Sunitinib, an oral small-molecule multitargeted tyrosine kinase inhibitors (TKIs), exerts dual effects on anti-tumor angiogenesis and anti-tumor cell proliferation via inhibiting the vascular endothelial growth factor-1, 2, 3 (VEGF-1, 2, 3), the platelet-derived growth factor-α, β (PDGFr-α, β), the stem-cell growth factor receptor (KIT), colony-stimulating factor (CSF)-1R, FMS-like tyrosine kinase-3 (FLT-3), and the rearranged during transfection (RET) (Mena et al., 2010). The agent has been approved by the Food and Drug Administration (FDA) for the treatment of metastatic renal cell carcinoma (mRCC), gastrointestinal stromal tumor (GIST) refractory to imatinib therapy, and advanced pancreatic neuroendocrine tumor (PNET) (Raymond et al., 2011; Savard et al., 2020; Li et al., 2024). The available data suggests that it can significantly prolong the median progression-free survival and overall survival of patients with mRCC or GIST (Hopkins et al., 2008; Moran et al., 2019). Encouraging results have also been shown in clinical trials of sunitinib alone or in combination with other anti-tumour agents for the treatment of solid tumours such as lung and breast cancer (Crown et al., 2013; Tanday, 2015).
However, despite its significant clinical benefits, the widespread clinical use of sunitinib inevitably leads to adverse effects in patients. In clinical phase II and phase III studies of sunitinib, the most common adverse events (AEs) occurring in at least 25% of recipients include fatigue, rash, diarrhea, mucositis, loss of appetite, hand-foot syndrome, hypertension, hemorrhages, taste disturbances, and dyspepsia (Cella et al., 2008; Escudier et al., 2009). Additionally, sunitinib has an FDA “black box” warning for hepatotoxicity, which has the potential to be fatal (Amaya et al., 2018). Therefore, it is highly desirable to utilize data mining algorithms to identify potential safety signals of sunitinib in real-world settings.
The Food and Drug Administration Adverse Event Reporting System (FAERS) is one of the largest post-marketing safety monitoring databases that document real-world standardized data, which can be used for identifying and analyzing potential drug-AEs associations (Michel et al., 2017). We performed a retrospective pharmacovigilance study to detect the AEs signals of sunitinib based on disproportionality analysis methods. This research aimed to find unexpected AEs that were not described on the drug’s label and offer valuable reference for clinical practices.
2 Materials and methods2.1 Data source and processingFAERS is the primary system in the United States for conducting post-marketing adverse drug reaction surveillance and is one of the main avenues for current pharmacovigilance research (Dhodapkar et al., 2022). In our pharmacovigilance study, the ASCII data packages submitted from the first quarter of 2006 to the first quarter of 2024 were retrieved from the database. All data analyses were imported into SAS 9.4 and Excel software for data cleaning and analyses. The sample group was chosen by screening for DRUGNAME and PROD_AI, using both the generic and brand names (sunitinib, Sutent®) as keywords, with the suspicion level for reporting limited to “primary suspect” drugs. Based on the latest version of the Medical Dictionary for Regulatory Activities (MedDRA) 26.0 dictionary, AEs of sunitinib are coded on preferred terms (PT) and system organ class (SOC) levels, and the toxicity spectrum of sunitinib was investigated. According to the recommended approach by the FDA to remove duplicate reports, choose the PRIMARYID, CASEID, and FDA_DT fields of the DEMO table, sort them by CASEID, FDA_DT, and PRIMARYID, and keep the report with the highest FDA_DT value for individual case safety reports (ICSRs) with the same CASEID. Additionally, keep the one with the highest PRIMARYID value for reports with both the same CASEID and FDA_DT. Ensure retention of the one with the largest PRIMARYID value. Since Q1 2019, a roster of erased reports has been included in every quarterly packet. Following data de-duplication, reports are deleted using the CASEID included in the roster above. The comprehensive screening procedure was depicted in Figure 1.
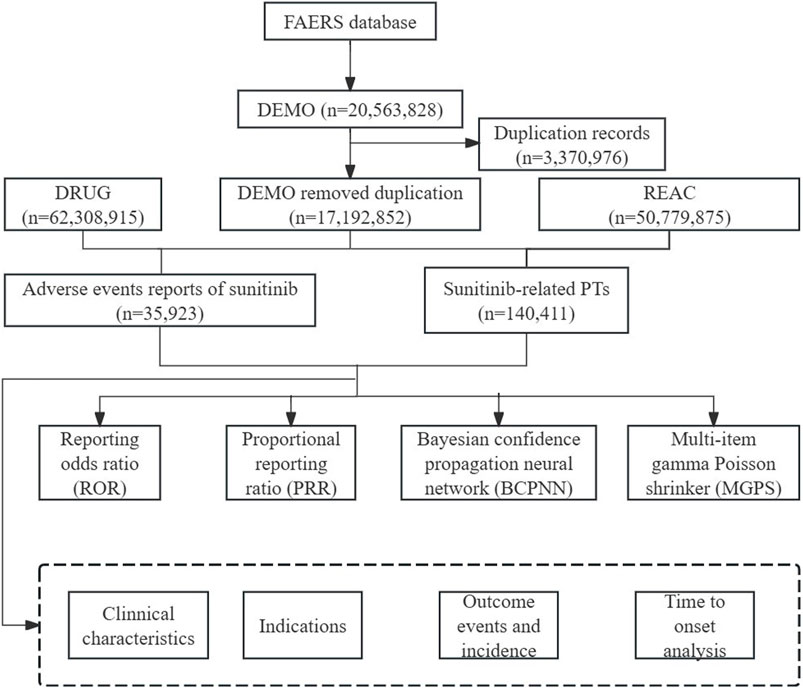
Figure 1. Flowchart of identifying AE cases of sunitinib from the FAERS database.
2.2 Onset time analysisThe onset time of sunitinib-related events was calculated by subtracting the date of AE occurrence from the date of sunitinib initiation. It is crucial to highlight that the study excluded erroneous ICSRs whose occurrence date preceded the initiation of the drug or ICSRs of unknown time of onset. The median and interquartile ranges were employed to characterize the time to onset.
2.3 Statistical analysisDescriptive analyses were used to present all sunitinib-related AEs reporting characteristics. In our study, disproportionate analyses, including reporting odds ratio (ROR) (Sakaeda et al., 2013), proportional reporting ratio (PRR) (Kelly et al., 2007), bayesian confidence propagation neural network (BCPNN) (Bate et al., 1998), and multinomial gamma Poisson shrinkage (MGPS) (Szarfman et al., 2004), were performed to identify signals indicating a possible increased risk of sunitinib-related AEs. The ROR and PRR are classified as frequency methods, and the methods demonstrate high sensitivity but low specificity. The BCPNN and MGPS are categorized as Bayesian methods which are suitable for handling complex variables, but with low sensitivity (Zou et al., 2023). Accordingly, multiple algorithms are combined to ensure the stability and reliability of the research results. The larger the value of the four parameters, the stronger the signal strength is. The relevant numerical values are the signal strength. The formulas and criteria for the four algorithms are shown in Table 1. (Liu et al., 2024; Tang et al., 2024). The AEs uncovered in the latest version of the sunitinib label issued by the FDA were defined as unexpected AEs. (FDA LABEL, 2021).
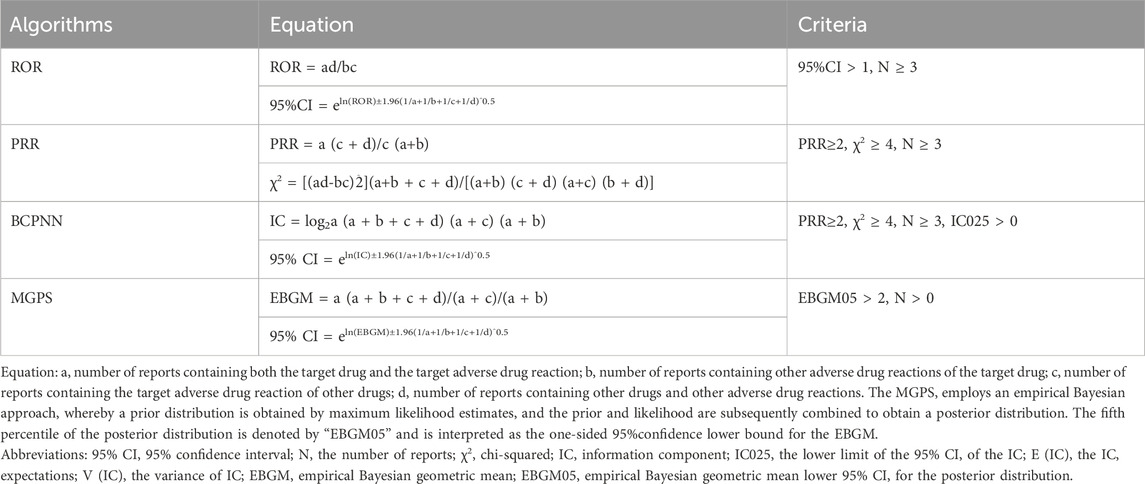
Table 1. The specific formulas for the four algorithms.
3 Results3.1 Descriptive analysisA total of 20,563,828 ICSRs were submitted to the FAERS database during the study period, among which there were 35,923 ICSRs on sunitinib of PS. The clinical characteristics of events regarding sunitinib were presented in Table 2 and Figure 2. Among all ICSRs, more males (59.35%) than females (31.85%) were reported. In terms of age, patients aged>65 contributed to the majority of ICSRs (38.55%). Death (28.92%) and hospitalization-initial or prolonged (28.38%) were the most common serious outcomes, and the high proportion of deaths might be more related to cancer progression in AEs. The country that reported the most was the United States (48.87%), and the primary ICSRs were consumers (38.13%) and physicians (30.93%). In terms of year of reporting, ICSRs were concentrated in 2010 (3085 cases), 2015 (3,760 cases), and 2016 (3,167 cases).

Table 2. Clinical characteristics of reports with sunitinib from the FAERS database.
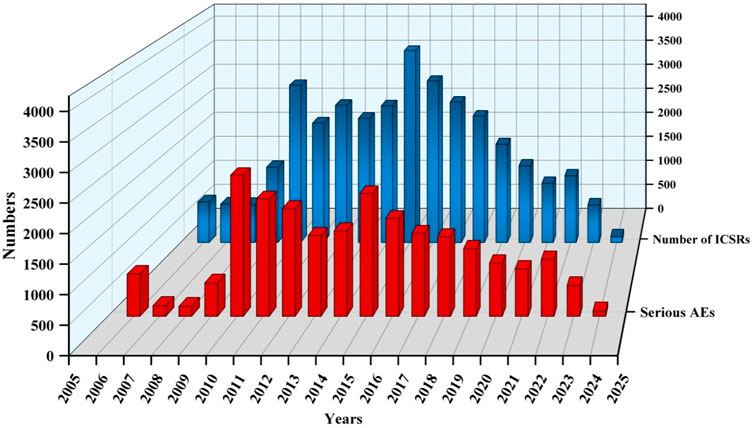
Figure 2. Annual trends in sunitinib reporting.
3.2 Potential safety signal detection resultsWe performed signal detection of sunitinib-associated AEs at the SOC level which was shown in Table 3. AEs of sunitinib involved a total of 27 organ systems. Statistically, the SOC that met all four criteria simultaneously and showed significant association with sunitinib AEs were gastrointestinal disorders (n = 24452, ROR 2.27, PRR 2.04, IC 1.03, EBGM 2.04) and endocrine disorders (n = 1128, ROR 3.21, PRR 3.21, IC 1.67, EBGM 3.18). Additionally, general disorders and administration site conditions (n = 28170) were the most common SOCs. Of note, skin and subcutaneous tissue disorders (n = 10290), blood and lymphatic system disorders (n = 4,123), and vascular disorders (n = 3864) were also common and noteworthy SOC categories.

Table 3. Signal strength of AEs of sunitinib at the SOC level in the FAERS database.
Based on the signal frequency and the signal strength (adopting the most sensitive ROR algorithm results), we respectively ranked these AEs which satisfied all four screening methods, as detailed in Tables 4, 5. Excepting in AEs of death (n = 6873), in our results, diarrhea (n = 4,049), fatigue (n = 3793), asthenia (n = 2249), decreased appetite (n = 2222), hypertension (n = 1678), and dysgeusia (n = 1606) were the most common AEs, which were consistent with the label and clinical trials. The AEs with significant potential risk signals included diffuse uveal melanocytic proliferation (ROR = 131.15, PRR = 131.15, IC = 6.59, EBGM = 96.44), salivary gland fistula (ROR = 98.36, PRR = 98.36, IC = 6.28, EBGM = 77.50), yellow skin (ROR = 67.81, PRR = 67.50, IC = 5.83, EBGM = 57.02), eyelash discoloration (ROR = 64.03, PRR = 64.02, IC = 5.77, EBGM = 54.52), scrotal inflammation (ROR = 39.20, PRR = 39.20, IC = 5.15, EBGM = 35.46), among others. Furthermore, comparing with the latest package insert of released by the FDA, unexpected significant AEs were discovered, such as including diffuse uveal melanocytic proliferation (ROR = 131.15, PRR = 131.15, IC = 6.59, EBGM = 96.44), salivary gland fistula (ROR = 98.36, PRR = 98.36, IC = 6.28, EBGM = 77.50), scrotal inflammation (ROR = 39.20, PRR = 39.20, IC = 5.15, EBGM = 35.46), thyroid atrophy (ROR = 36.07, PRR = 36.07, IC = 5.04, EBGM = 32.88), esophagobronchial fistula (ROR = 36.07, PRR = 36.07, IC = 5.04, EBGM = 32.88), perihepatic discomfort (ROR = 15.03, PRR = 15.03, IC = 3.85, EBGM = 14.47) and vena cava injury (ROR = 14.24, PRR = 14.24, IC = 3.78, EBGM = 13.73). Of note, nausea, vomiting, malaise, pain in extremity, pyrexia, constipation, and abdominal pain in the drug label did not meet the criteria for at least one of the four algorithms.
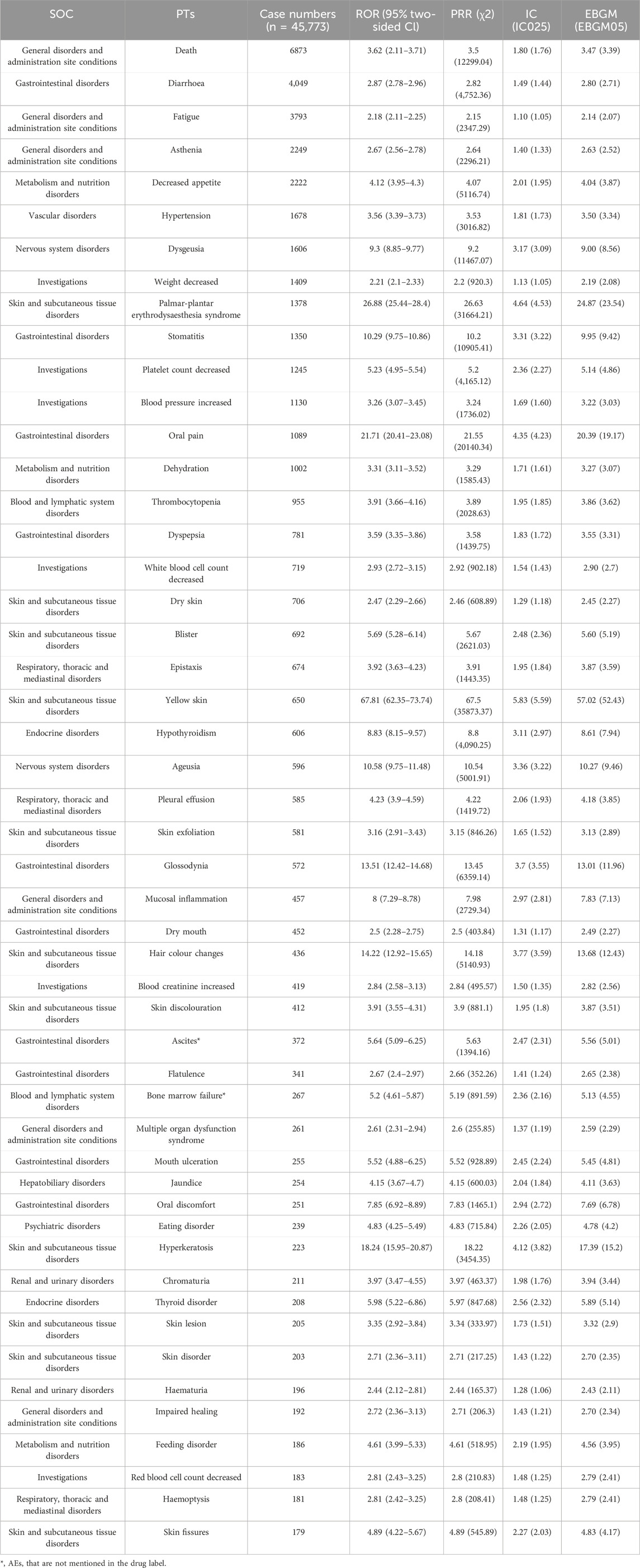
Table 4. The top 50 AEs of sunitinib ranked by the frequency at the PTs level.

Table 5. The top 50 signal strength of AEs of sunitinib ranked by the ROR at the PTs level.
3.3 Time-to-onset analysisThe onset time of sunitinib-related events was collected, with unreported onset time reports or erroneous reports excluded from the analysis. A total of 11,534 ICSRs were eligible for the inclusion criteria, and the mean time to onset was 207 days, with a median onset time of 51 days (interquartile range [IQR] 16–170 days). Our data revealed that 39.73% of ICSRs occurred within the first month following sunitinib administration (n = 4,582). Notably, AEs might still occur after 1 year of sunitinib treatment, accounting for 13.39% of the total cases, as illustrated in Figure 3.
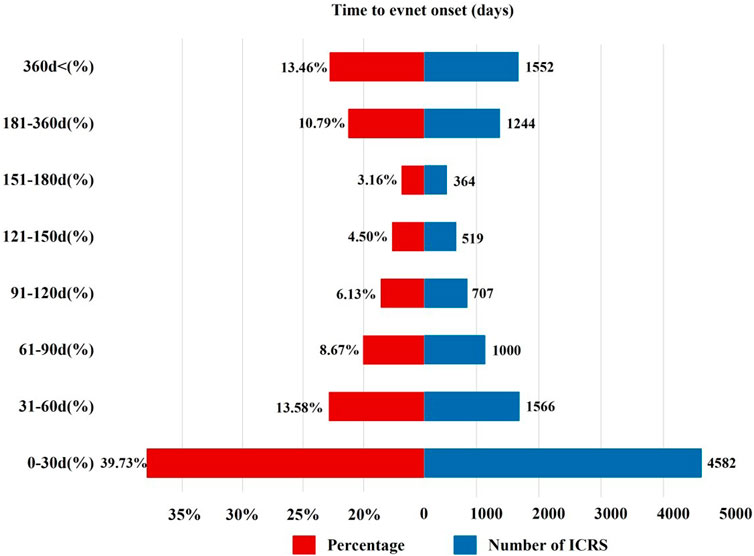
Figure 3. Time to onset of sunitinib-related AEs.
4 DiscussionIn recent years, several studies have carried out pharmacovigilance analyses of TKIs on electrolyte abnormalities, psychiatric disorders, and neuropsychiatric events (Raschi et al., 2022; Barbieri et al., 2023; Wang et al., 2023; She et al., 2024). However, the comprehensive safety profiles of sunitinib-induced AEs have not been reported. In this study, we systematically collect and assess the post-marketing AEs of sunitinib based on real-world pharmacovigilance data. The study aimed to identify new and significant risk signals and enhance the safety of clinical drug therapy. The ICSRs of sunitinib occurred more commonly in males (59.53%) than in females (31.85%), and a higher ICSRs proportion in elderly individuals over the age of 65 (38.83%), which could be attributed to the higher incidence of RCC in elderly males. It has been reported that the prevalence of renal cell carcinoma in males is approximately twice that in females and tends to increase with age (Scelo et al., 2018; Scelo and Larose, 2018). Besides, the median age of most patients at diagnosis is around 75 years old (Bukavina et al., 2022). Regarding reporting countries, the United States stands out as the most prominent reporting country (49.16%), possibly due to the earlier availability and higher prescription volume of the drug in the country. Notably, consumers (38.20%) were the predominant group reporting ICSRs, followed by physicians (30.98%). This indicates the need for heightening vigilance among clinical doctors and pharmacists in monitoring sunitinib-related AEs, particularly in the elderly population, to reduce the occurrence of life-threatening AEs.
Our disproportionality analyses identified that the significant SOCs were gastrointestinal disorders and endocrine disorders, and the most common SOC was general disorders and administration site conditions. As shown in Table 4, common AEs included diarrhea, fatigue, asthenia, decreased appetite, hypertension, dysgeusia, hand-foot syndrome, and stomatitis, which were mostly consistent with the insert and clinical trials (Saltz et al., 2007; Baumann et al., 2012; Jonasch et al., 2018). Hypertension is most common upon treatment with sunitinib (Pal et al., 2021). All-grade hypertension has been documented in up to 30% of mRCC patients receiving sunitinib, with grade 3 hypertension in 12% of cases (Motzer et al., 2009). Besides, hypertension was also a relatively common AE among other vascular endothelial growth factor TKIs (Zhu et al., 2009; Bæk Møller et al., 2019). The underlying mechanism is currently believed to be the activation of the endothelin-1 pathway and the disruption of endothelial cell survival signaling, resulting in reduced capillary density and diminished nitric oxide secretion (Kappers et al., 2010; Rini et al., 2011). It has been reported that hypertension often occurs as a complication in the early stages of treatment with TKIs, such as sorafenib and axitinib (Motzer et al., 2009). Therefore, it is recommended that blood pressure is regularly monitored at least once a week during the initial 6 weeks of sunitinib treatment (Zhu et al., 2009). In case of severe or persistent hypertension, it is advisable to withdraw sunitinib immediately and initiate antihypertensive treatment.
Some AEs with high signal intensity were also examined, such as yellow skin, eyelash discoloration, hand-foot syndrome (HFS), genital injury (including anal injuries, genital rashes, desquamation, ulcers, etc.), and bleeding events. Approximately 24% of patients suffered from skin discoloration, including yellow skin and pigmentation after sunitinib therapy (Rosenbaum et al., 2008). Of note, the yellow color of the medication may be a contributing factor to the development of yellow skin. Hair depigmentation occurred in 15.2% of patients, and the inhibition of multiple signaling pathways, such as platelet-derived growth factor receptors (PDGFR), seemed to have a role in the pathogenesis (Joensuu et al., 2011). The incidence of HFS was reported between 13.5% and 25%, of which 4%–7% of cases were grade 3 to 4. The onset of HFS was 2–4 weeks. The potential mechanism behind HFS is not completely understood, but it is hypothesized to involve the inhibition of VEGFR and PDGFR, resulting in vascular deformation and cell apoptosis in the dermis (Terada et al., 2015). Billemont et al. reported that 12.5% of patients experienced genital rash and anal injuries, with a median onset time of 66 days (Billemont et al., 2008). The mechanism of this AE is still largely unclear, and it may be related to VEGF and hypoxia-inducible factor-1a (Billemont et al., 2008; Chou et al., 2013). Lastly, various bleeding events such as splinter haemorrhage, umbilical haemorrhage, mediastinal haemorrhage, and scleral haemorrhage were also detected in our study. In a meta-analysis of patients with sunitinib and sorafenib, the incidence was 16.7% for all-grade bleeding events and 2.4% for grade 3/4 toxicity (Je et al., 2009). The increased risk of bleeding may be mediated by VEGF inhibition and concomitant administration with antiplatelet agents (Je et al., 2009). In addition, sunitinib-associated thrombocytopenia, with an incidence of 22% (Lee et al., 2014), was hypothesized to be an intriguing explanation for bleeding events.
Furthermore, unexpected and significant safety signals, such as diffuse uveal melanocytic proliferation, vena cava injury, esophagobronchial fistula, perihepatic discomfort, and thyroid atrophy, were detected in our analysis. Regardless, medical staff should note that patients on sunitinib are at risk of these unexpected AEs. So far, there have been no documented reports about diffuse uveal melanocytic proliferation and vena cava injury Therefore, it is necessary to further investigate the pathogenesis of these incidents. Basille et al. described a 40-year-old male with renal cell carcinoma who developed an esophagotracheal fistula after the administration of sunitinib for 2 months (Basille et al., 2010). However, the exact mechanism of esophagotracheal fistula remained unclear. In our study, we observed that sunitinib-induced hepatoxicity AEs mainly included perihepatic discomfort, liver failure, and jaundice. Approximately 40% of patients with sunitinib experienced elevated liver enzymes, and 3% of patients developed grade 3 or 4 hepatotoxicity (Ibrahim et al., 2013). Additionally, similar AEs have also been observed in other VEGF-TKIs (Teo et al., 2013). The onset time is 1–3 weeks after initiation of treatment or even several months later (Lammert et al., 2008). Toxic intermediate metabolites, mitochondrial dysfunction, and glycolysis inhibition have been described as possible mechanisms (Teo et al., 2013; Paech et al., 2017). Furthermore, several cases of sunitinib-related fulminant acute liver failure have been reported (Aqsa et al., 2021; Casas Deza et al., 2021). In 2010, FDA issued a black box warning that sunitinib-associated hepatotoxicity may be severe, and even fatal. Clinicians should be aware that liver failure is a complication unrelated to dosage or tumor progression, and liver function should be regularly monitored during the first year of treatment. According to a prospective study, the incidence of hypothyroidism as a common complication following sunitinib treatment was found to be 36% (Desai et al., 2006). However, our research findings suggested that sunitinib-induced thyroid atrophy represented a novel, unexpected, and rarely reported thyroid toxicity. A long-term study has indicated that the thyroid volume decreased by approximately 30% in metastatic renal cell carcinoma patients treated with sunitinib, and those with a reduction of over 50% in thyroid volume experienced a dramatically increased incidence of hypothyroidism (Shinohara et al., 2011). Thyroid atrophy induced by sunitinib may be attributed to the induction of follicular atrophy and apoptosis of thyroid cells, with its toxicity being a temporal relationship (Sakurai et al., 2010; Grosse et al., 2014). It has been observed that patients with sunitinib-induced hypothyroidism may show improvement upon suspension of the medication and initiation of levothyroxine therapy. However, in cases where severe thyroid atrophy caused by sunitinib leads to secondary hypothyroidism, it may be deemed irreversible (Desai et al., 2006). Therefore, regular monitoring of thyroid toxicity, particularly thyroid volume, is crucial during sunitinib treatment.
In this study, there is a wide variance between the onset of AEs and sunitinib application. The median onset time of reported AEs was 51 days, and 39.73% of ICSRs occurred within the first month after sunitinib initiation. However, 13.39% of ICSRs developed with a delayed onset, occurring 1 year after sunitinib therapy. Consistently, in a phase III clinical trial involving 312 GIST patients, the median onset time of AEs caused by sunitinib was 56 days, and the onset time of some AEs was longer. For example, the average onset time of hypothyroidism was 350 days (Joensuu et al., 2011). Therefore, it is imperative to be vigilant about the AEs throughout the whole course of treatment with sunitinib, and long-term follow-up for some AEs may be needed.
The pre-marketing drug safety studies are characterized by the small number of enrolled cases, brief observation periods, stringent medication usage conditions, etc. Thus, the AEs observed in pre-marketing trials of a drug may not reflect all AEs observed in practice. The spontaneous reporting database, with a wide monitoring range and early detection of suspected AE signals, was extensively used to carry out post-marketing pharmacovigilance on drug safety. In recent years, with the wide use of sunitinib in clinical practice there is an emerging need to make further evaluation of its safety profiles in a real-world environment. Our study first comprehensively and scientifically explores the post-marketing safety profiles of sunitinib based on AE reports from the FAERS database. Furthermore, some unexpected AEs were detected and the onset time of all AEs was analyzed, which guides the safe clinical use of sunitinib.
5 ConclusionIn conclusion, our study conducted a systematic and comprehensive exploration of the signals associated with sunitinib based on the FAERS database. The common AEs detected in this study were consistent with the manufacturer’s labeling and clinic trials. Some unexpected AEs were also revealed, such as diffuse uveal melanocytic proliferation, thyroid atrophy, esophagobronchial fistula, vena cava injury, and perihepatic discomfort. Furthermore, the median onset time of AEs was analyzed, which enables clinicians and pharmacists to make informed decisions regarding sunitinib. However, given the exploratory character of our work, future prospective clinical trials and long-term data are needed to validate these results and provide valuable evidence for the safety profile of sunitinib.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributionsXZ: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. XR: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. TZ: Data curation, Investigation, Writing–original draft. WZ: Data curation, Writing–original draft. CS: Conceptualization, Formal Analysis, Supervision, Writing–review and editing. CL: Conceptualization, Formal Analysis, Supervision, Writing–review and editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Shandong Province, China (Nos ZR2022QH109, ZR2023LSW013, and ZR2023MH077).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAmaya, G. M., Durandis, R., Bourgeois, D. S., Perkins, J. A., Abouda, A. A., Wines, K. J., et al. (2018). Cytochromes P450 1A2 and 3A4 catalyze the metabolic activation of sunitinib. Chem. Res. Toxicol. 31, 570–584. doi:10.1021/acs.chemrestox.8b00005
PubMed Abstract | CrossRef Full Text | Google Scholar
Aqsa, A., Droubi, S., Amarnath, S., Al-Moussawi, H., and Abergel, J. (2021). Sunitinib-induced acute liver failure. Case Rep. Gastroenterol. 15, 17–21. doi:10.1159/000511249
PubMed Abstract | CrossRef Full Text | Google Scholar
Bæk Møller, N., Budolfsen, C., Grimm, D., Krüger, M., Infanger, M., Wehland, M., et al. (2019). Drug-induced hypertension caused by multikinase inhibitors (sorafenib, sunitinib, lenvatinib and axitinib) in renal cell carcinoma treatment. Int. J. Mol. Sci. 20, 4712. doi:10.3390/ijms20194712
PubMed Abstract | CrossRef Full Text | Google Scholar
Barbieri, M. A., Sorbara, E. E., Russo, G., Cicala, G., Franchina, T., Santarpia, M., et al. (2023). Neuropsychiatric adverse drug reactions with tyrosine kinase inhibitors in gastrointestinal stromal tumors: an analysis from the European spontaneous adverse event reporting system. Cancers 15 (6), 1851. doi:10.3390/cancers15061851
PubMed Abstract | CrossRef Full Text | Google Scholar
Basille, D., Andrejak, M., Bentayeb, H., Kanaan, M., Fournier, C., Lecuyer, E., et al. (2010). Bronchial fistula associated with sunitinib in a patient previously treated with radiation therapy. Ann. Pharmacother. 44, 383–386. doi:10.1345/aph.1M469
PubMed Abstract | CrossRef Full Text | Google Scholar
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54 (4), 315–321. doi:10.1007/s002280050466
PubMed Abstract | CrossRef Full Text | Google Scholar
Baumann, K. H., du Bois, A., Meier, W., Rau, J., Wimberger, P., Sehouli, J., et al. (2012). A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann. Oncol. 23, 2265–2271. doi:10.1093/annonc/mds003
PubMed Abstract | CrossRef Full Text | Google Scholar
Bukavina, L., Bensalah, K., Bray, F., Carlo, M., Challacombe, B., Karam, J. A., et al. (2022). Epidemiology of renal cell carcinoma: 2022 update. Eur. Urol. 82, 529–542. doi:10.1016/j.eururo.2022.08.019
PubMed Abstract | CrossRef Full Text | Google Scholar
Casas Deza, D., Gascón Ruiz, M., Lamuela Calvo, L. J., Sierra Gabarda, O., Betoré Glaria, E., and Bernal Monterde, V. (2021). Fulminant liver failure in a patient treated with Sunitinib for metastatic gastrointestinal stromal tumor. Gastroenterol. Hepatol. 44, 424–425. doi:10.1016/j.gastrohep.2020.08.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Cella, D., Li, J. Z., Cappelleri, J. C., Bushmakin, A., Charbonneau, C., Kim, S. T., et al. (2008). Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: results from a phase III randomized trial. J. Clin. Oncol. 26, 3763–3769. doi:10.1200/JCO.2007.13.5145
PubMed Abstract | CrossRef Full Text | Google Scholar
Chou, C. Y., Wang, K. H., Lin, Y. H., Lin, Y. T., and Tsai, H. H. (2013). Sunitinib-induced scrotal cutaneous side-effect. J. Dermatol 40, 67–68. doi:10.1111/j.1346-8138.2012.01681.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Crown, J. P., Diéras, V., Staroslawska, E., Yardley, D. A., Bachelot, T., Davidson, N., et al. (2013). Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J. Clin. Oncol. 31 (23), 2870–2878. doi:10.1200/JCO.2012.43.3391
PubMed Abstract | CrossRef Full Text | Google Scholar
Desai, J., Yassa, L., Marqusee, E., George, S., Frates, M. C., Chen, M. H., et al. (2006). Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann. Intern. Med. 145, 660–664. doi:10.7326/0003-4819-145-9-200611070-00008
PubMed Abstract | CrossRef Full Text | Google Scholar
Dhodapkar, M. M., Shi, X., Ramachandran, R., Chen, E. M., Wallach, J. D., and Ross, J. S. (2022). Characterization and corroboration of safety signals identified from the US Food and drug administration adverse event reporting system, 2008-19: cross sectional study. BMJ 379, e071752. doi:10.1136/bmj-2022-071752
PubMed Abstract | CrossRef Full Text | Google Scholar
Escudier, B., Roigas, J., Gillessen, S., Harmenberg, U., Srinivas, S., Mulder, S. F., et al. (2009). Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J. Clin. Oncol. 27, 4068–4075. doi:10.1200/JCO.2008.20.5476
PubMed Abstract | CrossRef Full Text | Google Scholar
George, S., Reichardt, P., Lechner, T., Li, S., Cohen, D. P., and Demetri, G. D. (2012). Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Ann. Oncol. 23, 3180–3187. doi:10.1093/annonc/mds179
PubMed Abstract | CrossRef Full Text | Google Scholar
Grosse, J., Warnke, E., Wehland, M., Pietsch, J., Pohl, F., Wise, P., et al. (2014). Mechanisms of apoptosis in irradiated and sunitinib-treated follicular thyroid cancer cells. Apoptosis 19, 480–490. doi:10.1007/s10495-013-0937-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Hopkins, T. G., Marples, M., and Stark, D. (2008). Sunitinib in the management of gastrointestinal stromal tumours (GISTs). Target. Oncol. 34 (8), 844–850. doi:10.1016/j.ejso.2007.10.011
PubMed Abstract | CrossRef Full Text | Google Scholar
Ibrahim, E. M., Kazkaz, G. A., Abouelkhair, K. M., Bayer, A. M., and Elmasri, O. A. (2013). Sunitinib adverse events in metastatic renal cell carcinoma: a meta-analysis. Int. J. Clin. Oncol. 18, 1060–1069. doi:10.1007/s10147-012-0497-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Je, Y., Schutz, F. A., and Choueiri, T. K. (2009). Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet. Oncol. 10, 967–974. doi:10.1016/S1470-2045(09)70222-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Joensuu, H., Trent, J. C., and Reichardt, P. (2011). Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat. Rev. 37, 75–88. doi:10.1016/j.ctrv.2010.04.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Jonasch, E., Slack, R. S., Geynisman, D. M., Hasanov, E., Milowsky, M. I., Rathmell, W. K., et al. (2018). Phase II study of two weeks on, one week off sunitinib scheduling in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 36, 1588–1593. doi:10.1200/JCO.2017.77.1485
PubMed Abstract | CrossRef Full Text | Google Scholar
Kappers, M. H., van Esch, J. H., Sluiter, W., Sleijfer, S., Danser, A. H., and van den Meiracker, A. H. (2010). Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 56, 675–681. doi:10.1161/HYPERTENSIONAHA.109.149690
PubMed Abstract | CrossRef Full Text | Google Scholar
Kelly, W. N., Arellano, F. M., Barnes, J., Bergman, U., Edwards, R. I., Fernandez, A. M., et al. (2007). Guidelines for submitting adverse event reports for publication. Drug Saf. 30 (5), 367–373. doi:10.2165/00002018-200730050-00001
PubMed Abstract | CrossRef Full Text | Google Scholar
Lammert, C., Einarsson, S., Saha, C., Niklasson, A., Bjornsson, E., and Chalasani, N. (2008). Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology 47, 2003–2009. doi:10.1002/hep.22272
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, S. H., Bang, Y. J., Mainwaring, P., Ng, C., Chang, J. W., Kwong, P., et al. (2014). Sunitinib in metastatic renal cell carcinoma: an ethnic Asian subpopulation analysis for safety and efficacy. Asia Pac. J. Clin. Oncol. 10, 237–245. doi:10.1111/ajco.12163
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, J., Zhang, J., Zhang, Y., Qiu, H., Zhou, Y., Zhou, Y., et al. (2024). Efficacy and safety of ripretinib vs. sunitinib in patients with advanced gastrointestinal stromal tumor previously treated with imatinib: a phase 2, multicenter, randomized, open-label study in China. Eur. J. cancer (Oxford, Engl. 1990) 196, 113439. doi:10.1016/j.ejca.2023.113439
CrossRef Full Text | Google Scholar
Liu, M., Gu, L., Zhang, Y., Zhou, H., Wang, Y., and Xu, Z. X. (2024). A real-world disproportionality analysis of mesalazine data mining of the public version of FDA adverse event reporting system. Front. Pharmacol. 15, 1290975. doi:10.3389/fphar.2024.1290975
PubMed Abstract | CrossRef Full Text | Google Scholar
Mena, A. C., Pulido, E. G., and Guillén-Ponce, C. (2010). Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer. Drugs. 21 (Suppl. 1), S3–S11. doi:10.1097/01.cad.0000361534.44052.c5
PubMed Abstract | CrossRef Full Text | Google Scholar
Michel, C., Scosyrev, E., Petrin, M., and Schmouder, R. (2017). Can disproportionality analysis of post-marketing case reports be used for comparison of drug safety profiles? Clin. Drug Investig. 37 (5), 415–422. doi:10.1007/s40261-017-0503-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Moran, M., Nickens, D., Adcock, K., Bennetts, M., Desscan, A., Charnley, N., et al. (2019). Sunitinib for metastatic renal cell carcinoma: a systematic review and meta-analysis of real-world and clinical trials data. Target. Oncol. 14 (4), 405–416. doi:10.1007/s11523-019-00653-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Motzer, R. J., Hutson, T. E., Tomczak, P., Michaelson, M. D., Bukowski, R. M., Oudard, S., et al. (2009). Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27 (22), 3584–3590. doi:10.1200/JCO.2008.20.1293
PubMed Abstract | CrossRef Full Text | Google Scholar
Paech, F., Bouitbir, J., and Krähenbühl, S. (2017). Hepatocellular toxicity associated with tyrosine kinase inhibitors: mitochondrial damage and inhibition of glycolysis. Front. Pharmacol. 8, 367. doi:10.3389/fphar.2017.00367
PubMed Abstract | CrossRef Full Text | Google Scholar
Pal, S. K., Tangen, C., Thompson, I. M., Balzer-Haas, N., George, D. J., Heng, D. Y. C., et al. (2021). A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 397, 695–703. doi:10.1016/S0140-6736(21)00152-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Raschi, E., Fusaroli, M., Giunchi, V., Repaci, A., Pelusi, C., Mollica, V., et al. (2022). Adrenal insufficiency with anticancer tyrosine kinase inhibitors targeting vascular endothelial growth factor receptor: analysis of the FDA adverse event reporting system
留言 (0)