.
4 DiscussionThe present study revealed a relatively low adherence (52%) to the 2020 WHO PA recommendations among a sample of urban patients with ChD during the COVID-19 pandemic. Additionally, three variables—race, presence of dyslipidemia, and self-reported depression—were independently associated with overall levels of PA.
Studies assessing PA levels in the general population during the COVID-19 pandemic have reported conflicting estimates (19–21). For example, a cross-sectional study in Brazil, which online surveyed 1,853 individuals of both sexes, noted a decline in the proportion of physically active individuals during the COVID-19 pandemic compared to the pre-pandemic period (76.5% vs. 90.5%, respectively) (12). In contrast, another cross-sectional study with 771 students from a Brazilian public university, using an online questionnaire, found that 50.2% were classified as physically active during the COVID-19 pandemic (22), a lower percentage in comparison to the previously mentioned study. Further supporting the decreased levels of PA during the COVID-19 pandemic, a Canadian online survey involving 1,098 adults reported an even lower prevalence (36.6%) of physically active individuals during that period (23), with 40.5% of the physically inactive participants reporting that became less active after the implementation of pandemic restrictions recommendations, underscoring the detrimental impact of social isolation on overall PA levels.
The existing literature on the PA levels of individuals with ChD is limited, particularly in the context of the COVID-19 pandemic. In a prior cross-sectional observational study conducted by our research group between 2014 and 2017, encompassing 361 individuals with ChD under the care of the same institution, 74.2% were identified as physically active based on the same WHO’s PA definition (24). Therefore, considering that participants were selected from the same population base and presented similar clinical and demographic characteristics, a decrease in PA levels was observed when comparing data before and after the COVID-19 pandemic period, which may be attributed to the social isolation measures adopted during this period (19).
Three variables were independently associated with the total PA: race, dyslipidemia, and self-reported depression during quarantine. In our study, non-white race was associated with an increased total PA level (25, 26). In Brazil, non-white individuals often occupy blue-collar occupations due to their lower socioeconomic status. These occupations, characterized by physically demanding tasks, may contribute to elevated levels of total PA. Throughout the COVID-19 pandemic, many of these individuals lacked the financial resources to stay home, as their livelihoods depended on continued work, and they were unable to engage in remote work. In some cases, there was even an increase in workload during the pandemic period. These factors may explain the observed higher levels of PA levels observed in the non-white participants in the present study (27, 28).
In our study, the presence of dyslipidemia was significantly associated with decreased PA levels. Individuals with dyslipidemia usually present inadequate health habits, such as an unhealthy diet and low levels of PA (29). Since dyslipidemia is often a silent condition that is frequently underestimated, it is possible to speculate that individuals with dyslipidemia may have further decreased their levels of PA during the pandemic due to their usual poor lifestyle habits. However, due to the cross-sectional design of the present study, the direction of this association is difficult to establish, with most of previous examining the inverse direction, suggesting that reduced PA levels were associated with a higher risk of dyslipidemia (30–32).
Finally, our study showed that self-reported depression during quarantine was independently associated with decreased total PA levels. The COVID-19 pandemic has exacerbated mental health challenges (33–35). A major contributor to this scenario has been social distancing, a successful measure in controlling the transmission of COVID-19, but with several negative repercussions on people’s well-being due to the loss of social support and increased feelings of loneliness and isolation (8, 10). A cross-sectional study carried out in Colombia with 431 individuals assessing the effects of the COVID-19 lockdown on PA levels and mental health found a substantial negative compromise of the psychological well-being (36). Moreover, another cross-sectional study conducted in Spain with 483 individuals found that individuals who complied with the WHO’s 2020 PA recommendations showed greater resilience, positive affect, and fewer depressive symptoms that may have positively contributed to situations of isolation (21).
The present study has some limitations. The cross-sectional design of the study prevents us from drawing conclusions about the causal relationship between the investigated variables with total PA levels. Our sample was composed of patients regularly followed-up in a national reference center, which may limit external validity. Moreover, self-reported measurements of mental health aspects (anxiety, depression, fear, and sadness) may have suffered some influence from motivational, cultural, and social factors, such as the low education levels of the studied sample. On the other hand, this is the first study that evaluated the level of PA during the COVID-19 pandemic in patients with ChD.
In conclusion, we found a relatively low compliance with the WHO PA recommendations during the COVID-19 pandemic period in a sample of patients with ChD. Non-white race was positively associated with total levels of PA, while dyslipidemia, and self-reported depression during quarantine were negatively associated with total levels of PA. In this scenario, identifying patient characteristics associated with lower PA levels may facilitate the development of tailored approaches to encourage more ChD patients to become physically active.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Ethics statementThe studies involving humans were approved by Research Ethics Committee of the Evandro Chagas National Institute of Infectious Diseases. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsIX: Data curation, Writing – original draft, Writing – review & editing. PA: Writing – review & editing. RV: Writing – review & editing. TB: Writing – review & editing. LP: Writing – review & editing. MH: Writing – review & editing. LS: Writing – review & editing. GS: Methodology, Writing – review & editing. FM-R: Writing – review & editing. FM: Methodology, Writing – review & editing. AC: Writing – review & editing. MQ: Formal analysis, Conceptualization, Writing – review & editing. AH-M: Methodology, Writing – review & editing. IA: Writing – review & editing. AJ: Methodology, Writing – review & editing. RP: Writing – review & editing. IG: Writing – review & editing. VP: Writing – review & editing. TG: Writing – review & editing. RS: Methodology, Supervision, Writing – review & editing, Funding acquisition. MM: Writing – review & editing, Methodology, Supervision, Writing – original draft.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 and by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (process number E-26/201.436/2021).
AcknowledgmentsThe authors would like to thank the Evandro Chagas National Institute of Infectious Diseases for its clinical and logistical support. We also thank the Brazilian agency that supported this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Schmunis, GA, and Yadon, ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. (2010) 115:14–21. doi: 10.1016/j.actatropica.2009.11.003
PubMed Abstract | Crossref Full Text | Google Scholar
3. Barker-Davies, RM, O'Sullivan, O, Senaratne, KPP, Baker, P, Cranley, M, Dharm-Datta, S, et al. The Stanford hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. (2020) 54:949–59. doi: 10.1136/bjsports-2020-102596
PubMed Abstract | Crossref Full Text | Google Scholar
5. Wang, H, Paulson, KR, Pease, SA, Watson, S, Comfort, H, Zheng, P, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. (2022) 399:1513–36. doi: 10.1016/S0140-6736(21)02796-3
Crossref Full Text | Google Scholar
6. Szwarcwald, CL, Boccolini, CS, De Almeida, DS, Filho, AMS, and Malta, DC. COVID-19 mortality in Brazil, 2020-21: consequences of the pandemic inadequate management. Arch Public Health. (2022) 80:255. doi: 10.1186/s13690-022-01012-z
PubMed Abstract | Crossref Full Text | Google Scholar
7. Pan, A, Liu, L, Wang, C, Guo, H, Hao, X, Wang, Q, et al. Association of Public Health Interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. (2020) 323:1915–23. doi: 10.1001/jama.2020.6130
PubMed Abstract | Crossref Full Text | Google Scholar
9. Sayin Kasar, K, and Karaman, E. Life in lockdown: social isolation, loneliness and quality of life in the elderly during the COVID-19 pandemic: a scoping review. Geriatr Nurs. (2021) 42:1222–9. doi: 10.1016/j.gerinurse.2021.03.010
PubMed Abstract | Crossref Full Text | Google Scholar
10. Hwang, TJ, Rabheru, K, Peisah, C, Reichman, W, and Ikeda, M. Loneliness and social isolation during the COVID-19 pandemic. Int Psychogeriatr. (2020) 32:1217–20. doi: 10.1017/S1041610220000988
PubMed Abstract | Crossref Full Text | Google Scholar
12. Puccinelli, PJ, Da Costa, TS, Seffrin, A, De Lira, CAB, Vancini, RL, Nikolaidis, PT, et al. Reduced level of physical activity during COVID-19 pandemic is associated with depression and anxiety levels: an internet-based survey. BMC Public Health. (2021) 21:613. doi: 10.1186/s12889-021-10684-1
Crossref Full Text | Google Scholar
13. Matsudo, S, Araújo, T, Matsudo, V, Andrade, D, Andrade, E, Oliveira, LC, et al. (2012). Questionário internacional de atividade física (IPAQ): estudo de validade e reprodutibilidade no Brasil. Revista Brasileira De Atividade Física & Saúde, 6:5–18. doi: 10.12820/rbafs.v.6n2p5-18
Crossref Full Text | Google Scholar
14. Craig, CL, Marshall, AL, Sjöström, M, Bauman, AE, Booth, ML, Ainsworth, BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
Crossref Full Text | Google Scholar
15. Ainsworth, BE, Haskell, WL, Whitt, MC, Irwin, ML, Swartz, AM, Strath, SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. (2000) 32:S498–516. doi: 10.1097/00005768-200009001-00009
PubMed Abstract | Crossref Full Text | Google Scholar
16. Poggio, R, Serón, P, Calandrelli, M, Ponzo, J, Mores, N, Matta, MG, et al. Prevalence, patterns, and correlates of physical activity among the adult population in Latin America: cross-sectional results from the CESCAS I study. Glob Heart. (2016) 11:81–88.e1. doi: 10.1016/j.gheart.2015.12.013
PubMed Abstract | Crossref Full Text | Google Scholar
17. World Health Organization. WHO guidelines on physical activity and sedentary behaviour: At a glance. Geneva: World Health Organization (2020). 2020 p.
18. Dias, JCP, Ramos, AN Jr, Gontijo, ED, Luquetti, A, Shikanai-Yasuda, MA, Coura, JR, et al. 2nd Brazilian consensus on Chagas disease, 2015. Rev Soc Bras Med Trop. (2016) 49:3–60. doi: 10.1590/0037-8682-0505-2016
PubMed Abstract | Crossref Full Text | Google Scholar
19. Sonza, A, da Cunha de Sá-Caputo, D, Sartorio, A, Tamini, S, Seixas, A, Sanudo, B, et al. COVID-19 lockdown and the behavior change on physical exercise, pain and psychological well-being: an international multicentric study. Int J Environ Res Public Health. (2021) 18:3810. doi: 10.3390/ijerph18073810
PubMed Abstract | Crossref Full Text | Google Scholar
20. Obradović, J, Radulović, N, Cvijović, D, and Jurišić, MV. Physical activity before and during the COVID-19 pandemic in Vojvodina, Serbia. Front Public Health. (2022) 10:10. doi: 10.3389/fpubh.2022.993035
Crossref Full Text | Google Scholar
21. Carriedo, A, Cecchini, JA, Fernandez-Rio, J, and Méndez-Giménez, A. COVID-19, psychological well-being and physical activity levels in older adults during the Nationwide lockdown in Spain. Am J Geriatr Psychiatry. (2020) 28:1146–55. doi: 10.1016/j.jagp.2020.08.007
PubMed Abstract | Crossref Full Text | Google Scholar
22. Garcia, MC, Paravidino, VB, Lopes, CS, Mediano, MFF, Gonçalves, TR, de Oliveira, AJ, et al. Sleep duration and quality during the COVID-19 pandemic and the association with physical activity and screen time among Brazilian college students. Am J Hum Biol. (2024) 36:e24035. doi: 10.1002/ajhb.24035
Crossref Full Text | Google Scholar
23. Lesser, IA, and Nienhuis, CP. The impact of COVID-19 on physical activity behavior and well-being of Canadians. Int J Environ Res Public Health. (2020) 17:3899. doi: 10.3390/ijerph17113899
PubMed Abstract | Crossref Full Text | Google Scholar
24. Santos-Filho, JCL, Vieira, MC, Xavier, IGG, Maciel, ER, Rodrigues Junior, LF, Curvo, EOV, et al. Quality of life and associated factors in patients with chronic Chagas disease. Trop Med Int Health. (2018) 23:1213–22. doi: 10.1111/tmi.13144
PubMed Abstract | Crossref Full Text | Google Scholar
25. Hawes, AM, Smith, GS, McGinty, E, Bell, C, Bower, K, LaVeist, TA, et al. Disentangling race, poverty, and place in disparities in physical activity. Int J Environ Res Public Health. (2019) 16:1193. doi: 10.3390/ijerph16071193
PubMed Abstract | Crossref Full Text | Google Scholar
26. Jones, SA, Moore, LV, Moore, K, Zagorski, M, Brines, SJ, Diez Roux, AV, et al. Disparities in physical activity resource availability in six US regions. Prev Med. (2015) 78:17–22. doi: 10.1016/j.ypmed.2015.05.028
PubMed Abstract | Crossref Full Text | Google Scholar
27. Robinson, E, Boyland, E, Chisholm, A, Harrold, J, Maloney, NG, Marty, L, et al. Obesity, eating behavior and physical activity during COVID-19 lockdown: a study of UK adults. Appetite. (2021) 156:104853. doi: 10.1016/j.appet.2020.104853
PubMed Abstract | Crossref Full Text | Google Scholar
28. Chen, IS, and Fellenz, MR. Personal resources and personal demands for work engagement: evidence from employees in the service industry. Int J Hosp Manag. (2020) 90:102600. doi: 10.1016/j.ijhm.2020.102600
PubMed Abstract | Crossref Full Text | Google Scholar
29. Grundy, SM, Stone, NJ, Bailey, AL, Beam, C, Birtcher, KK, Blumenthal, RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of Blood Cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 73:3168–209. doi: 10.1016/j.jacc.2018.11.002
PubMed Abstract | Crossref Full Text | Google Scholar
30. Pérez-Gisbert, L, Torres-Sánchez, I, Ortiz-Rubio, A, Calvache-Mateo, A, López-López, L, Cabrera-Martos, I, et al. Effects of the COVID-19 pandemic on physical activity in chronic diseases: a systematic review and Meta-analysis. Int J Environ Res Public Health. (2021) 18:12278. doi: 10.3390/ijerph182312278
PubMed Abstract | Crossref Full Text | Google Scholar
31. Ali, N, Kathak, RR, Fariha, KA, Taher, A, and Islam, F. Prevalence of dyslipidemia and its associated factors among university academic staff and students in Bangladesh. BMC Cardiovasc Disord. (2023) 23:366. doi: 10.1186/s12872-023-03399-1
PubMed Abstract | Crossref Full Text | Google Scholar
32. Zou, Q, Su, C, Du, W, Wang, H, Zhang, B, Luo, S, et al. Longitudinal association between physical activity, blood lipids, and risk of dyslipidemia among Chinese adults: findings from the China health and nutrition surveys in 2009 and 2015. Nutrients. (2023) 15:341. doi: 10.3390/nu15020341
PubMed Abstract | Crossref Full Text | Google Scholar
33. Grocke-Dewey, M, Hardison-Moody, A, Haynes-Maslow, L, Maras, S, Webber, E, Andress, L, et al. Examining the relationship between physical activity and mental health during the COVID-19 pandemic across five U.S. States Prev Med Rep. (2021) 24:101537. doi: 10.1016/j.pmedr.2021.101537
Crossref Full Text | Google Scholar
34. Capasso, A, Jones, AM, Ali, SH, Foreman, J, Tozan, Y, and DiClemente, RJ. Increased alcohol use during the COVID-19 pandemic: the effect of mental health and age in a cross-sectional sample of social media users in the U.S. Prev Med. (2021) 145:106422. doi: 10.1016/j.ypmed.2021.106422
PubMed Abstract | Crossref Full Text | Google Scholar
35. Magnavita, N, Soave, PM, and Antonelli, M. Prolonged stress causes depression in frontline workers facing the COVID-19 pandemic-a repeated cross-sectional study in a COVID-19 hub-Hospital in Central Italy. Int J Environ Res Public Health. (2021) 18:7316. doi: 10.3390/ijerph18147316
Crossref Full Text | Google Scholar
36. De la Rosa, A, Monterrosa Quintero, A, Camacho-Villa, MA, Arc-Chagnaud, C, Andrade, AGP, Reyes-Correa, S, et al. Physical activity levels and psychological well-being during COVID-19 lockdown among university students and employees. Int J Environ Res Public Health. (2022) 19:11234. doi: 10.3390/ijerph191811234
留言 (0)