Chronic total occlusion (CTO) is a common occurrence in patients with coronary artery disease (CAD), affecting a third of patients with CAD (1, 2). The main treatment for CTO is percutaneous coronary intervention (PCI) to achieve revascularization. Previous studies have shown several clinical benefits of successful CTO recanalization, including angina relief, decreased ischemic burden, and even increased survival (3). Coronary collateral circulation (CCC) plays a vital role in maintaining myocardial perfusion in the presence of coronary artery occlusion (4). Previous studies have suggested that well-developed collaterals could reduce infarct size and improve ventricular function, benefit CTO-PCI revascularization, and be related to a better long-term prognosis in patients with CAD (4, 5).
Metabolic syndrome (MetS), a disease related to multiple factors, could lead to a poor prognosis for cardiovascular disease (6, 7). It has been reported that diabetic patients with CTO are associated with a higher incidence of revascularization and total adverse cardiovascular events over a period of 5 years (8). Successful CTO revascularization in diabetic patients may be related to better long-term survival benefits, but this is not observed in the non-diabetic population (9–11). Yilmaz et al. (12) found that the incidence of MetS was higher in patients with poor circulation compared to those with good CCC. As MetS is similar to diabetes, we speculate that poor CCC and MetS may also adversely affect the long-term clinical prognosis of CTO patients after PCI.
In this study, we aimed to investigate the effect of CCC on major adverse cardiovascular and cerebrovascular events (MACCEs) in patients with and without MetS after successful CTO-PCI. The findings from this study may have important implications for risk stratification and treatment strategies for patients with CTO-PCI.
Methods Study populationThis is a retrospective cohort study conducted at the People's Hospital of Liaoning Province between February 2021 and September 2023. The inclusion criteria were as follows: (1) aged ≥18 years, (2) diagnosed with CTO, (3) without a history of PCI or coronary artery bypass grafting (CABG), and (4) with complete clinical data. Patients were excluded based on at least one of the following conditions: (1) contraindications for PCI or contrast agent injection; (2) concurrent cardiac diseases like heart failure or pulmonary heart disease; (3) severely impaired liver or kidney functions; and (4) malignant tumors or immune system diseases.
CTO was defined as arteries occluded for a documented duration of occlusion ≥3 months with absolutely antegrade flow through the lesion [thrombolysis in myocardial infarction (TIMI) grade 0 flow] (13). The Syntax score served as a reproducible angiographic tool to quantify the extent of coronary artery disease. MetS was determined based on the criteria of the International Diabetes Federation (14). Participants were required to have a waist circumference of ≥94 cm (men) or ≥80 cm (women). Meanwhile, participants needed to meet at least two of the following criteria: (1) glucose levels ≥5.6 mmol/L or diagnosed diabetes; (2) low high-density lipoprotein cholesterol (HDL-C) levels <1.0 mmol/L (men), <1.3 mmol/L (women), or receiving drug treatment for low HDL-C; (3) triglyceride (TG) levels ≥1.7 mmol/L or receiving drug treatment for high TG; (4) blood pressure ≥130/85 mmHg or receiving drug treatment for hypertension. The study protocol was reviewed and approved by the Ethics Committee of the People's Hospital of Liaoning Province (Approval No. 2023-K063). All participants signed written informed consent.
Data collectionTrained physicians or nurses collected the following information about patients: demographic data, disease characteristics, treatment-related data, and occurrences of MACCEs. Demographic data included age, gender, height, weight, smoking and drinking habits, family history of CAD, history of myocardial infarction (MI), cerebral infarction, diabetes mellitus (DM), and MetS. Before the coronary interventions were performed, information on the characteristics of the disease was collected, including the number of occluded vessels, location of the CTO lesion, left ventricular ejection fraction, number of recanalized vessels in the CTO lesion, complete revascularization, and number of implanted stents. Treatment-related data included the type of therapeutic drugs, such as angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), β-blockers, statins, and hypoglycemic drugs.
Assessment of collateral circulationThe Rentrop scoring system was used to evaluate the grading of coronary collateralization: grade 0 indicates no visible filling of any collateral vessel, grade 1 indicates filling of the side branches by collateral vessels without filling of the epicardial arteries, grade 2 indicates partial filling of the epicardial artery by collateral vessels, and grade 3 indicates complete filling of the epicardial artery by collateral vessels (15). The Rentrop classification, categorized as grade 0 or 1, was defined as a poor coronary collateralization group, and grade 2 or 3 was considered a good group.
Outcomes and follow-upThe outcome was MACCEs, consisting of all-cause death, cardiac death, non-fatal MI, target vessel revascularization (TVR), and non-fatal stroke (15). Cardiac death was defined as any death for which a definite non-cardiac cause could not be determined. MI was defined as participants with typical chest pain, ST-segment deviation, T wave changes, and creatine kinase-myocardial band levels at least three times the upper limit of normal (16). TVR, which included interventions on the target and non-target vessels by PCI or CABG, was performed in patients with severe in-stent restenosis or newly emerged coronary lesions (70% luminal diameter stenosis) (15). The study population was followed up at 3, 6, 9, and 12 months after discharge through office interviews, outpatient visits, telephone consultations, and a review of medical records.
Statistical analysisThe normality of continuous variables was tested by skewness and kurtosis, while homogeneity was detected by the Levene test. Continuous variables with a normal distribution were described by the mean ± SD (standard deviation), while variables without a normal distribution were described by the median (interquartile range). Categorical variables were expressed as numbers and percentages. Student's t-test was used to compare group differences for continuous variables satisfying normal distribution and homogeneity of variance. A Satterthwaite t-test was used for continuous variables exhibiting normal distribution but lacking homogeneity of variance. For continuous variables that did not exhibit a normal distribution or homogeneity of variance, the Wilcoxon rank-sum test was used to evaluate differences between the two groups. The chi-squared test and Fisher's exact test were conducted to assess categorical variables between different groups, while the Wilcoxon rank-sum test was used for rank data. Covariates with P < 0.05 on univariate logistic analysis were considered potential confounders. Multivariable logistic regression analyses were conducted to investigate the relationship between the status of CCC and MACCEs. The results were presented as odds ratios (ORs) with their corresponding 95% confidence intervals (CIs). Survival curves were plotted for the two groups using the Kaplan–Meier method. Subgroup analyses stratified by MetS were also performed to explore the association between CCC and MACCEs. Model 1 was the crude model. Model 2 was adjusted for history of MI, number of occluded vessels, ACEI or ARB, and statins. The association of CCC with MACCEs was also explored in different DM and Syntax score subgroups. A two-sided P < 0.05 was used to indicate statistical significance. All analyses were performed using R version 4.2.3 (2023-03-15 ucrt).
Results Characteristics of CTP patientsA total of 342 CTO patients undergoing PCI were enrolled, with an average age of 61.43 years. Among them, 151 patients were classified as having a good CCC. There was statistical significance between the two groups in terms of smoking (P < 0.05). The demographic, clinical, and treatment information is presented in Table 1. Figure 1 illustrates the participants selection process.
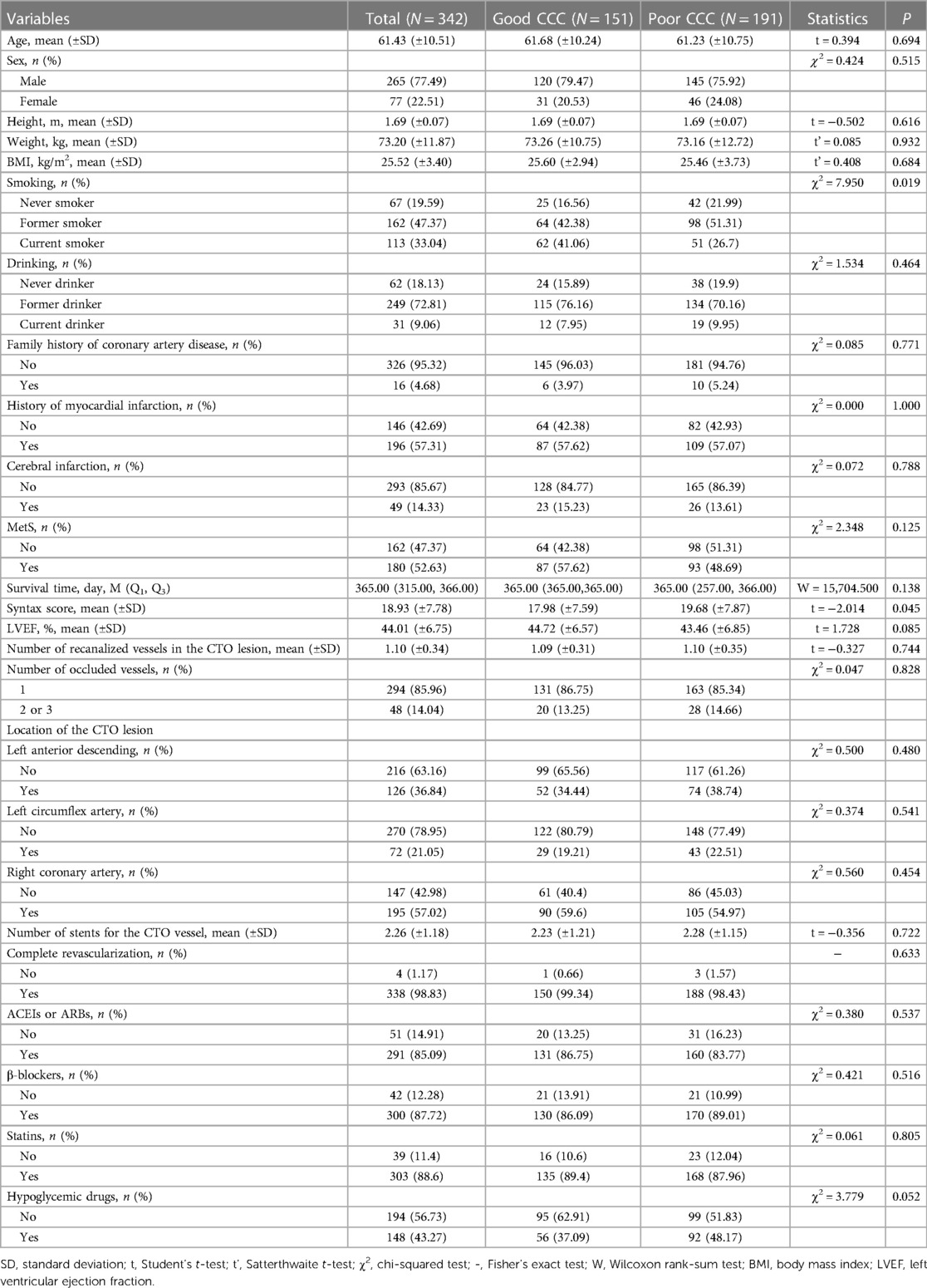
Table 1 Characteristics of CTO patients with good and poor CCC.
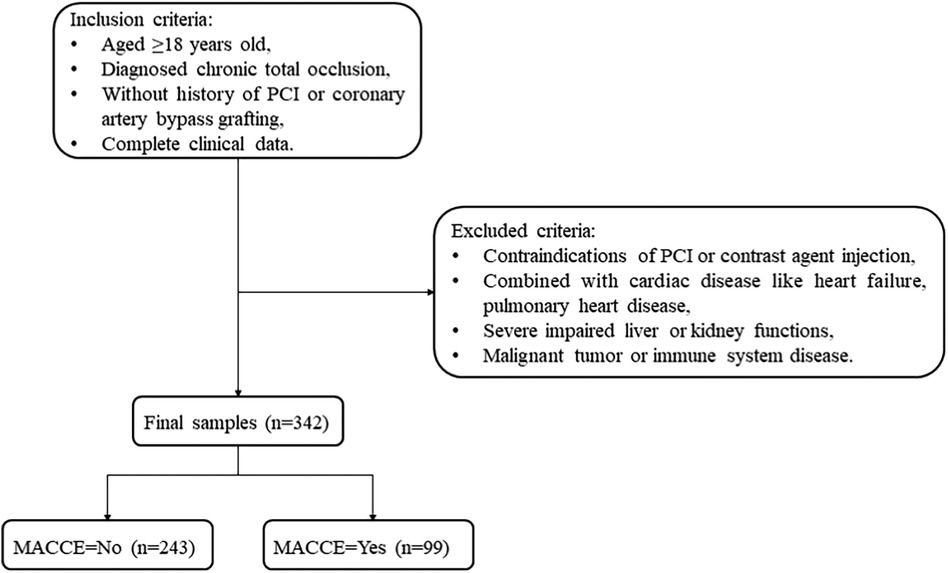
Figure 1 Flowchart of CTO patient screening.
MACCEs in CTO patientsTable 2 presents the clinical outcomes of CTO patients with good or poor CCC. During the 1-year follow-up period, 99 CTO patients experienced MACCEs. In total, 18 CTO patients succumbed to all-cause death, with 17 of them being attributed to cardiac death. In addition, 78 CTO patients experienced non-fatal MI, while 15 CTO patients suffered a non-fatal stroke. The survival curve of the MetS group was significantly lower than that of the non-MetS group (Figure 2). All participants received coronary angiography during follow-up, with 77 of them undergoing repeat revascularization. Overall, the rate of MACCEs and their components was higher in patients with poor CCC compared to those with good CCC.

Table 2 Clinical outcomes of CTO patients with good or poor CCC.
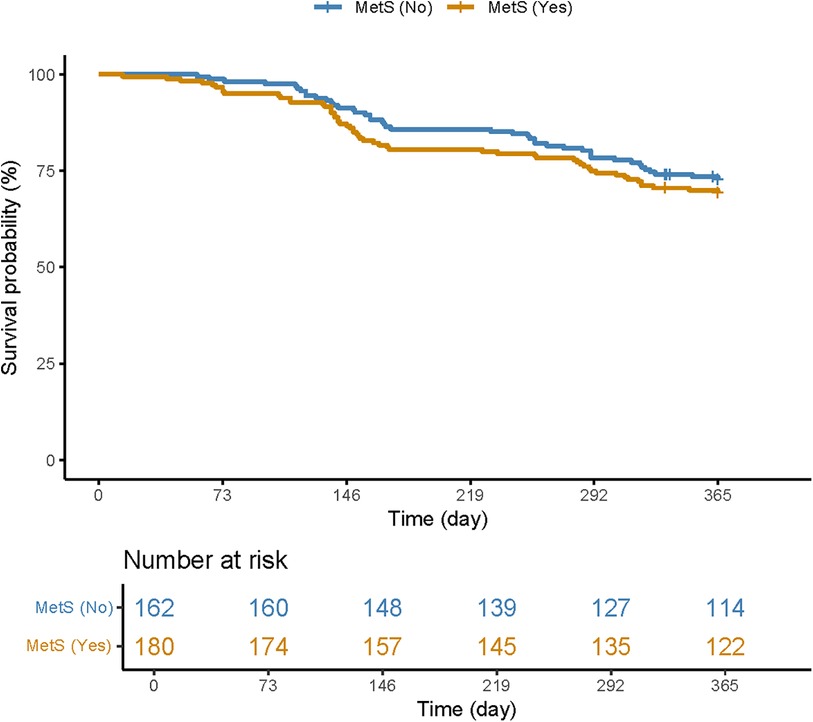
Figure 2 Kaplan–Meier curve plotted for patients with and without MetS.
Association between CCC and MACCEs in CTO patientsIn model 2, confounders were adjusted, including history of MI, number of occluded vessels, ACEI or ARB, and statin use. Poor CCC was related to a higher incidence of MACCEs (OR = 3.33, 95% CI: 1.93–5.72), non-fatal MI (OR = 3.11, 95% CI: 1.73–5.58), TVR (OR = 3.06, 95% CI: 1.70–5.53), and stent thrombosis (OR = 6.14, 95% CI: 2.76–13.65) (Table 3).
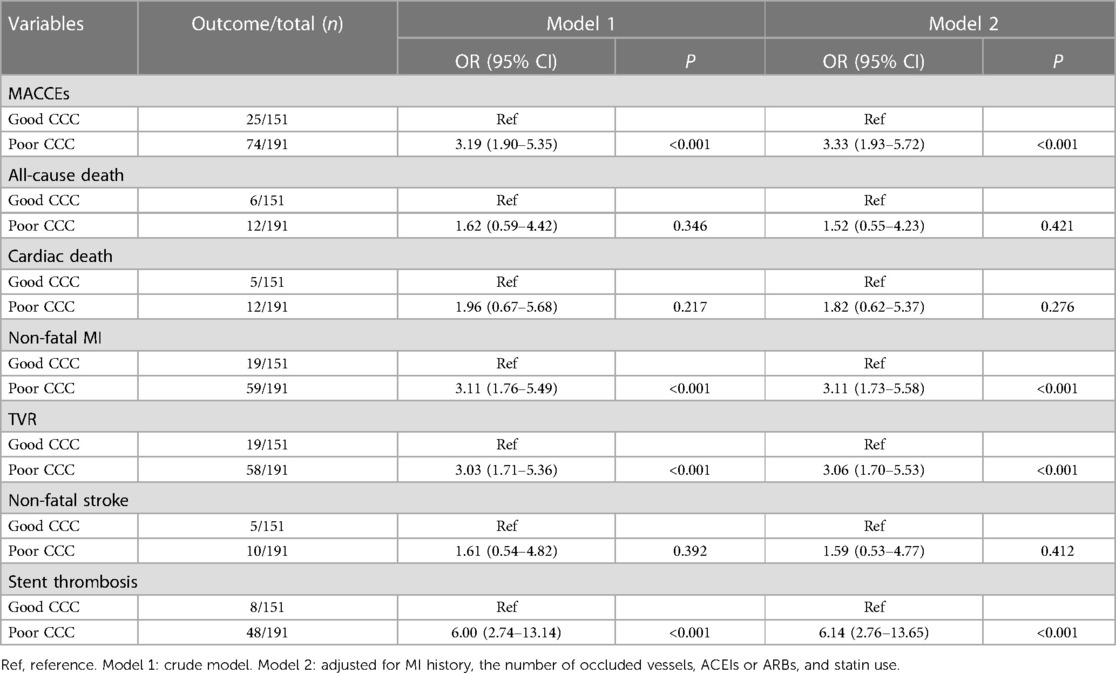
Table 3 Association of CCC with MACCEs in CTO patients.
The relationship of CCC status with MACCEs was further assessed in CTO patients with or without MetS. Poor CCC in patients with MetS was associated with higher odds of MACCEs (OR = 4.21, 95% CI: 2.05–8.65), non-fatal MI (OR = 4.44, 95% CI: 2.01–9.83), TVR (OR = 3.28, 95% CI: 1.51–7.11), and stent thrombosis (OR = 10.80, 95% CI: 3.11–37.54) (Table 4).
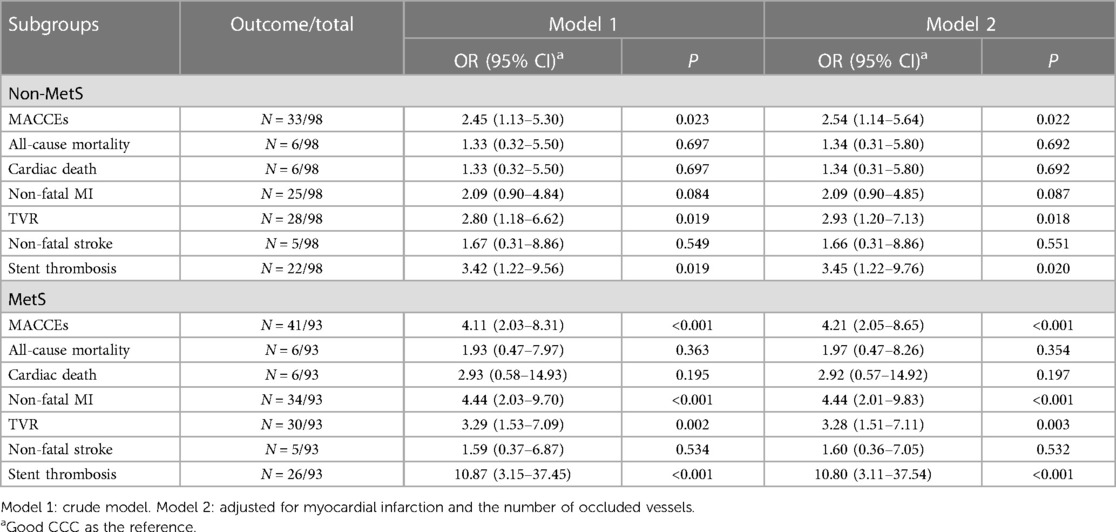
Table 4 Association of coronary collateral circulation with MACCEs and their components in patients with or without MetS.
Association between CCC and MACCEs in CTO patients with different DM and Syntax score subgroupsTable 5 illustrates the relationship between CCC and MACCEs in different DM and Syntax score subgroups. In DM patients, poor CCC was related to higher odds of MACCEs (OR = 4.42, 95% CI: 1.96–10.97), non-fatal MI (OR = 4.12, 95% CI: 1.70–11.39), TVR (OR = 3.09, 95% CI: 1.34–7.83), and stent thrombosis (OR = 10.98, 95% CI: 2.97–71.98). In CTO patients with Syntax score ≥23, poor CCC was associated with a higher incidence of MACCEs (OR = 3.83, 95% CI: 1.43–11.72), non-fatal MI (OR = 5.89, 95% CI: 1.77–27.28), TVR (OR = 3.45, 95% CI: 1.19–11.89), and stent thrombosis (OR = 11.49, 95% CI: 2.64–89.60).
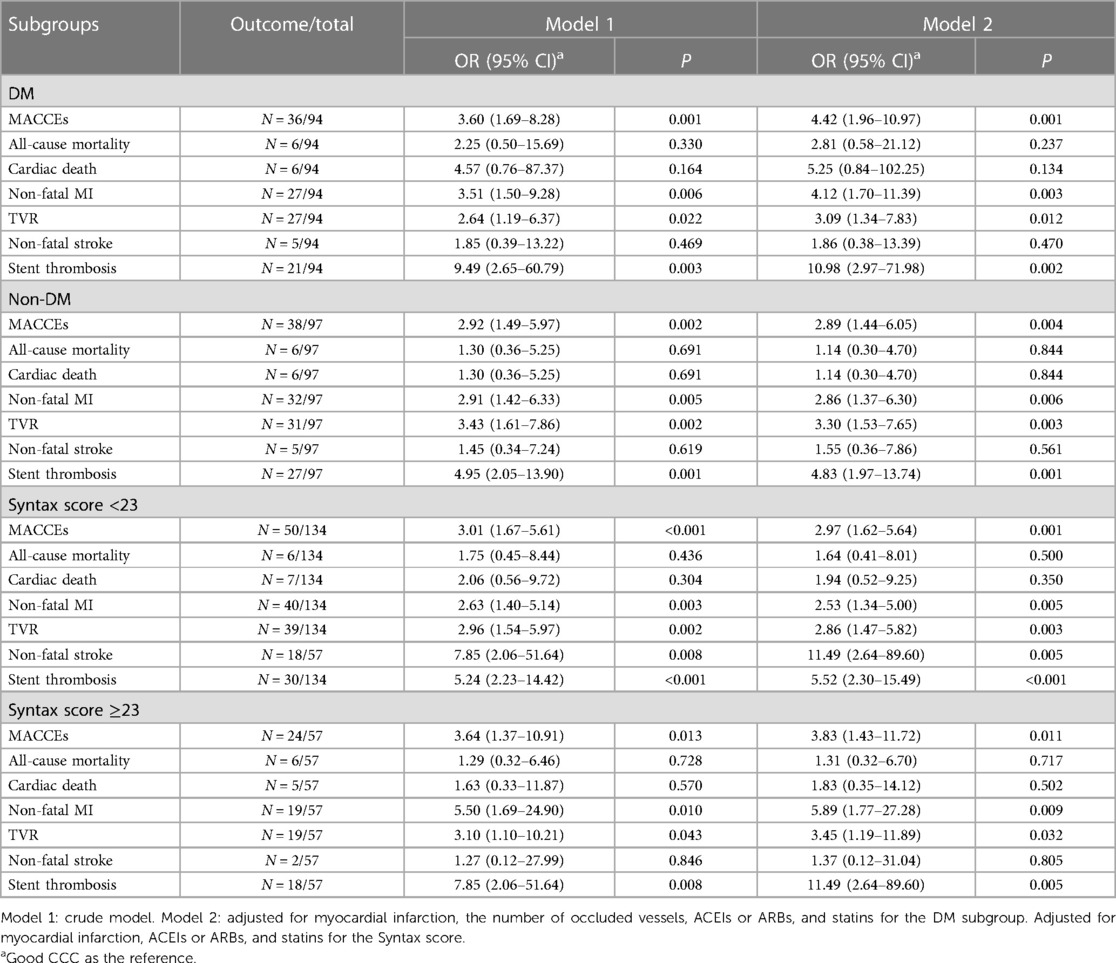
Table 5 Association of coronary collateral circulation with MACCEs in different DM and syntax score subgroups.
DiscussionOur study investigated the relationship between CCC and MACCEs in patients who underwent PCI for CTO. The results suggested that poor CCC was associated with MACCEs, non-fatal MI, TVR, and stent thrombosis in CTO patients. Similar findings were observed in CTO patients with MetS, with even significantly higher odds of MACCEs. This suggests that the CCC status of CTO patients and MetS may have a combined effect on MACCEs.
Our findings were consistent with previous studies on the impact of CCC status on the prognosis of CTO patients (15, 17, 18). CCC is a beneficial prognostic factor (19). Collateral vessels provide an important alternative route for blood flow, especially in vessel occlusion, and are associated with improved outcomes and reduced ischemic injury (20). Conversely, poor collateralization has been related to adverse events such as myocardial infarction and mortality (21). In contrast, Li et al. (22) reported that good CCC was not associated with a lower risk of cardiac death or MACCEs in CTO patients. Some factors, such as coronary steal, microcirculation dilation, and endothelial dysfunction, may offset the potential benefits of collateral vessels, thus leading to inadequate oxygen and flow supply through collateral vessels, concomitant with an elevated predisposition to arrhythmias in patients with good CCC. Future studies are needed to clarify the relationship between CCC and MACCEs in CTO patients.
In addition, the impact of poor CCC on MACCEs was particularly pronounced in patients with MetS. MetS constitutes a constellation of risk factors, including central obesity, insulin resistance, hypertension, and dyslipidemia, and is associated with poor coronary collateralization and increased cardiovascular risk (6, 23). Our results revealed that poor CCC in CTO patients with MetS was associated with higher odds of MACCEs and related events.
The mechanisms underlying the association between poor CCC and MACCEs in CTO patients with MetS involve complex pathophysiological interactions. In patients undergoing CTO-PCI, poor CCC may reflect a higher burden of coronary artery burden, with impaired development of collateral vessels unable to sufficiently compensate for the occluded vessel (24). This impaired collateralization may result from genetic predisposition, microvascular dysfunction, or inadequate release of pro-angiogenic factors (25, 26). Inadequate collateral support resulted in ongoing myocardial ischemia, impaired myocardial function, and increased susceptibility to adverse events (27, 28). Furthermore, consistent with our results, stent thrombosis was more prevalent in poor CCC, as collateral flow has been shown to protect against thrombus formation and facilitate myocardial reperfusion (29).
In patients with MetS, poor CCC may further exacerbate the cardiovascular effects associated with the syndrome. The presence of MetS is associated with endothelial dysfunction, chronic inflammation, and a prothrombotic state, all of which may contribute to impaired collateral vessel formation and function (30, 31). The presence of poor CCC in MetS patients may signify an inability to adequately respond to ischemic insults, leading to an increased risk of adverse events (32). There may be a combined effect between MetS and CCC.
In CTO patients with DM or a Syntax score ≥23, poor CCC was also related to high odds of MACCEs. It is well established that individuals with diabetes exhibit impaired collateral vessel development due to factors such as endothelial dysfunction, abnormal angiogenesis, and impaired growth factor signaling (33). These factors collectively contribute to reduced collateral vessel formation, resulting in compromised vascular supply to the ischemic myocardium. In the context of CTO patients with diabetes, this impaired collateralization may further aggravate the ischemic burden, leading to a higher risk of adverse cardiovascular events. A higher Syntax score indicates more severe and complex coronary artery disease, indicating the presence of multiple lesions or diffuse disease. In patients with a Syntax score ≥23, the extent of atherosclerotic burden is substantial, potentially leading to impaired collateral vessel formation and poorer perfusion to the myocardium. Moreover, the high complexity of lesions in this subgroup may increase the risk of stent thrombosis and TVR.
In patients who underwent CTO-PCI, the extent of CTO disease, blood glucose, blood lipids, and blood pressure should be monitored closely. Identifying patients with poor CCC following CTO-PCI and those with MetS could contribute to risk stratification in patients and guide targeted therapeutic interventions. More attention should be paid to CTO patients with DM and a Syntax score ≥23. Strategies aimed at enhancing CCC, such as physical activity, pharmacological interventions, and targeted revascularization strategies, may prove beneficial in these high-risk patient populations. In addition, close monitoring and aggressive management of modifiable risk factors may be warranted for individuals with poor CCC and MetS to mitigate their heightened risk of MACCEs and related events.
The current study has several limitations that need to be considered. First, the study population consisted of a single-center cohort, which may limit the generalizability of our findings. Multicenter, large-sample studies are needed in the future. Moreover, there were potential confounding factors that were not accounted for in this analysis, such as medication and calcification of blood vessels. Finally, clinical follow-up was relatively short, and the long-term prognostic relationship between CCC, MetS, and MACCEs was not fully investigated.
ConclusionPoor CCC has been associated with an increased risk of MACCEs in CTO patients, particularly those with MetS. Comprehensive risk evaluation and individualized management strategies are essential for patients with poor CCC. Further prospective multicenter studies are needed to confirm our results and investigate potential therapeutic interventions.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statementThe studies involving humans were approved by the People’s Hospital of Liaoning Province. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsYS: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. BZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. XinZ: Formal Analysis, Funding acquisition, Methodology, Project administration, Writing – review & editing. XiaZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. WB: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. HB: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. BL: Conceptualization, Project administration, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Kearney K, Hira RS, Riley RF, Kalyanasundaram A, Lombardi WL. Update on the management of chronic total occlusions in coronary artery disease. Curr Atheroscler Rep. (2017) 19:19. doi: 10.1007/s11883-017-0655-0
PubMed Abstract | Crossref Full Text | Google Scholar
3. Azzalini L, Karmpaliotis D, Santiago R, Mashayekhi K, Di Mario C, Rinfret S, et al. Contemporary issues in chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc Interv. (2022) 15:1–21. doi: 10.1016/j.jcin.2021.09.027
PubMed Abstract | Crossref Full Text | Google Scholar
4. Cetin MS, Ozcan Cetin EH, Balcı KG, Aydin S, Ediboglu E, Bayraktar MF, et al. The association between whole blood viscosity and coronary collateral circulation in patients with chronic total occlusion. Korean Circ J. (2016) 46:784–90. doi: 10.4070/kcj.2016.46.6.784
PubMed Abstract | Crossref Full Text | Google Scholar
5. Wu K, Huang Z, Zhong Z, Liao H, Zhou Y, Luo B, et al. Predictors, treatment, and long-term outcomes of coronary perforation during retrograde percutaneous coronary intervention via epicardial collaterals for recanalization of chronic coronary total occlusion. Catheter Cardiovasc Interv. (2019) 93:800–9. doi: 10.1002/ccd.28093
PubMed Abstract | Crossref Full Text | Google Scholar
6. Liu T, Wu Z, Liu J, Lv Y, Li W. Metabolic syndrome and its components reduce coronary collateralization in chronic total occlusion: an observational study. Cardiovasc Diabetol. (2021) 20:104. doi: 10.1186/s12933-021-01297-4
PubMed Abstract | Crossref Full Text | Google Scholar
7. Li X, Zhai Y, Zhao J, He H, Li Y, Liu Y, et al. Impact of metabolic syndrome and its components on prognosis in patients with cardiovascular diseases: a meta-analysis. Front Cardiovasc Med. (2021) 8:704145. doi: 10.3389/fcvm.2021.704145
PubMed Abstract | Crossref Full Text | Google Scholar
8. Mashaly A, Rha S-W, Choi BG, Baek MJ, Ryu YG, Choi SY, et al. Impact of diabetes mellitus on 5-year clinical outcomes in patients with chronic total occlusion lesions. Coron Artery Dis. (2018) 29:119. doi: 10.1097/MCA.0000000000000562
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zhao S, Chen Y, Wang Q, Zhu B, Wei Z, Wang Z, et al. Benefits of successful percutaneous coronary intervention in chronic total occlusion patients with diabetes. Cardiovasc Diabetol. (2022) 21:271. doi: 10.1186/s12933-022-01708-0
PubMed Abstract | Crossref Full Text | Google Scholar
10. Zhu Y, Meng S, Chen M, Liu K, Jia R, Li H, et al. Long-term prognosis of chronic total occlusion treated by successful percutaneous coronary intervention in patients with or without diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. (2021) 20:29. doi: 10.1186/s12933-021-01223-8
PubMed Abstract | Crossref Full Text | Google Scholar
11. Tsai C-T, Huang W-C, Teng H-I, Tsai Y-L, Lu T-M. Long term clinical impact of successful recanalization of chronic total occlusion in patients with and without type 2 diabetes mellitus. Cardiovasc Diabetol. (2020) 19:119. doi: 10.1186/s12933-020-01093-6
PubMed Abstract | Crossref Full Text | Google Scholar
12. Yilmaz MB, Caldir V, Guray Y, Guray U, Altay H, Demirkan B, et al. Relation of coronary collateral vessel development in patients with a totally occluded right coronary artery to the metabolic syndrome. Am J Cardiol. (2006) 97:636–9. doi: 10.1016/j.amjcard.2005.09.103
PubMed Abstract | Crossref Full Text | Google Scholar
13. Ybarra LF, Rinfret S, Brilakis ES, Karmpaliotis D, Azzalini L, Grantham JA, et al. Definitions and clinical trial design principles for coronary artery chronic total occlusion therapies: CTO-ARC consensus recommendations. Circulation. (2021) 143:479–500. doi: 10.1161/CIRCULATIONAHA.120.046754
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ebrahimi H, Emamian MH, Khosravi A, Hashemi H, Fotouhi A. Comparison of the accuracy of three diagnostic criteria and estimating the prevalence of metabolic syndrome: a latent class analysis. J Res Med Sci. (2019) 24:108. doi: 10.4103/jrms.JRMS_858_18
PubMed Abstract | Crossref Full Text | Google Scholar
15. Yang ZK, Shen Y, Dai Y, Wang XQ, Hu J, Ding FH, et al. Impact of coronary collateralization on long-term clinical outcomes in type 2 diabetic patients after successful recanalization of chronic total occlusion. Cardiovasc Diabetol. (2020) 19:59. doi: 10.1186/s12933-020-01033-4
PubMed Abstract | Crossref Full Text | Google Scholar
16. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. doi: 10.1161/CIR.0000000000000617
PubMed Abstract | Crossref Full Text | Google Scholar
17. Allahwala UK, Kott K, Bland A, Ward M, Bhindi R. Predictors and prognostic implications of well-matured coronary collateral circulation in patients with a chronic total occlusion (CTO). Int Heart J. (2020) 61:223–30. doi: 10.1536/ihj.19-456
PubMed Abstract | Crossref Full Text | Google Scholar
18. Wang B, Han Y-L, Li Y, Jing Q-M, Wang S-L, Ma Y-Y, et al. Coronary collateral circulation: effects on outcomes of acute anterior myocardial infarction after primary percutaneous coronary intervention. J Geriatr Cardiol. (2011) 8:93–8. doi: 10.3724/SP.J.1263.2011.00093
PubMed Abstract | Crossref Full Text | Google Scholar
19. Seiler C, Meier P. Historical aspects and relevance of the human coronary collateral circulation. Curr Cardiol Rev. (2014) 10:2–16. doi: 10.2174/1573403×113099990028 23859295
PubMed Abstract | Google Scholar
21. Allahwala UK, Nour D, Bhatia K, Ward MR, Lo S, Weaver JC, et al. Prognostic impact of collaterals in patients with a coronary chronic total occlusion: a meta-analysis of over 3,000 patients. Catheter Cardiovasc Interv. (2021) 97:E771–7. doi: 10.1002/ccd.29348
PubMed Abstract | Crossref Full Text | Google Scholar
22. Li Z, Wang Y, Wu S, Xiao J, Guo L, Meng S, et al. Good coronary collateral circulation is not associated with better prognosis in patients with chronic total occlusion, regardless of treatment strategy. Hellenic J Cardiol. (2023) 69:9–15. doi: 10.1016/j.hjc.2022.12.001
PubMed Abstract | Crossref Full Text | Google Scholar
23. Mouquet F, Cuilleret F, Susen S, Sautière K, Marboeuf P, Ennezat PV, et al. Metabolic syndrome and collateral vessel formation in patients with documented occluded coronary arteries: association with hyperglycaemia, insulin-resistance, adiponectin and plasminogen activator inhibitor-1. Eur Heart J. (2009) 30:840–9. doi: 10.1093/eurheartj/ehn569
PubMed Abstract | Crossref Full Text | Google Scholar
24. Allahwala UK, Khachigian LM, Nour D, Ridiandres A, Billah M, Ward M, et al. Recruitment and maturation of the coronary collateral circulation: current understanding and perspectives in arteriogenesis. Microvasc Res. (2020) 132:104058. doi: 10.1016/j.mvr.2020.104058
PubMed Abstract | Crossref Full Text | Google Scholar
27. Seiler C. Assessment and impact of the human coronary collateral circulation on myocardial ischemia and outcome. Circ Cardiovasc Interv. (2013) 6:719–28. doi: 10.1161/CIRCINTERVENTIONS.113.000555
PubMed Abstract | Crossref Full Text | Google Scholar
28. Ozdemir S, Barutcu A, Aksit E, Duygu A, Ozturk FK. Contradictory effect of coronary collateral circulation on regional myocardial perfusion that assessed by quantitative myocardial perfusion scintigraphy. Cardiol Res. (2021) 12:193–200. doi: 10.14740/cr1262
PubMed Abstract | Crossref Full Text | Google Scholar
29. Vural A, Kurt D, Karagöz A, Günaydın ZY. Well-developed coronary collateral circulation is associated with higher thrombus burden in the setting of ST-segment elevation myocardial infarction. Tex Heart Inst J. (2022) 49:e217574. doi: 10.14503/THIJ-21-7574
PubMed Abstract | Crossref Full Text | Google Scholar
30. Nair J, Kakkar VV, Shanker J. Comparative analysis of inflammatory gene expression levels in metabolic syndrome & coronary artery disease. Indian J Med Res. (2017) 145:777–85. doi: 10.4103/ijmr.IJMR_1678_14
PubMed Abstract | Crossref Full Text | Google Scholar
31. Jia G, Hill MA, Sowers JR. Vascular endothelial mineralocorticoid receptors and epithelial sodium channels in metabolic syndrome and related cardiovascular disease. J Mol Endocrinol. (2023) 71:e230066. doi: 10.1530/JME-23-0066
PubMed Abstract | Crossref Full Text | Google Scholar
32. Chilian WM, Penn MS, Pung YF, Dong F, Mayorga M, Ohanyan V, et al. Coronary collateral growth—back to the future. J Mol Cell Cardiol. (2012) 52:905–11. doi: 10.1016/j.yjmcc.2011.12.006
PubMed Abstract | Crossref Full Text | Google Scholar
33. Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. (2001) 49:554–60. doi: 10.1016/s0008-6363(00)00228-5
留言 (0)