The sense of smell enriches our daily lives in subtle yet profound ways, such as the comforting scent of home-cooked food or the invigorating aroma of ocean air. This seemingly simple sensory input is actually a highly complex neurological process governed by specialized systems. Mammalian olfaction relies on three main systems (Slotnick and Weiler, 1990): the main olfactory system (Keverne, 1999), the accessory olfactory (vomeronasal) system, and the septal organ (Baum and Cherry, 2015). The main olfactory system is the sole functional olfactory mechanism in humans (Cherry and Baum, 2020) thus has been the primary research focus. As a pivotal structure of this system and initial site of synaptic processing olfactory signals, the olfactory bulb (OB) receives odorant signals from olfactory sensory neurons (OSNs) in the olfactory epithelium and transmits these signals to various downstream processing centers in the temporal lobe, a key part of the limbic system (Sobel et al., 1998; Krusemark et al., 2013; Hackländer and Bermeitinger, 2017). The OB anatomical and cellular organization has been summarized in multiple excellent reviews (Nagayama et al., 2014; Ennis et al., 2015; Burton, 2017). Briefly, this intricate structure comprises multiple layers: glomerular layer (GL) where OSNs synapse with mitral and tufted cells (MTCs) and interneurons; external plexiform layer (EPL) containing synapses among MTCs and local interneurons as well as granule cells; mitral cell layer (MCL) and internal plexiform layer (IPL) respectively housing mitral cell bodies and tufted cell axonal terminations; and the granule cell layer (GCL) that is populated by GABAergic granule cells and axon terminals from cortical and subcortical neurons (Imamura et al., 2020). While the OB neuronal organization and functions have been extensively studied, it is crucial not to overlook the glial cells, the unsung heroes: which have historically been considered as mere neural “support.” The recent research underscores their profound roles in optimizing functionality of and resilience as well as adaptability within the nervous system. Depending on the distribution region and species, glial cells make up ~33% to 66% of the total brain mass (Azevedo et al., 2009; Herculano-Houzel, 2014). Nevertheless, recent data even refute the erstwhile notion that the ratio of glia to neurons in a mouse brain is < 1:1 ratio (Von Bartheld et al., 2016), revealing a nearly balanced presence in humans. Among glial subtypes, astrocytes are the most abundant accounting for 20% to 40% of all glial cells and are instrumental in diverse functions like neuronal survival, ionic regulation, and synaptic modulation (Verkhratsky and Butt, 2013; Vasile et al., 2017). Microglia constitute ~10%−15% of brain cells, serving as primary immune defenders of the central nervous system (CNS) (Dos Santos et al., 2020). Oligodendrocytes (OLs), making up about 20% of brain cells, serve as axonal insulators and contribute to myelin sheath formation, axonal maintenance, and neuronal sustenance (Valério-Gomes et al., 2018). Olfactory ensheathing cells (OECs), as specialized macroglia exhibiting phagocytic and immunoprotective properties, assist in the regeneration and guidance of OSN axons (Huang et al., 2010). Lastly, radial glia cells (RGCs) as multifunctional progenitors are essential for neural development and tissue organization so that disruption of their functions potentially causes developmental disorders (Kriegstein and Alvarez-Buylla, 2009).
In this review, we comprehensively summarize the current knowledge on the multifaceted roles of glial cells in the main OB by focusing on their origination, migration, distribution, morphology, and molecular markers or signaling pathways. We also emphasize their pivotal functions in regulating synapse formation, function, plasticity, and elimination within the OB, as well as their roles in neurodegenerative diseases. By elucidating these specific aspects, we aim to delineate how glial cells contribute to the formation and function of a healthy nervous system, particularly their impact on neuronal development and activity. These insights could be instrumental in advancing our understanding of brain health, developing early intervention strategies to treat neurodegenerative diseases, paving the way for innovative therapeutic applications for neuroregeneration, and ultimately improving therapeutic outcomes in patients.
Origination, migration and distribution AstrocytesAstrocytes originate from neural progenitor cells and engage in a meticulously structured journey toward the OB during neural development (Zhan et al., 2017). This migration is facilitated through the protrusion of astrocytic extensions that cling to radial glial strands. These strands serve as a structural framework imperative for cellular motility (Ling et al., 1989). The linkage with radial fibers provides not merely guidance for astrocytic trajectory but also contributes to their differentiation process. As they mature, astrocytes assimilate critical characteristics needed to fulfill their duties in preserving cerebral functionality (Figure 1) (Bayraktar et al., 2015).
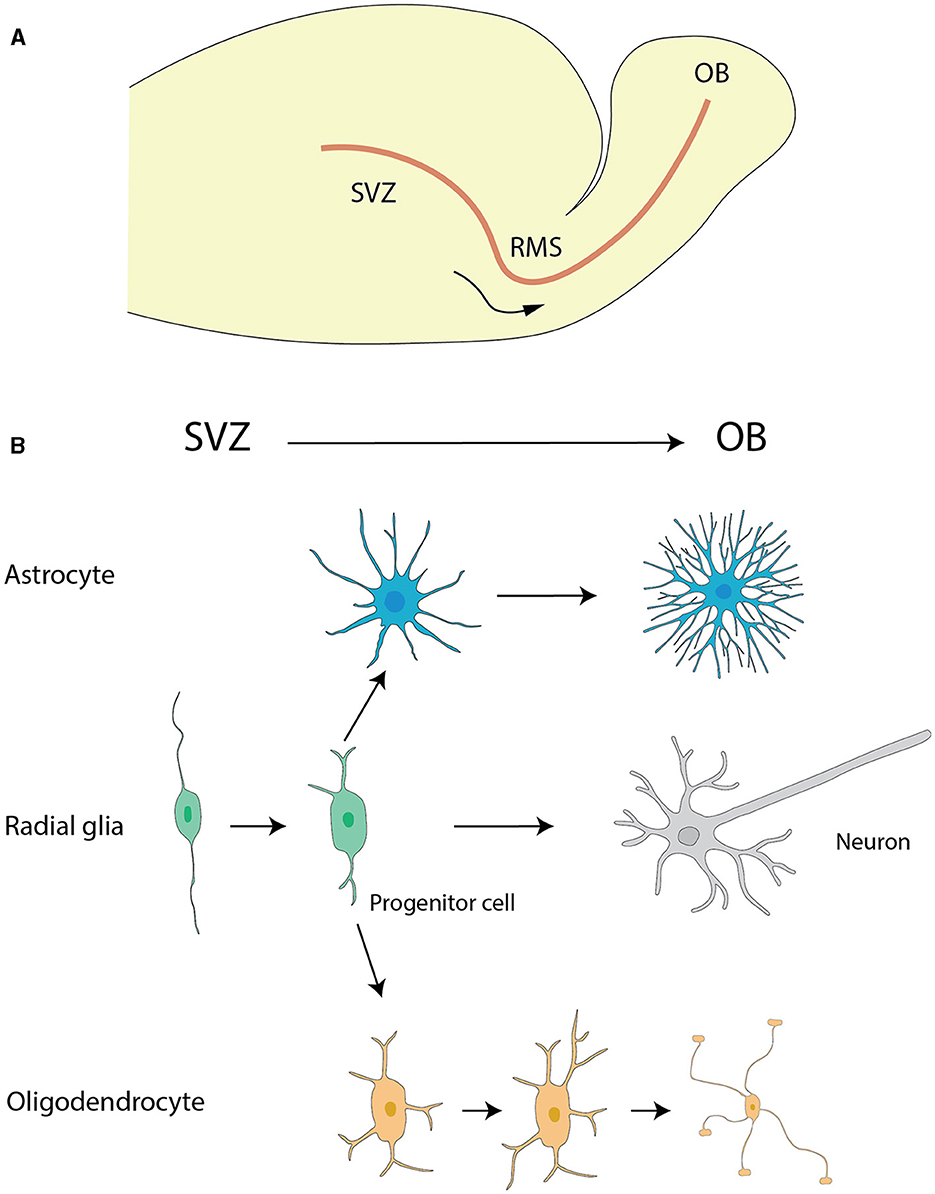
Figure 1. The migration of astrocytes, radial glia and oligodendrocytes. (A) The migration pathway of neural progenitor cells from the SVZ to the OB via the RMS. (B) Differentiation pathways of radial glial cells and the maturation of astrocytes and oligodendrocytes. Radial glial cells in the SVZ generate neural progenitor cells, which migrate to the OB via the RMS. During their migration, these progenitor cells differentiate into neurons, specifically granule cells and periglomerular cells, within the OB. Additionally, radial glial cells in the SVZ can differentiate into astrocytes and oligodendrocytes. Astrocytes and oligodendrocytes further mature in the SVZ and nearby regions, contributing to the overall functionality and support of the neural environment. The graphic drawings in this figure are based on data from Puche and Shipley (2001) and Belvindrah et al. (2011).
The rostral migratory stream (RMS) (Figure 1), frequently depicted as a “neuronal highway,” harbors a unique cohort of astrocytes. These varieties of astrocytes serve a pivotal role in orchestrating a combined physical and biochemical corridor that directs the immature neurons toward their terminal—the OB (Peretto et al., 1997; Alonso et al., 2008). This direction is promoted by the emission of chemo-attractive agents such as netrins, slit proteins, semaphorins, ephrins, chemokines, and vascular endothelial growth factor (VEGF) (Alvarez-Buylla and Lim, 2004; Thiriet, 2008; Cayre et al., 2009; Buffo et al., 2010), which act as navigational signals for the neuronal precursors charting their course to the OB.
Upon reaching the OB, astrocytes persist in delivering critical contributions. They are integral to preserving the framework's architectural cohesion and actively engage in the oversight of synaptic activity. Their influence extends through facilitating the integration and fine-tuning of synapses linking incoming neurons to pre-established neural circuits (Zhan et al., 2017). These processes through a range of mechanisms including their interactions with neurons, promote synapse development and maturation, modulate synaptic activity and gliotransmission, regulate ionic balance, and provide energy substrates for neurons (Takano et al., 2006).
The overall migration of astrocytes originating from the subventricular zone (SVZ) of the lateral ventricles to OB (Figure 1A) is a key event for paving the way for their diversified role in neurogenesis and synaptic process. This cell migration highlights the significance of studying astrocyte development and function, which has critical implications for therapeutic approaches targeting neurodevelopmental disorders.
In the OB, astrocytes are identified in all layers but have prominent presence in the glomerular layer (Bailey and Shipley, 1993; Roux et al., 2011; Klein et al., 2020). The glomerular astrocytes exhibit distinctive morphological and functional characteristics that distinguish them from counterparts in other cerebral locales. In layers deep to the glomerular layer, astrocytes take on heteromorphic features with noticeable variations. These celestial-shaped cells has been categorized into three identifiable clusters based on the characteristics of their cytoplasmic extensions emanating from the somas (Bailey and Shipley, 1993; Klein et al., 2020). It is postulated that such alignment divergences among cellular appendages indicate segregated functionalities linked to the OB framework: potentially ranging from spearheading axonal pathfinding during neurodevelopmental intervals to modulating synaptic interconnectivity amid neuronal assemblies (Bailey and Shipley, 1993; Zhang and Barres, 2010; Oberheim et al., 2012).
MicrogliaMicroglia, the resident immune cells constituting 5%−12% of all glial cells in the brain, were first identified by Río-Hortega, who intriguingly called them 'third elements' (Lawson et al., 1990). Río-Hortega described and interpreted the presence of microglia in his pioneer effort which detected their existence even at early stages of development. This led him to hypothesize that microglia could be of mesodermal origin found in the pia mater, an innermost layer of meninges lining the CNS (Río-Hortega, 1919; Rio-Hortega, 1932). Following his discovery, scientists went on to undertake considerable scientific studies concerning the exact lineage and derivation of microglial cells, a topic that has continued to generate constant debate among professionals (Rio-Hortega, 1932; Murabe and Sano, 1982). In the beginning, microglia were thought to be of hematopoietic nature due to their morphological and phagocytic similarities to peripheral monocytes, macrophages, and dendritic cells (DCs) (Rio-Hortega, 1939; Murabe and Sano, 1983). Pioneering studies have provided evidence suggesting that microglia derive from embryonic hematopoietic precursors, which begin to colonize in the CNS before the conceptus becomes a fetus (Hutchins et al., 1990; Alliot et al., 1991). This entry into the CNS officially precedes the onset of bone marrow hematopoiesis (Eglitis and Mezey, 1997; Simard and Rivest, 2004). Subsequent research has challenged long held views and instead proposes that microglia are embryonically derived from a cell lineage that gives rise to cells expressing key macrophage-microglial markers, such as CD11b (Mac-1), F4/80 and FcγRIII (Fc-R) (Alliot et al., 1991; Morris et al., 1991). Among others, the origin of this lineage is the early, uncommitted erythromyeloid progenitor (EMP), i.e., the yolk sac (YS) macrophage (Alliot et al., 1999; Ginhoux and Prinz, 2015; Ferrero et al., 2018; Utz et al., 2020). Primitive macrophages originating from the yolk sac (YS) invade the embryo proper by circulating through the cardiovascular campaign and growing through mesenchyme of the brain. Pial surfaces and the developing fourth ventricle are the gateway across, allowing this immigration to the site in the brain rudiment (Cho et al., 2013). Once these original agents of the immune system have breached the barrier, they divide prolifically over a subsequent period of days and maintain themselves in the CNS for the life of the adult. Alternatively, it has been proposed that circulating monocytes are not the only path by which the microglia make their entrance. Instead, sub-ependymal and/or pericyte-based precursors have been joined to the venous system at the end of the lateral ventricles in the brain and even to the vasculature outside of the blood-brain barrier (BBB) (Lewis, 1968; Mori and Leblond, 1969; Barón and Gallego, 1972; Lawson et al., 1992).
Microglia migration to the OB is a characterized multi-step process involving appropriate signaling molecules and cell interactions. Although the modalities are unexplored, many factors have emerged as determinants. The currently most consensual hypothesis is the migration of microglia from the SVZ to the OB through the RMS (Figure 1A), which extend from lateral ventricle and cavum septum pellucidem (Caggiano and Brunjes, 1993; Xavier et al., 2015). Once entering the bulb, microglia go to the deep laminae in particular the GCL close to subependymal area. An alternative hypothesis involves CX3CL1, a critical chemokine that is concurrently proposed to contribute significantly toward the microglial migration to the OB (Ruitenberg et al., 2008). CX3CL1 activates microglia via CX3CR1 receptors, a process leading to subsequent cytoskeletal/membrane changes (Nishiyori et al., 1998; Maciejewski-Lenoir et al., 1999). This is vital for microglial migration to the OB and helps in preserving the brain homeostasis and functions. Additionally, another mechanism has been proposed based on more enthralling discoveries transcending a thorough insight of cells which make up the bone marrow. It should be noted that this analysis entailed the methodical transplant of stem cells expressing green fluorescent protein from the bone marrow to fatally irradiated mice. Astonishingly, these transplanted cells showed an impressive ability to traverse the BBB and invade the brain parenchyma with remarkable accumulations in various regions, including the OB (Simard and Rivest, 2004).
OLsThe origin and migration of OLs in the OB is a vibrant mosaic of cellular development and intricately interwoven with diverse progenitor cell populations and differentiation cues. OLs in both the OB and subcortical white matter (SCWM) are primarily generated from distinct NG2 progenitor subpopulations residing in the anterior subventricular zone (aSVZ). These include NG2/Er81/Dlx/DC and NG2/Nkx2.2 progenitors, which contribute extensively to neurons and OLs (Aguirre and Gallo, 2004). The OB also harbors a specialized population of oligodendrocyte progenitor cells (OPCs) derived from Nkx2.1-positive progenitors in the medial ganglionic eminence (MGE) and anterior entopeduncular area (AEP) (Kessaris et al., 2006). This adds an additional level of complexity to the source of OB OLs.
Apart from these progenitors, OLs in the OB also originate from proteolipid protein (Plp)-expressing ventricular progenitors within the rostral pallium, forming an independent lineage unaffected by platelet-derived growth factor receptors (PDGFRs) signaling. Intriguingly, the generation of these cells relies on sonic hedgehog signaling akin to their spinal counterparts, but with a delayed differentiation of 4–5 days in the telencephalon, indicating an inherent oligodendrogenic capacity in the OB influenced by heterotopic heterochronies during transplantation (Spassky et al., 2001).
Stem cell-like multipotential precursors in the SVZ and its rostral extension (RE), including its distal portion in the OB, are crucial for the generation of OB OLs. These cells differ from migratory neuroblasts and exhibit diverse functional properties. For instance, cells from the proximal RE more efficiently give rise to OLs, while cells from the distal RE proliferate more slowly, suggesting a stratification of stem cell populations along the SVZ-RE axis (Gritti et al., 2002). In the adult brain, the ventricular-SVZ (V-SVZ), which is rich in cells expressing epidermal growth factor receptor (EGFR) and fibroblast growth factor receptor (FGFR), serves as an indispensable neurogenic incubator (Galvez-Contreras et al., 2016). This area hosts neural stem cells that continually generate neuroblasts and OL progenitor cells (Figure 1) (Menn et al., 2006).
At a fundamental level, the conversion of human OB neural stem cells (OBNSCs) to OPCs and ultimately into OLs is a process centered around differentiation. This involves the upregulation of OPC/OL markers (CNPase, Galc, NG2, MOG, OLIG1, OLIG2, and MBP) and a simultaneous decline in pluripotency markers (Oct4, Sox2, and Nestin) (Marei et al., 2018). Factors like PDGFs, basic fibroblast growth factor (bFGF), and hepatocyte growth factor guide OPC migration, promoting motility and maintaining bipolar migration along the PDGF gradient. In light of these complexities, elucidating the precise mechanisms dictating OL origination and migration within the OB remains a captivating research frontier.
OECsThe nomenclature of OECs has evolved with the knowledge advancement in neurosciences. In the late 1800s to early 1900s, these cells were referred to as “olfactory Schwann cells.” By the mid-1900s, this designation had changed to “olfactory nerve ensheathing cells” (ONECs). From there, terminology progressed to “olfactory ensheathing glia” (OEG) and olfactory Schwann cells in the late 1900s to 1990s (Barber and Lindsay, 1982; Wozniak, 1993; Franceschini and Barnett, 1996). Today, the most common term is OECs, a name fitting more of the cells' glial character and ensheathing capacity on the olfactory nerve fibers. Indeed, the progression of nomenclature reflected the proliferation of knowledge regarding these remarkable cells in our neural systems.
OECs originate from a source distinct from other glial cells and emerge either from neural crest (PNS glia) or the neural tube (CNS glia). OECs are delivered to OB by the olfactory placode, a transient tissue that forms the olfactory epithelium and olfactory nerves as the nasal cavity is sculpted during early embryogenesis. OECs travel with these olfactory nerves as they stream toward the OB and ultimately settle in the inner two layers of the nascent structure. During this developmental period, the wave of cells, including OEC progenitors, decants from the base of the invaginating olfactory placode. These cells migrate to invest the telencephalic vesicles and frequently move in intimate apposition to an advancing olfactory nerve. They then participate in the assembly of OB at their destination, another distinction between OECs and other glia (Mendoza et al., 1982; Doucette, 1990).
Proper development, maturation and neuronal regeneration of the olfactory system depend on directional migration of OECs. During development, OEC progenitor cells and developing olfactory sensory axons are generated from the olfactory placode and migrate toward the telencephalic vesicle. The primitive OB does not grow in size once the regions of the telencephalon vesicle have been invaded (called evagination) by the growing nerve fibers from the olfactory lobe; these growing nerve fibers then begin to stripe its outer surface. Collectively, these events eventually lead to the formation of a developing OB, which is a crucial structure for the proper functioning of the olfactory system (Mendoza et al., 1982; Farbman and Squinto, 1985; Doucette, 1989). OEC migration is regulated by different factors like calcium channels, GDNF/GFRa-1/Ret pathway, glycoprotein fibulin-3, and the lysophosphatidic acid (Liu et al., 1995; Buckland and Cunningham, 1998; Yan et al., 2003; Windus et al., 2007, 2010; Vukovic et al., 2009). Another key role in influencing OEC migration in addition to dynamic membrane protrusions is lamellipodial waves. These waves on the cells really help them move, because they enable dynamic modulation of membrane protrusions (Lohr et al., 2005, 2007). The cells can easily crawl by utilizing this type of wave on the actin rich membrane. These findings have important implications for our understanding how OECs are able to stimulate the regeneration of nerves in the affected areas and will provide insights into the development and repair of the olfactory system, a unique brain structure continuing to develop new nerve cells throughout an animal's life. Additionally, they may give us some clues on how we might apply them to neural repair in spinal cord injuries, or other types of traumas or diseases involving the nervous system.
OECs are predominantly distributed in the olfactory nerve layer (ONL), the most outer layer of OB. This layer signifies the point of entry for axons from the OSNs into the bulb. OECs are found interspersed among these axonal bundles within the ONL acting as a sheath to direct their growth into the bulb. Consistently, the distribution of these cells across this layer corresponds with the course of the entering OSN axons (Doucette, 1984, 1990; Au et al., 2002).
Some OECs may be seen abutting onto the adjacent GL comprising a smaller population. This would place these cells at the ONL/GL interface. However, this is rare and at other times the GL is not considered a key location for OECs (Au and Roskams, 2003). Additionally, OECs are usually not found in all other OB layers.
RGCsAs classified neural stem cells (NSCs), RGCs extend uniformly from the ventricle to the pia in most developing cortical structures. In addition to their major contribution to the formation of the cerebral cortex by acting as progenitor of neurons and neuroglia, RGCs play a role in neuronal migration. They are of particular interest in the context of the RMS (Figure 1). The transformation of RGCs into astrocytes deeply affects the migration of neuroblasts from the SVZ to the OB (Merkle et al., 2004) (Figure 1).
In the developing OB, RGCs have processes reaching most of OB layers radially from the GCL to GL (Puche and Shipley, 2001). Interestingly, a group of RGC-like cells were identified in the adult OB with morphological and immunochemical similarities to radial glia present in the OB during embryonic and early postnatal development. They have large circular somata located in the GCL outside the outer border of the RMS/SVZ of the OB with processes radially extending through the GCL toward and into the MCL (Emsley et al., 2012). The recent concept of the RGCs in the OB is expected to clarify the basic mechanism of the neural development and function.
Morphology AstrocytesThe generally star-shaped astrocytes, exhibit notable morphological diversity dependent on their location within the nervous system. In the rat OB, astrocytes were classified into six distinct categories according to their precise locations and their morphological features. Two types of astrocytes found in the glomerular stratum exhibited differentiated shapes, one of which assumed a radial configuration while the other adopted an elongated shape, with extensions pervading multiple or singularly one or two glomeruli respectively. The remaining types of astrocytes were located sequentially in deeper layers EPL, IPL, and GCL, each presenting singular morphologies concomitant with their projected cellular processes (Bailey and Shipley, 1993) (Figure 2).
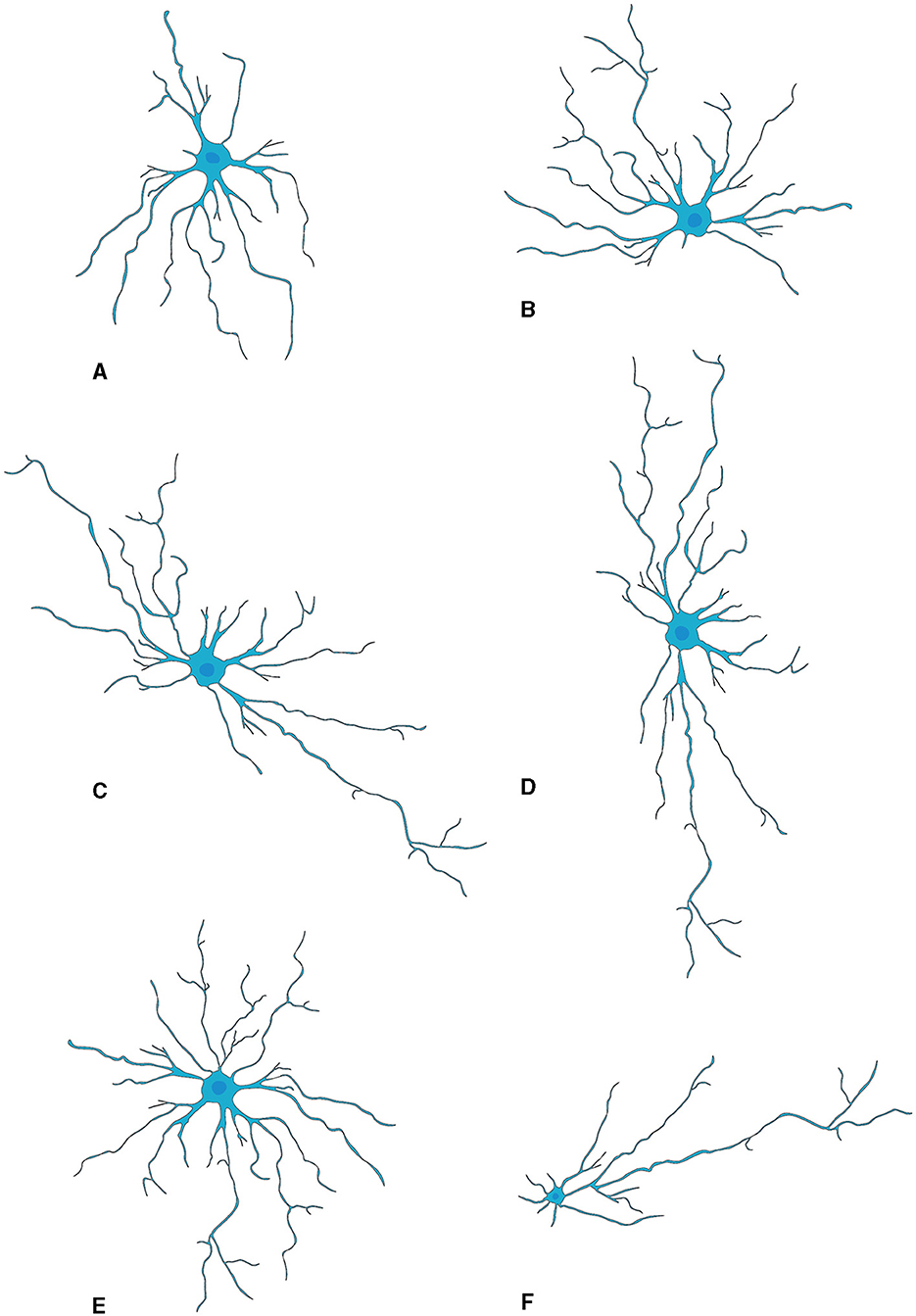
Figure 2. Morphological subtypes of astrocytes in OB. (A) Wedge-shape astrocyte. (B) Semicircular astrocyte. (C) Irregular astrocyte. (D) Elongate astrocyte. (E) Circular astrocyte. (F) Unipolar astrocyte. This figure is conferred from Bailey and Shipley (1993).
Recently, Marcel Klein's study delineated three categorically distinct clusters of OB astrocytes (Klein et al., 2020). In the inaugural category, “stellate” astrocytes are typified by a diminutive soma with emerging profuse, finely divided protrusions that intricately wrap around the glomeruli. The next group encompasses “periglomerular” astrocytes, characterized by an ample soma spawning scarcer yet elongated projections that weave into a tight network extending tangentially to the glomerular boundary. The third type involves “protoplasmic” astrocytes which have a formidable soma core flanked by sparser branching tendrils that radiate broadly in assorted directions, integrating into the dense network characteristic of their counterparts. Intriguingly, varied alterations were observed within these defined groups during postnatal neurogenesis: augmentations in length and complexity were observable within both stellate and periglomerular varieties; conversely, protoplasmic forms evidenced negligible modification in morphology.
Moreover, the structural configuration of astrocytes within the OB is subject to modulation as a function of the animal's senescence (Klein et al., 2020). Employing Sholl analytic techniques, the diversity of these glial cells has been discerned in relation to temporal progression (Tavares et al., 2017). A notable transformation, i.e., cellular extensions transition from cephalopod-like formations to more radiating and star-shaped arrangements, is primarily ascribed to alterations within vimentin-positive astrocytic projections. Such findings indicate the presence of persistent structural adaptability in OB resident astrocytes throughout an animal's life course, with potential repercussions on neuronal networks and olfactory function during advanced chronological stages. Nevertheless, elucidation of the underpinning processes precipitating these morphogenic variations and their functional implications necessitates exhaustive exploration.
Appreciating the morphological diversity of astrocytes and their distinct responses to postnatal neurogenesis is critical to understanding their contribution to olfactory processing and other CNS functions. Consequently, continued research in this domain is necessary.
MicrogliaMicroglia are highly abundant in the OB with an average cell density of 127 cells/mm2 across all layers, surpassing the density observed in the cortex or neostriatum (Lawson et al., 1990). Two morphological variants of microglia are observed in the OB (Figure 3): a larger, extensively branching, ramified type distributed throughout all layers, and a round-shaped, virtually process-free, amoeboid type principally present in the subependymal zone (Caggiano and Brunjes, 1993). They may represent two different states because microglia with branching have traditionally been considered “resting” while the spherical ones without processes are believed to be “activated” (Rio-Hortega, 1932; Streit et al., 1988, 1999; Stence et al., 2001; Nelson et al., 2002; Rezaie et al., 2002). Researchers used staining techniques involving B4 isolectin derived from Griffonia simplicifolia to gain insights into the development of microglia, in the rat OB (Caggiano and Brunjes, 1993). The glomerular layer contained considerable number of ramified cells having cell bodies and processes confined strictly within the boundaries of glomeruli. A fair quantity of amoeboid cells is present in both the olfactory nerve layer and the periglomerular zone (Wu et al., 1997). The ramified microglia with much arborization are scattered throughout the EPL. Also, microglial cell bodies were almost exclusively found in the inner and peripheral layers; microglial processes reach out among the mitral cells. The GCL contains numerous microglial cells, the morphologies of which range from almost ameboid to wholly ramified (Kaplan et al., 1985).
OLsAs the major myelin-forming glial cells, the OB OLs display differential spatial organization and morphological heterogeneity, thus reflecting their most important roles in modulating neuronal activities, synaptic transmission and axonal conductivity. Two types of OLs, dark- and medium-shaded, can be distinguished by their size and staining characteristics; dark OLs are smaller and constitute approximately 20% of the neuroglial cell population surrounding the glomeruli (Valverde and Lopez-Mascaraque, 1991). Within the GL, they form myelin-like coverings around dendrites and cell bodies within the olfactory glomeruli, potentially modulating synaptic transmission (Zhang et al., 2021) (Figure 4). Such particular distribution highlights the functional importance of OB OLs to rapid processing and conduction of olfactory information.
OECsAlthough they are almost exclusively associated with axons in the nerve fiber layer of the rat OB, OECs exhibit morphological characteristics and plasticity. For example, two morphological subtypes of OECs have been recognized as discrete, characterized mainly by the shape of their structural forms (Figure 5). One subtype has Schwann-like features with processes. In contrast, the other subtype represents the flattened form of astrocytes (Doucette, 1984). Nevertheless, these are not strict classifications but rather points of reference in the continuum of OECs morphological adaptations (Huang et al., 2008).
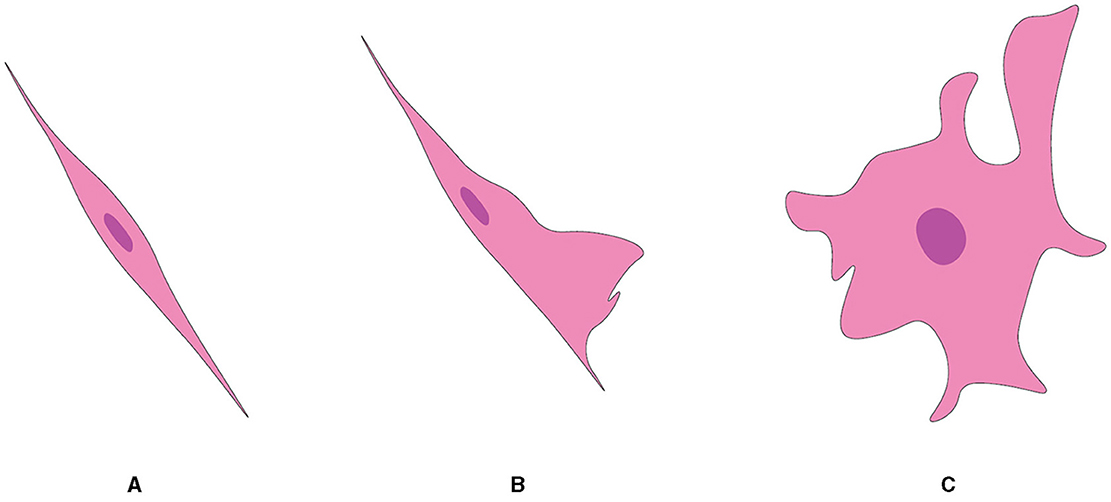
Figure 5. Two subtypes of OEC from OB, referred from Huang et al. (2008). (A) Schwann cell-like OEC. (B) Type 1 astrocyte-like OEC. (C) Type 2 astrocyte-like OEC.
What distinguishes OECs from other cell types is at the ultrastructural level, which consists of a minimal cytoplasm with a notably electron-dense nucleus. These unique cells typically have a somewhat lobulated nucleus, with the majority of chromatin distributed irregularly around the nuclear envelope. OECs generally contain one or two nucleoli. The electron density of the cytoplasm in an OEC is obviously greater than that in surrounding astrocytes, which do not feature freely scattered intermediate filaments. In terms of overall cellular appearance and as demonstrated best by cells ensheathing olfactory axons in fibers of the nerve layer of the rat OB, OECs look astrocyte-like. However, what renders them unique is that they are able to maintain an embryonic state (Barber and Lindsay, 1982; Doucette, 1984, 1990, 1991; Valverde and Lopez-Mascaraque, 1991).
During their developmental journey, OECs transform in a way that enables some to elongate and extend layered projections. This helps demonstrate the next step in their transformation. At the point where these cells are now mature OECs, they will be seen to surround bundles of small axons. The precursor cells, which are distinguished only as round cells, will eventually turn into mature OECs in the final frame (Valverde et al., 1992).
The ultrastructure of OECs is consistent both in vivo and in vitro. Mature OECs are attached to the basal lamina in certain areas where they contribute to form external glia limiting membrane within the OB even though they do not have a basal lamina within their plasma membranes, unlike other PNS to CNS transitional zones (Barber and Lindsay, 1982; Doucette, 1984, 1990, 1991). However, they do envelop blood vessels with their processes in a fashion resembling astrocytes.
The essential feature of OECs is their inherent plasticity, which is reflected in their ability to undergo rapid and reversible morphological changes. This dynamic feature provides smooth changes between the two referred subtypes (Li et al., 2018). Thus, the existence of morphological subtypes in OECs is considered as the evidence of their plasticity and versatility. Such plastic alterations are not only spontaneous or arbitrary but also adaptive responses to environmental stimuli, implying a great amount of OEC interaction with the environment. Although this type of flexibility in response to outside factors is a characteristic of these glial cells' dynamic nature and their potential use in neuroregenerative studies, we can uncover other functional aspects of OECs in the olfactory system and elsewhere by investigating their subtypes and dynamic transitions.
RGCsRGCs within the OB display an intricate and multifaceted trajectory that contrasts markedly with the uniform extension of their counterparts in other cortical structures (Puche and Shipley, 2001). Specifically, they are characterized by extensive branching and projection patterns that span multiple regions throughout the bulb (Figure 1). Immunohistochemical staining using the RGC cell marker 2 (RC2) antibodies provides an enlightening depiction of the exceedingly branched and elaborate RGCs network in the OB (Hajós and Gallatz, 1987; Anton et al., 1997; Emsley et al., 2012). The stained processes clearly demonstrate RGCs's extensively branched tree-like structure as well as the complex anastomosis formed between RGCs processes. However, the dense packing of the RC2-immunostained processes makes it difficult to unravel the individual branching patterns of these cells, revealing a shortfall in the reliance on immunohistochemistry as the sole investigative method.
To meet this challenge, Puche and Shipley (2001) used the lipophilic membrane dye DiI to label individual cells throughout development. This approach led to two important new revelations. First, individual RGCs can extensively branch within the bulb and able to extend their projections beyond a single glomerulus. Second, while glial glomeruli have prominent features of RGCs during development, stacking of RGCs processes within the glomerular layer appeared to be less prevalent than in other bulb layers. These observations demonstrate that an elaborate and diverse structural configuration of OB RGCs. The complexity of their morphology suggests a significant role in the neural development and functionality within this brain region, underscoring the need for further investigation into their intricate design and operation.
Delving further into the detailed structural morphology, it is important to note that RGCs manifest in two distinct categories, which bear distinctive characteristics and may serve specific functional roles in the development and organization of the OB. One category known as type I RGCs is uniquely defined by a single apical process traveling from the olfactory ventricle to the OB glomerular layer and eventually forming a specialized structure termed “glial glomerulus.” The precise structure of this tuft strongly correlates with the OB glomeruli as well as the burgeoning axons of OSNs. This intricate relationship leads to the speculation that type I RGCs are instrumental in the creation and/or fortification of mammalian OB glomeruli (Puche and Shipley, 2001). In contrast, the second category termed type II RGCs have multiple apical processes that, instead of reaching the glomerular layer, ramify more deeply within the bulb in the EPL. This distinctive feature suggests that type II RGCs play a crucial role in structuring the architecture lamination of the OB. This role may encompass providing a scaffold for the migratory patterns of primary projection neurons, including mitral and tufted cells.
Molecular markers and signaling AstrocytesAstrocytes are distinguishable from analogous cell types by virtue of specific proteins that they express. They are well known to produce glial fibrillary acidic protein (GFAP), a type of intermediate filament protein essential for cytoarchitectural maintenance and cellular response modulation upon harmful stimuli, making it a reliable astrocytic indicator (Barber and Lindsay, 1982; Doucette, 1984; Bailey and Shipley, 1993). Furthermore, the presence of S100 Calcium-Binding Protein B (S100β) within the cytoplasm acts as another definitive signature of astrocytes. This protein orchestrates diverse intracellular functions and stands as an indicative marker in contexts such as neurotrauma and neuroinflammation (Pixley, 1992; Henriksson and Tjälve, 2000; Haratizadeh et al., 2023). The employment of GFAP staining is predominantly directed toward discerning the nuanced morphological attributes of astrocytes; whereas S100 immunostaining—regularly appearing in tandem with GFAP signals—distinctly delineates the central region or soma of these glial cells.
Aldehyde dehydrogenase 1 family member L1 (ALDH1L1), a protein vital to the body's folate metabolism, serves as another reliable indicator for astrocyte identification (Otsu et al., 2015; Ung et al., 2020, 2021). Furthermore, the protein GLT-1 commonly accompanying ALDH1L1 and facilitating glutamate neurotransmission, is also considered indicative of astrocytic presence. Within the context of the OB, astrocytes often manifest connexin 30—a protein integral to cellular communication through gap junctions, rendering it an efficacious marker for their locative identification in this region (Otsu et al., 2015) even though some juxtaglomerular neurons also express this gap junction protein.
Immunoreactivity of aquaporin-4 (AQP4), a water channel protein vital for maintaining water homeostasis in the CNS (Saadoun and Papadopoulos, 2010) is present in the astrocytic perivascular end-feet around blood vessels in all OB layers. But its intense expression is in astrocytic processes and end-feet surrounding capillaries in the glomerular layer, indicating maintaining water homeostasis is an essential process within the OB for odor signal transduction (Saadoun and Papadopoulos, 2010) (Table 1).
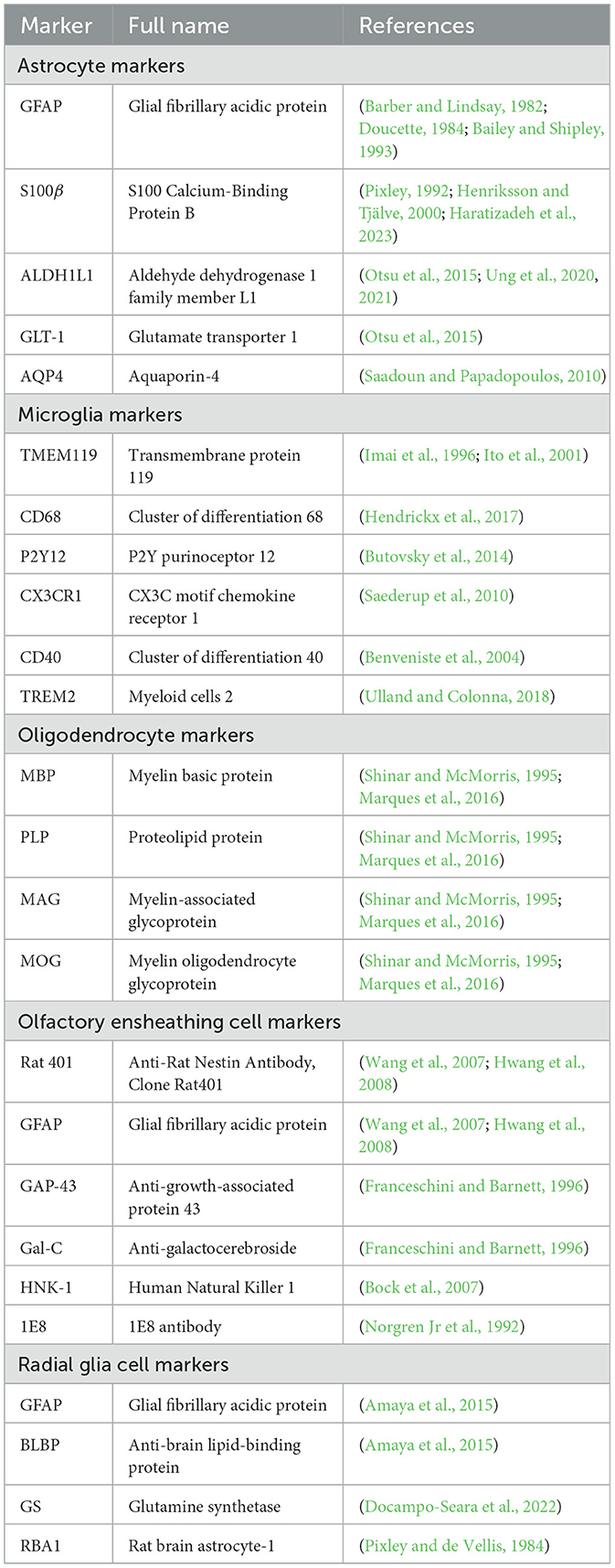
Table 1. Overview of glia cells maker proteins.
Identification of molecular biomarkers for astrocytes provide essential insights into their functionality, reactivity, and adaptability within the OB. These cell membrane-embedded molecules could serve not only as specific markers for determining astrocytic functions at the behavioral or physiological levels by selective manipulation or measures of astrocyte activities with chemogenetic approaches or calcium imaging but also as targets for modulation in diseases affecting the olfactory system. Furthermore, considering the diversity and complexity of astrocyte functions, future research should aim to uncover the precise mechanisms through which these cells influence OB functional dynamics and how these mechanisms may be affected or modulated in pathological conditions.
MicrogliaMicroglia have a wide array of molecular footprints, which help us understand the complexity of their functions and interplay of these cells within the neural environment. Iba1, a calcium binding protein closely associated with the activation of microglia and macrophages along with the transmembrane protein 119 (TMEM119) (Imai et al., 1996; Ito et al., 2001), acts as a marker to differentiate between resident microglia and infiltrating macrophages (Bennett et al., 2016). Cluster of differentiation 68 (CD68) (Hendrickx et al., 2017), a glycoprotein extremely upregulated in activated microglia, is used as an indicative marker for the initiation of neuroinflammatory conditions, whilst P2Y12, a purinergic receptor exclusively expressed by surveillant or “resting” microglia, illustrates vital insights to the microglial nature under quiescence (Butovsky et al., 2014). The receptors for fractalkine (CX3CR1) and antigen presenting molecule (CD40) expressed on microglia not only further enhance the complexity of this molecular architecture (Benveniste et al., 2004; Saederup et al., 2010) but also, due to their responses to the ligands on neurons, greatly complicate the communication between the two as well as the immune response potentiation (Ponomarev et al., 2006). Additionally, the triggering receptor expressed on myeloid cells 2 (TREM2) is a modulator of microglial activity and linked to neurodegenerative disorders, particularly AD (Ulland and Colonna, 2018) (Table 1). Collectively, these molecular markers completely underline certain roles microglia play in the CNS and initiate a huge amount of functional immune responses. A greater understanding of these biologic processes will assist in developing therapeutic strategies that address neurodegenerative and neuroinflammatory disorders.
OLsAn OL is identified by its expression of several key proteins that are essential for maintaining the integrity of compacted myelin sheaths. Myelin basic protein (MBP) comprises a family of proteins with various isoforms that play a crucial role in maintaining the structural integrity of the myelin sheaths. Proteolipid protein (PLP), the major component of myelin, is acylated and contributes to the stability of the myelin membrane. Myelin-associated glycoprotein (MAG) and myelin oligodendrocyte glycoprotein (MOG) are glycoproteins found in myelin, with MOG often being a target for autoimmune responses in demyelinating diseases. Collectively, these proteins maintain the integrity of compacted myelin sheaths (Shinar and McMorris, 1995; Marques et al., 2016) (Table 1).
As crucial components of the CNS, OLs are key to neuron survival and functionality through the secretion of a variety of molecules. Their secretory spectrum includes growth factors such as brain-derived neurotrophic factor (BDNF), which supports neuron survival and differentiation; cytokines including interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and transforming growth factor-beta (TGF-β), which play crucial roles in cell signaling; and structural proteins like laminin and fibronectin, contributing to the structural integrity of the nervous system (Muñoz-Fernández and Fresno, 1998). Additionally, OLs release glutathione, an antioxidant that helps protect cells from oxidative stress, the neurotransmitter glutamate essential for neuronal communication, adenosine triphosphate (ATP) as an energy source for cellular processes, and Nogo-A, a protein known for its role as a neurite outgrowth inhibitor, influencing the growth and connectivity of neurons (Anjum et al., 2020). Their functionality is heavily dependent on numerous external factors. The proliferation of OL precursor cells (OPCs) is driven by astrocyte-derived growth factors, with platelet-derived growth factor (PDGF) acting as a potent mitogen and stimulating their proliferation, while fibroblast growth factors (FGFs), a family of signaling proteins with diverse roles in development, tissue repair, and angiogenesis, particularly FGF2, significantly promote the proliferation of neonatal oligodendrocyte progenitors and influence oligodendrocyte development and myelination (Hinks and Franklin, 1999). Insulin-like growth factor-1 (IGF-1) is a critical facilitator of these cells' maturation and the remyelination process. Their survival, proliferation, differentiation, and myelinating activity depend on a host of factors including: neuregulins, ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), thyroid hormones, and neurotrophins (Domingues et al., 2016). During CNS development, PDGF, Netrin-1, and Shh are representative of long-range extracellular signals that guide OPCs' extensive migratory sojourns (Spassky et al., 2001; Zhang et al., 2004; Bin et al., 2013). Their motility and resultant migratory speed and direction are influenced by neurotransmitters, chemokines, and extracellular matrix proteins. With respect to OPC positioning within the CNS, their gestural interactions with neurons, astrocytes, and extracellular matrix proteins are pivotal. Notably, transcription factors Olig-1 and Olig-2 have pivotal roles in this context. Olig-2 is critical for OPC specification and proliferation, but Olig-1′s presence is required for the transition from OPC to matured OLs (Takebayashi et al., 2000). Dysregulation or mutations in these factors can lead to various neurological disorders, including multiple sclerosis and leukodystrophies, characterized by myelin formation and maintenance defects. CARNS1, a protein participating in the synthesis of histidine-containing dipeptides (HCDs), is identified in OLs of human white matter. Double-labeling of CARNS1 and OLIG2, an OL lineage marker, confirms the presence of CARNS1 within these cells (Van der Stede et al., 2023). OLs secrete extracellular vesicles (EVs) containing molecules such as unprocessed proteolipid protein (PLP) and DM20, two abundant myelin proteins produced through alternative splicing from the PLP gene (Krämer-Albers et al., 2007). Additionally, these EVs can influence neurons by supporting their metabolism and improving their viability under stress conditions, including oxidative stress, starvation, and oxygen and glucose deprivation (OGD) (Frühbeis et al., 2013; Fröhlich et al., 2014).
OECsOECs in embryos, neonates, and adults contain nestin (Rat 401) and GFAP (Wang et al., 2007; Hwang et al., 2008). The recent development of two monoclonal antibodies that label the OEC cytoskeleton suggests that they may target a protein within the OEC cytoskeleton that has not yet been identified. Researchers have used antibodies such as anti-growth-associated protein 43 (GAP-43), anti-galactocerebroside (Gal-C), HNK-1 (Human Natural Killer), and 1E8 to study OEC properties (Verhaagen et al., 1989; Franceschini and Barnett, 1996; Bock et al., 2007). The 1E8 antibody, which detects a subset of migratory neural crest cells, Schwann cells, and OECs, has been utilized to further characterize OECs (Norgren Jr et al., 1992) (Table 1). Despite the continuously growing list of membrane proteins known in OECs, identification of new specific markers and molecules expressed in OECs during various developmental stages will help us depict their unique contribution to the neural development of the olfactory system.
Throughout the growth of OECs, there is considerable regulation in expression of the low-affinity nerve growth factor receptor (L-NGFR). Studies have found that axotomy boosts L-NGFR expression in adult olfactory nerves (Barnett et al., 1993, 2000; Turner and Perez-Polo, 1993; Gong et al., 1994; Franceschini and Barnett, 1996; Barnett and Roskams, 2008). OECs consistently express membrane molecules, such as neural cell adhesion molecule L1, laminin, polysialylated neuronal cell adhesion molecule (PSA-N-CAM), and adult neural cell adhesion molecule (N-CAM), which are implicated in cell adhesion and axonal growth across all developmental stages (Miragall et al., 1988; Au et al., 2002; Ramer et al., 2004; Kumar et al., 2005; Planagumà et al., 2011; Witheford et al., 2013; Lazzari et al., 2014; Li et al., 2018). Among them, N-CAM and L1 are specifically localized in the axon-associated segment of the glial membrane, while PSA-N-CAM is only detectable in OECs that constitute the glia limitans, particularly in the membrane region without basal lamina contact (Miragall et al., 1988). Type IV collagen and fibronectin are associated with the OEC membrane region that interacts with the basal lamina (Au and Roskams, 2003; Lakatos et al., 2003; Teng et al., 2008). In developing and maturing OECs, vimentin is the main component of intermediate filaments (Oprych et al., 2017; Pellitteri et al., 2017).
RGCsRGCs in the OB display particular glial markers, primarily GFAP, anti-brain lipid-binding protein (BLBP), and glutamine synthetase (GS) (Amaya et al., 2015; Docampo-Seara et al., 2022). These markers have been observed in cells with a radial morphology lining the OB ventricle during embryonic development, and their expression continues into the early juvenile stage, notably in ependymal cells or tanycytes.
The GFAP marker which is most commonly employed for the identification of astrocytes, is expressed by RGCs in the developing brain including the OB (Ventura and Goldman, 2007; Zhu et al., 2018). The presence of GFAP in RGCs assists in discriminating and characterizing them. This marker is typified from cells with a radial layout lining the OB ventricle throughout embryonic development and broadens its expression into tanycytes during early juvenile stages.
Similarly, the marker BLBP, known to be specific to RGCs, is also expressed in the RGCs of the OB's ventricular zone. BLBP expression supports the identification and further analysis of these cells (Theofilas et al., 2017). GS, an enzyme integral to glutamate metabolism, is another marker expressed by RGCs in the OB (Guerrero-Cázares et al., 2011). The GS expression in RGCs contributes to the identification and further characterization of these cells. Echoing the pattern observed with GFAP, both BLBP and GS are expressed in radially oriented cells in the OB ventricle during embryonic stages, this expression is carried forward into tanycytes in the early juvenile stage.
Furthermore, the intermediate filament protein Vimentin is remarkably expressed in RGCs (Pixley et al., 1984). Evidence of this expression is provided by the immunoreactivity of cells labeled with the rat brain astrocyte-1 (RBA1) antibodies, which reportedly identify vimentin (Table 1). The distinct patterns of RBA1-positive cells, including RGCs, spark intriguing theories and questions about the function of vimentin in astrocytes and its possible regulation during recovery from injury or during developmental processes (Pixley and de Vellis, 1984).
Upon differentiation into mature astrocytes, RGCs display a fascinating protein expression dynamic. Typically occurring during the second and third postnatal weeks, it is characterized by a large decline in vimentin expression. Simultaneously, an increase in the intermediate filament protein, GFAP, is noted. Thus, RGCs display a way of altering the appearance of vimentin while they transition into mature astrocytes. This shift emphasizes that proteins in cellular development interact in complicated ways.
Functions AstrocytesAstrocytes are strategically positioned to spans across crucial cerebral regions encompassing the SVZ and RMS with extension into the OB. This endows them not only with a pivotal role in the intricate process of neurogenesis, the framework, movement, and assimilation of nascent neurons into the OB (Doetsch et al., 1999) but also with their multifaceted functions across varied neurological circumstances (Lim and Alvarez-Buylla, 1999).
Structural and metabolic supportAstrocytes are the main type of glial cells making up to ~50% of the mammalian brain. At the ultrastructural level, astrocytes' ultrathin processes as “tripartite synapse” architectural elements enwrap the synaptic elements of neurons (Araque et al., 1999). Tripartite synapses in the CNS are estimated to be from 30% to 60% in the neocortex and reaching up to 90% in hippocampus by studies with EM three-dimensional reconstruction. These numbers are very likely underappreciated given the ultra-thinness of astrocytic processes beyond the diffraction limit of conventional EM. In addition, the astrocyte morphology is more complex (especially in the human brain) that, one astrocyte is estimated to monitor an average range of 20,000–160,000 or 270,000–2 million individual synapses in rodent and human brain, respectively (Verkhratsky and Nedergaard, 2018). Moreover, astrocyte processes reach toward blood vessels (mostly capillaries) and assist in building up the BBB by enclosing capillary walls using their process terminals termed end-feet. This structurally arrangement helps them to regulate both synaptic activities and blood flow in the brain. Taken together, the ubiquitous distribution, complex morphologies, physical bridging synapses and blood vessels, expression and releasing of diverse signaling molecules, and strategical presence in the pathways of neuronal migration underlie astrocyte's myriad functions in the CNS including the OB (Figure 6).
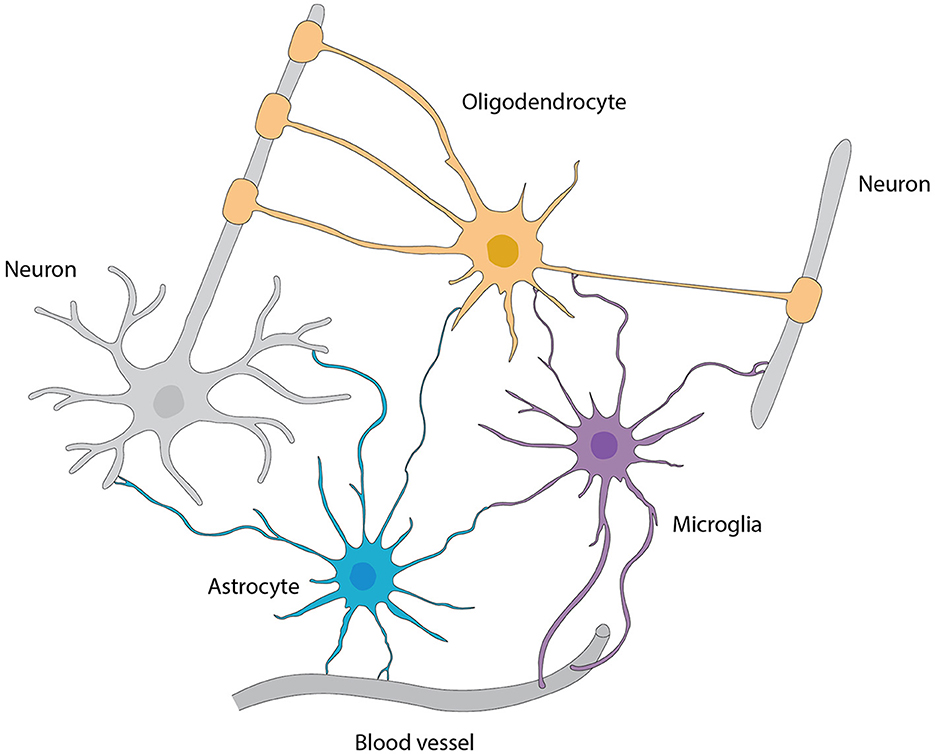
Figure 6. Interactions among various types of glial cells, neurons, and blood vessels, referred from Allen and Lyons (2018).
Additionally, astrocytes in the OB not only form networks of processes that surround synapses, blood vessels and peri-vascular macrophages for stability (Sofroniew and Vinters, 2010; Ponath et al., 2018; Lawal et al., 2022) but also function in providing structural support to migrating neurons and in shaping connections between newborn neurons and existing neural circuits (Piet et al., 2004; Verkhratsky and Nedergaard, 2014).
Regulation of neuronal excitability and synaptic transmissionA series of synaptic-relevant proteins including Na/K ATPase (NKA), glutamate transporters (GLAST and GLT1),
留言 (0)