Coronary heart disease (CHD) is caused by the narrowing or occlusion of coronary arteries, resulting in ischemia, hypoxia, or necrosis of the myocardium. It has the highest death rate (1), and anxiety and depression can make it more likely that bad prognostic events will happen. A relevant summary analysis indicates that anxiety and depression increase the incidence of adverse cardiac outcomes in acute myocardial infarction prognosis by 36% (2). Treating patients with CHD combined with anxiety or depression is based on combining anxiolytic-depressants, which include selective serotonin reuptake inhibitors (SSRIs) and benzodiazepines (BZD), with cardiovascular drugs. Even so, side effects and negative effects can include granulocyte insufficiency, elevated heart rate, endocrine disturbances, and upright hypotension (3). The accompanying medical costs and poor prognosis have greatly burdened society and families, so how to effectively prevent and treat them has become a hot issue.
Current studies suggest that the combined pathological mechanisms of anxiety and depression in CHD are linked to inflammatory factors, neurotransmitters, the hypothalamic-pituitary-adrenal (HPA) axis, endocrine markers, and high-sensitivity C-reactive protein (hs-CRP) (4, 5), where the inflammatory response is a critical component of the combined anxiety and depressive disorder episodes in coronary artery disease (6). The main components of omega-3 PUFAs include alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), which can only be consumed through food and cannot be generated by the human body, with deep-sea fish oil being its most important source, and whose antidepressant effect is linked to reduced production of pro-inflammatory cytokines (7), but also participate in neuromodulatory transmission and play a positive role in depression (8); Furthermore, it possesses cardioprotective properties, such as avoiding sudden cardiac death and ameliorating heart failure (9, 10). Several trials have proven its efficacy in preventing cardiovascular disease and depression. Nevertheless, several studies do not provide significant evidence that taking omega-3 PUFA supplements improves cardiovascular disease and depression (11).
It can be seen that the efficacy of omega-3 PUFAs in treating anxiety or depression in patients with CHD is debatable, and there is no relevant meta-analysis. Therefore, the value of omega-3 PUFAs in treating anxiety or depression must be demonstrated further. We compiled the relevant published clinical research literature and hope this meta-analysis will resolve the controversy and provide clinicians and patients with new treatment options.
2 Materials and methods2.1 Literature searchThe PubMed, Cochrane Library, Embase, and Web of Science receipt databases were chosen and searched for the period from each database’s inception until February 2023. The inquiry was conducted primarily with subject phrases and free words. For instance, the terms Coronary Disease [mesh]; Coronary Diseases [Title/Abstract]; and Disease, Coronary [Title/Abstract] were used to search PubMed.
2.2 Inclusion and exclusion criteria2.2.1 Inclusion criteriaThe included research was randomized controlled trials (RCTs); patients with coronary artery disease and anxiety or depression, whose diagnosis was based on criteria described in the literature, were required to constitute the study population, no matter their age or gender; The intervention group received omega-3 PUFAs supplements, which contained EPA, DHA, or ALA. There were no limitations on the kind, amount, duration, or frequency of the supplements, and they could be combined with conventional psychotropic medications, while the control group received a placebo; the underlying treatment measures and outcomes were measured consistently and statistically correctly in the trial and control groups; The main results obtained consisted of alterations in depression scores and the omega-3 index. The clinical trial met ethical specifications and had an exact time and place to be conducted.
2.2.2 Exclusion criteriaExclude conference abstracts, systematic reviews, case reports, animal experiments, errors and omissions in the primary outcome indicators, and the article for which accurate data were unavailable.
2.3 Data extractionTwo researchers individually searched and evaluated the relevant literature based on the inclusion criteria, eliminating studies that did not meet the requirements and viewing the entire text for issues that might fulfill the inclusion criteria. In cases of conflicting opinions, a consensus was formed through conversation, or a third investigator was invited to weigh in and render a decision. Only the literature with the most complete reported data was included. After the initial data extraction using the self-designed data extraction form, a secondary verification was conducted to confirm the data’s legitimacy and dependability. The specific extracts were as follows: the name of the first author, publication date, nation, sample size, gender, mean age, intervention, length of therapy, and outcome indicators. Two researchers examined the final included literature independently using the Cochrane system’s risk of bias evaluation technique for RCTs.
2.4 Quality evaluationUtilizing the Cochrane Handbook for Systematic Reviewers 5.4.0 quality assessment criteria, the included RCTs’ methodology was assessed, which included randomization, allocation concealment, blinding, data bias, selection bias, reporting of results, and the presence of other tendencies. The effects of each item were evaluated as “yes” (low bias risk), “unclear,” or “no” (high bias risk).
2.5 Statistical analysisStata 15.1 was used to process the extracted information. The odds ratio (OR) was selected to characterize dichotomous variables, the standard mean difference (SMD) was used to describe continuous variables, and the 95% confidence interval (CI) was used to analyze the data estimates of the outcome indicators. The I2 and P values were selected to investigate the heterogeneity between study outcomes: if I2 ≤50% and P≥0.1 indicated good data homogeneity, a fixed-effects model was selected; if I2>50% and P<0.1 indicated heterogeneous data, a random-effects model was selected, and the source of heterogeneity was actively sought. Using a funnel plot and Egger’s test, publication bias was evaluated; if P > 0.05, the risk of publication bias is low; if not, publication bias may exist. A sensitivity analysis was evaluated to assess the results’ dependability.
3 Result3.1 Study selection flowchart and resultThe preliminary literature search yielded a total of 502 articles, of which 234 were obtained after eliminating duplicate publications. After perusing the titles and abstracts of 12 studies, 6 were included in this study after further evaluation Figure 1.
Figure 1 Flowchart of literature search.
3.2 Characteristics of included studiesA total of 6 (12–17) randomized controlled trials were included. Included were 2570 patients with coronary artery disease combined with anxiety or depression; compared to the placebo group, there were 1,290 in the trial group Table 1.
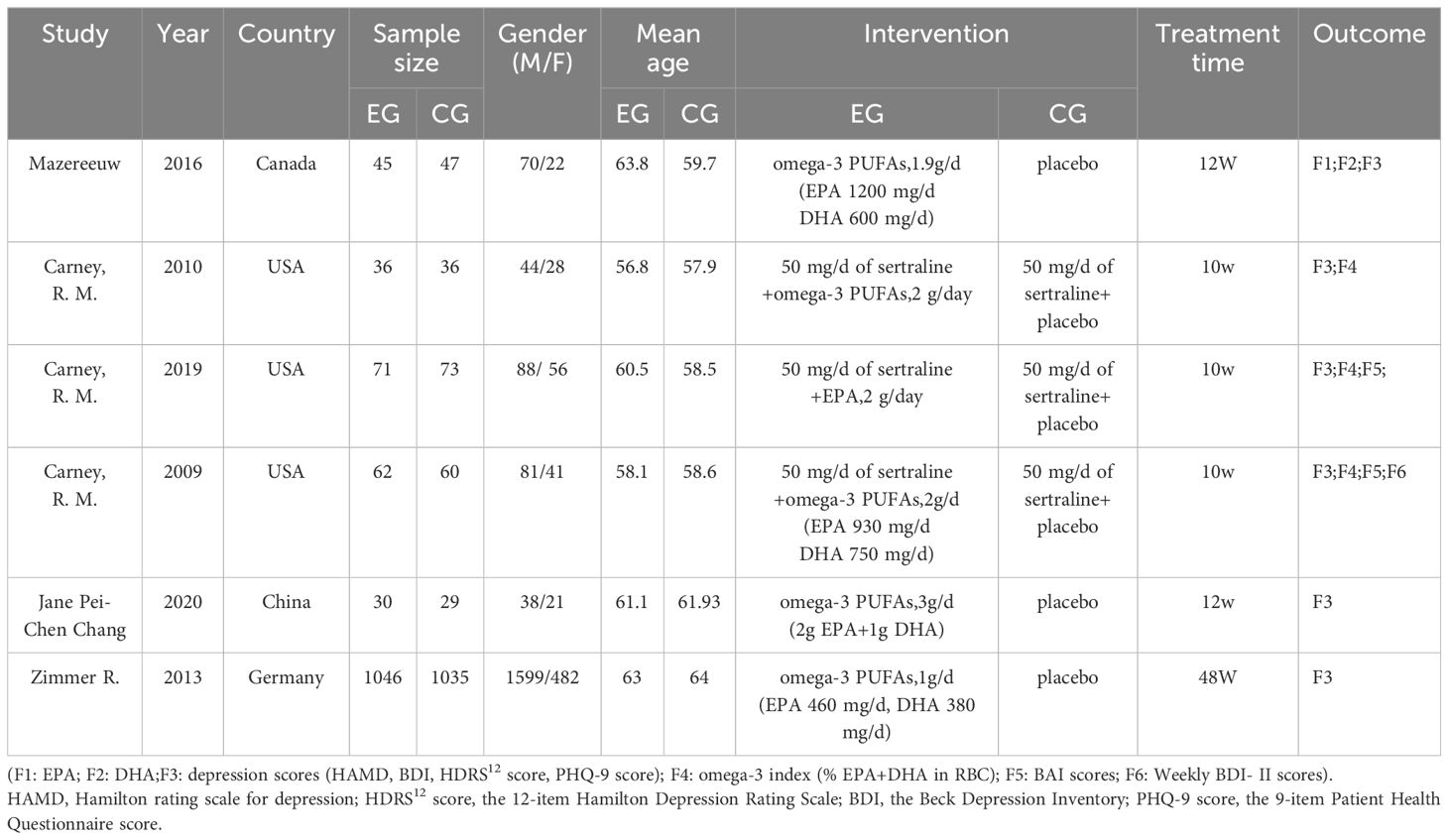
Table 1 Basic characteristics of the literature.
3.3 Bis risk assessment mapAll 6 studies were RCTs; 1 article (12) mentioned that “the central office performed randomization”; 5 articles (13–17) mentioned “randomization” but did not describe the specific randomization method. Among the randomized scheme allocation hides, 4 articles (12, 13, 15, 16) mention allocation hiding. Among the blind methods, 4 articles (12, 13, 15, 16) explicitly stated that blinding was used for subjects, investigators, and outcome evaluators. 2 articles (14, 15) did not report outcome indicators in full. As shown in Figure 2.
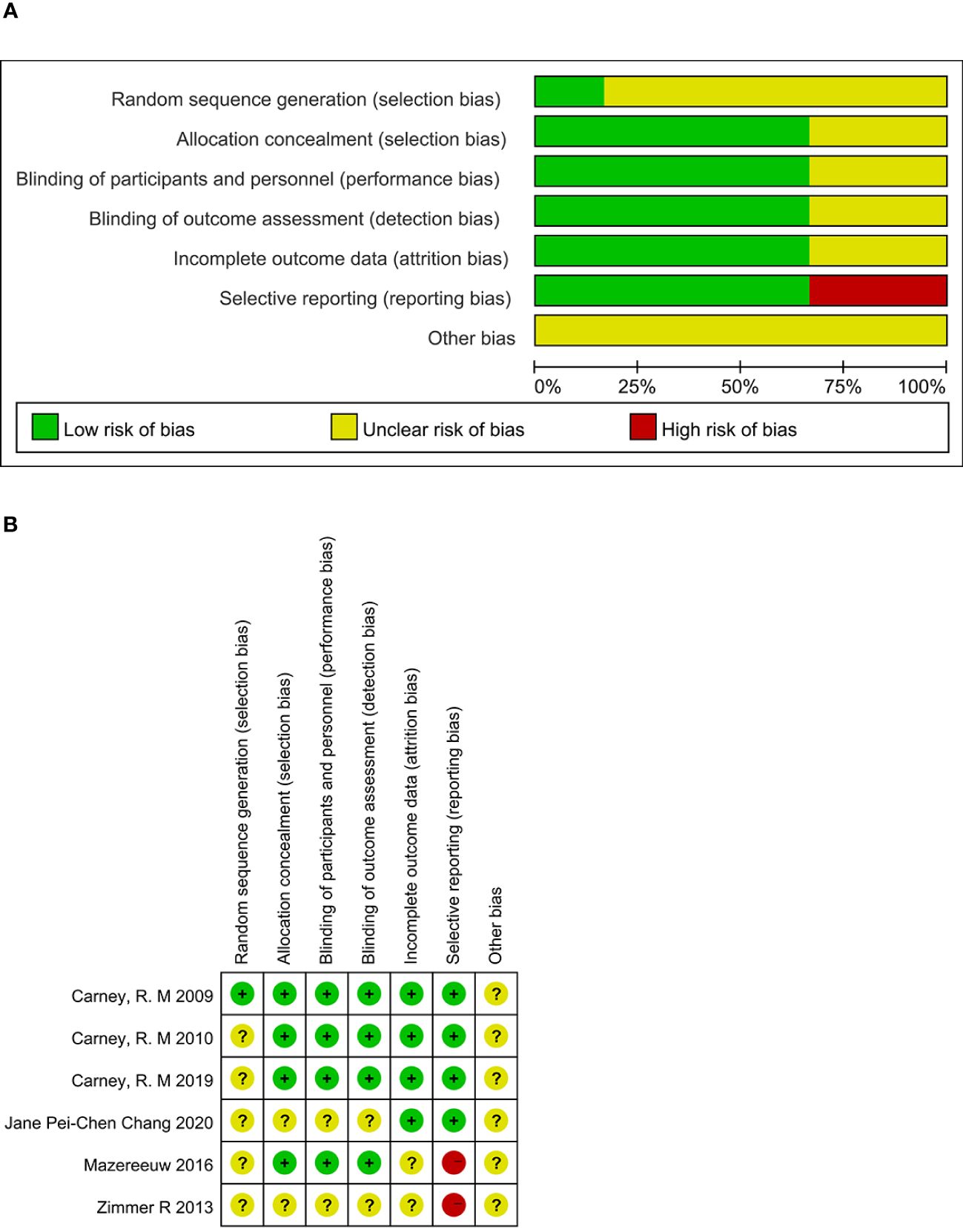
Figure 2 Risk of bias. (A) Risk of bias graph. (B) Risk of bias summary.
3.4 Results of meta-analysis3.4.1 Depression scoresThe depression scores were included in six studies, with a total of 2570 cases. They were tested for heterogeneity, which was significant across studies (P = 0.016, I2 = 57.3%); employing a random effects model revealed that the test group was not able to reduce depression scale scores in contrast to the placebo group [SMD = 0.09 (95% CI: - 0.07, 0.26)] Figure 3.
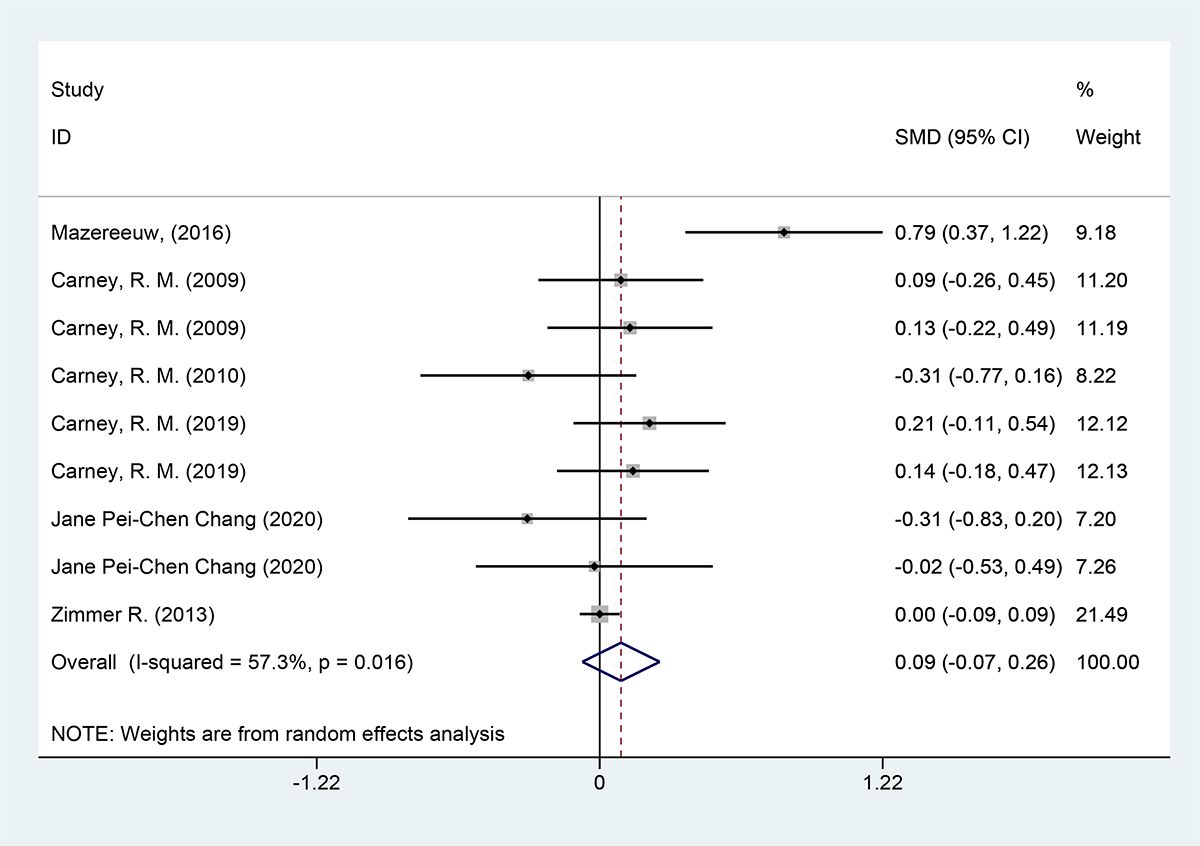
Figure 3 Forest plot of depression scores.
3.4.2 BAI scoresBAI scores were included in 2 studies with a total of 266 cases, tested for heterogeneity with good homogeneity between the results of the studies (P = 0.522, I2 = 0%), and using a fixed effects model indicated that it suggested that the test group could not reduce the scores of the anxiety scale in contrast to the placebo group. [SMD = 0.07 (95% CI: -0.17, 0.32)] Figure 4.
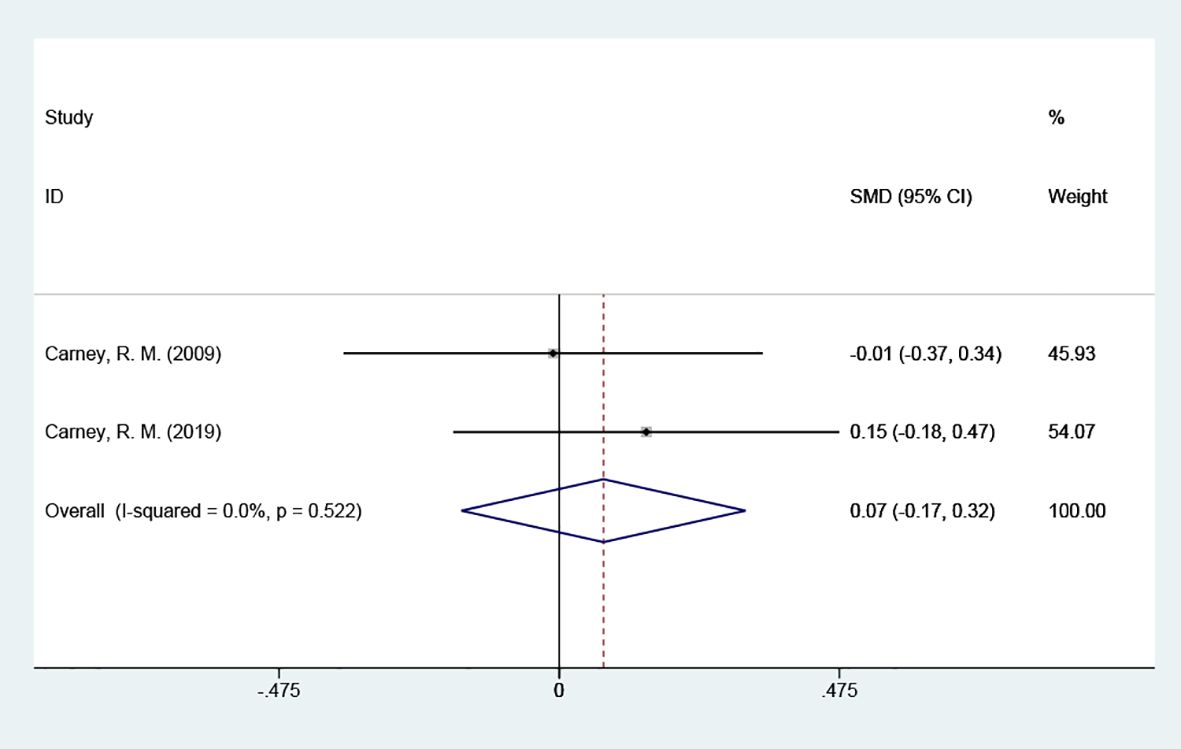
Figure 4 Forest plot of BAI scores.
3.4.3 Omega-3 indexThe omega-3 index was included in 3 studies with 388 cases and tested for heterogeneity; there was variability and heterogeneity between the groups (P = 0.000, I2 = 95.4%), and using a random effects model showed that the omega-3 index was elevated. [SMD = 1.33 (95% CI: 0.18, 2.49)] Figure 5.
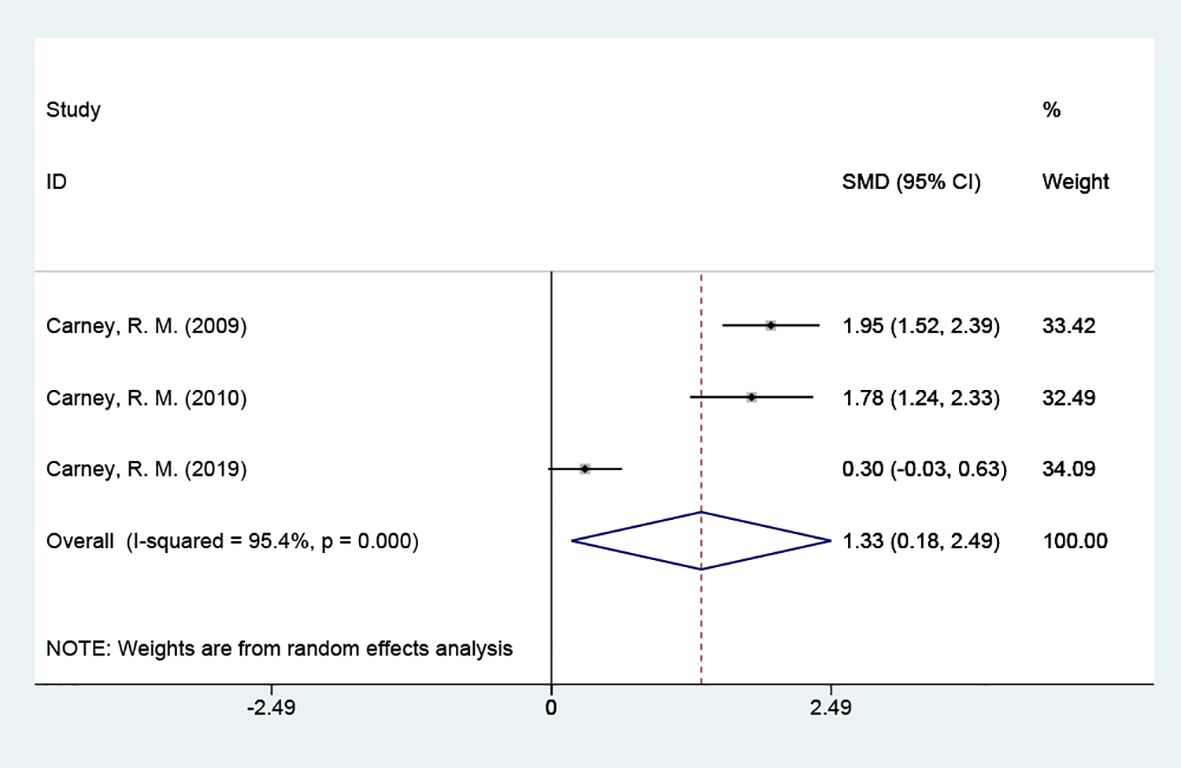
Figure 5 Forest plot of omega-3 index.
3.4.4 Subgroup analysis of depression scores3.4.4.1 Grouped by different scale types4 of the studies used the HAMD scale. The results indicated that the test group was not able to reduce depression scale scores in contrast to the placebo group [SMD = 0.19 (95% CI: -0.20, 0.58)]; 5 studies used the BDI scale, and the results indicated that the test group was not able to reduce depression scale scores in contrast to the placebo group [SMD = 0.01 (95% CI: -0.07, 0.09)]. Patients with coronary artery disease did not show an improvement in their depressive symptoms. Tracing back to the original literature, different studies used different versions of depression scales (HAMD, BDI, HDRS12 score, and PHQ-9 score), which may be the main reason for the significant heterogeneity Figure 6.
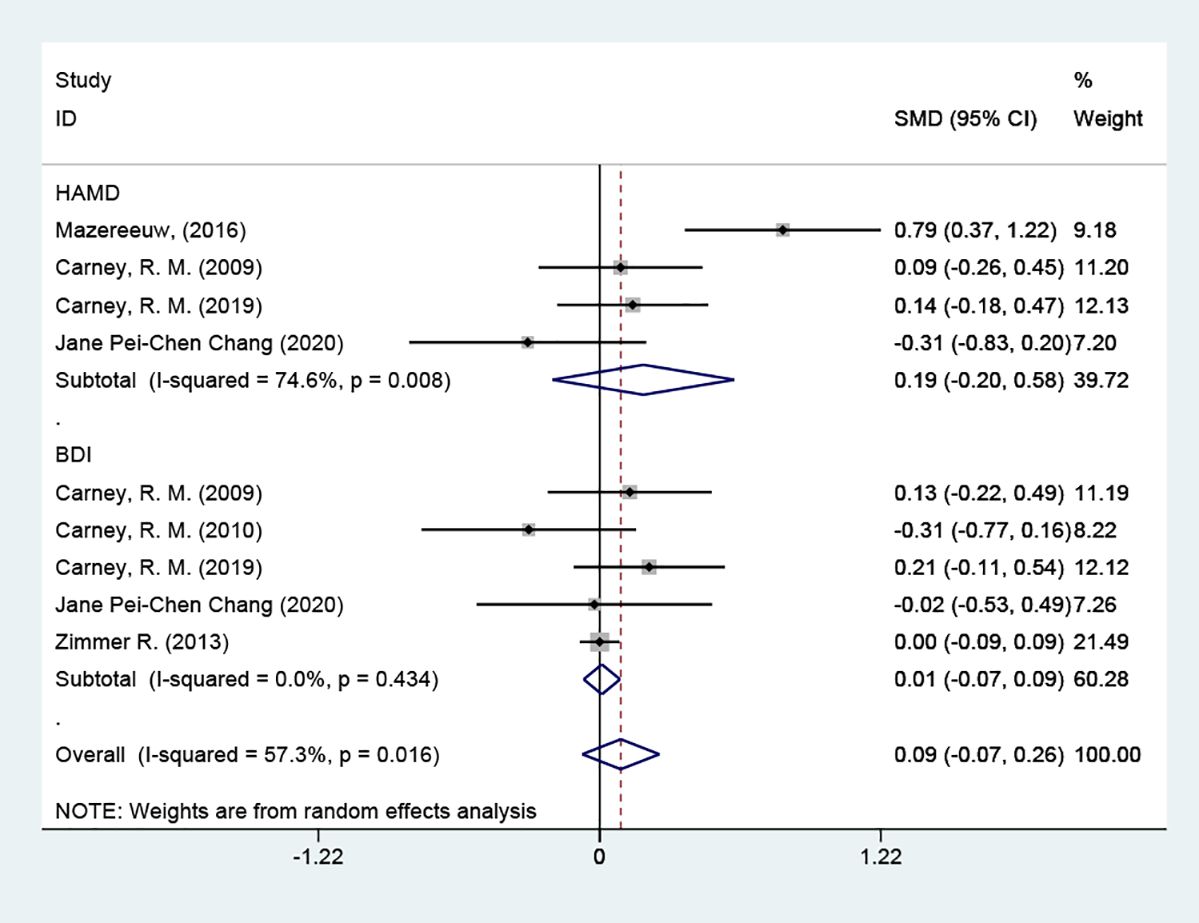
Figure 6 Subgroup analysis: depression scores.
3.4.4.2 Grouped according to different types of intervention3 of the studies used omega-3 PUFAs, and the results suggested that the omega-3 PUFAs group was not able to reduce depression scale scores in contrast to the placebo group [SMD = 0.24 (95% CI: -0.26, 0.74)]; 2 of these papers used sertraline + omega-3 PUFAs, and the results showed that, in contrast to the placebo group, it was also not able to reduce depression scale scores [SMD = -0.08 (95% CI: -0.46, 0.31)] Figure 7.

Figure 7 Subgroup analysis: Types of intervention.
3.4.4.3 Grouped according to the different courses of treatment3 of the studies had a 10-week course of treatment. In contrast to the placebo group, depression scores did not decrease after 10 weeks [SMD = 0.02 (95% CI: -0.23, 0.26)]; 2 of the articles had a 12-week course of treatment, and the results also indicated no reduction in depression scores after 12 weeks. [SMD = 0.40 (95% CI: -0.40, 1.20)] Figure 8.
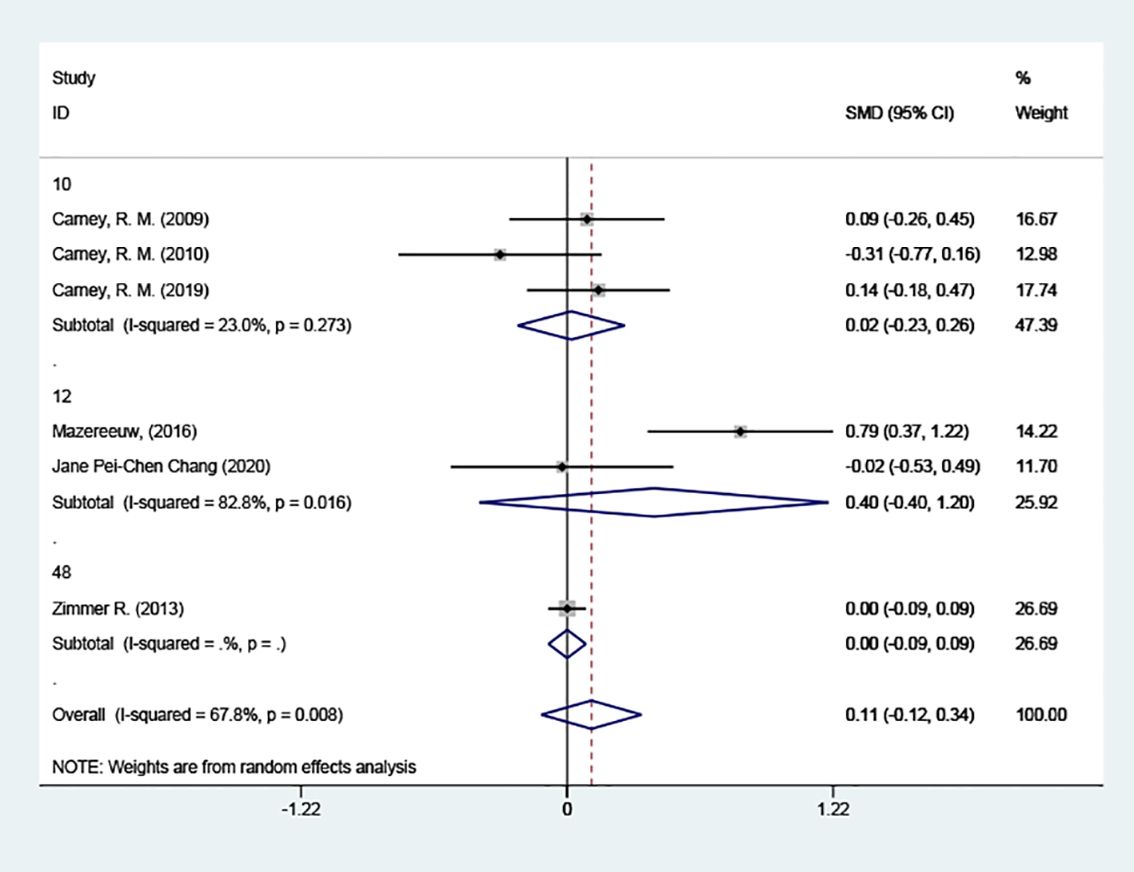
Figure 8 Subgroup analysis: Course of treatment.
3.5 Sensitivity and publication bias analysisBecause there was still significant heterogeneity within subgroups of depression scale scores, further sensitivity analyses were conducted to reassess the combined effect values using the one-by-one exclusion method. The findings of the analysis demonstrated that effect sizes, after one-by-one exclusion from the literature, remained within the boundaries, indicating good stability of the analysis results (Figures 9A, B). We used Egger’s test for assessing publication bias. Using the depression scale score as an indicator, the risk of publication bias was evaluated for the included studies, p = 0.488, suggesting a higher likelihood of publication bias for this outcome indicator. Using the omega-3 index as an indicator, p = 0.355, also suggesting a higher likelihood of publication bias for this outcome indicator (Figures 9C, D).
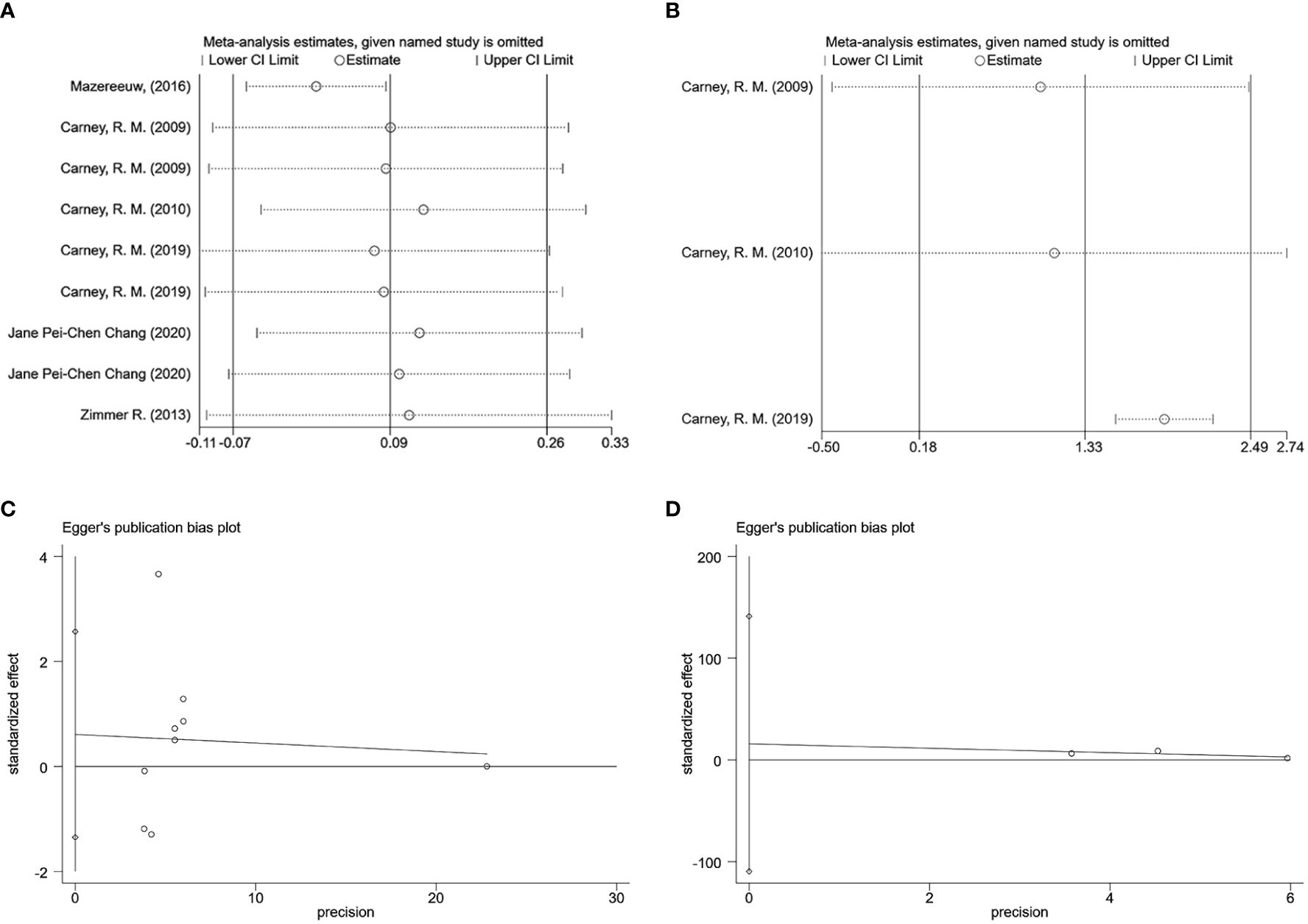
Figure 9 Sensitivity and publication bias analysis (A) Sensitivity analysis of depression scores. (B) Sensitivity analysis of omega-3 index. (C) Egger graph of depression scores. (D) Egger graph of omega-3 index.
4 DiscussionThis study sought to determine whether omega-3 PUFAs could reduce depression or anxiety in people with coronary artery disease. We discovered that omega-3 PUFAs did not help coronary artery disease patients with depression and anxiety in the meta-analysis of this randomized controlled trial. The omega-3 index indicates the status of the body’s EPA and DHA levels (18), and a lower omega-3 index tends to suggest a greater likelihood of cardiovascular disease and mental illness (19). The depressive states of patients with coronary artery disease are associated with a reduced amount of EPA and DHA in the red blood cell membranes, i.e., a lower omega-3 index (20). In the results of the present experiment, the omega-3 index was elevated [SMD = 1.33 (95% CI: 0.18, 2.49)], indicating that the concentration of omega-3 PUFAs in humans was replenished, but this did not improve the anxiety or depression states of the patients. 3 of the studies used the omega-3 PUFAs group (SMD = 0.24 (95% CI: -0.26, 0.74)); 2 of the studies used the sertraline+omega-3 PUFAs group [SMD = -0.08 (95% CI: -0.46, 0.31)]; neither of which improved depressive status. Consequently, neither as monotherapy nor as an addition to medication, omega-3 PUFAs did not significantly alleviate depression in the current study.
It is highly contentious whether omega-3 PUFAs alleviate anxiety or depression among patients with coronary artery disease. During our investigation, we discovered that the effects of omega-3 PUFAs were more skewed towards the antidepressant than the anxiolytic. Thus, we will focus on their impacts on depressive states in the following. There is a typical pathologic relationship between coronary artery disease and anxiety or depression that involves inflammation, and serum levels of inflammatory factors such as CRP and tumor necrosis factor-alpha (TNF-α) are considerably higher in coronary artery disease patients with negative emotions, such as anxiety or depression, than in those without such emotions. DHA and EPA may suppress the release of inflammatory cytokines, including TNF-α, Interleukin 1 (IL-1), IL-2, and IL-6, which may have an anti-anxiety or anti-depressive effect (21). In addition, pathophysiological alterations in depression are associated with neuronal apoptosis and reduced neurogenesis. At the same time, omega-3 PUFAs may positively affect the positive effects of depression via numerous mechanisms altering nerve cell membrane stability, neurotransmitter transmission, neurogenesis, and neuroprotection (22–24). Moreover, omega-3 PUFA supplementation may improve the architecture of the brain’s white matter and decrease the severity of depressive symptoms in patients with major depressive disorder (25). Relative to patients with cardiovascular disease alone, patients with cardiovascular disease and depression had lower levels of total omega-3 PUFAs (26), a declining trend in DHA levels with increasing dysphoric moods, and lower DHA levels correlated with higher state depression severity (27). Greater consumption of omega-3 PUFAs may reduce the likelihood of depressive illness, enhance the efficacy of antidepressant medications, and alleviate mild depressive symptoms in post-infarction patients (28). However, other studies have revealed that even if supplementation with omega-3 PUFAs exhibited antidepressant advantages, they were minor and hampered by small sample sizes, and the results require further investigation (29, 30). Several studies indicate that omega-3 PUFA supplementation does not alleviate depression (31, 32).
There are several possible reasons for the controversy. First, we hypothesize that the therapeutic effect of omega-3 PUFAs on depression depends on the variety of omega-3 supplements used and the ratio of EPA to DHA. According to several studies, the favorable effects of omega-3 PUFAs on depression symptoms appear to rely more heavily on EPA than DHA (33). When EPA is used alone or as the predominant therapy modality, improvements in depression are observed (34). EPA supplementation enhances brain N-acetyl-aspartate, which works as a neuroprotective agent and can reverse brain shrinkage in severe depression (35). Enhanced treatment with EPA has a more significant antidepressant effect, with one meta-analysis placing a 50% lower limit on the proportion of EPA in omega-3 PUFAs supplemented (36). Sublette et al. recommend doses of EPA or DHA between 1000 and 2000 mg/d, with at least 60% EPA, and cannot exclude the possibility of more significant antidepressant effects with higher doses of EPA or DHA (37). Regarding the specific ratio of EPA to DHA, Song et al. determined that the optimal balance of EPA to DHA for depression was 2:1 or 3:1 (38). In the current trial, omega-3 supplements comprising EPA and DHA were administered separately or in combination at various doses and ratios, which may have impacted the results.
To be sure, omega-3 PUFAs have a positive effect on reducing cardiovascular disease risk and improving anxiety or depression in patients with coronary artery disease. The REDUCE-IT (39) trial showed that taking 4 grams of a purified form of EPA every day lowered the risk of a number of cardiovascular events, such as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. Amin et al. (40) observed that there was a reduction in depression scale scores for each 4.5% increment in the omega-3 index among patients diagnosed with acute coronary syndrome (ACS). Haberka M et al. (41) discovered that administering low dosages of omega-3 PUFAs early on had a positive impact on lowering anxiety and depression in individuals with acute myocardial infarction. The above results show that omega-3 PUFAs can help improve anxiety or depression in people with coronary artery disease. They may also provide information and a clinical basis for the future use of omega-3 PUFAs in clinical trials and treatments for coronary artery disease combined with anxiety or depression, such as figuring out the best dosage or EPA/DHA ratio and the best way to supplement. Omega-3 PUFAs deficiency appears to play an important role in the network linking coronary heart disease, acute myocardial infarction, or other negative cardiovascular prognosis with adverse moods such as depression and anxiety, but the current research is still controversial, and longer, targeted studies are needed in the future.
And the possible synergistic benefits of mixing conventional antidepressants with omega-3 polyunsaturated fatty acids need additional research. It is unknown whether this is connected to the timing of medication administration or which antidepressant has the most significant benefit when combined with omega-3 PUFA supplements. Some studies indicate that using them as adjuvant therapy, such as in conjunction with antidepressants, is more effective than using them alone (42). However, our findings suggest that omega-3 PUFAs in combination with sertraline do not show a better therapeutic effect on depressed coronary artery disease patients.
In addition, our study found that neither a 10-week course of treatment [SMD = 0.02 (95% CI: -0.23, 0.26)] nor a 12-week course of treatment [SMD = 0.40 (95% CI: -0.40, 1.20)] was able to reduce scores on the anxiety or depression scales. This may be due to the longer time required for blood levels of omega-3 PUFAs to improve depression significantly and the lack of sufficient observation time in the trial (43). We anticipate further studies to figure out whether a longer treatment course can reduce depression scores, alleviate anxiety or depression, and reduce mortality in anxious or depressed coronary artery disease patients.
This study exhibited substantial heterogeneity and a high risk of bias. The magnitude of change in article heterogeneity is large after excluding this 2016 article, which is a source of significant heterogeneity Figure 10.
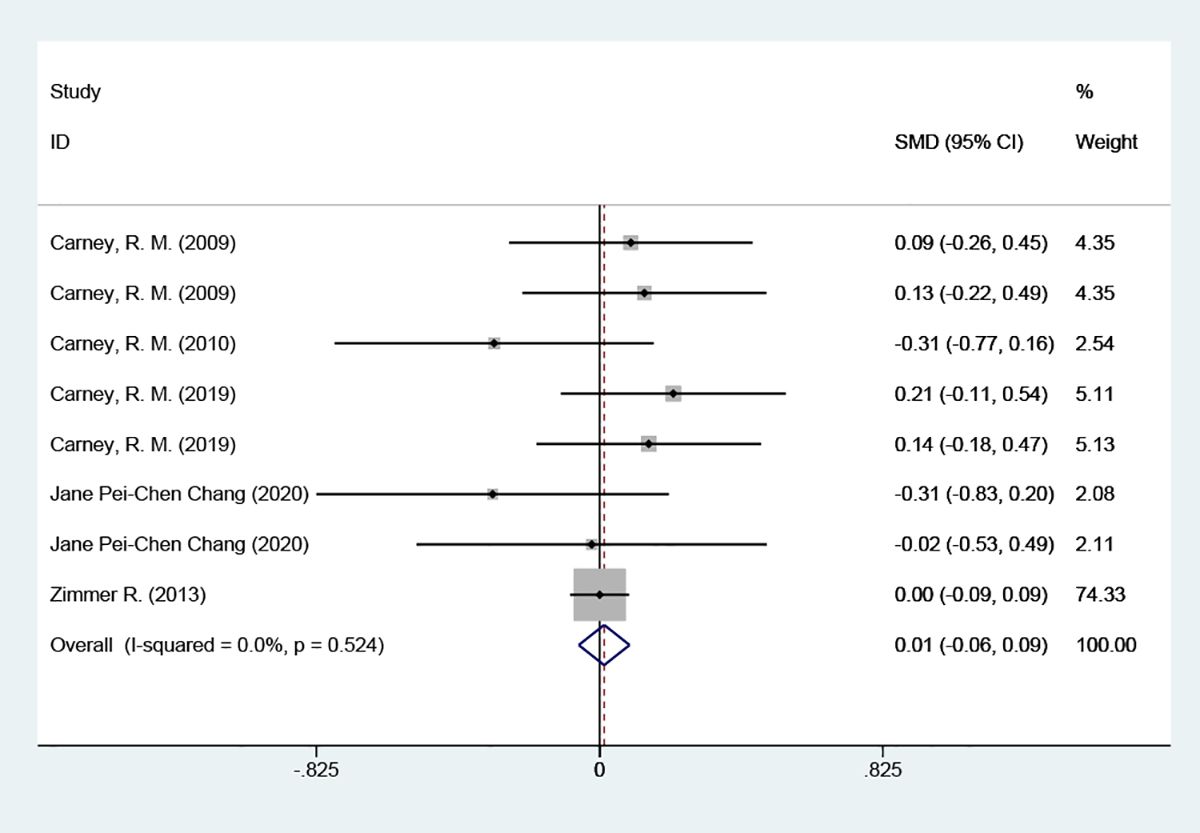
Figure 10 Forest plot of depression scores [After excluding Mazereeuw, G. 2016 (15)].
Firstly, the number of articles that met the inclusion criteria for this study was limited, and despite extensive systematic searches for topics and manual searches for relevant articles to retrieve as many eligible papers as possible, relevant studies were likely missed or excluded. Furthermore, an examination of the primary sources revealed differences in the severity of coronary artery disease and anxiety or depression among the patients in the analyzed studies, making it impossible to account for baseline levels. It’s possible that the study’s heterogeneity was caused by differences in the types and amounts of drugs given to the intervention groups, such as EPA and DHA doses and EPA/DHA ratios. There were also differences in the length of treatment and the depression scales used to measure outcomes. Although we performed detailed subgroup analyses to investigate potential sources of heterogeneity, we were unable to analyze other meaningful sources because we were limited by the original study design. Therefore, the findings should be generalized with caution. Future studies with large and representative samples are needed.
5 ConclusionCurrent research often supplements the treatment of coronary heart disease with anxiety or depression with two main types of omega-3 PUFAs, EPA and DHA. Studies have shown that using EPA alone or in combination with DHA can significantly reduce the risk of cardiovascular disease and alleviate the symptoms of anxiety or depression. However, our research shows that taking supplements with these two main types of omega-3 PUFAs does not help with anxiety or depression in people with coronary artery disease. Neither omega-3 PUFAs nor their combination with conventional antidepressants have been shown to be effective against depression. It is important to note that ALA is one of the components of omega-3 PUFAs. However, there are limited studies on the use of ALA as an omega-3 supplement for the treatment of coronary artery disease with comorbid anxiety or depression. Therefore, there is still a need to update and include high-quality research data to support supplementation in the future, and we eagerly anticipate further clinical studies.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributionsYG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Investigation, Writing – review & editing. DH: Software, Writing – review & editing. HZ: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by the National Natural Science Foundation of China (No. 82174332).
AcknowledgmentsThe findings and conclusions in this document are those of the authors, who are responsible for its contents.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1368007/full#supplementary-material
References1. Ozkan B, Örsçelik Ö, Uyar H, Ballı M, Güçer E, Aslan O, et al. Awareness of pleiotropic and cardioprotective effect of statins in patients with coronary artery disease. BioMed Res Int. (2018) 2018:1–7. doi: 10.1155/2018/8961690
CrossRef Full Text | Google Scholar
2. Huffman JC, Celano CM, Januzzi JL. The relationship between depression, anxiety, and cardiovascular outcomes in patients with acute coronary syndromes. Neuropsych Dis Treat. (2010) 6:123–36. doi: 10.2147/NDT
CrossRef Full Text | Google Scholar
3. Shively CA, Silverstein-Metzler M, Justice J, Willard SL. The impact of treatment with selective serotonin reuptake inhibitors on primate cardiovascular disease, behavior, and neuroanatomy. Neurosci Biobehav Rev. (2017) 74:433–43. doi: 10.1016/j.neubiorev.2016.08.037
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Tschorn M, Kuhlmann SL, Rieckmann N, Beer K, Grosse L, Arolt V, et al. Brain-derived neurotrophic factor, depressive symptoms and somatic comorbidity in patients with coronary heart disease. Acta Neuropsychiatr. (2021) 33:22–30. doi: 10.1017/neu.2020.31
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Wang B, Teng Y, Li Y, Lai S, Wu Y, Chen S, et al. Evidence and characteristics of traditional chinese medicine for coronary heart disease patients with anxiety or depression: A meta-analysis and systematic review. Front Pharmacol. (2022) 13:854292. doi: 10.3389/fphar.2022.854292
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Kalkman HO. The association between vascular inflammation and depressive disorder. Causality, biomarkers and targeted treatment. Pharmaceuticals. (2020) 13:92. doi: 10.3390/ph13050092
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. (2011) 25:1725–34. doi: 10.1016/j.bbi.2011.07.229
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Vancassel S, Leman S, Hanonick L, Denis S, Roger J, Nollet M, et al. n-3 Polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J Lipid Res. (2008) 49:340–8. doi: 10.1194/jlr.M700328-JLR200
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Siscovick DS, Barringer TA, Fretts AM, Wu JHY, Lichtenstein AH, Costello RB, et al. Omega-3 polyunsaturated fatty acid (Fish oil) supplementation and the prevention of clinical cardiovascular disease. Circulation. (2017) 135:e867–84. doi: 10.1161/CIR.0000000000000482
PubMed Abstract | CrossRef Full Text | Google Scholar
10. O’Connell TD, Block RC, Huang SP, Shearer GC. ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J Mol Cell Cardiol. (2017) 103:74–92. doi: 10.1016/j.yjmcc.2016.12.003
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk. Jama. (2020) 324:2268. doi: 10.1001/jama.2020.22258
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA. (2009) 302(15): 1615–57. doi: 10.1001/jama.2009.1487
CrossRef Full Text | Google Scholar
13. Carney RM, Freedland KE, Stein PK, Steinmeyer BC, Harris WS, Rubin EH, et al. Effect of omega-3 fatty acids on heart rate variability in depressed patients with coronary heart disease. Psychosom Med. (2010) 72:748–54. doi: 10.1097/PSY.0b013e3181eff148
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Zimmer R, Riemer T, Rauch B, Schneider S, Schiele R, Gohlke H, et al. Effects of 1-year treatment with highly purified omega-3 fatty acids on depression after myocardial infarction. J Clin Psychiatry. (2013) 74:e1037–45. doi: 10.4088/JCP.13m08453
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Mazereeuw G, Herrmann N, Oh PI, Ma DWL, Wang CT, Kiss A, et al. Omega-3 fatty acids, depressive symptoms, and cognitive performance in patients with coronary artery disease. J Clin Psychopharm. (2016) 36:436–44. doi: 10.1097/JCP.0000000000000565
CrossRef Full Text | Google Scholar
16. Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. A randomized placebo-controlled trial of omega-3 and sertraline in depressed patients with or at risk for coronary heart disease. J Clin Psychiatry. (2019) 80:19m12742. doi: 10.4088/JCP.19m12742
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Chang JP, Chang S, Yang H, Chen H, Chien Y, Yang B, et al. Omega-3 polyunsaturated fatty acids in cardiovascular diseases comorbid major depressive disorder – Results from a randomized controlled trial. Brain Behavior Immunity. (2020) 85:14–20. doi: 10.1016/j.bbi.2019.03.012
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Baghai TC, Varallo-Bedarida G, Born C, Häfner S, Schüle C, Eser D, et al. Major depressive disorder is associated with cardiovascular risk factors and low omega-3 index. J Clin Psychiatry. (2011) 72:1242–7. doi: 10.4088/JCP.09m05895blu
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Ali S, Garg SK, Cohen BE, Bhave P, Harris WS, Whooley MA. Association between omega–3 fatty acids and depressive symptoms among patients with established coronary artery disease: data from the heart and soul study. Psychother Psychosom. (2009) 78:125–7. doi: 10.1159/000203118
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Dogaru I, Puiu MG, Manea M, Dionisie V. Current perspectives on pharmacological and non-pharmacological interventions for the inflammatory mechanism of unipolar depression. Brain Sci. (2022) 12:1403. doi: 10.3390/brainsci12101403
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Dang R, Zhou X, Tang M, Xu P, Gong X, Liu Y, et al. Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur J Nutr. (2018) 57:893–906. doi: 10.1007/s00394-016-1373-z
留言 (0)