In 1947, an American plant pathologist Walter Burkholder first described Pseudomonas cepacia as a plant pathogen capable of causing onion rot (Burkholder, 1948). Later, additional P. cepacia isolates were detected and it became clear that this group of bacteria is occupying a wide array of ecological niches including rhizosphere of plants and freshwater environments (Vandamme and Dawyndt, 2011). With the advent of rRNA–DNA hybridization and subsequently the rRNA gene sequencing methods, taxonomists revised the initial classification and transferred P. cepacia and six other species of the so-called Pseudomonas rRNA group II to the new genus Burkholderia (Yabuuchi et al., 1992). In 1997, Peter Vandamm et al. showed that Burkholderia cepacia originally classified as single species included at least five different genomovars. Thus, the collective of the genomovars was named as the B. cepacia complex (Bcc) (Vandamme et al., 1997). To date, Bcc includes at least 24 phylogenetically related, but genetically different species (Tavares et al., 2020; Wang et al., 2020). Bcc bacteria can thrive in the diverse range of environments (such as pharmaceutical solutions, hospital equipment, industrial setting, shampoo, oil, fuel) and is capable of a variety of complex interactions (Mahenthiralingam et al., 2008; Tavares et al., 2020). Thus, several Bcc bacteria can degrade different xenobiotic compounds and organic pollutants, including constituents of crude oils, pesticides, phthalates, and solvents (Tavares et al., 2020). For example, B. vietnamiensis strain G4 is one of the most efficient degraders of trichloroethylene (O'Sullivan and Mahenthiralingam, 2005). Bcc bacteria can proliferate within the rhizosphere and promote the growth of many important crops such as peas (colonized by B. ambifaria), maize (B. cenocepacia), rice (B. vietnamiensis) and wheat (B. cepacia and B. cenocepacia), protecting seedling plants against other bacteria, protozoa, nematodes, and fungal diseases, such as root rot or seed-damaging infections. Some of Bcc species are also capable of N2 fixation, thereby contributing to plant growth (Parke and Gurian-Sherman, 2001; Tavares et al., 2020). Unfortunately, all these fascinated bacterial species can cause opportunistic infection in immunocompromised individuals, including cystic fibrosis (CF) patients and those with chronic granulomatous disease (CGD) (Greenberg et al., 2009; Coutinho et al., 2011).
Cystic fibrosis is an autosomal recessive disease associated with the mutation in CF transmembrane conductance regulator (CFTR) gene, that encodes a protein of c-AMP gated chloride channel. Impairment of ionic transport across the apical surface of epithelia leads to a high absorption of natrium and water by epithelial cells. The result of this process is increased viscosity of cellular secrets, which disturbs the function of respiratory system, pancreatic gland, hepatobiliary system, intestine and urogenital tract (Shteinberg et al., 2021). Mucociliary clearance impairment and CFTR deficiency related immune disturbances make respiratory tract an ideal environment for bacterial colonization (Ribeiro et al., 2023; Thornton and Parkins, 2023). Microbiological landscape in CF airways changes drastically through age and stage of disease. Staphylococcus aureus and Haemophilus influenzae dominate in early childhood and at early stages of CF lung disease. These bacteria “pave the way” for other microorganisms, such as P. aeruginosa and the Burkholderia cepacia complex, which prevail in the sputum samples in the later stages of the disease through adolescence and early adulthood (Ribeiro et al., 2023; Thornton and Parkins, 2023). Chronic lung inflammation is characterized by non-productive exuberant neutrophil infiltration and increased cytokine (TNF-α, IL-8, IL-17), neutrophil elastase, and MMPs production. Recurrent lung exacerbations lead to structural destruction of CF airways, respiratory tract obstruction and lung tissue remodeling. Progressive lung disease remains the leading cause of mortality in CF patients (Caverly et al., 2022; Ribeiro et al., 2023).
Burkholderia species are naturally resistant to many antibiotics (aminoglycosides, quinolones, β‐lactams, and host antimicrobial peptides including β‐defensins) making their eradication very difficult (Rhodes and Schweizer, 2016; Scoffone et al., 2017; Ganesh et al., 2020). Though only a small proportion of CF patients are infected with Bcc (1.0% in USA (Cystic Fibrosis Foundation Patient Registry, 2021) and 0.0-6.1% in European countries (Orenti et al., 2023), they represent a major concern, since individual outcomes are unpredictable and can vary from asymptomatic carriage to “cepacia syndrome” (Sfeir, 2018). This is an acute necrotizing pneumonia with an almost inevitable fatal outcome. Cepacia syndrome can be developed soon after the first acquisition of Bcc but it can also occur many years after the first organism isolation (Blackburn et al., 2004; Gilchrist et al., 2012). There is extensive literature on Bcc virulence factors including host response induction and mechanisms of drug resistance (Ganesan and Sajjan, 2012; Scoffone et al., 2017; Sfeir, 2018; Ganesh et al., 2020). However, most studies concerning host-pathogen interactions have been performed using cell cultures (Wright et al., 2011; Guadalupe Cabral et al., 2017; Dorrington et al., 2021) or animal models (mice, zebrafish) (Zgair, 2012; Mesureur et al., 2017; Loeven et al., 2021). At the same time, data on the inflammatory process in CF patients with chronic Bcc infection are largely missing in the literature. We found only a few references to cytokine levels in plasma and sputum samples of CF patients with chronic Bcc infection. These studies compared inflammatory markers of CF subjects with different microbiological characteristics and included a limited number of Bcc infected participants (n≤12) (Watt et al., 2005; Brazova et al., 2006; Hansen et al., 2010).
The primary objective of the present study was to carry out cross-sectional analyses of local and systemic biomarkers in Bcc infected CF patients (n=47) and in CF subjects who were Bcc free (n=69). In addition, the rate of non-pulmonary complications and concomitant diseases in two patient groups had been compared. The secondary aim was to assess prospectively overall survival of the study participants during up to 8 years of follow-up.
2 Methods2.1 Study designThere were 116 CF paediatric patients (age less than 18 years) who had attended the Cystic Fibrosis Department of the Federal State Budgetary Institution «Russian Paediatric Clinical Hospital» in Moscow between January 2013 and December 2014 enrolled in the study. Current clinical, microbiological and functional data were obtained. Blood and sputum samples of the patients had been collected and cross-sectional analyses of local and systemic biomarkers were performed. Then mortality rate had been prospectively evaluated during up to 8 years of follow-up period (Figure 1).
Figure 1 Study design.
2.2 Patient assessmentCF was diagnosed by positive sweat test (chloride concentrations >60 mmol/L), typical clinical symptoms, and/or detection of CF causing variants. The CFTR genotype was determined in 111 patients: 44 individuals were homozygous for the F508del, 42 were heterozygous for the F508del, 25 subjects were carriers of other CFTR mutations and 5 had unknown/missing genotypes.
Spirometric tests were also carried out in children >6 years of age during periods of clinical stability. Spirometric data (forced vital capacity (FVC) and forced expiratory volume exhaled during the first second (FEV1), % predicted) were obtained from 110 of 116 patients. The limiting factors for spirometry performance were age less than 6 years old (4 patients), neurological problems (1 patient) and hemoptysis (1 patient). Respiratory microbial flora was determined by microscopy and culture of lower respiratory tract secretions or throat swabs performed at every routine visit to the CF Department. Bacterial identification and genotyping technology were conducted according to standardized protocols established for CF patients (Chernukha et al., 2014; Voronina et al., 2018). Chronic airway colonization was defined by the persistence of the pathogen in at least three airway samples for a period of at least 12 months.
According to European Cystic Fibrosis Society Standards (Smyth et al., 2014), cystic fibrosis-related liver disease was defined by at least one of the following criteria: 1) documented persistent increase in serum concentrations of the liver enzymes alanine aminotransferase (ALT, at least twice normal), aspartate transaminase (AST, at least 1.5 times normal), alkaline phosphatase (at least 1.5 times normal), or gamma-glutamyltransferase (GGT, at least 1.5 times normal); 2) persistent hepatomegaly (a percussed liver span greater than 1 SEM for age) for more than 6 months; 3) splenomegaly (a palpable spleen greater than 2.0 cm below the left costal margin); 4) abnormalities on ultrasound scan (liver with increased size, dishomogeneous echogenicity, or nodules with irregular margins; splenomegaly) for more than 6 months (Smyth et al., 2014).
Disturbances of glucose metabolism were assessed using the routine oral glucose tolerance test (OGTT) in accordance with European Cystic Fibrosis Society Standards (Smyth et al., 2014). All patients were clinically stable at the time of the OGTT, with no recent pulmonary exacerbations or symptoms suggestive of acute infection. Fasting plasma glucose (FPG) and 2-hour plasma glucose (2hPG) levels were measured after an oral glucose load of 1.75 g/kg of body weight (maximum 75g). FPG levels ≤5.5 mmol/L and 2hPG levels ≤7.8 mmol/L were considered to be consistent with normal glucose tolerance. Impaired glucose tolerance (IGT) was diagnosed if the 2hPG was from 7.9 to 11.0 mmol/L, and CF-related diabetes (CFRD) was diagnosed if the 2hPG was >11.0 mmol/L (Smyth et al., 2014).
All CF patients were treated with basic therapy following the European Cystic Fibrosis Society Standards (Smyth et al., 2014); treatment included mucolytics (dornase-alpha), multivitamins, high calorie diet, and microspheric pancreatic enzymes. In the case of acute pulmonary exacerbation, patients received antibacterial treatment which depended on the sputum microbiological analysis.
2.3 Blood collection and sputum processingBlood and sputum collecting was performed during the years 2013-2014 (see Figure 1). At the time of blood and sputum sampling, all patients were clinically stable, without any recent pulmonary exacerbations or symptoms of acute infection. Venous blood was collected in EDTA tubes by venipuncture. The tubes were centrifuged at 400 × g for 10 min at 4°C to pellet the cells. Plasma was harvested, aliquoted and stored at –70°C for up to 3 months.
The sputum samples were placed in ice and delivered to the laboratory within 1 h. Each sputum sample was weighted, and double the weight of phosphate-buffered saline without Ca2+ and Mg2+ was added to the sputum specimen. The mixture was put on vortex for 40 s and then on the rocker for 30 min. Then sputum samples were filtered through 100 μm filters to get rid of mucus, and centrifuged at 400 x g for 10 min at 4°C to pellet the cells. The supernatants were collected, aliquoted, and stored at –70°C for up to 3 months. Protein concentrations in the samples were evaluated by Bradford’s method (Bradford, 1976).
2.4 Cytokine, adrenocorticotropic hormone and matrix metalloproteinase assessmentsCytokine and ACTH assessments were performed in accordance with the protocol of the research program “Immunological Monitoring of Cystic Fibrosis Patients”, developed by Research Centre for Medical Genetics since 1998. MMP-8 (Quantokine® ELISA, R&DSystems), MMP-9 (Quantokine® ELISA, R&DSystems), MMP-12 (Cloud-Clone Corp.) in sputum; IL-2 (High Sensitivity ELISA), IL-6 (High Sensitivity ELISA), TGFβ1, IL-10HS, IL-18, IL-22, IL-23 (Bender MedSystems GmbH; Vienna, Austria) in plasma; neutrophil elastase (Bender MedSystems GmbH; Vienna, Austria); TNF-α, IL-10, IFN-γ, IL-4, IL-8 (CYTOKINE; St. Petersburg, Russia), IL-17A and IL-17F (Bender MedSystems GmbH; Vienna, Austria) in plasma and sputum samples were analysed by ELISA technique with commercially available kits in accordance with the manufacturers’ instructions. Sputum cytokine levels were normalized to the protein content of each sample. Plasma ACTH was also measured using a commercially available kit (Diagnostic Systems Laboratories, Inc.; Webster, TX, USA). ELISA kits of the same lots were used for biomarker measurements.
2.5 Dexamethasone induced suppression of lymphocyte proliferationPeripheral blood lymphocytes (PBL) were isolated from heparinazed peripheral blood by Ficoll-hypaque density gradient centrifugation. The cells were washed twice in phosphate-buffered saline without Ca2+ and Mg2+, and resuspended in RPMI-1640 medium supplemented with 10% heat-inactivated donor horse serum, 2x10–3 M HEPES, 2 mM L-glutamine, 2.8x10–6 M 2-mercaptoethanol, and 20 mg/ml gentamycin. The cells (5x104 cells/well) were seeded in flat-bottomed 96-well plates and stimulated with PHA in the final concentration 5 mg/ml. Inhibition lymphocyte proliferation by dexametasone was evaluated at six different concentrations (10–10 to 10–6 M). Dexametasone was not added to the control wells containing a culture medium with or without PHA). The cells were incubated for 72 h at 37°C in humidified atmosphere containing 5% CO2. Four hours before the end of cultivation, each well was pulsed with 40 kBq of (3H)-thymidine (Isotope, Russia). The cells were harvested with a cell harvester and counted on a liquid scintillation counter. Triplicate wells of each concentration were assayed and the counts per minute (count/min) were averaged. Percentage inhibition was calculated by dividing the count/min in each inhibited sample by the count/min in the sample containing PHA only. The intensity of suppression was expressed as ED50. To evaluate individual susceptibility to glucocorticoids, the Δh parameters were calculated as described in our early studies (Pukhalsky et al., 1990, Pukhalsky et al., 1999; Shmarina et al., 2001). Previously the direct positive correlation between the level of PHA-induced lymphocyte proliferation and the inhibition degree of such stimulation by dexamethasone (ED50) has been shown. On the basis of this correlation the method of evaluation of individual susceptibility to the antiproliferative effect of glucocorticoids by Δh parameter calculation had been proposed (Pukhalsky et al., 1990).
Δh was calculated using formula:
where Y = lnED50, the experimental parameter; Y′ = lnED50′, the expected parameter; lnED50′= 0.447X – 4.399, X = ln ([count per min] in the samples treated with PHA only).
In this study we evaluated inhibitory effect of dexamethasone on proliferative response of PHA-stimulated lymphocytes obtained from 11 Bcc infected and 10 Bcc free CF subjects enrolled into the study. Their Δh values were compared with proper parameters of healthy volunteers and patients with chronic obstructive pulmonary disease (COPD). COPD group consisted of 47 patients (mean age, 48.4 ± 1.8 years; 27 males) who had attended the Clinical Department of Laboratory of Pulmonology, Moscow State University of Medicine and Dentistry named after A.I. Evdokimov, Moscow, Russia. Diagnosis of COPD confirmed by post-bronchodilator FEV1/EVC<0.7 during a stable state. The healthy group included 32 non-smoking volunteers (mean age, 28.2 ± 1.1 years; 15 males) with a negative history of respiratory disease or intercurrent illness.
2.6 Statistical analysisQuantitative characteristics are presented in the form: mean ± standard error of the mean. Descriptive statistics of cytokines are expressed as the median (minimum value ÷ maximum value). Qualitative variables are expressed in their respective frequencies. Comparison of patient characteristics by groups was performed using the Mann–Whitney U-Test, Fisher’s exact test and the χ2 test. The odds ratio (OR) and 95% confidence interval (95% CI) were calculated. The Kaplan-Meier method was used to generate survival curves, and differences between the groups were assessed using a log-rank test. For univariate analysis and to estimate the independent effects of variables, a Cox proportional hazards model was used with 95% confidence intervals. Four independent categorical variables were analyzed via univariate Cox regression analysis: female sex, Bcc infection, glucose metabolism disturbance, low-dose prednisolone therapy. P-values less than 0.05 were considered statistically significant. Statistical analyses were performed using the statistical package SPSS, version 22.0 (IBM Corp.; Armonk, NY, USA) and STATISTICA 10 (StatSoftInc.; Tulsa, OK, USA).
3 Results3.1 Bcc infected CF patients did not differ from non-infected ones in term of demographic and clinical dataDepending on microbiological status, all patients were divided into two groups: children who were chronically infected with Всс and those who were Bcc free. Demographic and clinical data are shown in Table 1. Bcc infected patients did not differ from Bcc free subjects in sex, age, and number of F508del mutation carriers. Similarly, clinical course of CF lung disease in Bcc infected children did not tend to be more severe than that in Bcc uninfected ones. Thus, mean values of FEV1, FVC and body mass index (BMI) did not differ between the patient groups. Bcc infected patients did not more frequently require prescribing of long-term low-dose prednisolone therapy (see Table 1). Nine patients with Bcc (18.4%) and 8 patients without the pathogen (11.6%) died during cross-sectional period of the study. Individual characteristics of dead patients are presented in Supplementary Table 1. The mean time from Bcc infection onset to death was 4.8 ± 0.5 years. Dead Bcc infected patients tended to be older than dead children from the Bcc free group (14.5 ± 1.3 vs 11.5 ± 1.3 years old, respectively, p=0.092) and had increased frequencies of CF-related complications such as diabetes and cirrhosis with portal hypertension (8/9 vs 2/8, respectively, p=0.013). In the same time all dead children from Bcc free group received oral steroids whereas there were 4 Bcc infected patients who were treated with prednisolone (p=0.020).
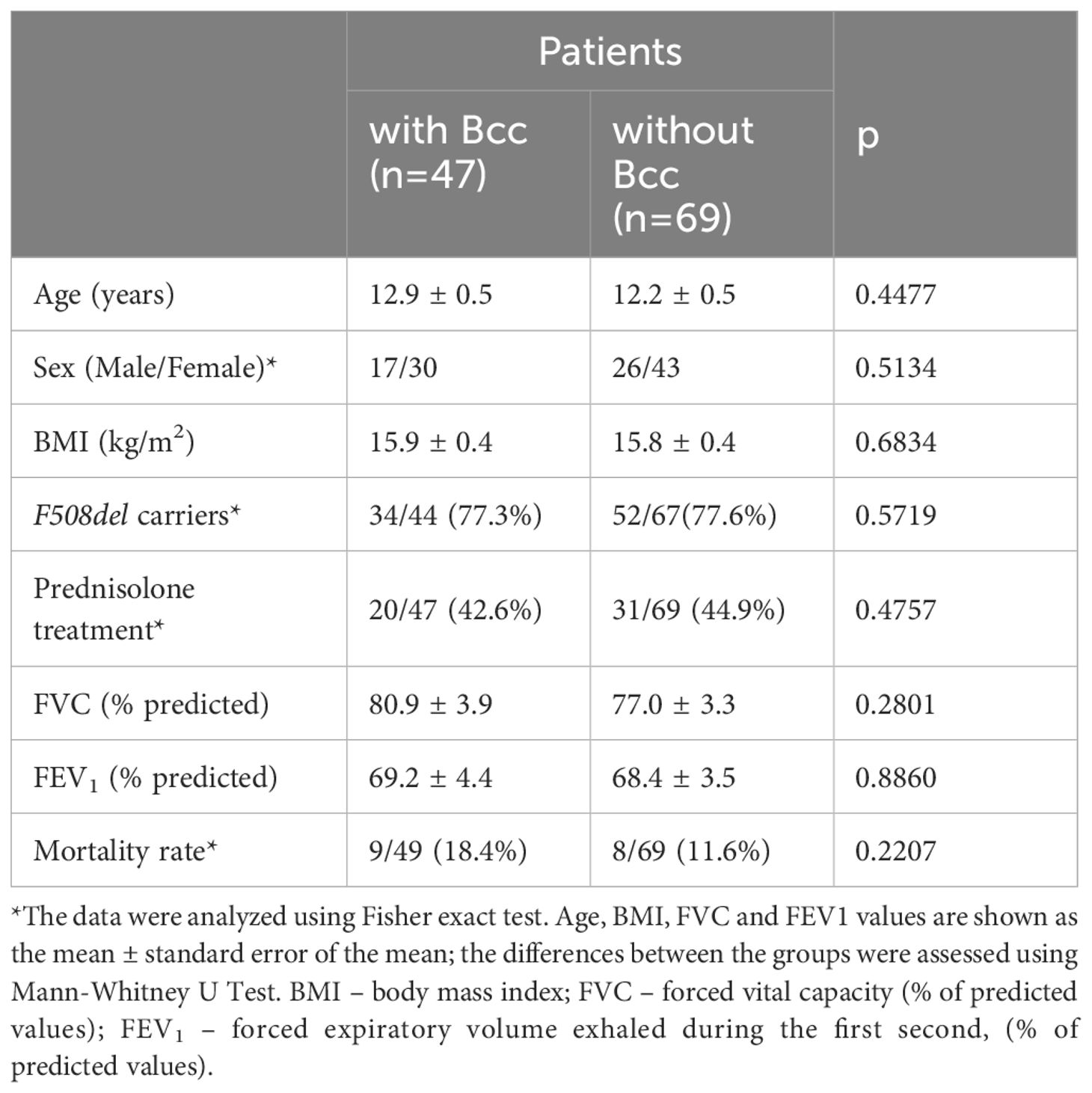
Table 1 Characteristics of the study subjects during cross-sectional period of the study.
3.2 Microbiological landscape in airways of Bcc infected/free patientsAirway microbial community has a great impact on CF lung disease progression. Therefore, the study of CF lung microbiology is as important as demographic and clinical data evaluation. Repeated microbiological studies revealed the presence of traditional CF pathogens (Table 2). The most common combinations were В. cepacia with S. aureus (16/47, or 34.0%) or P. aeruginosa with S. aureus in Bcc free group (43/69, or 62.3%).
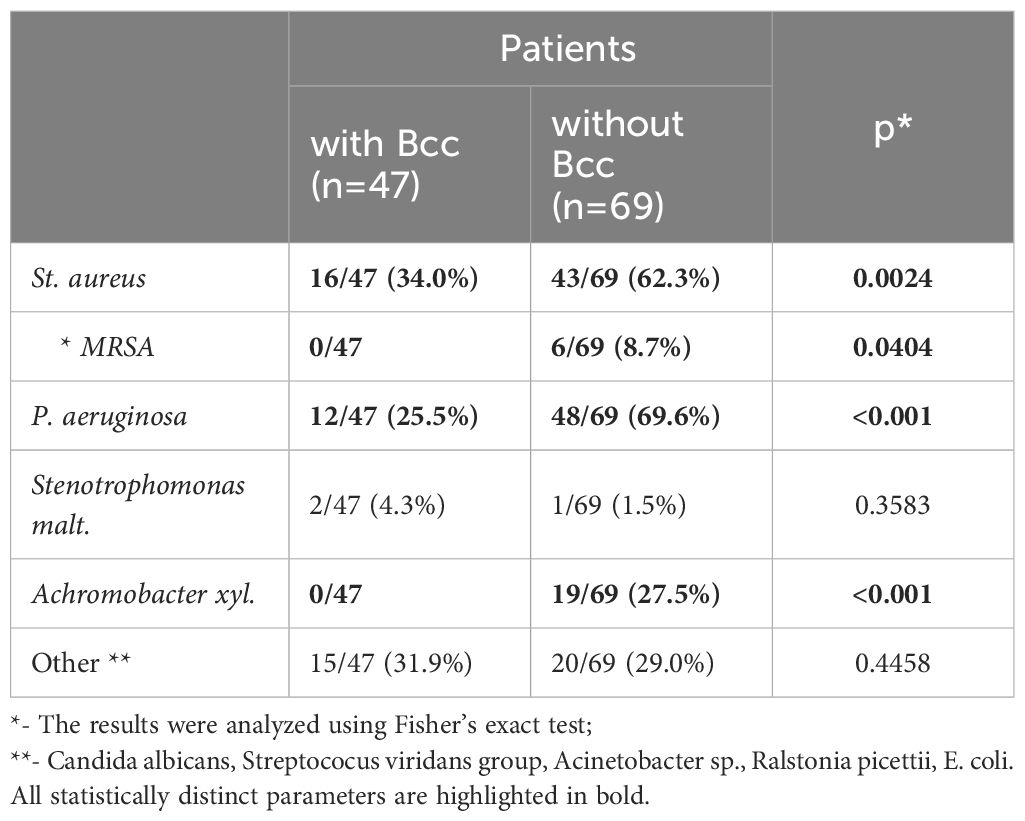
Table 2 Microbiological characteristic of the study subjects during a cross-sectional period of the study.
Twelve (25.5%) Bcc infected subjects and 48 (69.6%) Bcc free patients had chronic colonization with P. aeruginosa (p<0.001; Table 2). There were no cases of methicillin resistant Staphylococcus aureus (MRSA) infection among Bcc infected patients. In the same time Bcc free group included six patients with MRSA (p=0.0404). Achromobacter xyl. infection was revealed in 19 Bcc free subjects whereas nobody of Bcc-colonized patients were infected with the pathogen (p<0.001).
Most patients (45 of 47) were colonized with B. cenocepacia, the most abundant Bcc species which constituted 93.0% of all Bcc cases in Russia CF cohorts between 2012-2017 yrs (31). One patient (2%) had B. contaminans, the other one (2%) was infected with B. vietnamiensis (Supplementary Figure 1).
3.3 Frequency of CF complications and concomitant diseases during cross-sectional period of the studyCF complications and concomitant diseases contribute to lung disease progression, worsen the prognosis of the disease, and sometimes become a cause of death. Frequency of CF complications and concomitant diseases in the Bcc infected/free groups are presented in the Table 3. There were no significant differences in the frequencies of CF-related liver disease (including cirrhosis complicated with portal hypertension) between the Bcc infected and Bcc free patients. However, the patients colonized with Bcc were more likely than those in the Bcc free group to exhibit disturbances in glycemic control (X2 = 6.25, p = 0.012; OR 3.34 (95% CI 1.36–8.19)). The risks for abnormal glucose metabolism among Bcc infected patients who received long-term low-dose prednisolone treatment or who had this therapy in their recent history, were further increased (X2 = 5.30, p = 0.021; OR 4.17 (95% CI 1.19–14.54). There were no differences in number of patients receiving oral steroids between Bcc infected and Bcc free groups: 20/47 (42.6%) vs 31/69 (44.9%), respectively (see Table 1). However, 10 of 20 (50%) and 6 of 31 (19.4%) of the steroid-treated patients from Bcc infected and Bcc free groups, respectively, developed glucose metabolism disturbances.
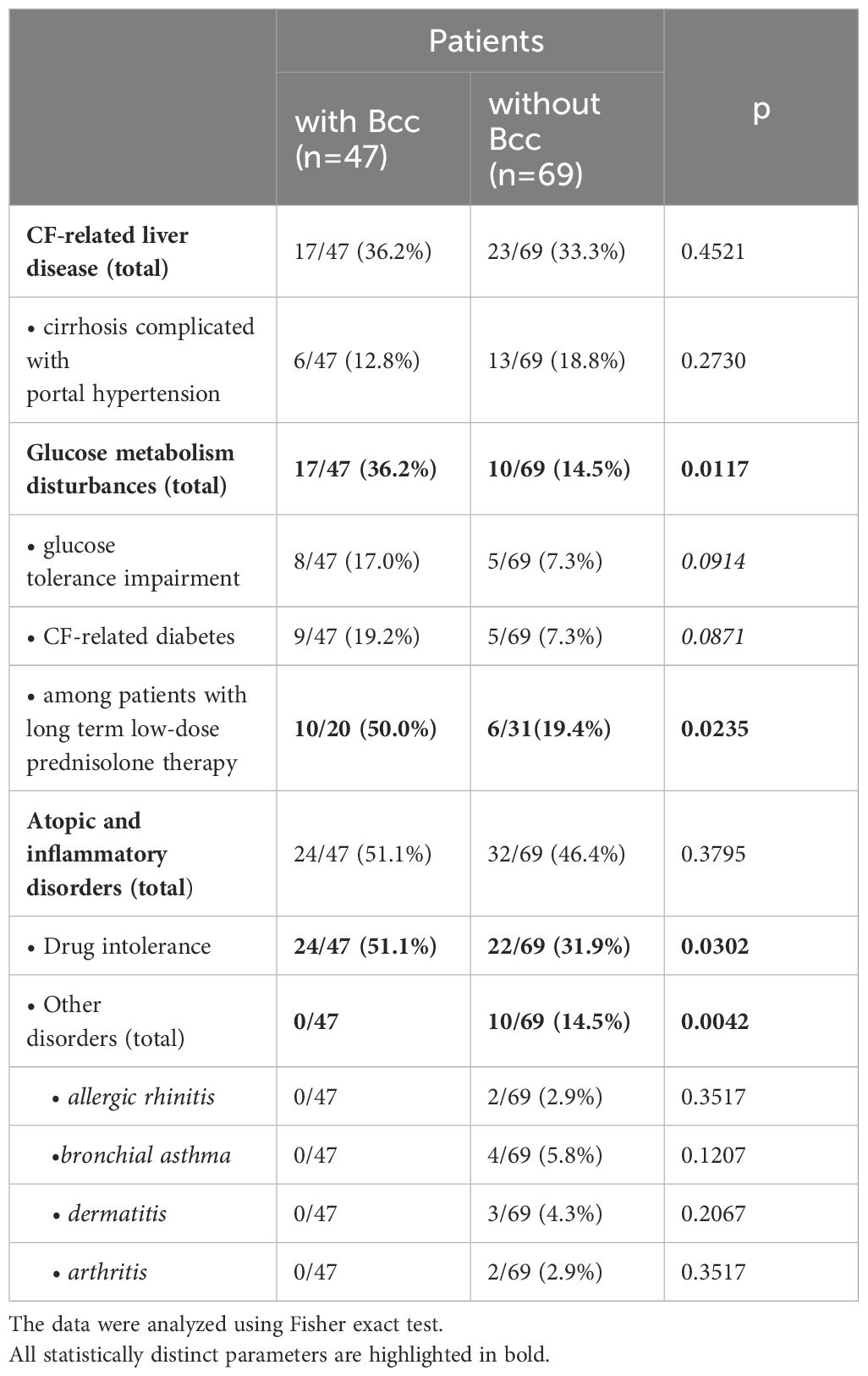
Table 3 CF complications and concomitant diseases during cross-sectional period of the study.
Atopic and inflammatory disorders were diagnosed in 24 of 47 (51.1%) patients with Bcc infection and in 32 of 69 (46.4%) Bcc free subjects. Drug intolerance episodes were more common in Bcc infected patients (24/47, 51.1%) than in Bcc free children (22/69, 31.9%; p=0.030). Also, remarkably, none of the patients with chronic Bcc infection developed or had recent history of arthritis and atopic diseases, but 10 Bcc free subjects were found to have concomitant inflammatory disorders including allergic rhinitis, bronchial asthma, dermatitis and arthritis as well (p=0.004; see Table 3).
3.4 Bcc infected CF patients demonstrated increase in TNFα and reduction in IL-17 F levels in their sputum samplesSputum samples were obtained from 107 CF participants (Table 4). The patients with chronic Bcc colonization had higher concentrations of sputum TNFα compared to those of non-infected subjects (27.1 ± 9.2 and 6.1 ± 1.2 pg/mg protein, respectively, p=0.032). Besides, patients from Bcc infected group tended to show almost 2-fold increase in MMP-9 level in their sputum samples: 1124.8 ± 247.8 vs 573.5 ± 74.4 ng/mg protein in Bcc free group (p=0.056). In the same time there was found a significant reduction in sputum IL-17F and clear trend to decline in sputum MMP12 in Bcc infected group compared to Bcc free one (7.1 ± 2.3 vs 23.1 ± 9.3 pg/mg protein, p=0.029 and 12.3 ± 3.9 vs 22.8 ± 4.5 ng/mg protein, respectively; p=0.074). Other biomarkers including neutrophil elastase, IL-8, IL-10, IFNγ, IL-4, MMP-8 were detected in the sputum samples, but no difference in concentrations was found when comparing the groups (see Table 4). The concentrations of IL-17A in the sputum samples of the most study participants were below detection limit (0.5 pg/ml).
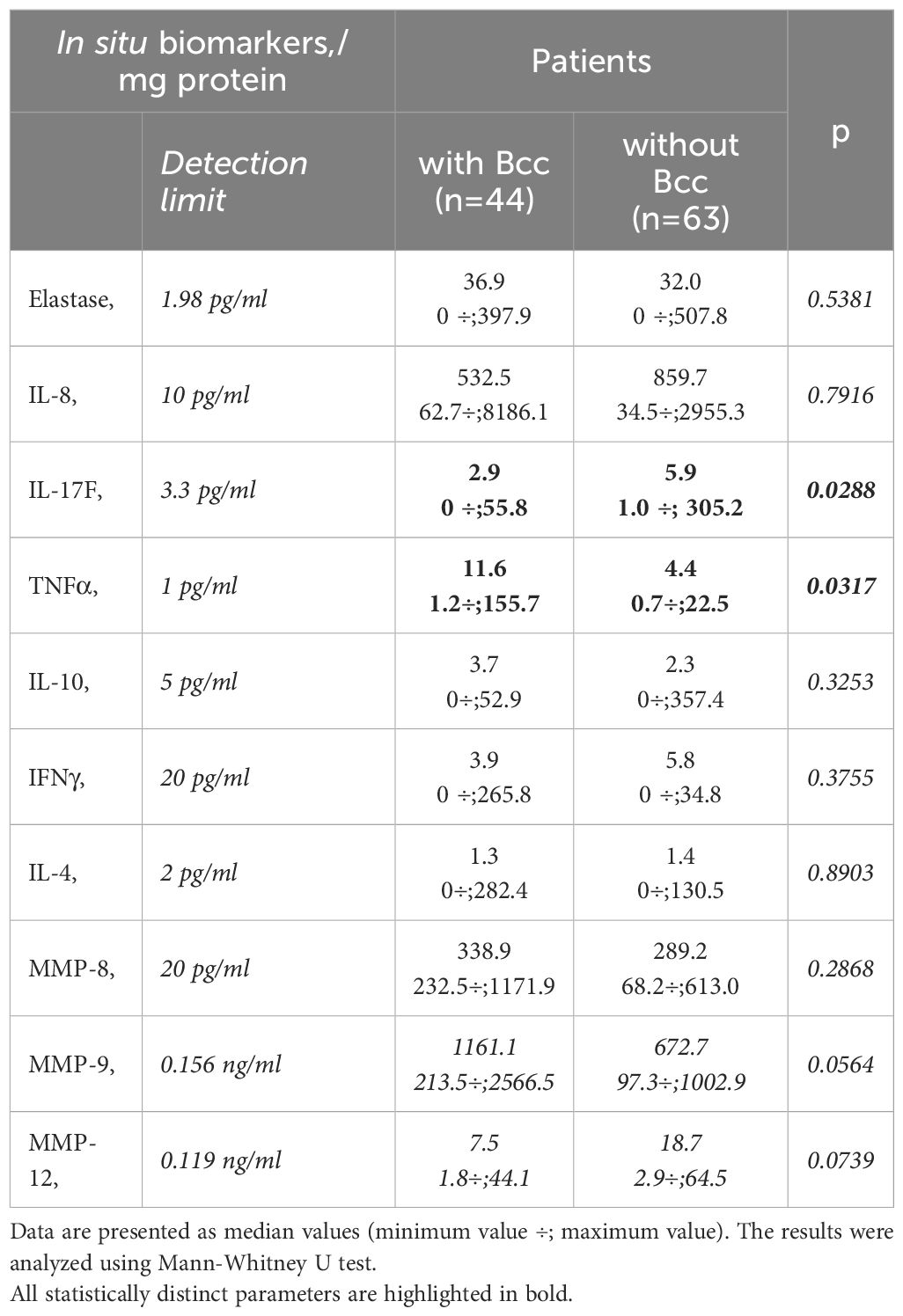
Table 4 Sputum biomarkers.
3.5 Bcc infected patients showed a reduction in both pro- and anti-inflammatory cytokine levels in their plasma samplesPlasma biomarkers were assessed for the 46 Bcc infected patients and 69 Bcc free persons. There were some differences in the biomarker patterns of the groups (Table 5). There were many in vitro and ex vivo studies showing exaggerated proinflammatory cytokines production by wild and CFTR-deficient cells treated with Bcc strains or Bcc LPS (Zughaier et al., 1999; Ganesan and Sajjan, 2012). Strikingly, in our study Bcc infected children showed a marked reduction in both pro- and anti-inflammatory cytokine levels in their plasma samples (Table 5). Thus, mean plasma IL-17F and IL-18 concentrations were, respectively, 34.3 ± 1.9 and 132.6 ± 36.4 pg/ml in Bcc infected group vs 50.8 ± 3.8 pg/ml and 415.8 ± 117.1 pg/ml in Bcc free patients (both p<0.031). Mean values for plasma IL-10 and TGF-β1 levels had amounted, respectively, 7.5 ± 1.5 pg/ml and 10.1 ± 1.1 ng/ml in Bcc infected group vs 34.9 ± 9.6 pg/ml and vs 14.5 ± 1.0 ng/ml in Bcc free group (both p<0.01).
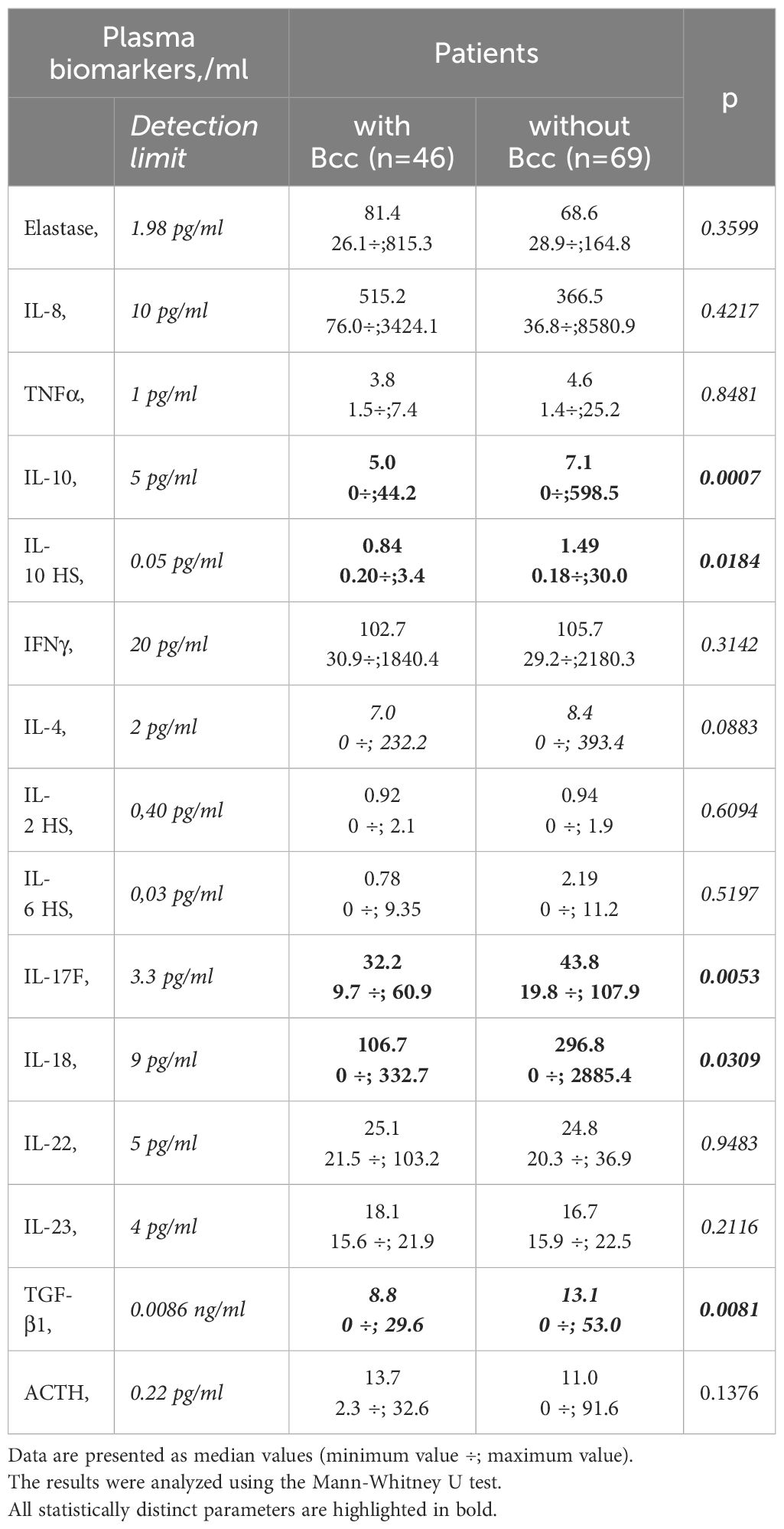
Table 5 Plasma biomarkers.
There were no significant differences between the groups in plasma levels of neutrophil elastase, IL-8, TNFα, IL-10, IFNγ, IL-2, IL-4, IL-6, IL-22, IL-23, and ACTH levels (see Table 5). The concentrations of IL-17A and TNFα in the plasma samples of the most study participants were below detection limit (0.5 pg/ml and 1.0 pg/ml, respectively).
3.6 Peripheral blood lymphocyte susceptibility to steroid suppressionDecreased cytokine concentrations in plasma samples of Bcc infected patients suggest the reduction in systemic inflammatory response. Since PBL susceptibility to glucocorticoids is strongly correlated with the severity of systemic inflammation (Shmarina et al., 2001; De et al., 2002; Quax et al., 2013), we evaluated inhibitory effect of dexamethasone on proliferative response of PHA-stimulated lymphocytes obtained from Bcc infected (n=11) and Bcc free (n=10) patients. Nobody of the patients received long-term low-dose prednisolone treatment or had this therapy in the recent history. The data are presented in Figure 2 and Supplementary Table 2.
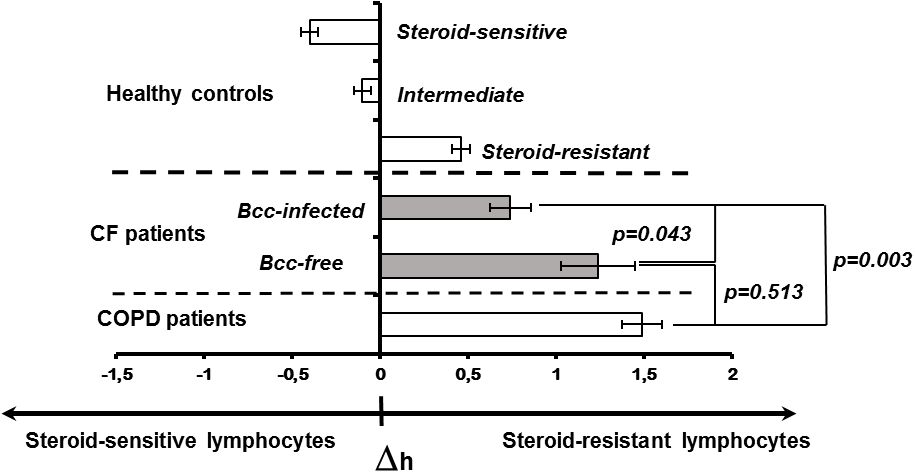
Figure 2 Peripheral blood lymphocyte susceptibility to steroid suppression. Inhibition degree of PHA-induced lymphocyte proliferation by different concentrations of dexamethasone was evaluated. Patients and healthy subjects were classified as steroid resistant if their Δh-parameters > 0 and steroid sensitive if their Δh-parameter > 0. The cell sensitivity is presented as the mean (± m) of the Δh value. The results were analyzed using the Mann-Whitney U test.
There were no significant differences in the levels of PBL proliferative response between the patient groups. In the same time, the mean ED50 value of dexamethasone in Bcc infected patients was about twice lower than that in Bcc free group (31.6 ± 11.2 vs 67.0 ± 28.8 µM, respectively), but this difference did not reach statistical significance (p=0.183).
To evaluate individual susceptibility to glucocorticoids, the Δh-parameters were accounted as described in ‘Material and Methods’. Figure 2 provides Δh values for healthy subjects, CF children and patients with severe COPD. Since Δh-parameters are not widely used in laboratory practice, the presented data can be a good illustration for changes of Δh values in health and disease. Besides, there are phenotypic similarities between COPD and CF pulmonary disease including airway surface liquid dehydration, mucus hypersecretion, pulmonary microbiome composition, as well as small airway disease with neutrophilic inflammation and lung remodelling (Mall et al., 2023; Miravitlles et al., 2024). Recent studies revealed acquired CFTR impairment in airways, sweat glands and intestines of COPD patients (Mall et al., 2023; Miravitlles et al., 2024). Similar to CF subjects with mutated CFTR, COPD patients demonstrated increased chloride concentrations in their sweat samples and elevated Beclin 1 level in peripheral blood (Raju et al., 2013; Schlemmer et al., 2018). The main reasons of acquired CFTR dysfunction include smoking, reactive oxygen species, inflammation and bacterial byproducts (Mall et al., 2023; Miravitlles et al., 2024). Local and systemic consequences of impaired CFTR expression and function in COPD have been discussed in comprehensive review by Miravitlles et al. Over the last decade, clinical studies of CFTR potentiators (ivacaftor and icenticaftor), being created to recover mutant protein activity in CF subjects with specific mutations, have been conducted in COPD patients (Solomon et al., 2016; Martinez et al., 2023).
There were statistically significant differences between the patient groups (CF, COPD) and steroid-sensitive, intermediate as well as steroid-resistant healthy controls (all p<0.035). However, CF subjects with chronic Bcc colonization were more steroid-sensitive then Bcc free CF children and COPD patients (both p<0.044). Thus, mean values for Δh-parameters in Bcc infected group had amounted 0.74 ± 0.12 abs. units vs 1.24 ± 0.21 (in Bcc free group) and 1.49 ± 0.11 abs. units (in COPD group). The values of Δh-parameters in the Bcc free CF patients and COPD subjects were statistically equivalent (p = 0.513).
Thus, during cross-sectional period of the study we revealed neither significant lung function failure nor survival disadvantage in Bcc infected patient group. Besides, these patients demonstrated the reduction in systemic inflammatory response in comparison with Bcc free CF subjects. To clarify the data obtained we continued to follow-up microbiological status and lifespan of the examined patients.
3.7 Outcomes: survival and Bcc colonization longevityDuring up to 8 years follow up, we observed 46 deaths. Of these, 25 deaths occurred in Bcc infected group and 21 deaths were documented in Bcc free cohort (Supplementary Table 3). Thereafter, survival rates in the patient groups were 46.8% and 69.6%, respectively (X2 = 6.05, p = 0.0139; OR 2.6 (95% CI 1.2–5.6)). During follow up period, patients infected with Bcc survived a median of 84.5 months, whereas there were insufficient death numbers in the Bcc free group to define a median. Kaplan-Meier survival analyses showed that Bcc infected patients had decreased overall survival compared with Bcc free ones (p=0.020, Log Rank test; Figure 3A).
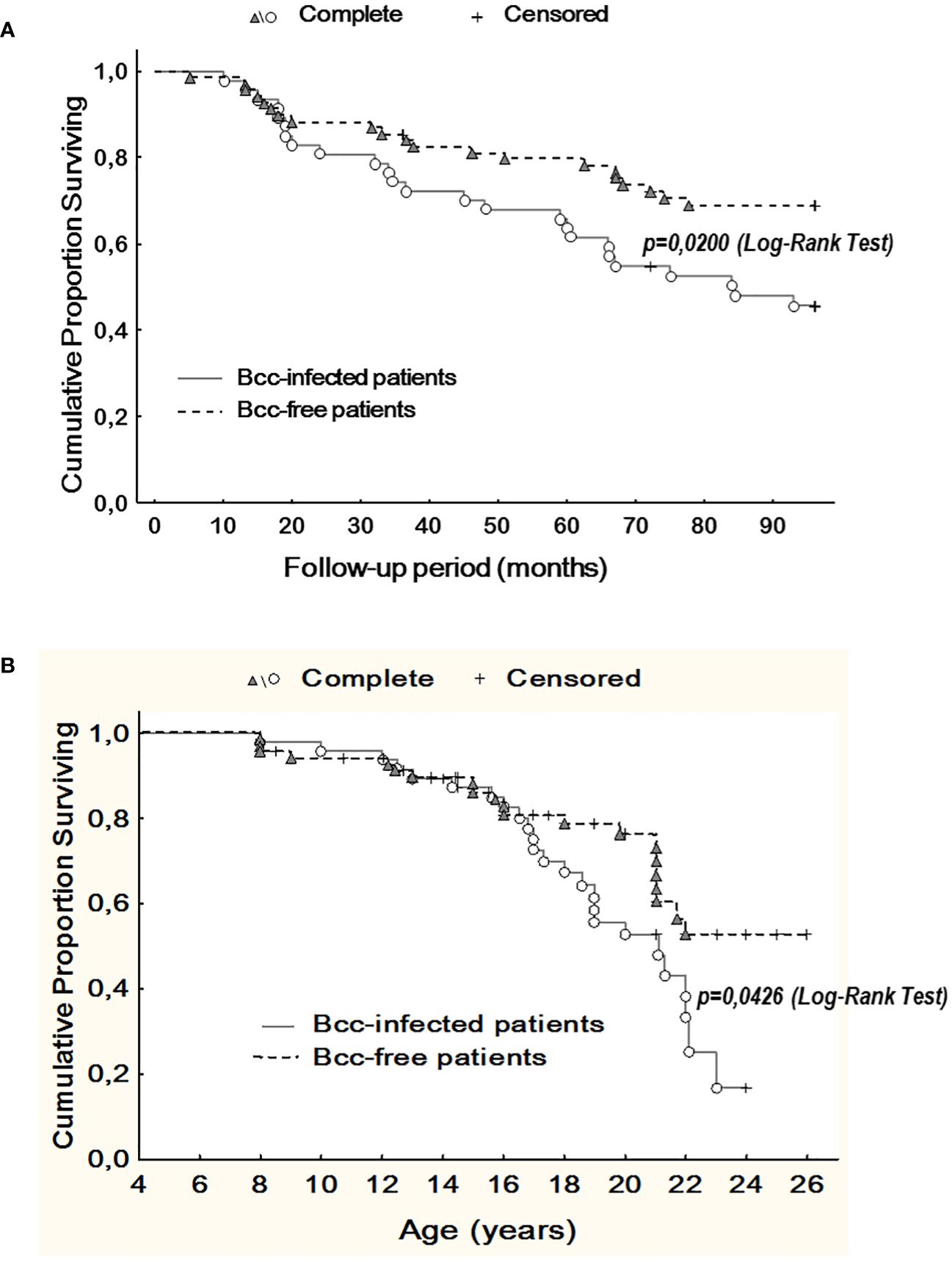
Figure 3 Kaplan-Meier survival analysis of Bcc infected and Bcc free patients. (A) Cumulative proportion surviving vs Follow-up period; (B) Cumulative proportion surviving vs Age of the patients. The plots indicate that chronic Bcc-colonization is associated with a worse outcome. The end points were considered as deaths. Bcc – Burkholderia cepacia complex.
Similarly, in Bcc free group, it was impossible to evaluate median survival time in years due to insufficient death numbers. In Bcc infected group median survival time in years was 21.1. Kaplan-Meier survival analysis demonstrated that Bcc-infected patients had a worse prognosis in overall survival than Bcc free CF subjects (p=0.043, Log Rank test; Figure 3B).
The Cox proportional hazards model was constructed to confirm the Kaplan-Meier survival analysis and determine the other variables that were associated with outcome (Table 6). Besides chronic Bcc colonization, glucose metabolism disturbance and low-dose long-term prednisolone therapy were also significantly associated with increased hazard ratios.
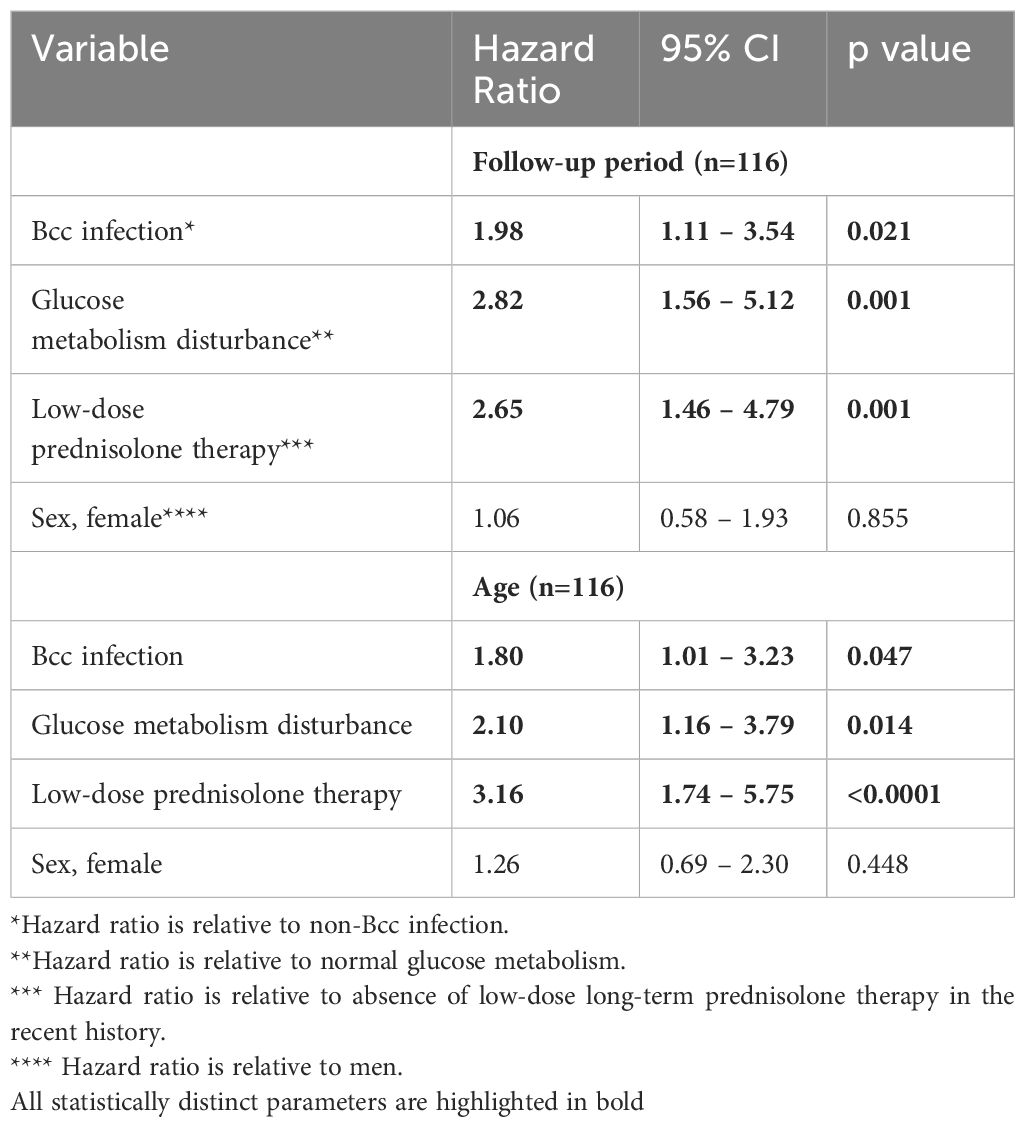
Table 6 Cox proportion hazards model for studied patients.
It was notably the mean age of Bcc acquisition in infected group was 8.9 ± 0.5 years. At the time of Bcc onset the survivors were about 2 years younger than dead patients: 7.9 ± 0.6 and 9.8 ± 0.7 years, respectively (p=0.0498; Supplementary Table 4). The mean value of Bcc colonization longevity was reached 9.2 ± 0.5 years. In the subgroup of survived Bcc subjects this parameter was 11.3 ± 0.3 years, in the cohort of dead Bcc patients – 7.4 ± 0.6 years. These data are in line with the recent study by Krasovsky et al. that included 138 Bcc infected and 419 Bcc free adult CF patients (Krasovskiy et al., 2019).
The microbiological follow-up did not reveal cases of chronic or intermittent Bcc colonization among uninfected patients.
4 DiscussionThe number of CF patients with chronic Bcc infection is small (3.5% in worldwide) and continues to decline due to preventive measures and modern therapies (Sousa et al., 2021). Since chronic Bcc infection is much more common in adult patients, the current literature provides data only for adults or mixed patient populations (children and adults) (Folescu et al., 2015; Zlosnik et al., 2015; Granchelli et al., 2018; Krasovskiy et al., 2019; Somayaji et al., 2020). Thus, our study is pretty unique one, as it includes CF children and adolescents with chronic Bcc colonization. As in the other studies (Jones et al., 2004; Brazova et al., 2006; Krasovskiy et al., 2019; Somayaji et al., 2020), immunological parameters and survival of Всс infected patients have been compared with the non-colonized CF peers who had the same stage of the lung disease. Most of the Bcc free patients were chronically infected with P. aeruginosa, a minority has recurrent lung exacerbations related to chronic MRSA, Stenotrophomonas maltophilia, Achromobacter xylosoxidans infections.
The study consists of two parts: cross-sectional analyses of demographic, microbiological and immunological characteristics of Bcc free/infected CF children and prospective assessment of overall survival of the study participants. Cross-sectional period of this study had been performed in years 2013-2014 when there were limited data on the survival, outcomes and inflammatory response severity even in patient cohorts infected with specific Bcc species nothing to say about patient cohorts infected with B. cenocepacia specific strains. Historically, any B. cenocepacia infection was considered to be connected with rapid decrease of lung function, weight loss, exaggerated inflammatory response and greater risk of death or transplantation (Jones et al., 2004; Drevinek and Mahenthiralingam, 2010; Folescu et al., 2015; Zlosnik et al., 2015; Somayaji et al., 2020). This opinion was mainly based on clinical data of patients infected with clones of epidemic ET-12 lineage, a highly transmissible B. cenocepacia species which causes aggressive inflammatory response, resulting in progressive lung function failure and high mortality rate (Jones et al., 2004; Drevinek and Mahenthiralingam, 2010; Somayaji et al., 2020). In addition, the majority of in vivo and in vitro studies on the molecular pathogenesis of B. cenocepacia have used ET-12 clone prototypic strains (for example, J2315, BC7 or k56-2) demonstrating extremely high severity and virulence of this Bcc species (Wright et al., 2011; Mesureur et al., 2017; Dorrington et al., 2021; Loeven et al., 2021). In our study nearly all patients (45 of 47) of Bcc group were infected with B.cenocepacia. Median colonization longevity at the moment of biomarkers assessment was 4.5 (1.0 ÷ 8.0) years (see Supplementary Table 4). However our data indicate that Bcc infected pediatric patients did not differ from Bcc free CF peers in terms of FVC, FEV1 and BMI values, mortality rate, and number of subjects who required prescribing of long-term low-dose prednisolone therapy (see Table 1). Moreover, Bcc infected patients demonstrated the signs of reduction in systemic inflammatory response, such as decreased plasma cytokine concentrations and improvement of PBL steroid-sensitivity (see Table 5 and Figure 2). Furthermore, none of the infected patients developed or had recent history of inflammatory or atopic diseases (excluding multiple episodes of drug intolerance) whereas 10 Bcc free subjects had concomitant inflammatory disorders. In the same time, there was found an increased rate of glucose metabolism disturbances in Bcc infected group (see Table 3). These results may seem somewhat unusual. We could not explain the inconsistency of our data to the generally accepted point of view and continued to follow-up microbiological status and lifespan of the examined patients. Nobody of uninfected individuals had been colonized with Bcc during follow-up period. Survival rates in Bcc infected/free groups were 46.8% and 69.6%, respectively (p=0.020). These data are completely in line with the results of two early studies demonstrating a survival disadvantage of Bcc infected patients in comparison with uninfected ones during prolong follow-up periods (Frangolias et al., 1999; Jones et al., 2004). In the same time the authors did not find any difference in annual changes of weight and spirometry parameters between the patient groups. Similarly, there were no differences in baseline FEV1% or the rate of FEV1% decline between the groups with and without Bcc infection in the recent retrospective cohort study by Somayaji et al (Somayaji et al., 2020). The median time from Bcc acquiring to death or transplantation for B. cenocepacia (excluding ET-12) group was 6.24 (7.0, in our study) vs 9.42 years for those without Bcc infection. In the univariate model, Burkholderia infection was associated with an HR of 1.48; 95% CI: 1.00 –2.19 (1.98; 95% CI: 1.11 – 3.54, in our study) for death or transplantation compared to those with no infection. For the patients infected with ET-12 clones, median time from Bcc onset to death and HR were 1.95 years and 3.92 (95% CI: 2.25 – 6.81), respectively (Somayaji et al., 2020).
Repeated microbiological examinations demonstrated limiting acquisition or persistence of other infections in children with Bcc colonization. Thus, during the steady-state period, nobody of Bcc colonized patients were infected with MRSA or Achromobacter xylosoxidans whereas Bcc free group included 6 patients with MRSA and 19 subjects with Achromobacter xylosoxidans infections in their recent history (see Table 2). These data are consistent with findings of previous studies (Folescu et al., 2015; Granchelli et al., 2018). Folescu et al. (Folescu et al., 2015) carried out a retrospective study of 56 patients (0-36 years) who were categorized into three groups (I: Bcc free, II: intermittent Bcc colonization, III: chronic Bcc colonization). Similar to our data, chronic MRSA colonization was found in 7.7% in group I, 7.4% in group II and 0% in group III. Using the data of Cystic Fibrosis Foundation Patients Registry, 2003-2011, Granchelli et al (Granchelli et al., 2018). performed analysis of 538,458 sputum cultures of 28,042 CF patients aged six and older from 257 accredited US Care Centers and Affiliates. All participants had sputum cultures for at least two consecutive years for MSSA, MRSA, Pseudomonas aeruginosa, Burkholderia cepacia complex, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, Candida and Aspergillus species. This study showed that Bcc-colonization is associated with lower odds for concurrent and subsequent MRSA and/or Achromobacter xylosoxidans acquisition and persistence. In addition, there were strong negative associations between Bcc and MSSA as well as between Bcc and P.aeruginosa infections (Granchelli et al., 2018). In agreement, in our study Bcc free group included 43 (62.3%) patients with chronic MSSA and 48 (69.6%) patients with chronic P.aeruginosa colonization, whereas in Bcc infected group these parameters were 16 (34.0%) and 12 (25.5%), respectively. It is widely known that decrease in airway microbiome diversity in CF patients is associated with severe lung function failure and poor prognosis (Granchelli et al., 2018; Thornton and Parkins, 2023). Indeed, repeated courses of antibiotic therapy for recurrent lung exacerbations inevitably reduce variety of CF microbial community. Eventually, patients with advanced lung disease usually have poor microbiological landscape with a predominance of 1-2 typical CF pathogens (Thornton and Parkins, 2023). In CF, co-colonization with several pathogens has its benefits and drawbacks. Thus, co-colonization can promote antibiotic resistance development and subvert the host immune response (Limoli and Hoffman, 2019; Oliveira et al., 2023). In addition, co-occurring species maintain virulence factor production resulting in development of exuberant inflammatory response and, as a consequence, more rapid lung tissue destruction (Bottery et al., 2024). In such a situation, lung inflammation caused by intermittent infection with highly virulent strain of a new pathogen can be easily stopped due to already activated anti-inflammatory mechanisms. At the absence of competing species, the dominant pathogen evolves under pressure of the host immune system towards the decrease or loss of virulence factor production (Thornton and Parkins, 2023; Bottery et al., 2024). This pathoadaptation mitigates systemic inflammation, leading to decrease of both pro- and anti-inflammatory cytokine levels and increases lymphocyte sensitivity to steroid suppression (see Table 5 and Figure 2). The destruction of lung tissue is slowed down. However, any accidental infection with a new highly virulent germ can break down peripheral tolerance to the dominant pathogen and trigger uncontrolled inflammatory reaction, quickly leading to death, as it occurs with cepacia syndrome (Blackburn et al., 2004; Gilchrist et al., 2012; Sfeir, 2018). It should be noted that the adapted poorly virulent pathogens are highly resistant to antibiotics and vaccination, and their complete eradication is impossible (Wang et al., 2020; Sousa et al., 2021).
In situ biomarkers assessment revealed decreased IL-17F and elevated TNFα levels in sputum samples of Bcc infected patients compared to those of uninfected ones. Besides, patients with Bcc tended to show an increase in sputum MMP-9 and reduction in MMP-12 concentrations (see Table 4). It is known, that both important opportunistic CF pathogens B. cenocepacia and P. aeruginosa induce NF-κB activation triggering TNFα and MMPs (including MMP-9 and MMP-12) expression in epithelial and immune cells (Zughaier et al., 1999; Bamford et al., 2007; McKeon et al., 2010; Wright et al., 2011; Park et al., 2015; Hendrix and Kheradmand, 2017). However, in vitro experiments had shown that alive Bcc strains and/or Bcc LPS elicit up to 25 times more TNF-α compared to strains and/or LPS of other CF pathogens including P. aeruginosa (McKeon et al., 2010; Cabral-Pacheco et al., 2020). It has been noted that unlike our study, Hansen et al. did not find any differences in TNFα levels in sputum samples of CF patients with Bcc (n=11) and P. aeruginosa (n=21) infections. However, the patients in this study were older (mean age, 29.5 and 28.5 years, respectively). In addition, the authors did not normalize TNF-α concentrations to the protein or urea content of each sputum sample (Hansen et al., 2010).
Several authors had reported a TNF-mediated induction of MMP-9 synthesis and release by non-CF immune and epithelial cells (Richardson, 2010; Cabral-Pacheco et al., 2020). Beeh et al. had shown that MMP-9 positively correlated with TNF-α in the sputum samples of lung transplant recipients and COPD patients, suggesting a regulatory role of TNF-α on MMP-9 expression and activation (Beeh et al., 2001, Beeh et al., 2003). In accordance with the above, in the study by Wright et al. B. cenocepacia strain BC7 (but not B. multivorans strain LMG13010, or P. aeruginosa strain PAO1) dramatically increased MMP-9 expression and activity in both CF and non-CF lung epithelial cells (Wright et al., 2011). The activation of MMP-9 correlated with an impairment of wound repair in confluent monolayers of lung epithelial cells. Specific inhibition of MMP-9 in medium from cells exposed to B. cenocepacia completely reversed this negative effect (Wright et al., 2011). Several studies have investigated the link between MMP-9 and CF lung disease severity. BAL fluid MMP-9 levels are elevated and negatively correlated with lung function, as measured by FEV1, in adults and children with CF (Sagel et al., 2005; Hilliard et al., 2007; Devereux et al., 2014; Hendrix and Kheradmand, 2017).
Our study is believed to be the first one reported sputum MMP-9 and MMP-12 levels in CF patients with chronic Bcc colonization. MMP-9 possessing high gelatinolytic activity can cleave collagen before prolyl endopeptidase further degrades the gelatin fragments to form neutrophil chemoattractant proline–glycine–proline (PGP), which contributes to the chronic neutrophilic inflammation in CF patients (Gaggar et al., 2008). In the same time, in vivo study by Hong et al. had shown that MMP-9 specifically inactivated the cytokine IL-17А, thereby terminating neutrophilia in lungs of mice with acute S. pneumoniae infection (Hong et al., 2011). MMP-12, a potent elastin-degrading proteinase, is secreted by lung macrophages (Shan et al., 2009). Previous studies had demonstrated that elevated levels of MMP-12 in the sputum were associated with emphysema severity in COPD and asthma patients. MMP-12 activity was found to be elevated on the surface of airway macrophages in bronchoalveolar lavage from young CF children with early lung disease. In addition, SNP in the MMP-12 promoter (rs2276109) and a tightly linked SNP (rs737693) were seemed to be positively associated with severity of CF lung disease (Trojanek et al., 2014). We did not find any data concerning effects of B. cenocepacia on MMP-12 expression and activity. In the same time in vitro investigation demonstrated that infection with P. aeruginosa strain K induced NF-κB mediated MMP-12 expression in human airway epithelial H292 cells (Park et al., 2015). Study by Shan et al. had shown that IL-17A, the canonical Th17 cell cytokine, enhanced secretion of MMP-12 from lung macrophages (Shan et al., 2009). So, specific immunological in situ profiles of Bcc infected group with increased TNF-α/MMP-9 and Bcc free group with elevated IL-17F/MMP-12 (see Table 4) are completely in line with the data of the recent studies.
IL-17A and IL-17 F are the most studied members of IL-17 cytokines family. They share more than 50% structural homology, form heterodimers and have two common receptors: IL-17RA and IL-17RC. Both cytokines possess similar pro-inflammatory activity, though IL-17A is the more potent than IL-17
留言 (0)