Two opposing clinical syndromes characterise the immune and inflammatory response to major traumatic and thermal injury: a pro-inflammatory systemic inflammatory response syndrome (SIRS) and a counteracting compensatory anti-inflammatory response syndrome (CARS). Evident within minutes of trauma, and persisting into the acute injury phase (1–4), a defining feature of the CARS response is reduced ex vivo production of pro-inflammatory cytokines by lipopolysaccharide (LPS) challenged monocytes (5). Associated with the development of nosocomial infections (NI) and sepsis (6), this post-injury induction of endotoxin tolerance is of clinical significance, yet the mechanisms underlying its development are poorly understood.
Offering a potential explanation for the state of systemic immune suppression that develops post-trauma is the concept of “damage associated molecular pattern (DAMP)-induced immune tolerance”. Culminating in impaired anti-microbial responses to secondary stimulation, this theory proposes that following their release from injured tissues, DAMPs, a heterogeneous collection of nuclear, cytosolic and mitochondrial-derived proteins, lipids and DNA, induce functional tolerance in circulating immune cells through binding to pathogen recognition receptors (PRR) (7, 8). Suggesting a role for endogenous ligands of toll-like receptor 4 (TLR4) in mediating post-injury endotoxin tolerance, pre-conditioning monocytes isolated from healthy volunteers with DAMPs detected by this PRR, such as high mobility group box-1 (HMGB-1), heat shock protein-70 (HSP-70) and calprotectin, has been shown to significantly reduce their production of tumour necrosis factor-alpha (TNF-α) upon subsequent LPS stimulation (9–12).
Involved in oxygen transport and energy production, heme is an iron containing intracellular porphyrin that serves as the prosthetic group for such haemoproteins as myoglobin, haemoglobin and cytochrome P450. However, in times of cellular and tissue damage, heme is released from haemoproteins into the extracellular environment, where it functions as a potent immunomodulatory molecule. In conditions associated with significant haemolysis or rhabdomyolysis, free heme, a ligand of TLR4 (13), has been proposed to promote systemic inflammation by triggering the generation of pro-inflammatory cytokines, reactive oxygen species (ROS) and extracellular traps by neutrophils and macrophages (13–16). Recently, immune tolerising properties have also been assigned to heme, with murine models of trauma haemorrhage and liver crush injury demonstrating that elevated circulating concentrations of this DAMP were associated with an increased susceptibility to, and severity of, pulmonary infections (17, 18). Attributed to impaired innate immune responses, heme was shown to suppress the phagocytic activity of alveolar macrophages (18) and promote the downregulation of TLR2 and TLR4 from the neutrophil surface (17). Whether heme induced a state of systemic endotoxin tolerance post-injury was not investigated.
At present, our understanding of how trauma impacts upon circulating heme levels is based on the results of a single study. Performed alongside a murine model of liver crush injury, in which an immediate (<30 minutes) elevation in plasma heme levels was detected, Lee et al. analysed post-hospital admission plasma samples from six trauma patients and reported a significant post-injury increase in heme concentrations (17). Notably, despite profound tissue injury and haemolysis (19), no study, to our knowledge, has measured circulating heme levels in thermally-injured patients. Rather, studies have focused upon burn-induced changes in the heme scavenging system (20–22). Comprised of the plasma proteins hemopexin, haptoglobin and albumin, and the inducible enzyme heme oxygenase-1 (HO-1), this scavenging system is responsible for the neutralisation, degradation and removal of free heme (23, 24). A prospective based study of five severe burns patients detected significantly lower concentrations of hemopexin in plasma samples obtained at days 1–5 post-injury (20), whilst an analysis of skin biopsies obtained from eleven patients revealed a post-burn increase in HO-1 expression (22). Whether traumatic injury leads to alterations in the scavenging of free heme is currently unclear, with results of human and murine-based studies reporting either no difference or a significant increase in plasma hemopexin levels post-injury (17). Severe trauma has however been shown to increase HO-1 expression in circulating leukocytes, with this post-injury induction preceding the diagnosis of sepsis (25, 26).
Investigating to what extent free heme contributes to immune dysfunction after traumatic injury was recently identified as a high priority research topic in a scoping review that discussed the role of hemolysis in the pathophysiology of trauma (27). It has been suggested that, by reducing anti-microbial immune responses, excessive heme release combined with a post-injury dysregulation in heme scavenging may contribute to the increased susceptibility of critically ill patients to NI and sepsis (25). To test this hypothesis, we have performed, for the first time, a comprehensive assessment of the impact of severe injury on heme biology. Combined with ex vivo analyses of LPS-induced cytokine production by whole blood leukocytes, and an assessment of HO-1 expression in peripheral blood mononuclear cells (PBMCs) of major trauma patients, we have measured the concentrations of total heme, hemopexin, haptoglobin and albumin in plasma samples obtained from over 200 trauma and burns patients across the ultra-early (≤1 hour), immediate (4–12 hours) and acute (48–72 hours) post-injury settings. Furthermore, we have examined whether a measurement of total heme levels on day 1 post-burn can distinguish between patients with differing clinical outcomes, specifically non-survival and the development of sepsis. Accompanying these ex vivo studies, we have investigated whether pre-conditioning monocytes with heme can induce endotoxin tolerance in vitro.
Materials and methodsPatient cohortsBurnsThis manuscript presents data acquired between November 2016 and September 2023 from adult (≥16 years) burns patients admitted to the West Midlands Regional Burns Centre (WMRBC) within 24 hours of sustaining a total body surface area (TBSA) burn ≥15%. Based at the Queen Elizabeth Hospital Birmingham, the WMRBC is one of three burns centres participating in the Scientific Investigation of the Biological Pathways Following Thermal Injury-2 (SIFTI-2) study, an ongoing prospective, longitudinal observational cohort study of children and adult patients with moderate and severe burn injury. Details relating to study design, exclusion criteria and the procedure of patient consent are described in the SIFTI-2 study protocol (28). The SIFTI-2 study (trial registration number:NCT04693442) received ethical approval from the West Midlands, Coventry and Warwickshire Research Ethics Committee (REC reference:16/WM/0217).
TraumaData generated from the analysis of peripheral blood samples acquired from subjects enrolled into the Brain Biomarkers after Trauma Study (BBATS) between May 2014 and August 2018 are presented in this manuscript. Conducted at a single Major Trauma Centre site in the UK (University Hospitals Birmingham NHS Foundation Trust (UHBFT), Birmingham), BBATS is an ongoing prospective longitudinal observational study of adult trauma patients. On a 24/7 basis, pre-hospital emergency care teams obtain blood samples from adult trauma patients (≥18 years) with a suspected injury severity score (ISS) ≥8 within 1 hour of injury (defined as the time of phone call to emergency services). Details relating to patient capacity and consent, enrolment and study exclusion criteria have been described previously (2, 29, 30). Ethical approval for the study was granted by the North Wales Research Ethics Committee - West (REC reference:13/WA/0399, Protocol Number: RG_13–164).
Clinical data collectionPatient and injury details were obtained prospectively from electronic and physical medical records. Data collected included patient age, gender, mechanism of injury, time of injury, severity of injury [Injury Severity Score (ISS), New ISS (NISS) and Glasgow Coma Scale (GCS)], percent TBSA, percent TBSA full thickness, baux score, revised baux score, abbreviated burn severity index, sequential organ failure assessment score and Denver score. Albumin concentrations were measured as part of routine biochemistry investigations during inpatient stays, with the results retrospectively extracted for each patient from the electronic clinical records system used by UHBFT.
Patient outcomesAdhering to the recommendations of the 2007 American Burn Association (ABA) consensus for the definition of sepsis and infections in burns patients (31), a diagnosis of sepsis was made when patients met ≥3 of the six sepsis ABA trigger criteria and had a documented infection, identified as either (i) a positive bacterial culture from wound swabs, blood, sputum or urine samples, or (ii) a clinical response to antimicrobials. Data regarding patient mortality and ICU and hospital-free days (calculated as 30 minus the number of days the patient stayed in ICU and hospital respectively) was extracted from electronic clinical records. Patients who died in the hospital or ICU setting within 30 days of admission were assigned a score of 0.
Blood samplingBlood samples were collected into BD Vacutainers® (BD Biosciences, UK) containing lithium heparin, z-serum clotting activator or a 1/10 volume of 3.2% trisodium citrate. For patients enrolled into BBATS, blood samples were obtained at three post-injury time-points; pre-hospital (≤1 hour), 4–12 and 48–72 hours. In the pre-hospital setting, blood samples were obtained during the intravenous cannulation of patients or by venepuncture. Vacutainers were stored at room temperature (RT) during transportation to hospital, where, upon arrival, they were stored at 4°C, and collected for analysis within 1 hour by a single researcher on a 24/7 basis. For subjects enrolled into the SIFTI-2 study, data presented in this manuscript were generated from the analysis of blood samples acquired at days 1 and 3 post-burn.
60 adults (mean age 34 years, range 19–69 years) served as a cohort of healthy controls (HC), who we defined as individuals not taking any regular medication for a diagnosed illness and who had not experienced an acute episode of infection prior to enrolment. HC were recruited in accordance with the ethical approval granted by the University of Birmingham Research Ethics Committee (Ref: ERN_12–1184).
Haematological analysisFull blood cell counts were performed on citrated or heparinsed anti-coagulated whole blood using the Sysmex XN-1000 haematology analyser (Sysmex UK, Milton Keynes, UK). Instrument performance was ensured by daily internal quality control measurements (XN check, Sysmex UK) and enrolment into a national external quality assurance scheme (UKNEQAS, Watford, UK).
Preparation of platelet free plasma and serumPFP was prepared from citrate anti-coagulated whole blood by a two-step centrifugation process. Blood samples were centrifuged at 2,000 × g for 20 minutes at 4°C, after which the top two-thirds of platelet-poor plasma (PPP) was carefully removed. PPP was then subjected to centrifugation at 13,000 × g for 2 minutes at 4°C to generate PFP, which was collected and stored at −80°C until analysed.
Serum was prepared from blood collected into BD vacutainers containing z-serum clotting activator. Following a 30 minute incubation at RT, samples were centrifuged at 1,620 x g for 10 minutes at 4°C, after which serum was removed and stored at −80°C until analysed.
Preparation of hemePrepared fresh on the day of experimentation, heme stock solutions were generated by dissolving 6.5 mg synthetic hemin (Stratech, Cambridge, UK) in dimethyl sulfoxide (Merck, Dorset, UK). Stock solutions and vehicle control were diluted in phosphate buffered saline or RPMI-1640 media supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (GPS) and 20% fetal calf serum (FCS) prior to cell treatments.
Cell culture and isolation of PBMCsHuman monocytic THP-1 cells were purchased from the American Type Culture Collection (Virginia, USA) and cultured at 37°C/5%CO2 in RPMI-1640 media supplemented with GPS and 10% heat-inactivated FCS [hereafter referred to as complete media (CM)]. PBMCs were isolated from heparin anti-coagulated blood samples by density gradient centrifugation using Ficoll-Paque PLUS media (Cytiva, Sheffield, UK).
Measurements of heme, hemopexin and haptoglobinFollowing manufacturer’s instructions, concentrations of total heme in PFP were determined using a heme assay kit (Abcam, Cambridge, UK). Enzyme linked immunosorbent assays (ELISAs) to quantify concentrations of hemopexin and haptoglobin in PFP were performed in accordance with manufacturer’s protocols (Abcam).
Cytokine and chemokine measurementsFollowing manufacturer’s guidelines, serum concentrations of IL-1 receptor antagonist (IL-1Ra), interleukin (IL)-6, IL-10, granulocyte colony stimulating factor (G-CSF) and monocyte chemoattractant protein-1 (MCP-1) were determined using a commercially available magnetic bead multiplex immunoassay (BioRad, Hertfordshire, UK).
LPS stimulation of whole blood and quantification of TNF-α and interleukin-6 levelsFor the ex vivo analysis of leukocyte function, 400 µl aliquots of heparinised whole blood were stimulated for 4 or 18 hours (37°C/5%CO2) with 10 ng/ml LPS from Escherichia coli (serotype 0111:B4; Merck) or vehicle control. Post-treatment, samples were centrifuged at 461 x g for 8 minutes at 4°C, after which supernatants were collected and stored at −80°C until analysed. Following manufacturer’s instructions, TNF-α and IL-6 concentrations were determined using commercially available ELISAs (R and D Systems, Oxford, UK), with results normalised to monocyte count.
Treatments of THP-1 cells and PBMCsTo examine the role of glycolysis and mitogen activated protein kinase (MAPK) signalling in TNF-α production by monocytes, 1x106 THP-1 cells or PBMCs in CM were treated for 1 hour (37°C/5%CO2) with 5–50 mM 2-Deoxy-D-Glucose (2-DG; Merck), 10 µM PD98059 (Cell Signalling Technology (CST) Leiden, Netherlands) or vehicle control, after which cells were stimulated for 4 hours with 100 ng/ml or 1 µg/ml LPS respectively at 37°C/5%CO2. Post-incubation, samples were centrifuged (1,500 x g, 2 minutes), and cell free supernatants collected for the determination of TNF-α concentrations by ELISA.
To investigate the effect of heme treatment on monocyte function, THP-1 cells (1–2x106) or PBMCs (1x106), resuspended in RPMI media supplemented with GPS (hereafter referred to as assay media) or RPMI + GPS + 20% FCS, were cultured for 1 or 4 hours (37°C/5% CO2) in the presence of 10–20 µM heme or vehicle control. Post-treatment, cells were pelleted (1,500 x g, 2 minutes) and resuspended in CM or assay media prior to a 30 minute, 2 hour or 4 hour stimulation with 100 ng/ml or 1 µg/ml LPS or vehicle control (37°C/5%CO2). Post-culture, samples were centrifuged (1,500 x g, 2 minutes), and either cell free supernatants collected for the determination of TNF-α and lactate concentrations, or cell pellets resuspended in RLT or SDS lysis buffer in preparation for RNA isolation and Western blotting respectively.
To ascertain whether serum collected from thermally-injured patients could modulate monocyte function, THP-1 cells (0.5–1x106) were cultured for 24 hours (37°C/5%CO2) in assay media supplemented with 10% sera pooled from 5 burns patients on day 1 of injury or HC. Post-treatment, cells were stimulated for 2 or 4 hours with 1 µg/ml LPS (37°C/5%CO2), after which cells were pelleted (1,500 x g, 2 minutes) and either cell free supernatants collected for the determination of TNF-α concentrations or cell pellets resuspended in RLT lysis buffer in preparation for RNA isolation. Endotoxin concentrations in patient and HC serum samples were determined using a commercially available limulus amebocyte lysate chromogenic endpoint assay (Hycult Biotech, Pennsylvania, USA).
Measurements of lactate concentration and lactate dehydrogenase activity in cell-free supernatantsIn accordance with manufacturer’s guidelines, lactate concentrations and LDH activity in 5–10 µl aliquots of cell-free supernatants collected from heme and/or LPS treated THP-1 cells or PBMCs were determined using commercially available lactate or LDH activity assay kits (Merck). For the LDH activity assay, a positive control was generated by treating 1x106 THP-1 cells with 10 µM staurosporine (Merck) for 4 hours (37°C/5% CO2).
Western blottingCell lysates prepared from 1 µg/ml LPS stimulated THP-1 cells pre-treated for 4 hours with 20 µM heme or vehicle control were separated on 10% SDS-polyacrylamide gels. Following protein transfer to polyvinylidene difluoride membranes (Bio-Rad, Hertfordshire, UK), blots were probed overnight at 4°C with rabbit anti-human antibodies (purchased from CST) directed against phosphorylated NF-κB p65 (Ser536) or ERK1/2 (Thr202/Tyr204). Post-incubation, membranes were washed in tris-buffered saline containing 0.001% tween (TBST) and incubated for 1 hour at RT with a goat anti-rabbit secondary antibody conjugated to horse radish peroxidase (HRP; diluted 1:4000 in TBST; GE Healthcare, Buckinghamshire). HRP activity was detected using enhanced chemiluminescence (Cytiva, Massachusetts, USA). To confirm equal loading of proteins, blots were stripped with harsh stripping buffer (Abcam) and probed with antibodies against total P65 or ERK1/2 (CST; diluted 1:1000 in TBST). Densitometry analysis was performed using Image J software (National Institute of Health, Maryland, USA).
Real time polymerase chain reactionFollowing manufacturer’s guidelines, total RNA was extracted from THP-1 cells or PBMCs using an RNeasy Mini kit (Qiagen Ltd) or TRIzol™ reagent (ThermoFisher Scientific UK, Chesire, UK), with concentrations quantified using a NanoDrop 2000 (ThermoFisher Scientific).
mRNA expression levels of TNF-α and HO-1 were determined, relative to 18S, by RT-PCR using the iTaq™ Universal SYBR® Green One-step kit mastermix (Bio-Rad), 5 ng total RNA and primers (TNF-α: Forward 5’CCT CTC TCT AAT CAG CCC TCT G3’, Reverse 5’GAG GAC CTG GGA GTA GAT GAG3’. HO-1: Forward 5’CAG GAT TTG TCA GAG GCC CTG AAG G3’, Reverse 5’TGT GGT ACA GGG AGG CCA TCA CC3’. 18S: Forward 5’GTA ACC CGT TGA ACC CCA TT3’, Reverse 5’CCA TCC AAT CGG TAG CG3’). All reactions had a total volume of 5 µl and were performed in triplicate. Data were acquired using a Bio-Rad sfx cycler (Bio-Rad) and analysed by the 2-ΔΔCt method using Bio-Rad CFX manager software (Bio-Rad). Gene expression was calculated relative to 18S. For cell treatment experiments, results are presented as fold change above untreated controls.
Statistical analysesStatistical analyses were performed using GraphPad PRISM software (GraphPad Software Ltd, California, USA) and R v4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). Data distribution was assessed using the Kolmogorov-Smirnov or Shapiro-Wilk normality tests. Data that followed a normal distribution were analysed using a one-way ANOVA with either a Dunnett or Bonferroni multiple comparison post-hoc test, an unpaired student t test or a paired student t test. To analyse non-normally distributed data, a Kruskal-Wallis test with Dunn’s multiple comparison post-hoc test, Mann-Whitney U test or Wilcoxon matched-pairs signed rank test was performed. Relationships between continuous variables were assessed using a Spearman’s correlation. For comparisons of continuous variables between survivors and non-survivors, and septic and non-septic patients, Mann-Whitney U tests or independent samples t tests were performed, whilst Chi-squared tests were conducted to compare categorical variables. Box and whisker plots are presented in Tukey style. The threshold for statistical significance was set at p≤ 0.05.
Associations between concentration levels in predictor variables and outcomes were modelled using logistic regression models. Initially, concentration is the only covariate included in the models (the unadjusted models). Further models, adjusted for age, gender, and TBSA are also fit to the data. Odds ratios, 95% CIs and p-values are reported for all models. Predicted probabilities, and 95% CIs, of outcomes are reported for the unadjusted models. The potential discriminatory ability of concentrations measured on day 1 to distinguish between survivors and non survivors was assessed through fitting logistic regression models. Discriminatory performance of the models is reported using area under the receiver-operating characteristic curve (AUROC) and Brier scores.
ResultsPatient demographicsA total of 98 thermally-injured patients were included in this study (Table 1). Patients had a mean age of 47 years (range 16–84 years), and presented with a mean TBSA burn of 35% (range 15–85%). Flame burn was the predominant mechanism of injury, with 43% of the cohort sustaining an inhalation injury. The incidence of sepsis and mortality was 53% and 20% respectively, with day 6 post-burn the median time to first septic episode (Table 1).
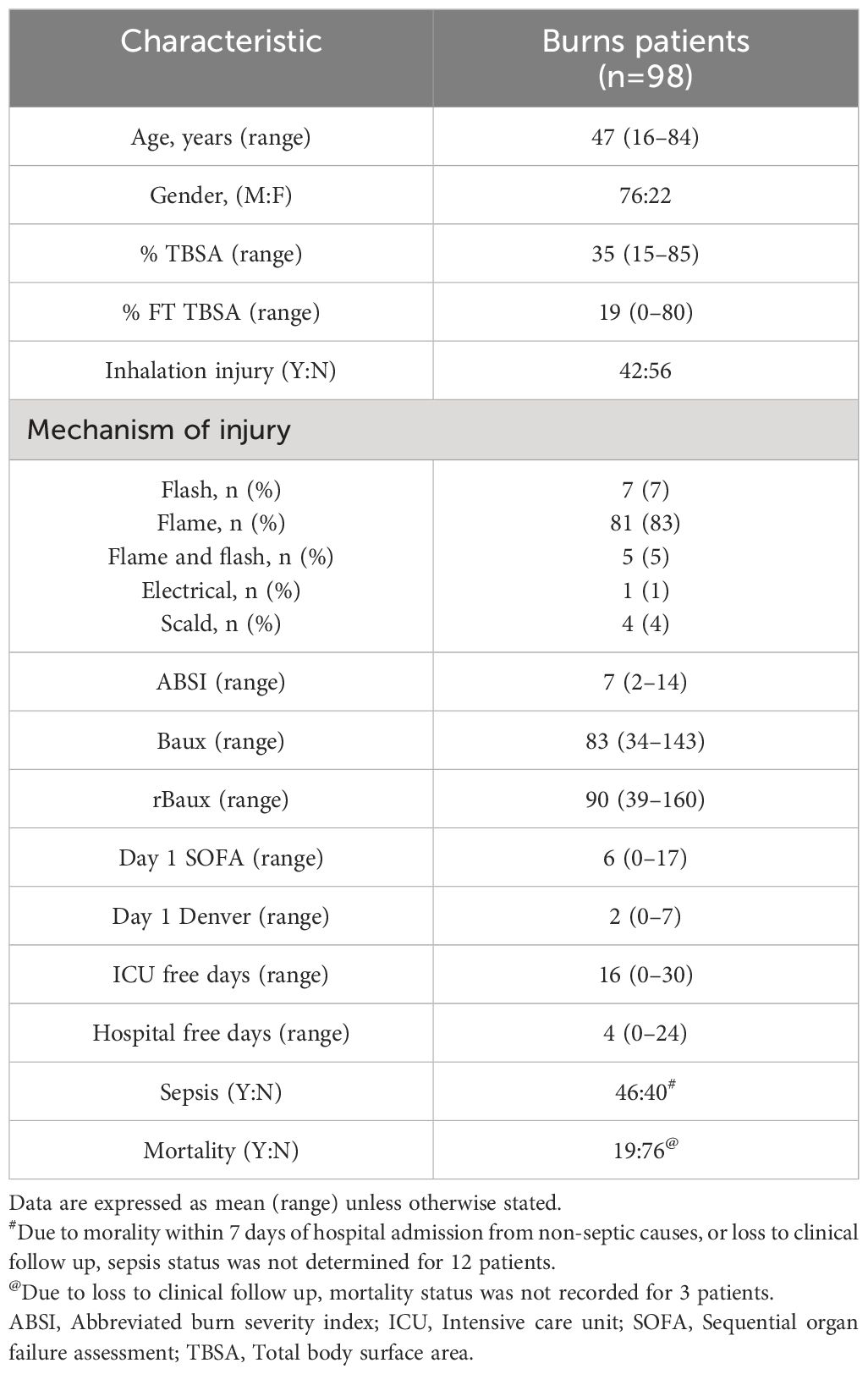
Table 1 Burns patients demographics.
Road traffic collisions (53.1%) were the predominant mechanism of injury in our cohort of 147 adult trauma patients who had a mean age of 42 years (range 18–95 years) and a mean ISS of 25 (range 9–66) (Table 2). The mean time of pre-hospital blood sampling was 42 minutes post-injury (range 13–60 minutes).
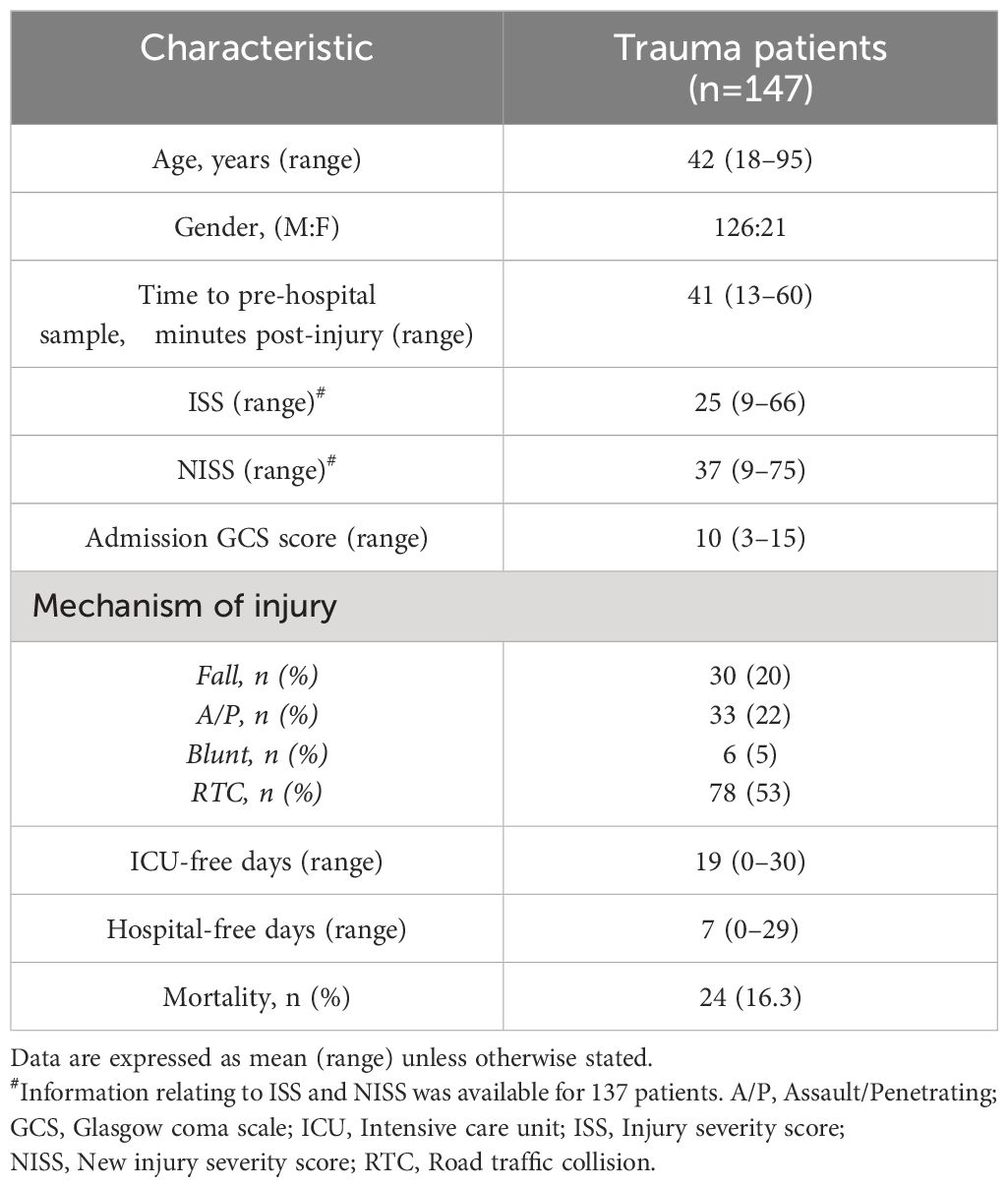
Table 2 Trauma patients demographics.
Severe thermal injury results in an immediate and sustained systemic inflammatory response that is accompanied by impaired ex vivo LPS-induced pro-inflammatory cytokine production by whole blood leukocytesIn line with our previous work in the setting of major trauma (2), analysis of serum samples obtained from thermally-injured patients revealed an early and persistent elevation in the circulating concentrations of inflammatory cytokines and chemokines, with the levels of IL-6, G-CSF, MCP-1 and IL1-Ra significantly elevated on day 1 and/or day 3 post-burn when compared to the concentrations measured in HC (Figures 1A–D). Thermal injury did not alter the circulating levels of IL-10 (Figure 1E).
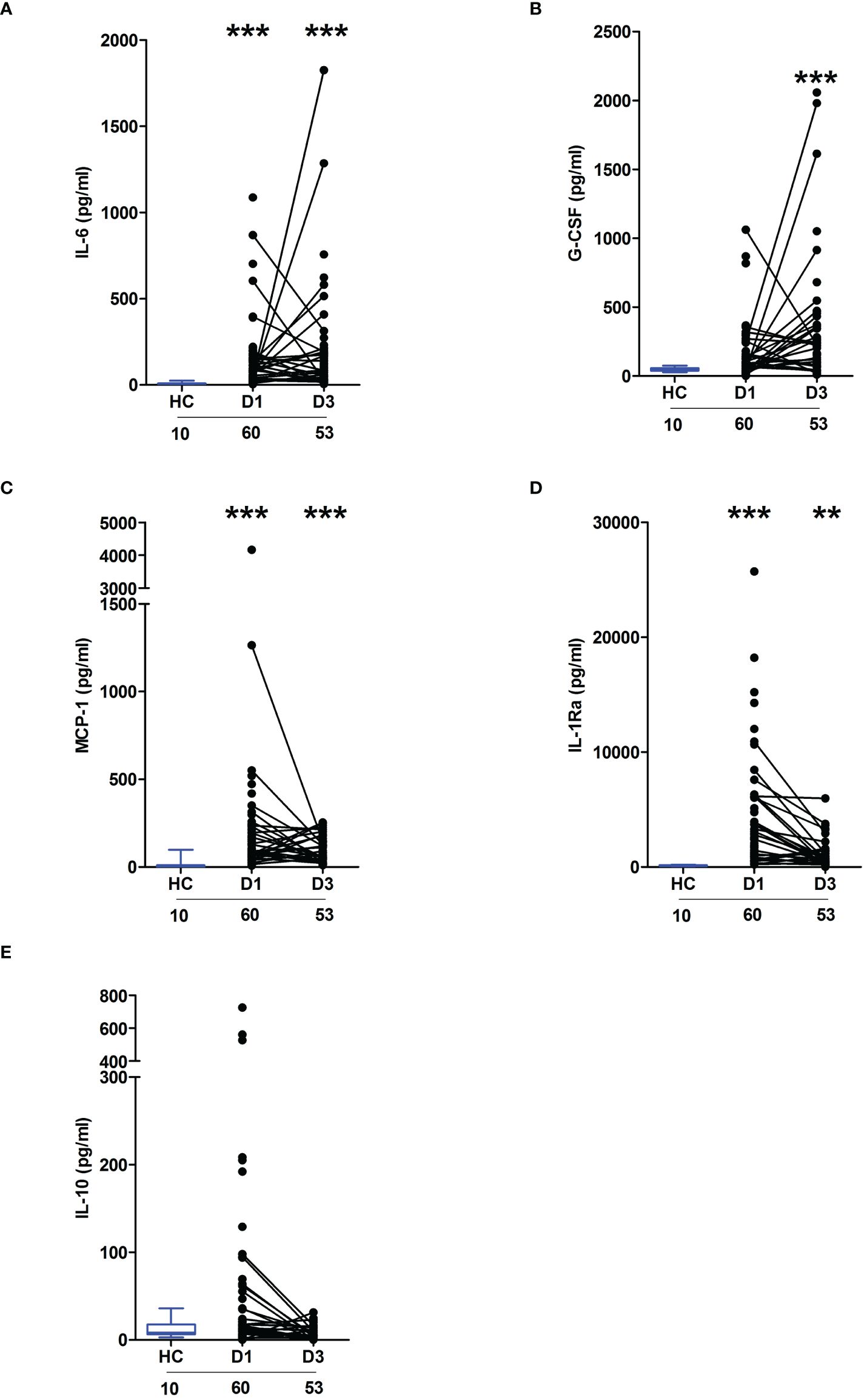
Figure 1 Severe thermal injury results in an immediate and sustained systemic inflammatory response. (A–E) Comparison of concentrations of interleukin (IL)-6 (A), Granulocyte colony stimulator-factor (G-CSF) (B), Monocyte chemoattractant protein-1 (MCP-1) (C), IL-1 receptor antagonist (IL-1Ra) (D) and IL-10 (E) in serum samples obtained from thermally-injured patients at days 1 and 3 post-burn and healthy controls (HC). The number of samples analysed are stated below each study time-point. **p<0.005, ***p<0.0005.
Across the ultra-early (<1 hour) and acute (2–72 hours) post-injury phase, we and others, have previously demonstrated that whole blood leukocytes from major trauma patients exhibit impaired ex vivo pro-inflammatory cytokine production upon LPS stimulation (1, 2). To determine whether thermal injury also resulted in peripheral endotoxin tolerance, whole blood leukocytes isolated from severe burns patients at days 1 and 3 post-injury were stimulated with LPS for 4 or 18 hours, after which the levels of pro-inflammatory cytokines in cell free supernatants were measured. Compared to samples from HC, significantly lower concentrations of TNF-α and IL-6 were detected in supernatants of LPS-challenged blood from burns patients at both post-injury time-points (Figures 2A, B).
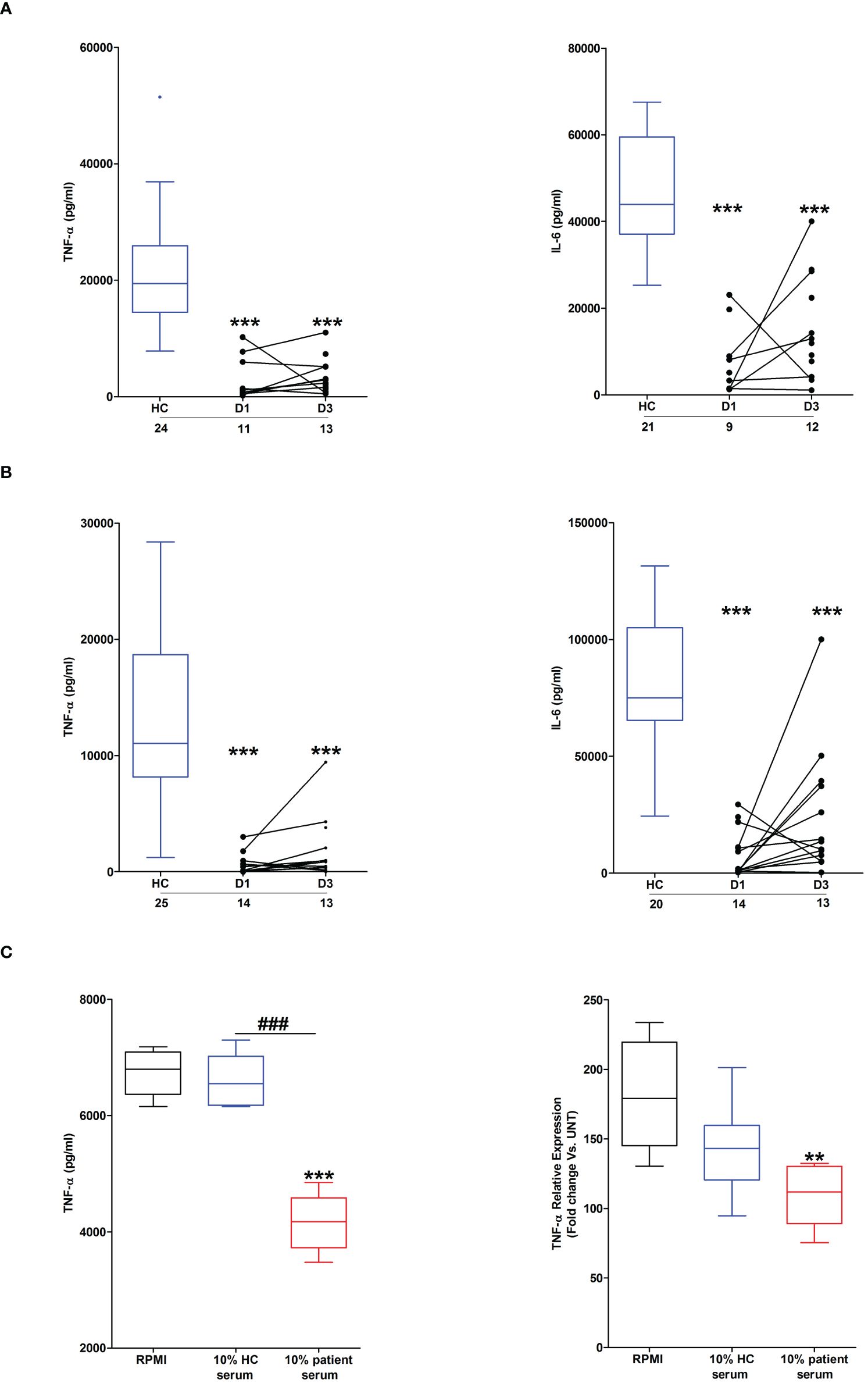
Figure 2 Effect of severe thermal injury on lipopolysaccharide (LPS)-induced pro-inflammatory cytokine production by whole blood leukocytes. (A, B) Tumour necrosis factor-alpha (TNF-α; left panel) and interleukin-6 (IL-6; right panel) concentrations measured in supernatants of whole blood samples obtained from burns patients at days 1 and 3 post-injury and healthy controls (HC) following a 4 hour (A) or 18 hour (B) ex vivo stimulation with 10 ng/ml LPS. The number of samples analysed are stated below each study time-point. (C) Following a 24 hour culture in media supplemented with 10% serum obtained from burns patients on day 1 of injury or HC (n=5), THP-1 cells were stimulated with 1 µg/ml LPS, after which TNF-α concentrations in cell free supernatants (left panel) or TNF-α mRNA levels (right panel) were measured respectively. For the measurement of TNF-α concentrations in culture supernatants, THP-1 cells were stimulated for 4 hours with LPS. TNF-α mRNA levels were examined in THP-1 cells following a 2 hour stimulation with LPS. **p<0.005, ***p<0.0005 Vs vehicle. ###p<0.0005.
Suggesting a role for circulating factors in promoting post-burn endotoxin tolerance, THP-1 cells cultured for 24 hours in media supplemented with 10% serum obtained from thermally-injured patients exhibited impaired TNF-α production and transcription upon LPS stimulation (Figure 2C). Compared to HC, endotoxin levels were significantly lower in serum samples of burns patients (Supplementary Figure 1).
Major thermal and traumatic injury results in elevated circulating concentrations of heme that are positively associated with systemic inflammationAs reported in Figure 3A, relative to HC, significantly higher concentrations of total heme were detected in PFP samples obtained from burns patients on day 1 of injury. At day 3 post-burn, no significant difference was detected in the circulating concentrations of total heme between patients and HC (burns patients, 17.03 ± 0.92 µM Vs HC, 17.59 ± 1.47 µM; p= n.s). Analysis of day 1 samples revealed total heme concentrations were positively associated with % TBSA (r(n=88)=0.456, p=<0.0001), % full thickness TBSA (r(n=88)=0.466, p=<0.0001), baux score (r(n=88)=0.350, p=0.0008) and revised baux score (r(n=88)=0.390, p=0.0002). A negative association was found between total heme levels and the time of sample acquisition post-burn (r(n=88)=-0.411, p=<0.0001). With our patient cohort ranging in age from 16–84 years, we examined whether a relationship existed between circulating heme levels and age. We found no association between patient age and plasma concentrations of heme at days 1 or 3 post-burn (Supplementary Table 1).
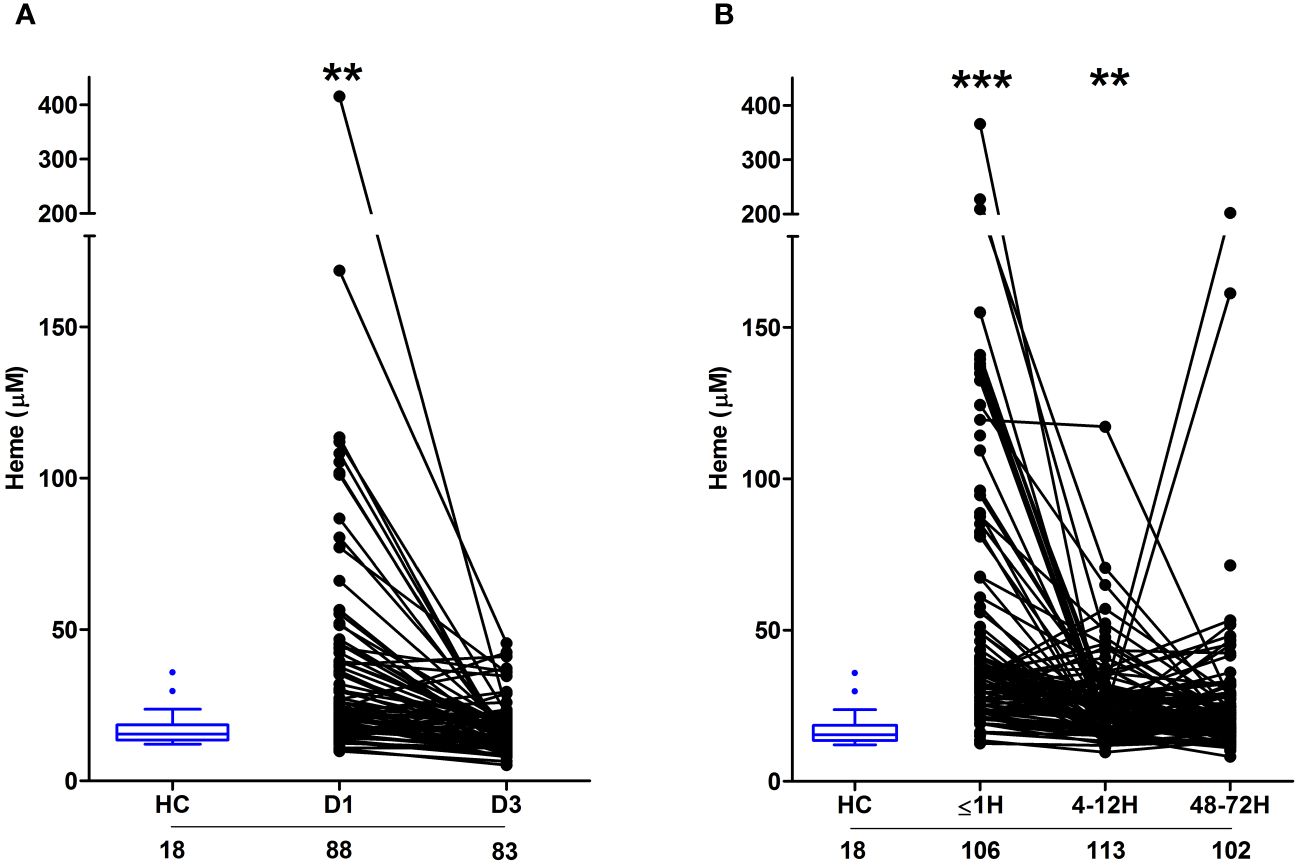
Figure 3 Severe burns and major traumatic injury result in an immediate elevation in plasma concentrations of total heme. (A, B) Concentrations of total heme measured in PFP samples of severe burns (A) and major trauma (B) patients across the ultra-early (≤1H) and acute (4–72H) post-injury setting. The number of samples analysed are stated below each study time-point. **p<0.005, ***p<0.0005. HC, Healthy control.
In our cohort of trauma patients, total heme concentrations were significantly elevated in PFP prepared from blood samples obtained ≤1 and 4–12 hours post-injury when compared to HC (Figure 3B). As observed with burns patients, heme levels negatively correlated with the time of sample acquisition post-injury (r(n = 321) =-0.452, p=<0.0001). Analysis of samples acquired within 1 hour of trauma revealed no relationship between heme concentrations and injury severity when assessed using either ISS (r(n = 101) =0.09, p=0.350) or NISS (r(n = 101) =0.130, p=0.194). An examination of the relationship between patient age and heme levels revealed a weak positive association between these two variables only at the 4–12 hour post-injury sampling time-point (Supplementary Table 1).
On day 1 of burn injury, we found circulating levels of IL-10 and MCP-1 were positively associated with heme concentrations (Table 3). At day 3 post-burn, plasma concentrations of heme positively correlated with the circulating levels of IL1-Ra and MCP-1 (Table 3). In our cohort of major trauma patients, in whom we have previously measured levels of circulating pro and anti-inflammatory cytokines (2), we found positive associations between plasma heme levels and the concentrations of IL1-Ra, IL-6, IL-10 and G-CSF at the 4–12 hour post-injury sampling time-point, and between heme and MCP-1 48–72 hours post-injury (Table 3).
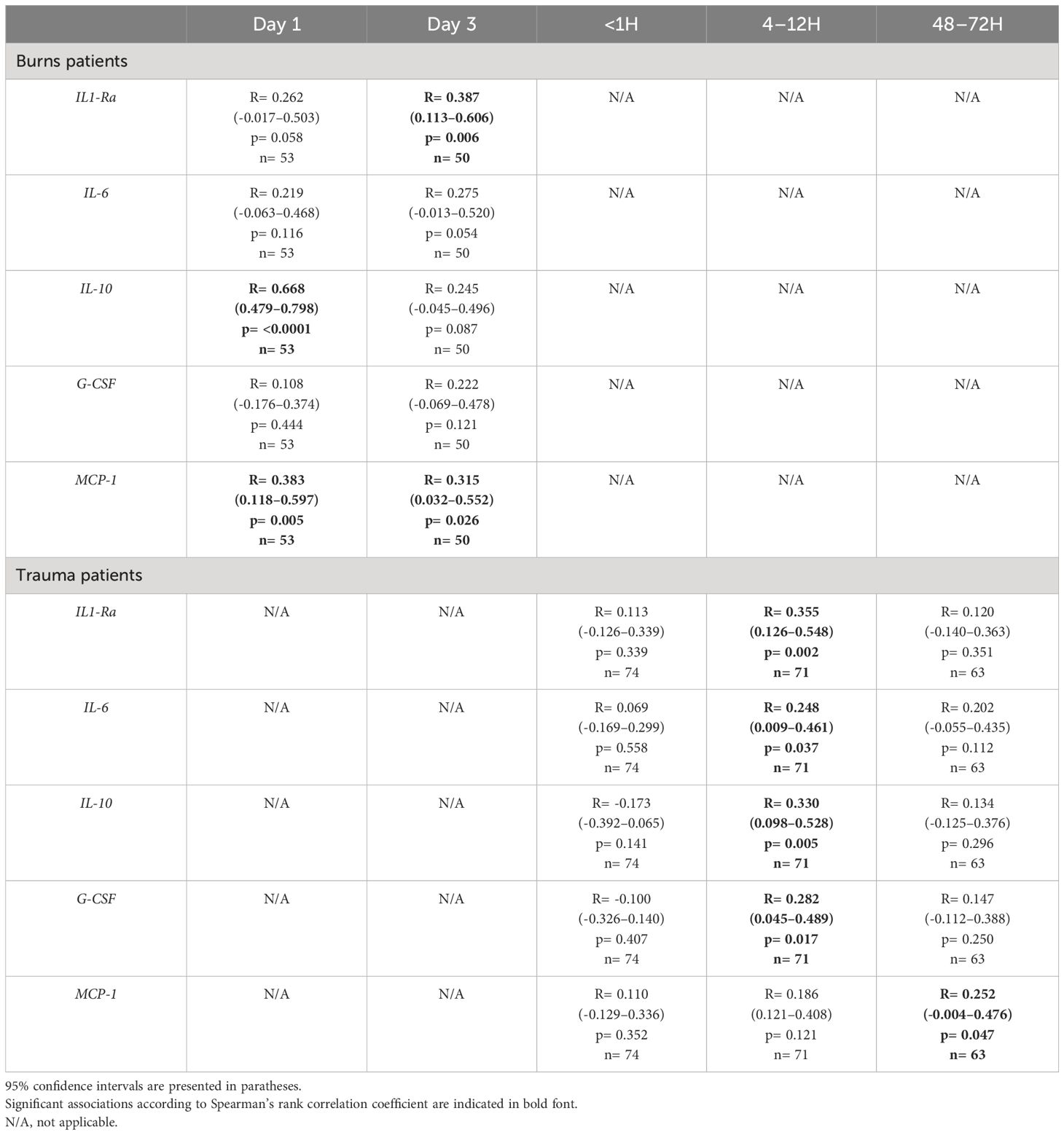
Table 3 Correlative analyses examining the relationship between plasma heme levels and the circulating concentrations of pro and anti-inflammatory cytokines in severe burns and major trauma patients.
Fragmented red cells and damaged tissue are potential sources of circulating heme in major burns and trauma patientsConfirming previous observations (19), thermally-injured patients presented on day 1 of injury with significantly elevated counts and frequencies of FRC, with readings returning to levels comparable to those recorded for HC by day 3 post-burn (Figure 4A). Both the absolute number and frequencies of FRC on day 1 of burn injury were positively associated with total heme concentrations (absolute number, r(n=88)=0.472, p= <0.0001; frequency, r(n=88)=0.471, p=<0.0001).
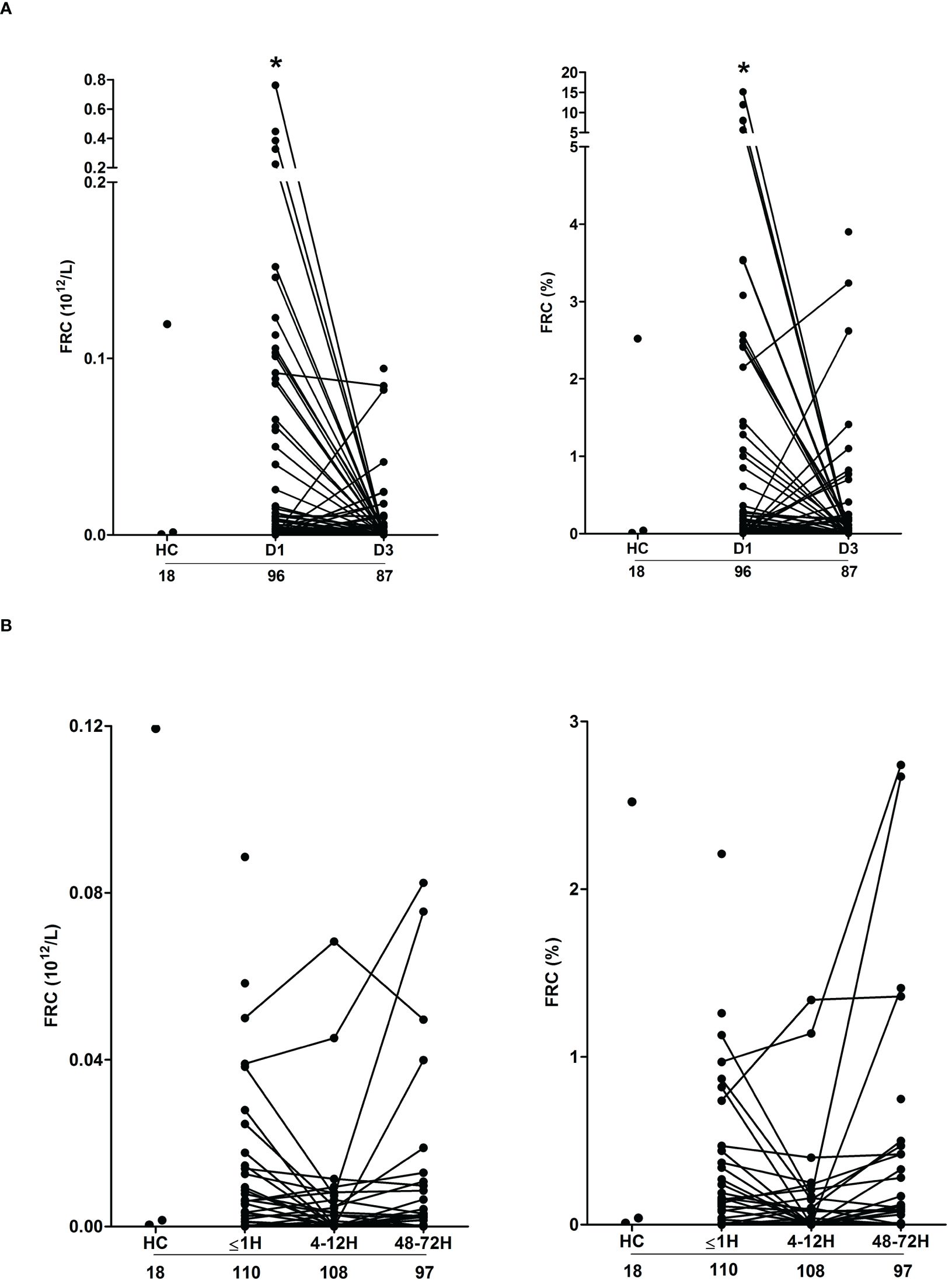
Figure 4 Severe thermal injury but not major trauma results in the generation of fragmented red blood cells. (A, B) Absolute number (left panel) and frequency (right panel) of fragmented red blood cells (FRC) in peripheral blood samples of burns patients on days 1 and 3 post-injury (A) and major trauma patients (B) in the ultra-early (≤1H) and acute (4–72H) post-injury setting. The number of samples analysed are stated below each study time-point. *p<0.05. HC, Healthy control.
Compared to HC, we detected no difference in the absolute number or frequency of FRC in blood samples obtained from trauma patients at any of our three study time-points (Figure 4B). Used as a marker of tissue injury, we previously reported that circulating concentrations of G-actin are elevated in the immediate post-injury setting (29). Analysis of plasma samples acquired from trauma patients ≤1 hour post-injury revealed a significant positive relationship between total heme levels and the circulating concentrations of G-actin (r(n=37)=0.366, p=0.02).
Severe thermal and major traumatic injury results in the depletion of plasma proteins involved in heme and haemoglobin scavengingCirculating heme levels are regulated by a scavenging system comprised of the plasma proteins hemopexin, haptoglobin and albumin (23, 24). Burns patients presented with significantly lower concentrations of hemopexin and albumin at days 1 and 3 post-injury (Figures 5A, B). Repeated measures analysis revealed a significant decrease in the concentrations of both proteins between the two study time-points (Supplementary Figure 2). On day 1 of burn injury, significant negative associations were found between % full thickness TBSA and the plasma levels of hemopexin (r(n=83)=-0.310, p=0.004) and albumin (r(n =95) =-0.404, p=<0.0001). Thermal injury had no effect upon the circulating concentrations of haptoglobin (Figure 5C).
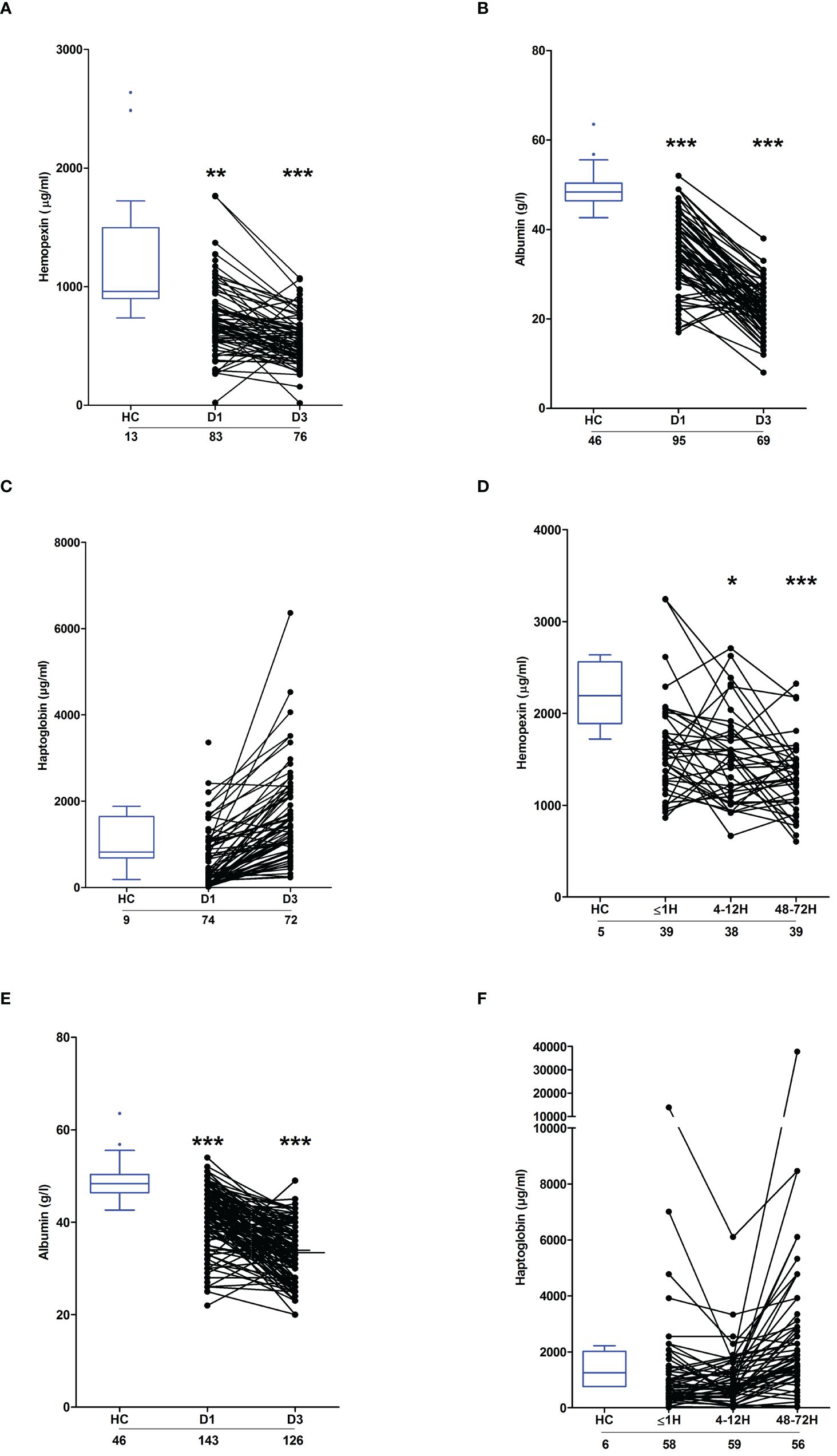
Figure 5 Effect of severe thermal injury and major trauma on the concentrations of plasma proteins implicated in heme and haemoglobin scavenging. (A‐C) Concentrations of hemopexin (A), albumin (B) and haptoglobin (C) measured in PFP samples acquired from thermally-injured patients on days 1 and 3 post-burn. (D‐F) Concentrations of hemopexin (D), albumin (E) and haptoglobin (F) measured in PFP samples acquired from major trauma patients during the ultra-early (≤1H) and acute (4–72H) post-injury phase. The number of samples analysed are stated below each study time-point. *p<0.05, **p<0.005, ***p<0.0005. HC, Healthy control.
Relative to HC, hemopexin levels were significantly lower in PFP samples obtained from trauma patients 4–12 and 48–72 hours post-injury (Figure 5D). Similarly, on days 1 and 3 post-trauma, patients presented with significantly reduced plasma concentrations of albumin (Figure 5E). Traumatic injury did not alter the circulating levels of haptoglobin (Figure 5F).
Major traumatic injury but not severe burns lead to an up-regulation in HO-1 gene expression in PBMCsHO-1 is a stress-inducible enzyme that catalyses the degradation of intracellular heme (24). Compared to HC, HO-1 gene expression was significantly increased in PBMCs isolated from major trauma patients 4–12 and 48–72 hours post-injury (Figure 6A). In contrast, we detected no difference in HO-1 mRNA levels between PBMCs isolated from burns patients at days 1 or 3 post-injury and HC (Figure 6B).
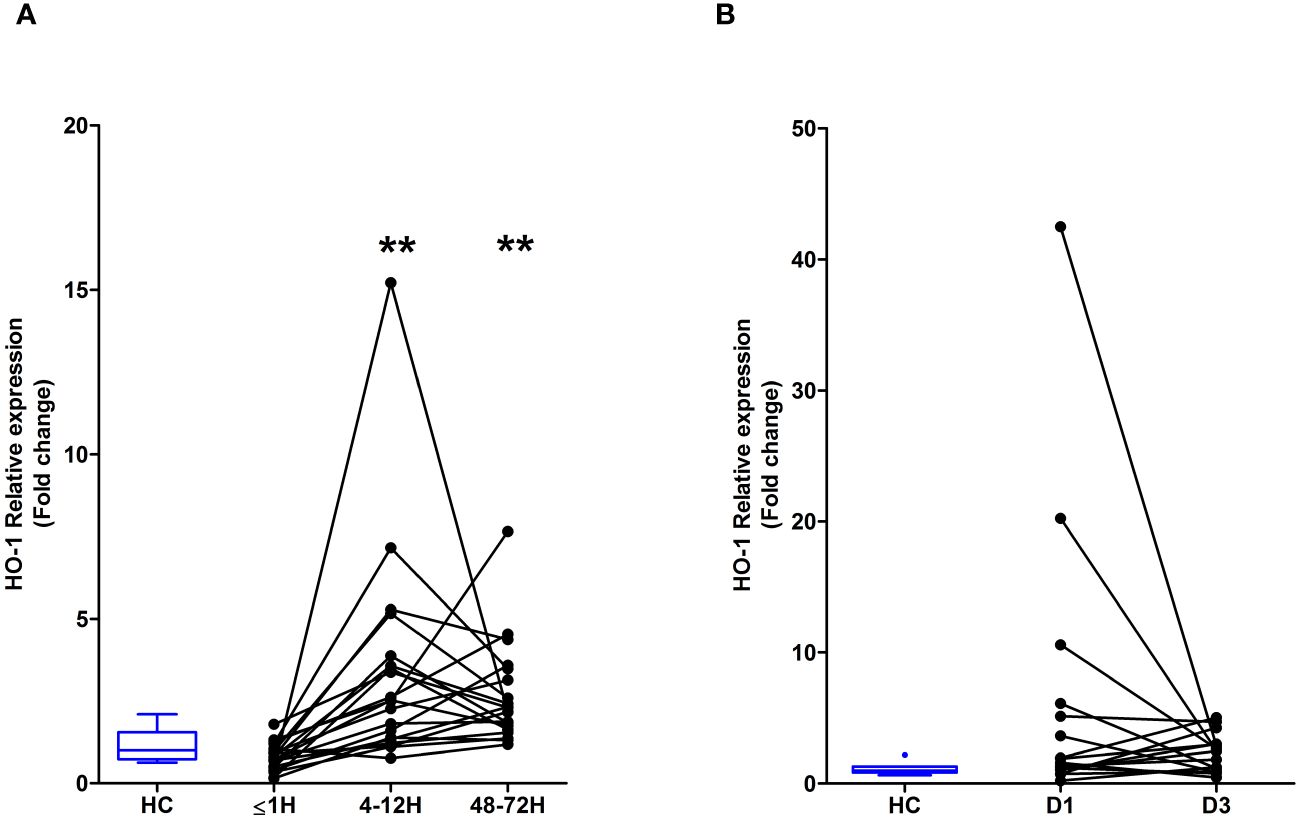
Figure 6 Major traumatic injury but not severe burns results in increased heme oxygenase-1 (HO-1) gene expression in peripheral blood mononuclear cells (PBMCs). (A) Comparison of HO-1 mRNA levels in PBMCs of healthy controls (HC; n=10) and major trauma patients (n=20) at three post-injury time-points. (B) HO-1 gene expression in PBMCs isolated from HC (n=8) and severe burns patients (n=16) at days 1 and 3 post-thermal injury. **p<0.005.
Elevated total heme concentrations are associated with poor clinical outcomes post-burnTo investigate whether elevated plasma concentrations of total heme on day 1 of thermal injury were associated with poor clinical outcomes, we performed exploratory analysis to examine if any associations existed between total heme levels and either patient mortality or the development of sepsis. Within our cohort of 98 burns patients, 4 died within 7 days of injury from non-septic causes and 8 withdrew from clinical follow up during their in-hospital stay. Of the remaining 86 patients, 7 did not have a measurement of total heme levels on day 1 of injury. Thus, sepsis status was determined for 79 patients, with 42 developing this secondary complication, a prevalence rate of 53%. The demographics of our septic and non-septic cohorts are summarised in Supplementary Table 2. Of the 95 patients for whom survival status was determined, 7 did not have a measurement of total heme levels on day 1 of injury. Mortality was therefore assessed in 88 patients, with 14 patients meeting this clinical endpoint. The demographic and clinical data of survivors and non-survivors are summarised in Supplementary Table 2.
Non-survivors of thermal injury and patients who developed sepsis post-burn presented on day 1 of injury with significantly elevated plasma concentrations of total heme (Figures 7A, B). To investigate this relationship further, we calculated the predicted probabilities of mortality and the development of sepsis in burns patients at days 1 and 3 post-injury using the quantiles of heme concentrations measured at these two sampling time-points (Table 4).
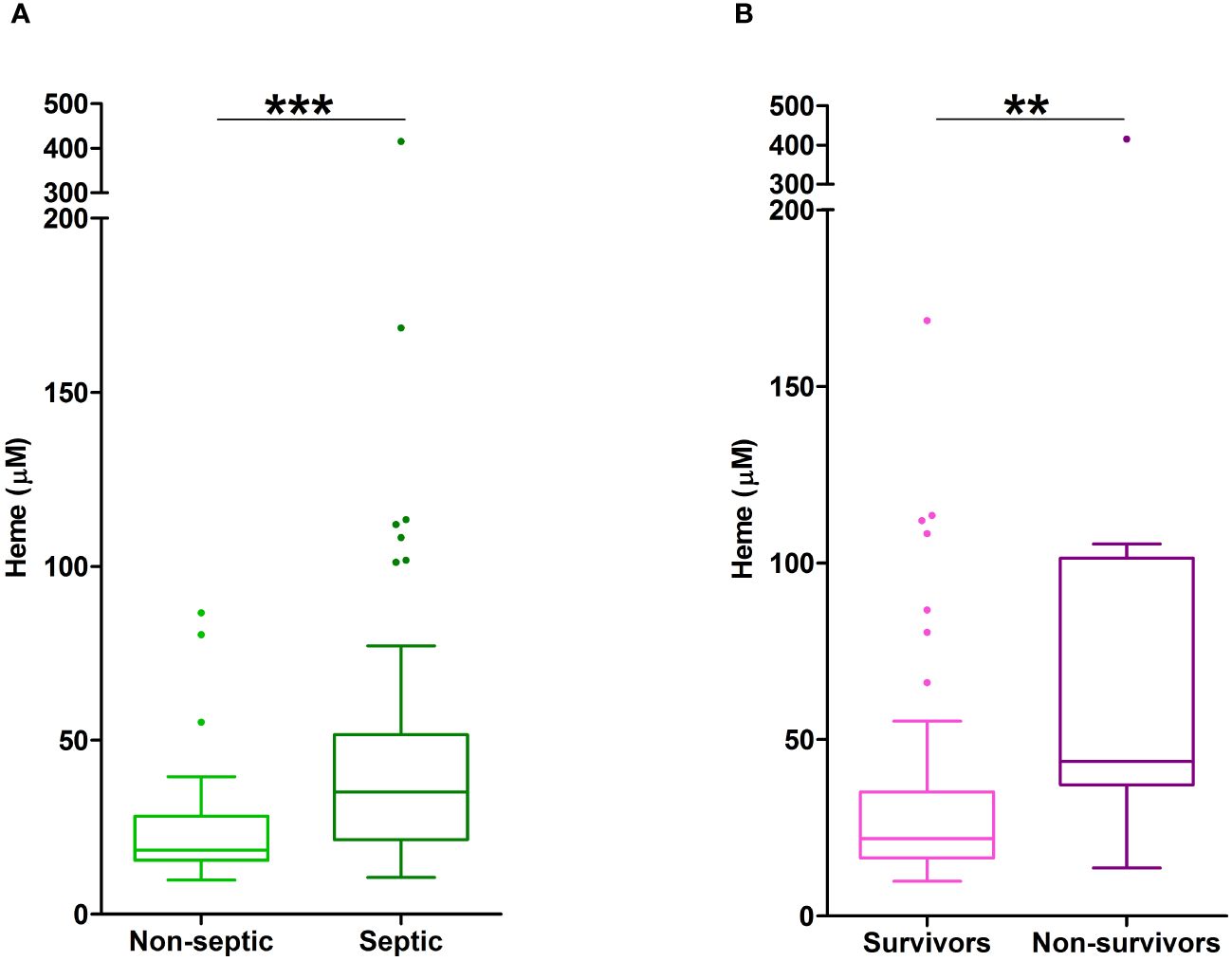
Figure 7 Elevated plasma concentrations of total heme are associated with poor clinical outcomes in severe burns patients. (A) Total heme concentrations measured in PFP samples obtained on day 1 of injury from thermally-injured patients who did (n=42) or did not (n=37) develop sepsis post-burn. (B) Comparison of day 1 total heme levels in PFP samples of survivors (n=74) and non-survivors (n=14) of severe thermal injury. **p<0.005, ***p<0.0005.
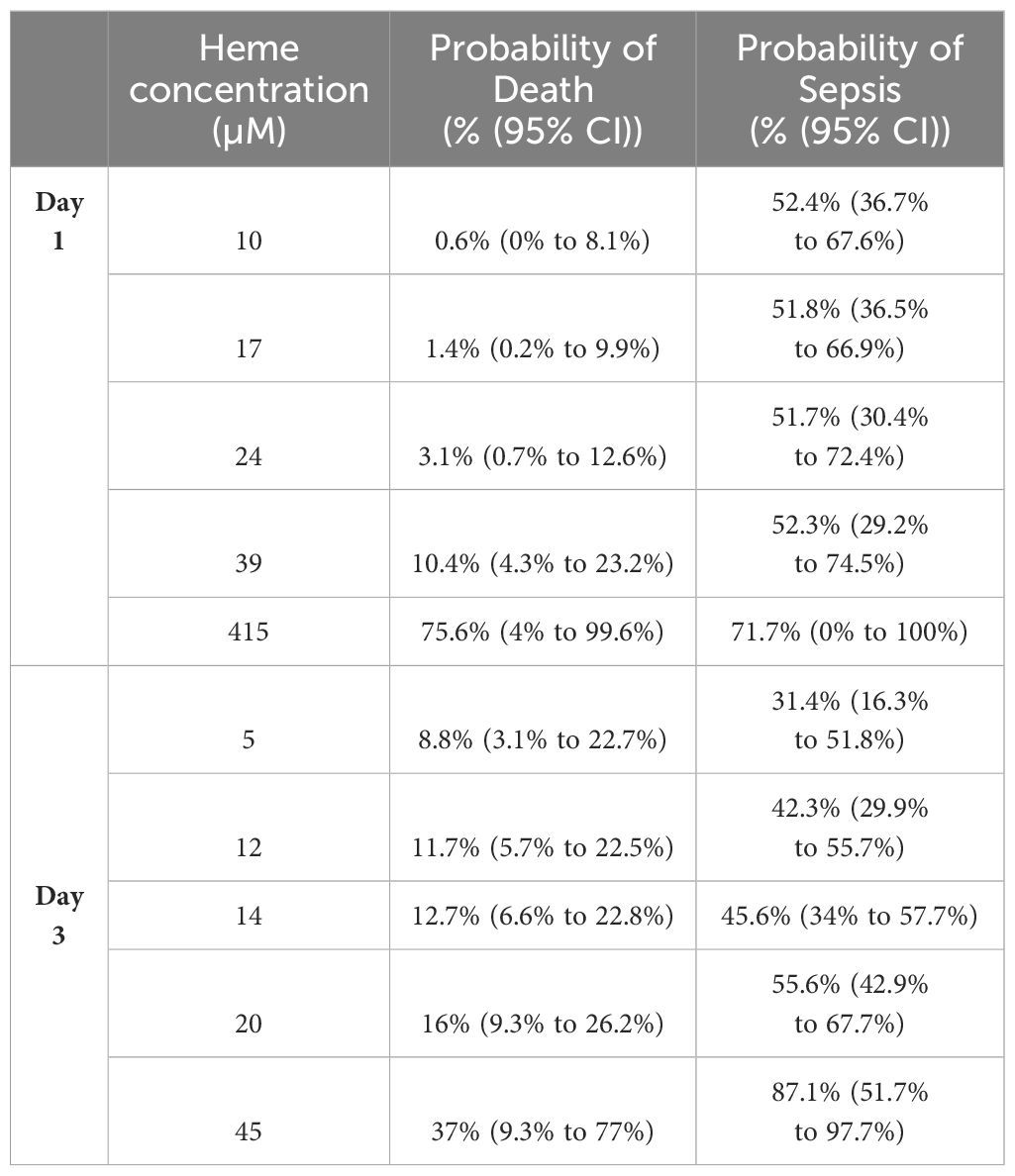
Table 4 Predicted probabilities of mortality and the development of sepsis in thermally-injured patients based on the quantiles of total heme concentrations measured on days 1 and 3 post-burn.
In unadjusted logistic regression models, we found that a difference of 6.5 µM in the circulating concentration of total heme corresponded to a relative increase in the odds of sepsis of 24% (OR, 1.24 (95% CI, 1.05, 1.46), p=0.013). However, when models were adjusted for age, gender and % TBSA, the size of this association was reduced (OR, 1.12 (95% CI, 0.95, 1.33), p=0.172). In terms of mortality, a difference of 6.5 µM in the circulating concentration of total heme corresponded to a relative increase in the odds of mortality of 63% (OR, 1.63 (95% CI, 1.12, 2.37), p=0.004), an association that remained in a model adjusted for age, gender and % TBSA (OR, 1.52 (95% CI, 1.02, 2.28), p=0.021).
To assess the potential discriminatory ability of day 1 total heme levels to distinguish between survivors and non survivors, prognostic models were examined. AUROC analyses revealed day 1 total heme levels had moderate power to discriminate between these two patient groups (AUROC, 0.768 (95% CI, 0.615–0.922), Brier Score 0.119). In comparison, a model built on rBAUX scores generated an AUROC value of 0.718 (95% CI, 0.587–0.848; Brier score 0.123).
Exposure to heme induces endotoxin tolerance in monocytesSuggesting a link between elevated heme concentrations and monocyte endotoxin tolerance post-burn, significant negative associations were detected between circulating total heme levels on day 1 of injury and the concentrations of TNF-α and IL-6 measured in supernatants of whole blood samples challenged ex vivo with LPS for 4 hours (TNF-α, r(n=11)=-0.736, p=0.013; IL-6, r(n=9)=-0.717, p=0.037) or 18 hours (IL-6, r(n=14)=-0.582, p=0.029).
In vitro exposure to heme did not trigger pro-inflammatory cytokine production by THP-1 monocytes (Supplementary Figure 3A). However, when compared to vehicle controls, THP-1 cells pre-treated for 1 or 4 hours with 10 or 20 µM heme generated significantly less TNF-α upon subsequent LPS challenge (Figure 8A), a finding we confirmed in primary human monocytes (Figure 8B). Demonstrating the specificity of this response, heme-induced, but not LPS-induced, endotoxin tolerance was prevented by culturing THP-1 cells in media supplemented with 20% FCS (Figure 8C). An assessment of cellular toxicity found no difference in the activity of lactate dehydrogenase (LDH) in supernatants derived from vehicle or heme treated THP-1 cells (Supplementary Figure 3B). Staurosporine treatment served as a positive control in this assay and resulted in significantly increased LDH activity in culture supernatants (Supplementary Figure 3B).
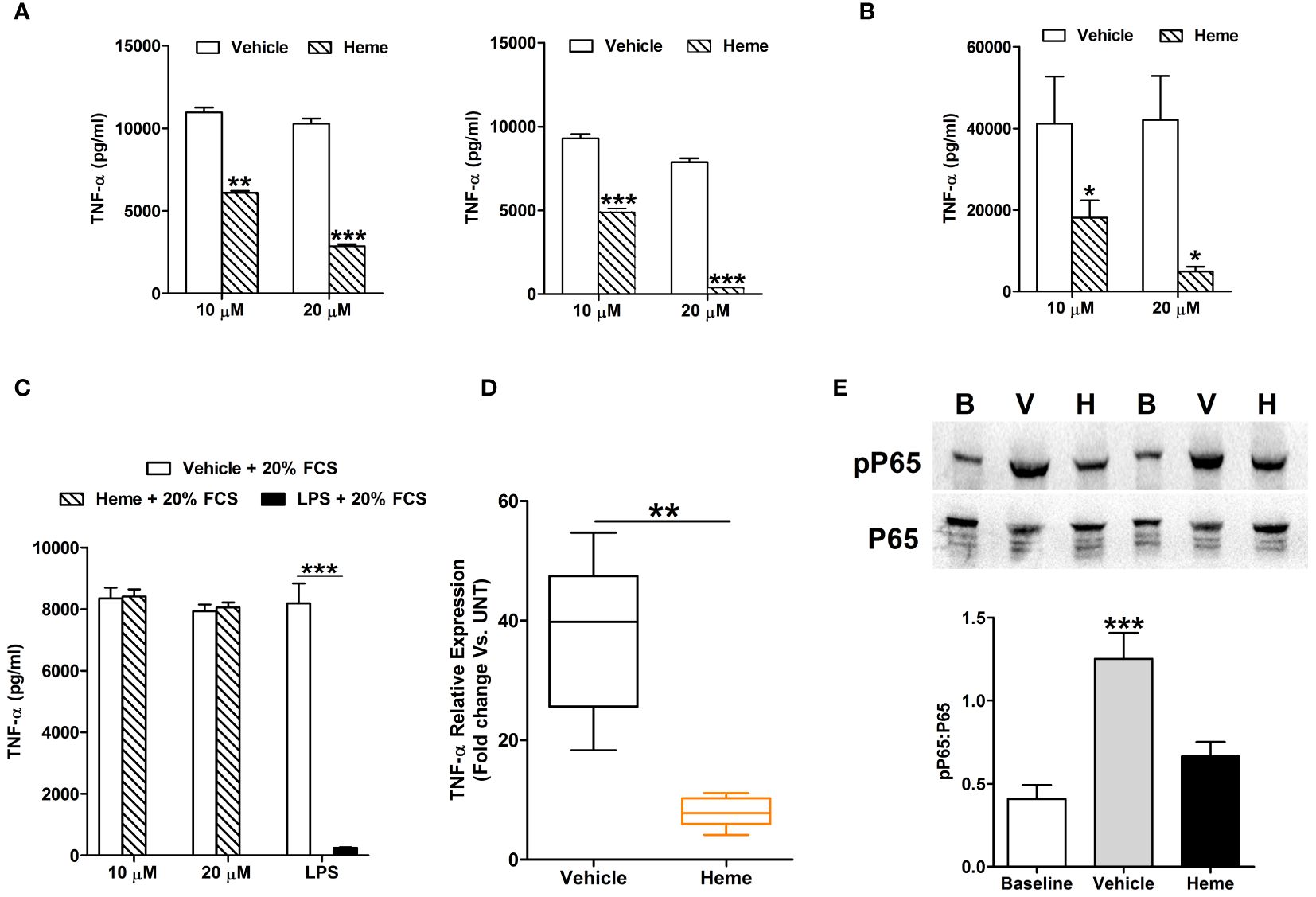
Figure 8 In vitro heme treatment induces endotoxin tolerance in human monocytes. (A) Concentration of tumour necrosis factor-alpha (TNF-α) detected in supernatants of lipopolysaccharide (LPS) challenged THP-1 cells (n=6) pre-treated for 1 hour (left panel) or 4 hours (right panel) with 10 or 20 µM heme. **p<0.005, ***p<0.0005. (B) Concentration of TNF-α measured in supernatants of LPS challenged PBMCs (n=8) pre-treated for 4 hours with 10 or 20 µM heme. *p<0.05. (C) TNF-α levels recorded in supernatants of LPS challenged THP-1 cells pre-treated for 4 hours with 20 µM heme (n=8) or 100 ng/ml LPS (n=3) in media supplemented with 20% fetal calf serum (FCS). (D) Comparison of TNF-α mRNA levels in LPS stimulated THP-1 cells pre-treated for 4 hours with 20 µM heme or vehicle control (n=5). (E) Representative Western blot (top panel) and collated densitometry data (bottom panel, n=7) showing the phosphorylation status of the NF-κB subunit P65 in LPS challenged THP-1 cells pre-treated for 4 hours with 20 µM heme or vehicle control. **p<0.005 Vs. Baseline. B, Baseline; V, vehicle control; H, heme-treated.
Analysis of gene expression found that, relative to vehicle treated controls, TNF-α mRNA levels were significantly lower in LPS challenged THP-1 cells pre-treated with heme (Figure 8D). This heme-induced impairment in TNF-α gene transcription was associated with reduced phosphorylation of the NF-κB subunit P65 (Figure 8E).
Heme pre-treatment results in impaired LPS-induced activation of the MAPK ERK1/2Activation of the MAPK ERK1/2 has been implicated in the production of TNF-α by LPS stimulated monocytes (32, 33). In line with this data, THP-1 monocytes pre-treated with the ERK1/2 inhibitor PD98059 exhibited significantly reduced TNF-α secretion upon LPS challenge (Figure 9A), with this impairment associated with decreased TNF-α gene transcription (Figure 9A).
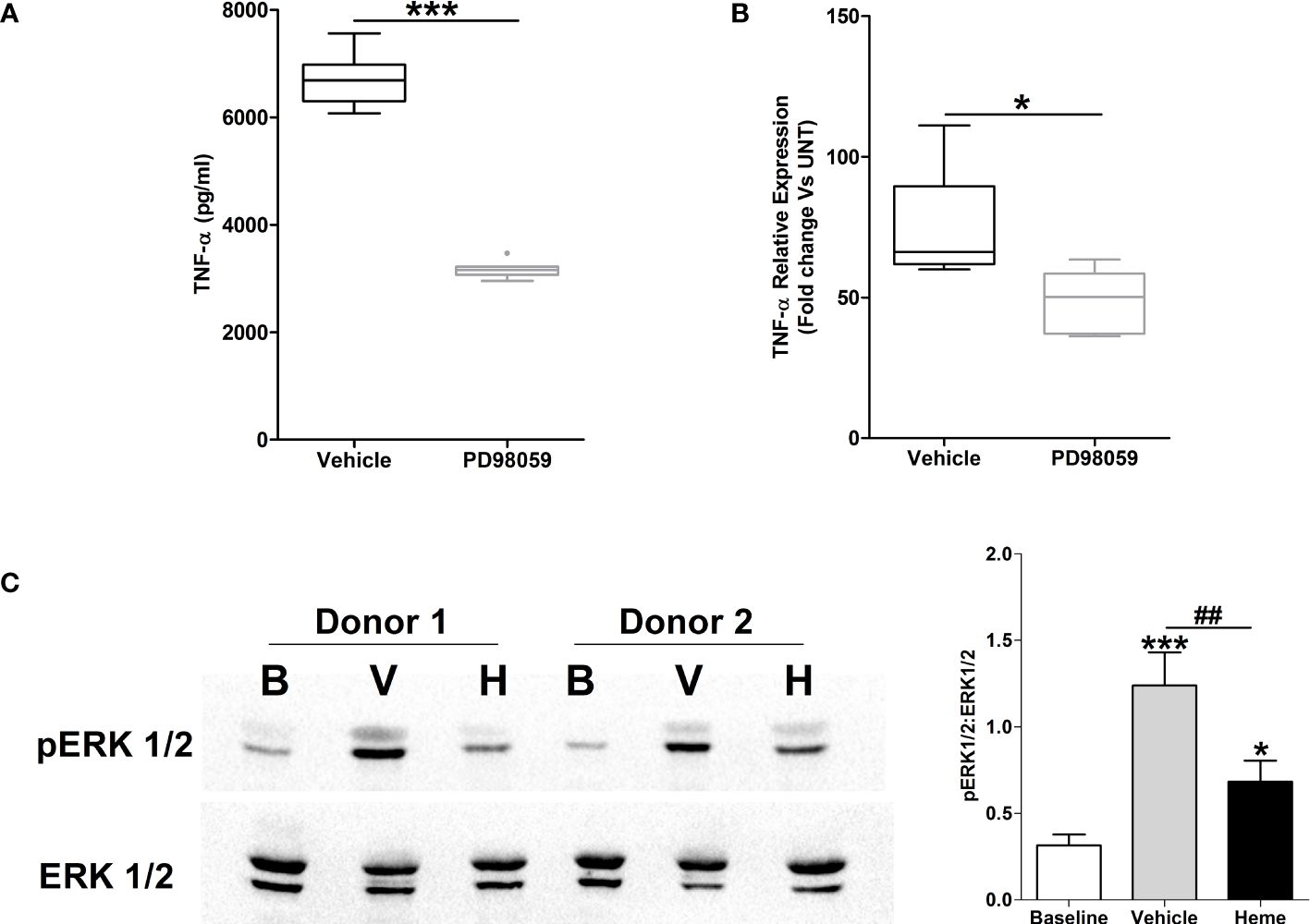
Figure 9 Impaired lipopolysaccharide (LPS)-induced activation of the MAPK extracellular signal regulated kinase 1/2 (ERK 1/2) in heme pre-treated THP-1 cells. (A, B) Following a 1 hour pre-treatment with 10 µM PD98059 or vehicle control, THP-1 cells were stimulated with 1 µg/ml LPS, after which tumour necrosis factor-alpha (TNF-α) concentrations in culture supernatants (n=10) (A) and mRNA expression (n=6) (B) was measured. *p<0.05, ***p<0.0005. (C) Representative Western blot (left panel) and collated densitometry data (right panel, n=6) showing the phosphorylation status of ERK1/2 in LPS challenged THP-1 cells pre-treated for 4 hours with 20 µM heme or vehicle control. *p<0.05, ***p<0.0005 Vs Baseline. ##p<0.005. B, Baseline; V, vehicle control; H, heme-treated.
To investigate whether exposure to heme influenced LPS-induced activation of ERK1/2, we analysed the phosphorylation status of this MAPK in LPS stimulated THP-1 cells pre-treated with heme. As shown in Figure 9B, THP-1 monocytes pre-exposed to heme exhibited significantly impaired activation of ERK1/2 following LPS challenge.
Monocytes pre-treated with heme exhibit impaired glycolytic responses upon LPS challengeConfirming that metabolic reprogramming towards increased glycolysis precedes TNF-α production by LPS stimulated monocytes (34), we detected significantly higher concentrations of lactate in supernatants of LPS challenged T
留言 (0)