Myocardial infarction (MI) is a frequent event that carries high morbidity and mortality worldwide. Bacterial endotoxins (lipopolysaccharides, LPSs) are pathogen-associated molecular patterns (PAMPs) that are part of the outer membrane of Gram-negative bacteria. LPSs have been described as exerting a noxious effect by triggering inflammation (TLR-4 recognition and NF-kB transcription) (1).
There is increasing evidence that endotoxemia is associated with MI through multiple underlying mechanisms. First, chronic low-grade endotoxemia has been reported in metabolic disorders (2, 3), defining the concept of metabolic endotoxemia (4). In addition, LPSs are closely linked to lipoprotein metabolism by reverse LPS transport (5, 6). These conditions are risk factors for MI, and emerging concepts suggest that endotoxemia has a role in coronary artery disease (CAD) and the genesis of MI (7, 8). Second, several studies have suggested an alteration of the gut barrier function at the acute phase of MI, resulting in acute endotoxemia (9, 10). Because endotoxins reduce cardiac performance (11) and cause vasoplegia (12), they might increase the risk of heart failure after MI and worsen short-term patient outcomes. Finally, inflammation is thought to extend myocardial injury (13) and promote recurrent cardiovascular (CV) events (14), so endotoxemia might also have negative effects on long-term recovery. Despite these potentially harmful effects, data regarding acute endotoxemia and MI are scarce and further evidence is needed (15).
The primary objective of the present study was to explore factors associated with endotoxemia at hospital admission in patients with MI and to determine whether endotoxemia at hospital admission was related to metabolic conditions (thus chronic low-grade translocation promoting atherosclerosis) or to acute gut barrier failure in the context of MI. The secondary objective was to determine the association of endotoxemia with inflammation, initial MI severity, and short-term and long-term outcomes.
Methods PatientsThis study is an ancillary analysis of a prospectively acquired database (RICO survey) (16). All consecutive patients admitted to the coronary intensive care unit (ICU) of the Dijon University Hospital (France) from January 2013 to April 2015 for type 1 MI were prospectively included. Patients with prior coronary arterial disease [MI, transluminal angioplasty, unstable angina, or coronary artery bypass graft (CABG) surgery] or chronic kidney disease were excluded. The present study agrees with the ethical guidelines of the Declaration of Helsinki. Informed consent was obtained from the participants before their inclusion in the study, and the Ethics Committee of the University Hospital of Dijon approved the protocol (BIOCARDIS-2016–9205AAO034S02117).
Data collectionPatient characteristics, including age and gender, were obtained at hospital admission, along with medical history (hypertension, diabetes, smoking, and family history of coronary artery disease, main treatments [antiplatelets, angiotensin 2 receptor blockers (ARB), angiotensin 2 converting enzyme (ACE) inhibitors, statins, and beta-blockers], and clinical data [left ventricular ejection fraction, heart rate (HR), blood pressure, catecholamine administration, and infarction location]. Shock index was defined as the heart rate divided by systolic blood pressure (SBP). Coronary artery disease burden at coronary angiography through multivessel disease and the SYNTAX (17) score were also collected. The Global Registry of Acute Coronary Events (GRACE) risk score (18), a robust prognosis tool following MI, was also calculated.
Determination of blood markersBlood samples were taken on admission. N-terminal pro-brain natriuretic peptide (Nt-ProBNP, normal value <125 pg/ml), blood lipids [normal range 1.20–2.4 g/L for cholesterol, 0.4–0.6 g/L for HDL cholesterol, and 0.4–1.5 g/L for triglycerides (TG)], glucose (normal range 4.3–6.4 mmol/L), glycated hemoglobin (HbA1c, normal value <6%), and C-reactive protein (CRP) (normal value <4 mg/L) were measured. The troponin Ic peak (normal value <0.1 µg/L) was obtained from three blood samples within 24 h following admission. The estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease-epidemiology collaboration formula (CKD-EPI).
Plasma was taken upon ICU admission in an EDTA (ethylenediaminetetraacetic acid) blood collection tube. Blood was centrifugated (2,000×g for 10 min at 4 °C), and plasma was stored at −80 °C. LPS, lipid transfer protein activities [phospholipid transfer protein (PLTP) and cholesteryl ester transfer protein (CETP)], and cytokines were retrospectively measured from this plasma collection.
LPS was quantified by measuring one of its components [3-hydroxy myristate (3HM)] using liquid chromatography coupled with tandem mass spectrometry, as previously described (19). Briefly, samples were hydrolyzed for 3 h at 90 °C in the presence of hydrochloric acid to release free fatty acids, which were subsequently extracted with water and ethane/ethyl acetate (3/2 v/v). Dried extracts were dissolved in ethanol and injected onto an SBC18 2.1 mm × 50 mm, 1.8 µm column, connected to an Infinity 1290 HPLC system (Agilent Technologies). Separation of hydroxylated fatty acids was achieved at 45 °C at a flow rate of 0.4 ml/min using ammonium formate (5 mM/0.1% formic acid) as eluent A (55% for 0.5 min) and 95% acetonitrile as eluent B (100% reached in 2.5 min and maintained for 5 min). MS/MS detection was performed in negative mode using a QqQ 6490 triple quadrupole mass spectrometer equipped with a JetStream ESI source to quantify the selected ions as follows: for 3HM, precursor ion 243.2 Da and product ion 59 Da; for 3-hydroxytridecanoic acid (IS), which was used as the internal standard, precursor ion 229.2 Da and product ion 59 Da.
PLTP and CETP activities were measured in undiluted plasma using commercially available fluorescence activity assays (Roar Biomedical), according to the manufacturer's instructions. Incubations were performed at 37 °C for 30 min (for PLTP) or 3 h (for CETP), with fluorescence monitoring (excitation, 465 nm; emission, 535 nm) throughout the incubation period with a Victor2 multilabel counter (PerkinElmer, Waltham, United States). Transfer activities were calculated from the slope of fluorescence increase between 1 and 30 min (for PLTP) or between 1 min and 1 h (for CETP) and expressed as arbitrary fluorescence units (AU).
Cytokines were measured using a Luminex® Human Magnetic assay (R&D Systems, Minneapolis, USA). The assays were performed according to the manufacturer's instructions (including for sample collection and preparation). Plasma was not diluted for this analysis. Standards and samples were analyzed on a Luminex® apparatus (BioPlex 200, Bio-Rad, Munich, Germany) using BioPlex Manager Software (Version 5, Bio-Rad, Hercules, CA, USA). For all cytokines, standard curves ranged from 3.2 to 10,000 pg/ml.
OutcomesPlasma markers included endotoxin plasma concentration, cytokine plasma concentration, and CRP.
Clinical outcomes included in-hospital major adverse CV events (MACEs), such as CV death, heart failure (defined as Killip class >1), and recurrent myocardial infarction, ventricular and supraventricular arrhythmia, and ICU length of stay. A follow-up at 1 year with the patient, next of kin, or the treating physician was conducted by mail or telephone. Occurrences of MACEs, defined as CV death, recurrent myocardial infarction, hospitalization for heart failure, unscheduled percutaneous coronary intervention (PCI), coronary artery bypass surgery, unstable angina or angina pectoris, were collected.
Statistical analysisNo sample size calculation was performed for this study. All the patients included in the cohort were analyzed. Dichotomous variables are expressed as n (%), and continuous variables are expressed as means ± standard deviations or medians [interquartile ranges (IQR)]. A Kolmogorov–Smirnov test was performed to assess the normality of continuous variables. Non-normally distributed variables were log-transformed when needed. The Mann–Whitney test or Student's t-test was used to compare continuous data, as appropriate. Pearson correlation analyses (for normally distributed variables) or Spearman correlation analyses (one or two non-Gaussian variables) were performed. The threshold for significance was set at p < 0.05. Multivariate linear regression models were built to estimate LPS levels based on significant variables in univariate analysis, with an inclusion threshold of p < 0.10. Variable selection was stepwise. The normality of residuals and homoscedasticity were checked graphically; the model was also checked for autocorrelations of residuals (Durbin–Watson test) and multicollinearity (variance inflation factor). Missing data were considered at random and were omitted. SPSS version 12.0.1 (IBM Inc., Armonk, NY, USA) was used for all analyses.
Results Baseline characteristicsTwo hundred and forty-five patients were analyzed. Baseline characteristics are presented in Table 1. The mean age was 62 ± 13 years, most patients were men (72%), and the median body mass index (BMI) was 27 (25–29). We identified 92 patients (37.6%) with hypercholesterolemia and 34 (14%) with diabetes. Most patients had ST-segment elevation (Table 2).

Table 1 Association between LPS plasma concentration and patient's characteristics.
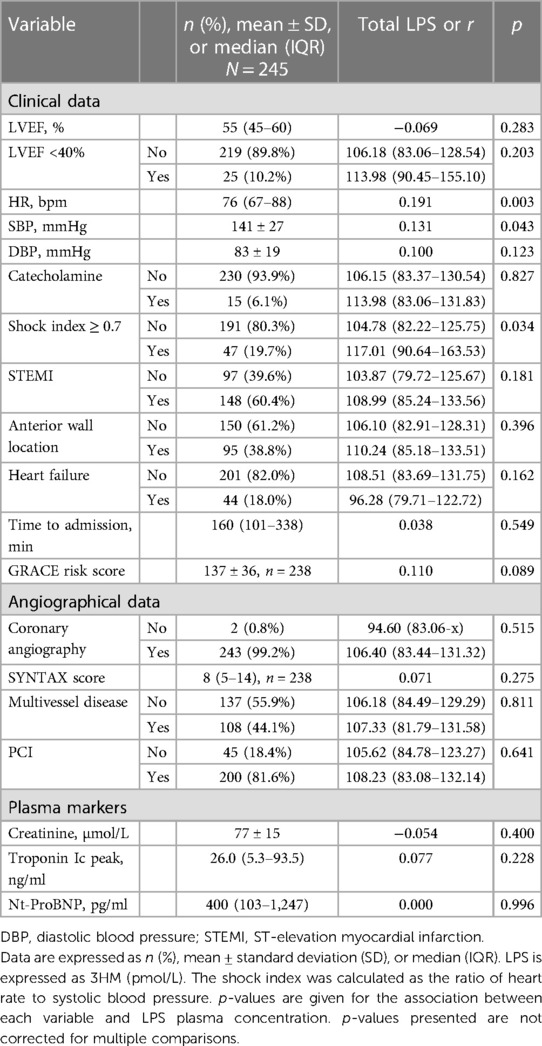
Table 2 Association between LPS plasma concentration and clinical data on admission.
Variables associated with endotoxemiaThe median LPS concentration was 106 (83–131) pmol/L of 3HM. The distribution of LPS concentration in our population is illustrated in Figure 1. Endotoxemia was associated with age, diabetes, and obesity (Table 1, Figure 2), as well as with total cholesterol (r = 0.191, p = 0.003), triglyceridemia (r = 0.201, p = 0.002), and glucose metabolism parameters (blood glucose, HbA1c) (Table 3). LPS was lower in smokers (102.06 pmol/L of 3HM (79.58–121.80) vs. 110.44 pmol/L of 3HM (91.60–132.65), p = 0.22). PLTP activity was positively correlated with LPS concentrations (r = 0.146, p = 0.022). At admission, patients with high endotoxemia had a faster heart rate (r = 0.19, p < 0.01), and a higher shock index (≥0.7) was associated with higher LPS concentrations. In multivariate analysis, triglycerides, total cholesterol, history of diabetes, and age were independently associated with LPS concentration (Table 4).
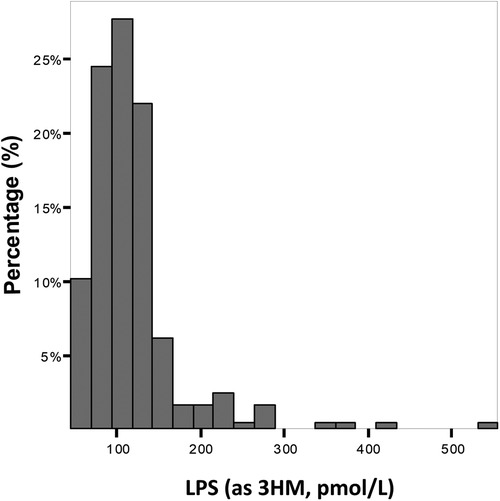
Figure 1 Distribution of the population according to LPS concentration at admission. Results are expressed as % of the population for each range of LPS concentration.
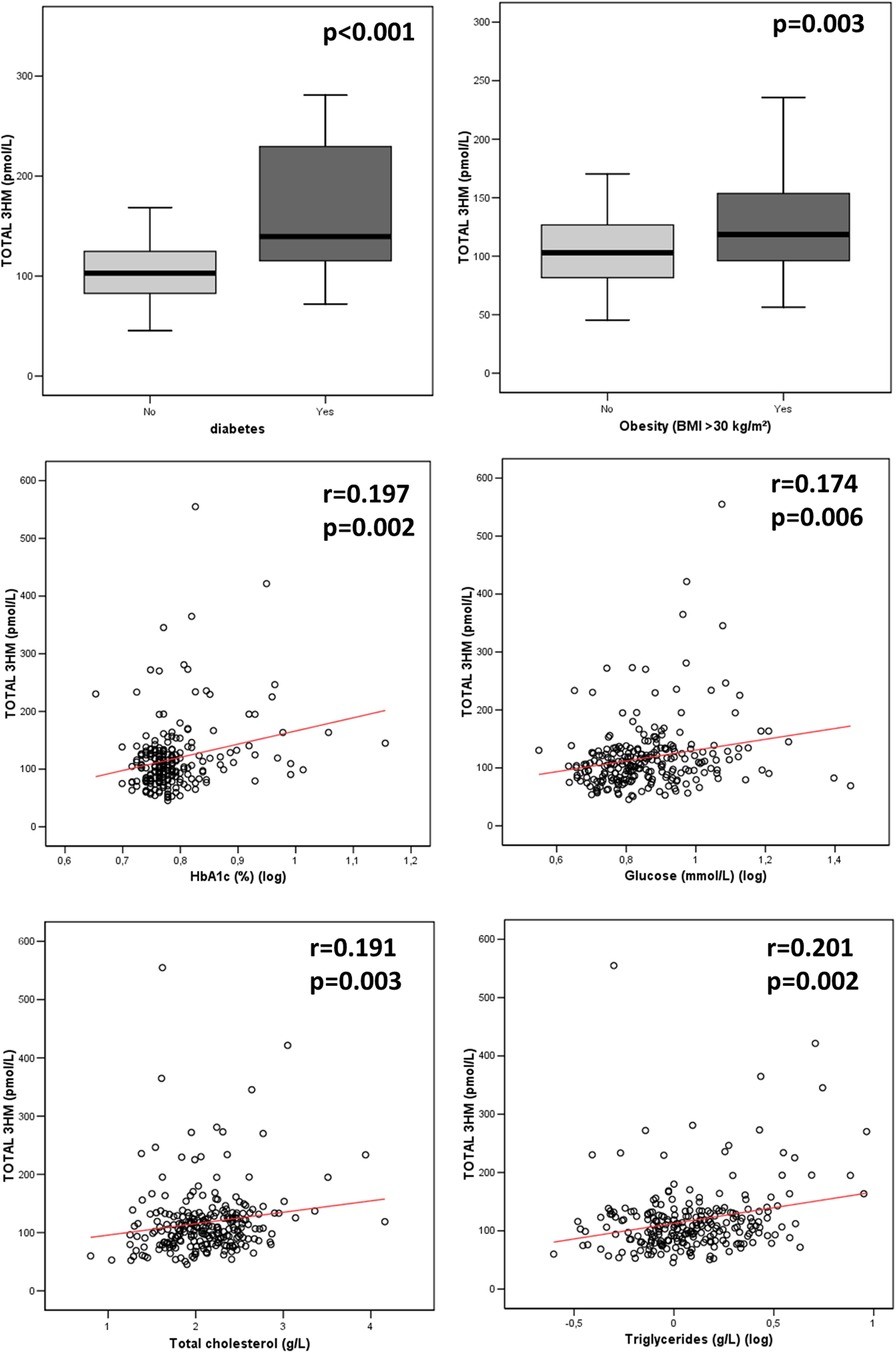
Figure 2 Association between LPS concentration and metabolic parameters at admission. p-values were computed using the Spearman test for correlation and the Mann–Whitney test for group comparisons. n = 235 for HbA1c, 245 for glycemia, 245 for diabetes, and 244 for total cholesterol, triglyceridemia, and obesity. 3HM, 3-hydroxy myristate.
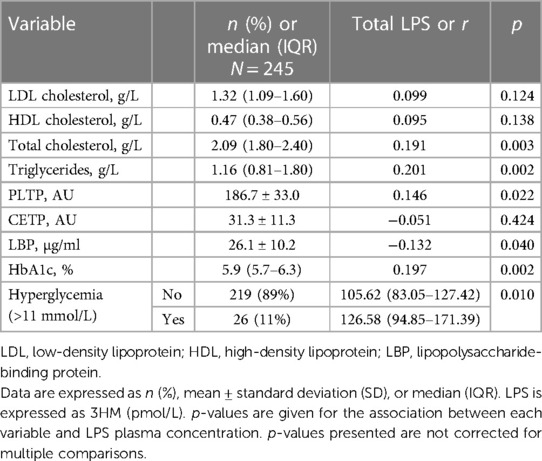
Table 3 Association between LPS plasma concentration and lipid and glucose metabolism.
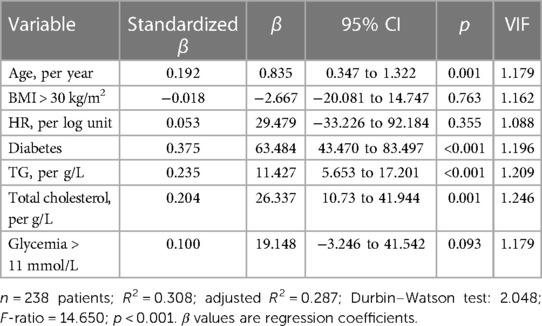
Table 4 Variables independently associated with LPS plasma concentration.
Relationship between endotoxemia and inflammationInterleukin (IL)-6 and CRP were below the detection threshold in most patients (74% and 84%, respectively). We found no association between the measured cytokines [IL-6, IL-8, tumor necrosis factor (TNF)-α] and LPS (p > 0.05). A level of CRP higher than 10 mg/L was not associated with LPS (Table 5).
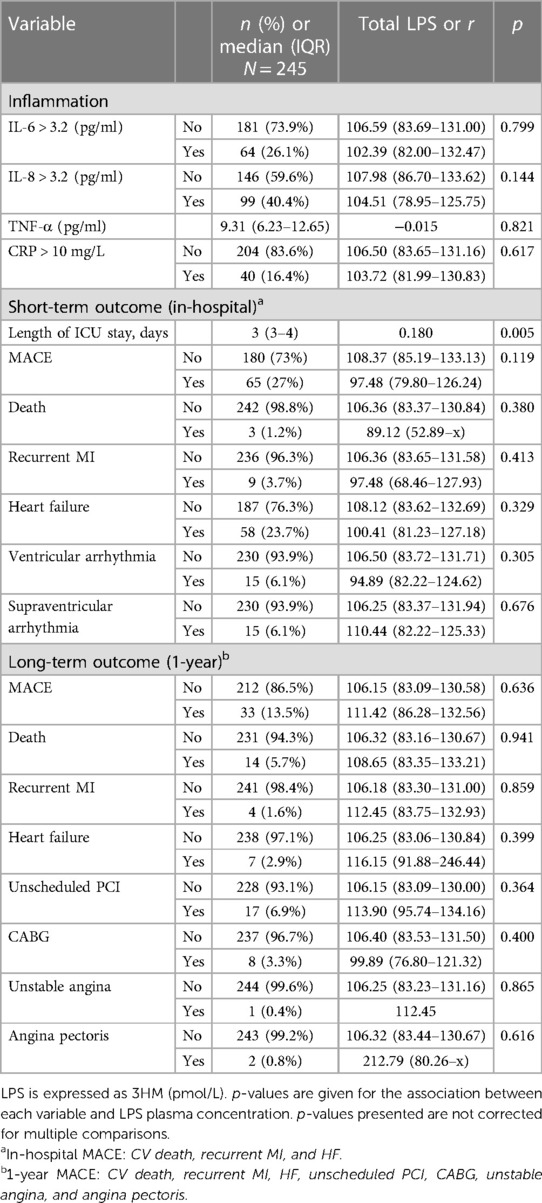
Table 5 Associations between LPS plasma concentration, inflammation, and short- and long-term outcomes.
Relationship between endotoxemia and short-term outcomeLPS blood concentration was not associated with initial severity (Table 2) nor with the occurrence of MACE (or any of its components) or all-cause mortality (p = 0.4) during hospital stay, as assessed by GRACE and SYNTAX scores. However, LPS was associated with a longer ICU stay (r = 0.18, p = 0.005) (Table 5).
Relationship between endotoxemia and long-term (1-year) outcomeLPS blood concentration was not associated with the occurrence of 1-year MACE (or any of its components) nor with all-cause mortality (p = 0.9, Table 5).
DiscussionLipopolysaccharide plasma concentration at hospital admission was associated with metabolic conditions (in particular cholesterol, triglyceride concentrations, and diabetes) and age rather than with MI severity. LPS concentrations were not associated with inflammatory biomarkers, coronary artery disease severity assessed by angiography, or short-term and long-term occurrence of MACE.
When compared with measurements obtained using the same method and in the same laboratory, the LPS plasma concentrations in our cohort were slightly higher than those reported in healthy volunteers [106 (83–131) vs. 96 (77–116)] but lower than in patients with septic shock [106 (83–131) vs. 134 (126–142)] (20). These findings are in line with previous studies that reported increased endotoxin concentrations in patients with coronary artery disease (10, 21), which led to a hypothesis about the role of LPS proinflammatory activity in atherosclerosis development (22, 23) and MI pathogenesis (7). However, whether low-grade endotoxemia in MI patients is related to pre-existing metabolic conditions or acute digestive translocation linked to an MI event remains to be determined. Moreover, as LPS is mostly inactive in human blood, the LPS burden is imperfectly captured by LPS activity measurement (i.e., an increase in activity could represent either higher translocation or a decreased host inactivation capacity) (24). Our findings, which suggest an increased LPS mass concentration, further suggest an increase in gut-derived absorption rather than a default in the inactivation process.
Endotoxemia has been shown to produce low-grade inflammation, which is involved in the pathogenesis of obesity (25). Lipid and carbohydrate metabolism are also associated with endotoxemia (26). In particular, LPS absorption might occur through chylomicron metabolism, which is closely related to triglyceride absorption (27). Obesity is associated with dysregulations in lipid (i.e., hypertriglyceridemia, low HDL cholesterol, high LDL cholesterol) (28) and carbohydrate (i.e., hyperglycemia, hyperinsulinemia, and insulin resistance) (29) metabolism, both of which were associated with endotoxemia in our cohort. Nevertheless, there was no association between obesity and endotoxemia after adjustment for these confounding conditions. Therefore, our results give new insight into the relationship between obesity and endotoxemia by suggesting that this association is driven by metabolic alterations. During the acute phase of gut barrier disruption, PLTP negatively correlates with LPS concentration, probably reflecting increased elimination capacity (30–32). In contrast, the positive association between PLTP activity and LPS reported here might be related to the increase in PLTP activity documented in patients with metabolic syndrome (33), further suggesting the presence of metabolic endotoxemia in our cohort. We did not observe associations between endotoxemia and inflammation in our cohort, probably due to the early measurement of inflammatory biomarkers (before the peak was reached). We observed lower LPS levels in smokers. Whereas smoking and endotoxemia have been described as increasing the risk of incident atherosclerosis (23), previous studies did not report an association between smoking and increased endotoxin activity (34).
In a previous study reporting on endotoxemia attributed to gut barrier failure in the acute phase of MI, the authors noted a peak value for LPS activity on day 2 and associations between LPS concentration (delta day 2 − day 1) and adverse post-MI CV events (9). In our cohort, LPS measured at admission was associated with heart rate and shock index, suggesting a link between LPS and hemodynamic changes in MI. LPS is known to trigger inflammation, and these hemodynamic changes might reflect inflammation-related vasoplegia. LPS was also correlated to longer cardiac ICU length of stay, suggesting the clinical consequences of acute translocation. Nevertheless, we found no association between LPS measured at admission and post-MI adverse events, thus suggesting that LPS concentration measured at admission has few clinical consequences.
Some limitations need to be underlined. This was a single-center study; therefore, our findings require external validation. There was no sample size calculation, and some analyses might lack power. The inflammation parameters were taken very early (at hospital admission), and many measurements were below the threshold for detection (probably measured before peak). As a consequence, the relationships between LPS, vascular tone, and acute inflammation warrant further evaluation. Repeated or daily measurements of LPS could have provided additional information on the translocation mechanisms in the acute phase of MI. A combination of LPS activity and LPS mass measurement may also have provided further insight. A comparison with patients undergoing percutaneous coronary intervention without evidenced atherosclerosis and with atherosclerosis but no myocardial infarction could have provided additional information on the respective roles of metabolic and acute endotoxemia. Describing the reasons for patients’ ICU length of stay duration could have provided additional interesting information. We measured the total 3HM; thus, it is possible that part of the 3HM measured might be derived from human metabolites. However, bacterial LPS is the major part of total 3HM, and total 3HM is highly associated with LPS concentration (19).
In conclusion, at admission, higher LPS levels were related to metabolic alterations (cholesterol, triglyceride, and diabetes) and age. They were also associated with hemodynamic modifications (higher heart rate and shock ratio) and longer cardiac ICU length of stay but were not associated with inflammation and short- or long-term MACEs. Altogether, endotoxins measured at admission in patients with MI seemed to reflect low-grade metabolic endotoxemia due to pre-existing conditions leading to myocardial infarction rather than acute clinical severity. Endotoxins, therefore, do not appear to be a relevant therapeutic target for treating acute MI, but targeting endotoxemia could represent a promising strategy to prevent metabolic disorders and subsequent coronary artery disease progression. However, these findings require further confirmation.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statementThe studies involving humans were approved by the Ethics Committee of the University Hospital of Dijon. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsMN: Writing – review & editing, Writing – original draft. AP: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. DM: Writing – review & editing, Writing – original draft, Supervision. YC: Writing – review & editing, Writing – original draft. TG: Supervision, Writing – review & editing, Writing – original draft. LT: Writing – review & editing, Writing – original draft. A-LR: Writing – review & editing, Writing – original draft. P-GG: Writing – review & editing, Writing – original draft. MM: Formal Analysis, Data curation, Writing – review & editing, Writing – original draft. J-PP: Writing – review & editing, Writing – original draft. VD: Writing – review & editing, Writing – original draft. MF: Writing – review & editing, Writing – original draft. LL: Writing – review & editing, Writing – original draft, Supervision. MZ: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Conceptualization.
FundingThe authors declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Dijon University Hospital, French Federation of Cardiology, Association de Cardiologie de Bourgogne and by grants from the Agence Régionale de Santé (ARS) de Bourgogne-Franche-Comté and from the Regional Council of Burgundy-Franche-Comté. This work was supported by a French Government grant managed by the French National Research Agency (Agence nationale de la recherche) as part of the “Investissements d'Avenir” program, reference: ANR-11-LABX-0021-01- LipSTIC Labex. It is also part of an integrated project funded by the French government (Regional Council of Bourgogne-Franche-Comté).
AcknowledgmentsThe authors thank Suzanne Rankin for the English revision of the manuscript and Morgane Laine for providing technical assistance.
Conflict of interestMF reports having received grants, consulting fees, and/or honoraria and delivering lectures for Abbott, Amarin, Amgen, AstraZeneca, Ajanta, Kowa, Merck and Co, Novartis, Organon, Pfizer, Recordati, Sanofi/Regeneron, Servier, SMB, Ultragenyx, and Viatris. YC reports receiving consultant or speaking fees for Bayer, BMS/Pfizer, Boehringer Ingelheim, Novartis, Sanofi, and Servier. MZ declares research grants from Amarin Corp and speaking fees from Organon. MN declares research grants and consulting fees from Baxter, educational fees from Fresenius, and congress fees from Pfizer. P-GG receive fees for lectures from Aguettant, AOP, Baxter, Medtronic, Edwards and Vygon. P-GG is a consultant for ABBOT.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. (2007) 56:1761–72. doi: 10.2337/DB06-1491
PubMed Abstract | Crossref Full Text | Google Scholar
3. Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. (2011) 34:392–7. doi: 10.2337/DC10-1676
PubMed Abstract | Crossref Full Text | Google Scholar
4. Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie. (2016) 124:11–20. doi: 10.1016/j.biochi.2015.06.020
PubMed Abstract | Crossref Full Text | Google Scholar
5. Gautier T, Deckert V, Nguyen M, Desrumaux C, Masson D, Lagrost L. New therapeutic horizons for plasma phospholipid transfer protein (PLTP): targeting endotoxemia, infection and sepsis. Pharmacol Ther. (2022) 236:108105. doi: 10.1016/J.PHARMTHERA.2021.108105
PubMed Abstract | Crossref Full Text | Google Scholar
6. Gautier T, Lagrost L. Plasma PLTP (phospholipid-transfer protein): an emerging role in “reverse lipopolysaccharide transport” and innate immunity. Biochem Soc Trans. (2011) 39:984–8. doi: 10.1042/BST0390984
PubMed Abstract | Crossref Full Text | Google Scholar
7. Carnevale R, Sciarretta S, Valenti V, di Nonno F, Calvieri C, Nocella C, et al. Low-grade endotoxaemia enhances artery thrombus growth via toll-like receptor 4: implication for myocardial infarction. Eur Heart J. (2020) 41:3156–65. doi: 10.1093/EURHEARTJ/EHZ893
PubMed Abstract | Crossref Full Text | Google Scholar
8. Asada M, Oishi E, Sakata S, Hata J, Yoshida D, Honda T, et al. Serum lipopolysaccharide-binding protein levels and the incidence of cardiovascular disease in a general Japanese population: the Hisayama study. JAHA. (2019) 8:e013628. doi: 10.1161/JAHA.119.013628
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zhou X, Li J, Guo J, Geng B, Ji W, Zhao Q, et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. (2018) 6:66. doi: 10.1186/S40168-018-0441-4
PubMed Abstract | Crossref Full Text | Google Scholar
10. Carrera-Bastos P, Picazo Ó, Fontes-Villalba M, Pareja-Galeano H, Lindeberg S, Martínez-Selles M, et al. Serum zonulin and endotoxin levels in exceptional longevity versus precocious myocardial infarction. Aging Dis. (2018) 9:317. doi: 10.14336/AD.2017.0630
PubMed Abstract | Crossref Full Text | Google Scholar
11. Soraya H, Masoud WGT, Gandhi M, Garjani A, Clanachan AS. Myocardial mechanical dysfunction following endotoxemia: role of changes in energy substrate metabolism. Basic Res Cardiol. (2016) 111:24. doi: 10.1007/s00395-016-0544-7
PubMed Abstract | Crossref Full Text | Google Scholar
12. Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. (1989) 321:280–7. doi: 10.1056/NEJM198908033210503
PubMed Abstract | Crossref Full Text | Google Scholar
13. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. (2002) 53:31–47. doi: 10.1016/S0008-6363(01)00434-5/2/53-1-31-GR3.GIF
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMOA1707914
PubMed Abstract | Crossref Full Text | Google Scholar
15. Nguyen M, Gautier T, Masson D, Bouhemad B, Guinot P-G. Endotoxemia in acute heart failure and cardiogenic shock: evidence, mechanisms and therapeutic options. J Clin Med. (2023) 12:2579. doi: 10.3390/JCM12072579
PubMed Abstract | Crossref Full Text | Google Scholar
16. Zeller M, Steg PG, Ravisy J, Lorgis L, Laurent Y, Sicard P, et al. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation. (2008) 118:482–90. doi: 10.1161/CIRCULATIONAHA.107.753483
PubMed Abstract | Crossref Full Text | Google Scholar
17. Serruys PW, Onuma Y, Garg S, Sarno G, van den Brand M, Kappetein AP, et al. Assessment of the SYNTAX score in the syntax study. EuroIntervention. (2009) 5:50–6. doi: 10.4244/EIJV5I1A9
PubMed Abstract | Crossref Full Text | Google Scholar
18. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. (2003) 163:2345–53. doi: 10.1001/ARCHINTE.163.19.2345
PubMed Abstract | Crossref Full Text | Google Scholar
19. de Barros J-P P, Gautier T, Sali W, Adrie C, Choubley H, Charron E, et al. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay. J Lipid Res. (2015) 56:1363–9. doi: 10.1194/jlr.D059725
PubMed Abstract | Crossref Full Text | Google Scholar
20. Dargent A, De Barros J-P P, Ksiazek E, Fournel I, Dusuel A, Rerole AL, et al. Improved quantification of plasma lipopolysaccharide (LPS) burden in sepsis using 3-hydroxy myristate (3HM): a cohort study. Intensive Care Med. (2019) 45(11):1678–80. doi: 10.1007/s00134-019-05749-0
PubMed Abstract | Crossref Full Text | Google Scholar
21. Simonsen JR, Järvinen A, Harjutsalo V, Forsblom C, Groop P-H, Lehto M. The association between bacterial infections and the risk of coronary heart disease in type 1 diabetes. J Intern Med. (2020) 288:711–24. doi: 10.1111/joim.13138
PubMed Abstract | Crossref Full Text | Google Scholar
22. Westerterp M, Berbée JFP, Pires NMM, Van Mierlo GJD, Kleemann R, Romijn JA, et al. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E–knockout mice. Circulation. (2007) 116:2173–81. doi: 10.1161/CIRCULATIONAHA.107.693382
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck study. J Am Coll Cardiol. (1999) 34:1975–81. doi: 10.1016/S0735-1097(99)00448-9
PubMed Abstract | Crossref Full Text | Google Scholar
24. Nguyen M, Pallot G, Jalil A, Tavernier A, Dusuel A, Le Guern N, et al. Intra-abdominal lipopolysaccharide clearance and inactivation in peritonitis: key roles for lipoproteins and the phospholipid transfer protein. Front Immunol. (2021) 12:1641. doi: 10.3389/FIMMU.2021.622935/BIBTEX
Crossref Full Text | Google Scholar
25. Dubinski P, Czarzasta K, Cudnoch-Jedrzejewska A. The influence of gut microbiota on the cardiovascular system under conditions of obesity and chronic stress. Curr Hypertens Rep. (2021) 23:31. doi: 10.1007/S11906-021-01144-7
PubMed Abstract | Crossref Full Text | Google Scholar
26. Määttä AM, Salminen A, Pietiäinen M, Leskelä J, Palviainen T, Sattler W, et al. Endotoxemia is associated with an adverse metabolic profile. Innate Immun. (2021) 27:3. doi: 10.1177/1753425920971702
Crossref Full Text | Google Scholar
27. Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. (2009) 50:90–7. doi: 10.1194/jlr.M800156-JLR200
PubMed Abstract | Crossref Full Text | Google Scholar
30. Nguyen M, Gautier T, Reocreux G, Pallot G, Maquart G, Bahr P-A, et al. Increased phospholipid transfer protein activity is associated with markers of enhanced lipopolysaccharide clearance in human during cardiopulmonary bypass. Front Cardiovasc Med. (2021) 8:756269. doi: 10.3389/FCVM.2021.756269
PubMed Abstract | Crossref Full Text | Google Scholar
31. Deckert V, Lemaire S, Ripoll P-J, de Barros J-PP, Labbé J, Borgne CC-L, et al. Recombinant human plasma phospholipid transfer protein (PLTP) to prevent bacterial growth and to treat sepsis. Sci Rep. (2017) 7:3053. doi: 10.1038/s41598-017-03285-9
PubMed Abstract | Crossref Full Text | Google Scholar
32. Payen D, Dupuis C, Deckert V, de Barros J-PP, Rérole A-L, Lukaszewicz A-C, et al. Endotoxin mass concentration in plasma is associated with mortality in a multicentric cohort of peritonitis-induced shock. Front Med (Lausanne). (2021) 8:749405. doi: 10.3389/FMED.2021.749405
PubMed Abstract | Crossref Full Text | Google Scholar
33. Lebrun LJ, Pallot G, Nguyen M, Tavernier A, Dusuel A, Pilot T, et al. Increased weight gain and insulin resistance in HF-fed PLTP deficient mice is related to altered inflammatory response and plasma transport of gut-derived LPS. Int J Mol Sci. (2022) 23:13226. doi: 10.3390/IJMS232113226
PubMed Abstract | Crossref Full Text | Google Scholar
34. Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. (2007) 86:1286–92. doi: 10.1093/AJCN/86.5.1286
留言 (0)