Upper tract urothelial carcinoma (UTUC) is a rare and highly heterogeneous disease which accounts for approximately 5% of all urothelial malignancies (1, 2). In China, the figure may be even higher, accounting for 17.9% of urothelial cancers (3). The incidence of this disease varies in different regions. UTUC is more likely to invade surrounding tissues and metastasize due to the thin ureteral wall and abundant periductal lymph. Because of this, it has a poor prognosis and often has more aggressive biological characteristics, and its five-year survival rate is about only 60% (4). Radical nephroureterectomy (RNU) has been the main treatment option because of the absence of standardized clinical diagnosis and treatment guidelines. Despite timely completion of standard radical surgery, the outlook remains grim, as 22% to 47% of patients experience intravesical recurrence postoperatively (5). Thus, it is necessary to investigate biomarkers that can be monitored after RNU.
An increasing amount of studies have concentrated on the involvement of the immune system in every stage of cancer progression (6). Inflammation may lead to alterations in the levels of different cells associated with inflammation in the body, including leukocytes, erythrocytes, and thrombocytes. Blood markers of inflammation like platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), and lymphocyte-monocyte ratio (LMR) have been utilized for assessing inflammation levels in the body and predicting the likelihood of recurrence and prognosis in urinary system cancers (7–10). The systemic immune-inflammation Index (SII), a novel inflammatory marker, has gained increasing recognition and acceptance in recent times. It is determined by the formula SII = platelet count × neutrophil count/lymphocyte count. It has more excellent clinical value than previous inflammatory indicators because it simultaneously contains three kinds of peripheral blood cells. To date, there have been no published meta-analyses examining the effects of SII on the prognosis of UTUC. Hence, the objective of this research was to methodically examine the predictive importance of SII in individuals diagnosed with this condition.
Materials and methodsProtocol and ethicsThe research was carried out following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (11) and was registered in PROSPERO (registration number CRD42022316333) in advance. All data in this study were secondary analyses of previous studies and therefore did not require ethical approval and signed patient informed consent.
Search strategyTwo researchers (Z.Y. and Z.X.) conducted an extensive search of various databases including PubMed, Cochrane Library, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI), WanFang database, and Chinese Scientific Journal Database (VIP) until March 10, 2022 independently, and discuss any differences with a third researcher (J.M.). This meta-analysis utilized the search terms (upper tract OR ureter OR ureteral OR renal pelvis OR renal pelvic OR ureteral neoplasms OR urothelium) AND (systemic immune-inflammation index OR SII). We carefully screened and reviewed the literature by title and abstract, and initially eliminated the nonconforming literature. Then we further screened the selected literature by reading the full text. Furthermore, we conducted a manual search of the reference lists of pertinent studies in hopes of identifying additional suitable articles.
Criteria for inclusion and exclusionAll studies had to meet specified criteria to be included in this meta-analysis, as follows: (i) The studies examined how the pretreatment systemic immune-inflammation index (SII) correlates with the prognosis of patients with upper tract urothelial carcinoma confirmed through histological analysis. (ii) The complete text of the articles included specified the exact threshold value for SII; (iii) Survival outcomes such as overall survival (OS), cancer-specific survival (CSS), or recurrence-free survival (RFS) for patients with UTUC are assessed for availability. (iv) Patient prognosis hazard ratio (HR) and 95% confidence interval (CI); (v) English or Chinese full‐text articles.
Publications will be excluded if they refer to the following situations: (i) Poster sessions, reviews, letters, case reports, conference abstracts, or comments; (ii) Incomplete or unavailable data; (iii) Animal experiments or basic research; (iv) Duplicate articles.
Extracting and analyzing dataFrom the included literature that met the criteria, the following information was extracted: author’s name, publication year, the region of study population, study design, study period, sample size, patient age, follow-up time, cut-off value, clinical stage, prognostic indicators (OS\CSS\RFS), and HRs with the corresponding 95% CIs.
In this meta-analysis, pooled HRs with 95% confidence intervals were assessed by two methods:(a) Extracting HRs with 95% CIs directly from articles through univariate and multivariate analysis; (b) Multivariate analysis was favored for its higher accuracy in selecting HRs.
The quality of the study was assessed using the Newcastle-Ottawa scale (NOS) (12). The NOS score is on a scale of 0 to 9, and studies with a score of 6 or higher were considered high quality researches.
Statistical analysisThe data collected was analyzed using Stata 17.0 software (Stat Corp, College Station, TX). The prognostic significance of SII on the survival of UTUC patients was assessed through pooled HRs and 95% CIs. SII was deemed a significant predictor if the pooled 95% confidence interval did not intersect with 1 and had a p-value less than 0.05. Pooled HRs>1 with 95% CIs, not including 1, means that patients with higher SII have a poorer prognosis. The study’s heterogeneity was assessed using Cochran’s Q-test and Higgin’s I² statistic. An I² value greater than 50% or a p-value less than 0.10 signified notable heterogeneity. A random-effect model was employed in this article. Furthermore, subgroup analysis and sensitivity analysis were employed to examine the possible sources of variation and evaluate the consistency of the findings. Publication bias was visually assessed using Begg’s and Egger’s tests. An examination was conducted to analyze the connection between SII and clinical and pathological characteristics by combining the odds ratios (ORs) and their corresponding 95% confidence intervals (CIs). Pooled ORs>1 with 95% CIs not including 1 means that higher SII is related to poorer pathological type. A P value less than 0.05 signifies the statistical importance of the findings in the study.
ResultsSearch resultsWe initially retrieved 263 articles, and after eliminating duplicates, 216 articles remained. After reviewing the title and abstract, articles that did not meet the criteria were eliminated, leaving 17 articles to be read in their entirety. Six studies were finally confirmed, including 3911 UTUC patients in seven cohorts (13–18). Figure 1 depicts the process of retrieving information.
Figure 1 Meta-analysis flow diagram of included studies.
Characteristics of the studies includedThe basic information description of the above-included literature is summarized in Table 1. All the articles included in the study were published within the past 5 years and were retrospective in nature. Two of the studies (14, 18) had three cohorts in China, two (13, 17) in Taiwan China, one (16) in Japan, and one (13) in Europe and the United States. Among the above six articles, there is one article in Chinese (18) and five in English (13–17). The total sample size for all studies was 3911, with included studies ranging from 103 to 2373. The average age of the patients was above 65 years, with thresholds varying from 410.3 to 672.44. Among them, five cohorts examined the correlation between OS and SII (13–16), while six cohorts analyzed the association between CSS and SII (13–17), and five cohorts investigated the link between RFS and SII (14, 15, 17, 18). Every study that was included in the analysis was deemed to be of excellent quality, scoring 6 or above on the Newcastle-Ottawa Scale (Table 2).
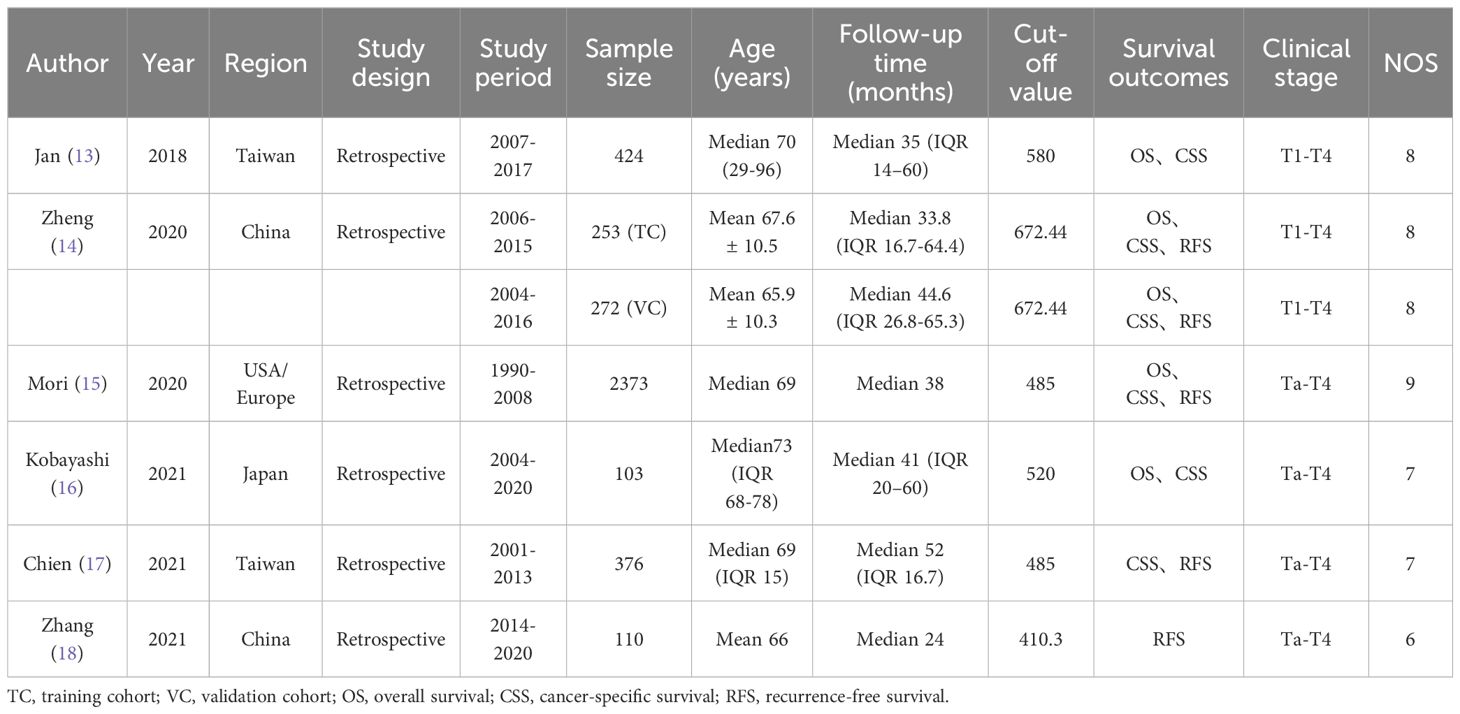
Table 1 Main characteristics of all included studies in the meta-analysis.
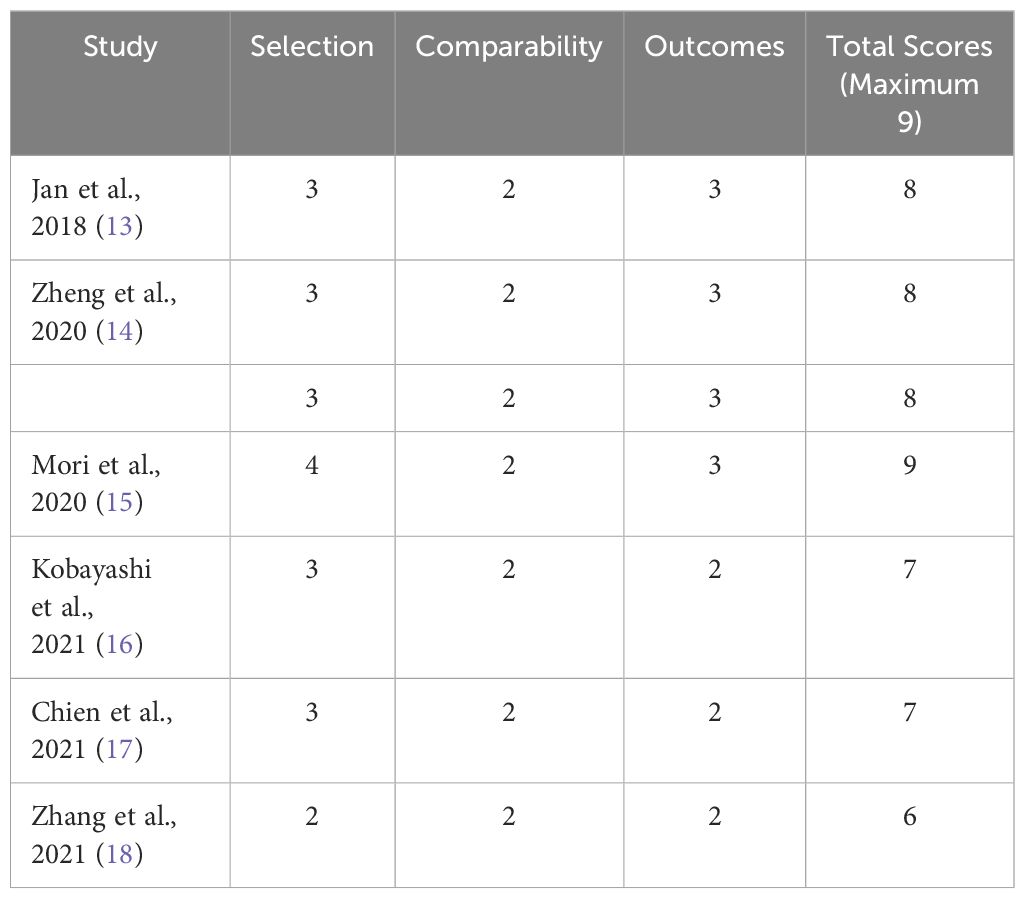
Table 2 The Newcastle-Ottawa Scale (NOS) for assessing the quality of cohort studies.
The impact of SII on OS in UTUCThe analysis included five groups consisting of 3425 patients to examine the correlation between preoperative SII and survival in UTUC patients (13–16).
The findings indicated that elevated systemic immune-inflammation index prior to therapy was associated with worse overall survival outcomes (HR =1.87, 95%CI: 1.20-2.92, p=0.005).
Because of the higher heterogeneity (I²= 68.4%, p=0.013), a random-effect model was performed (Figure 2 and Table 3). After that, subgroup analysis was performed by region, sample size, cut-off value and follow-up time. Table 3 shows that poorer OS was significantly associated with higher SII in subgroups with samples size >300 (p<0.001), cut-off value > 550 (p<0.001), and median follow-up time>40 months (p=0.001).
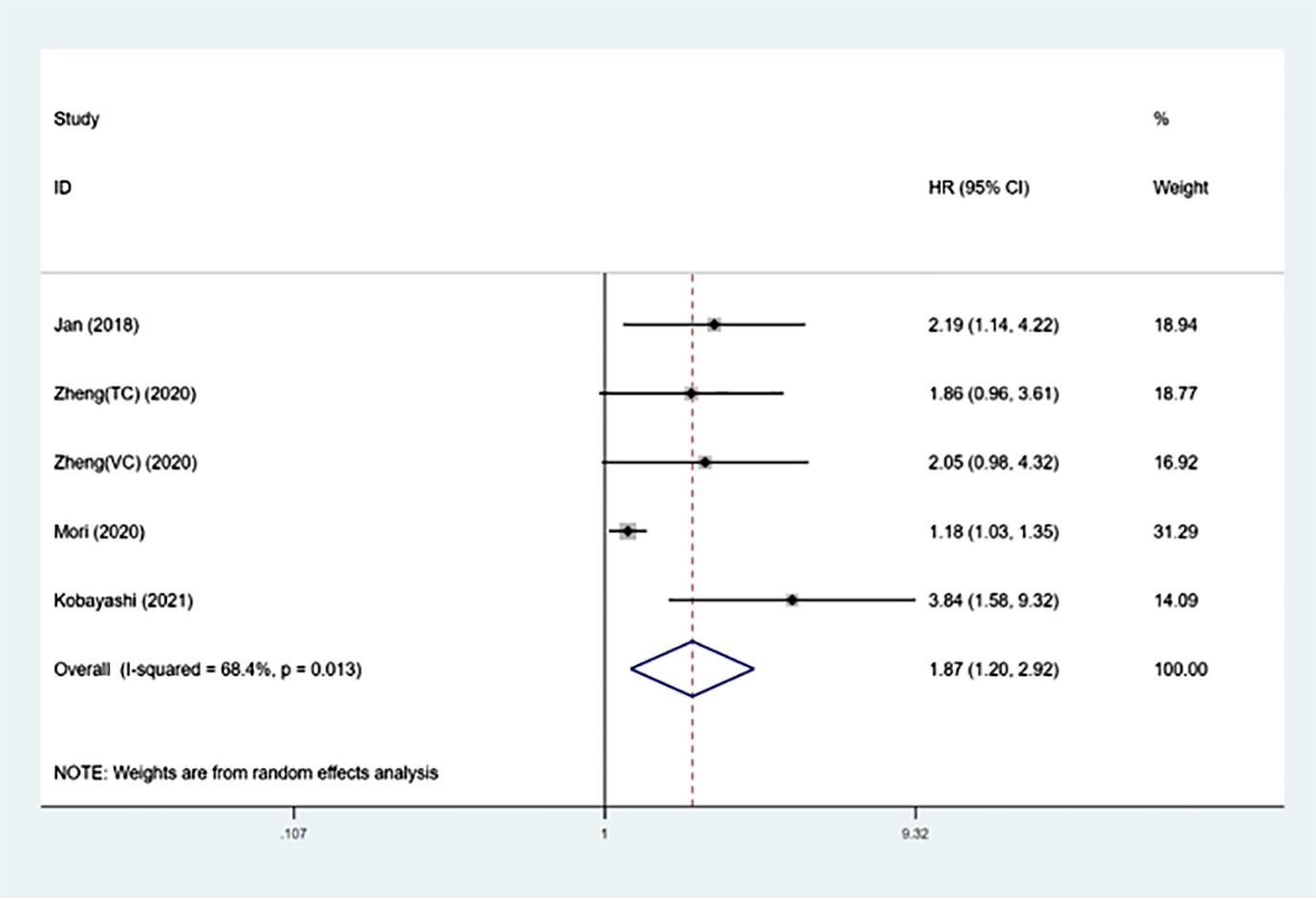
Figure 2 Forest plot of the association between SII and OS in patients with UTUC.
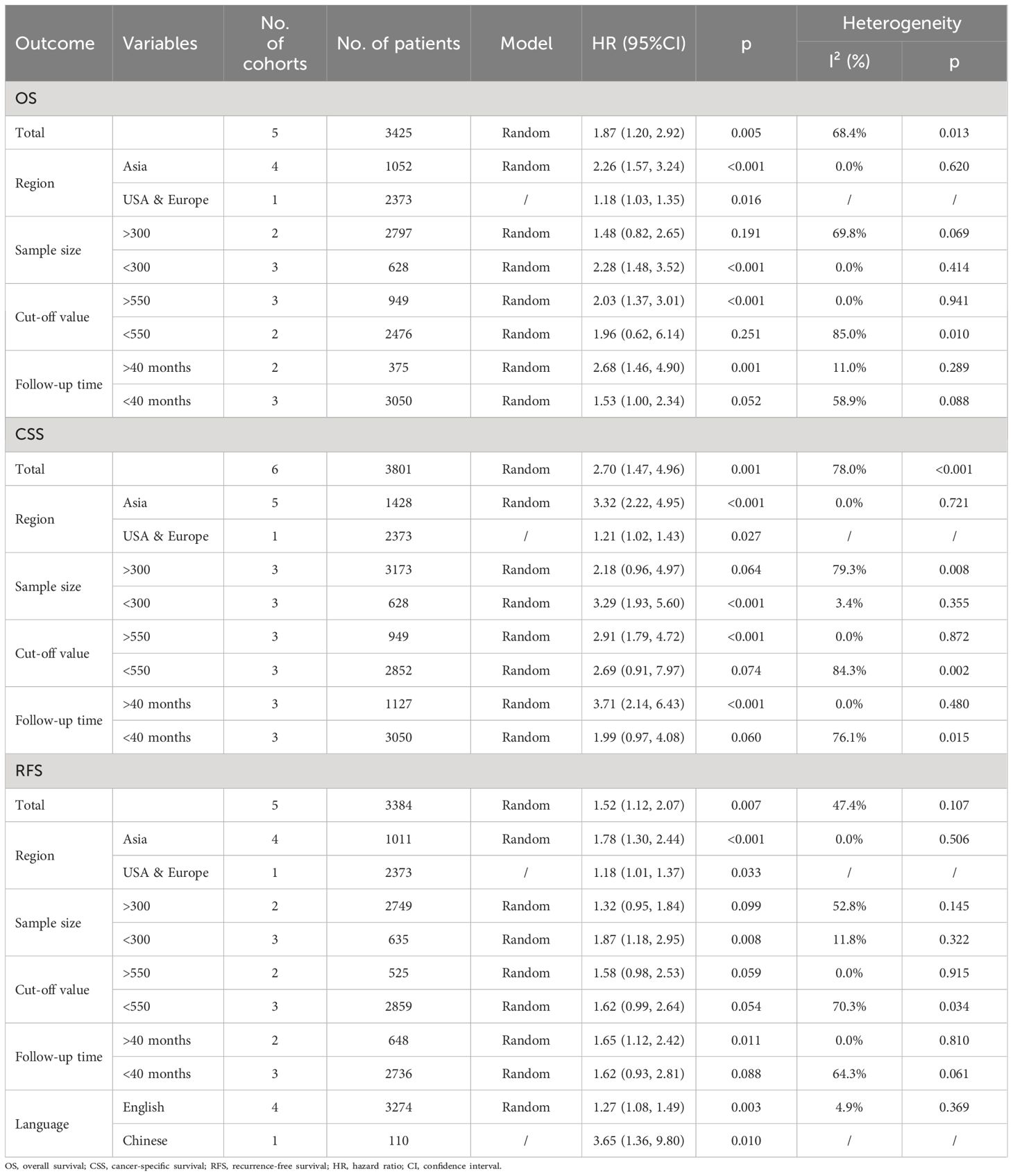
Table 3 Subgroup analysis of OS, CSS and RFS.
Prognostic significance of SII on CSS in UTUCThe prognostic importance of SII for CSS was evaluated by six groups involving 3801 patients (13–17), and the overall findings indicated that increased SII levels were linked to worse CSS outcomes (HR =2.70, 95%CI 1.47-4.96, P=0.001). Despite the notable heterogeneity (I²= 78.0%, p<0.001), we continued to utilize the random-effects model for the combination process (Figure 3 and Table 3). The findings in Table 3 demonstrate that increased SII was significantly linked to worse CSS in subgroups with sample sizes less than 300 (p<0.001), cut-off values greater than 550 (p<0.001), and median follow-up times exceeding 40 months.
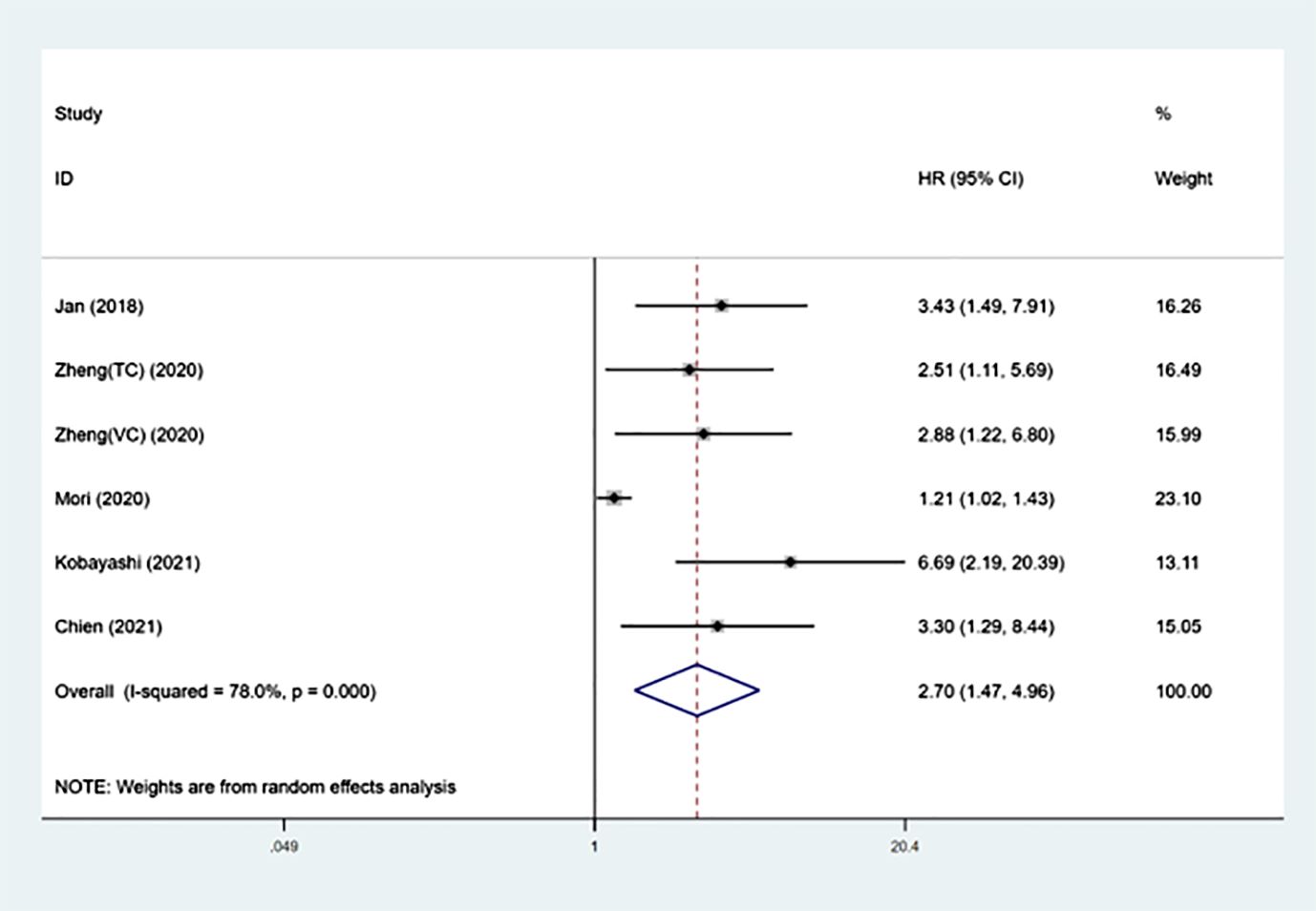
Figure 3 Forest plot of the association between SII and CSS in patients with UTUC.
The predictive importance of SII on RFS in UTUCFigure 4 shows how the SII affects RFS in UTUC patients, with a total of 5 groups comprising 3384 patients. Using a random-effects model, a combined hazard ratio of 1.52 was computed, with a 95% confidence interval ranging from 1.12 to 2.07 and a significance level of 0.007. This suggests that higher SII levels were linked to worse recurrence-free survival rates (I²= 47.4%, p=0.107) (Figure 4 and Table 3). Further subgroup analysis revealed that when sample size<300 (p=0.008), and median follow-up time > 40 months (p=0.011), SII increase was correlated with poor RFS.
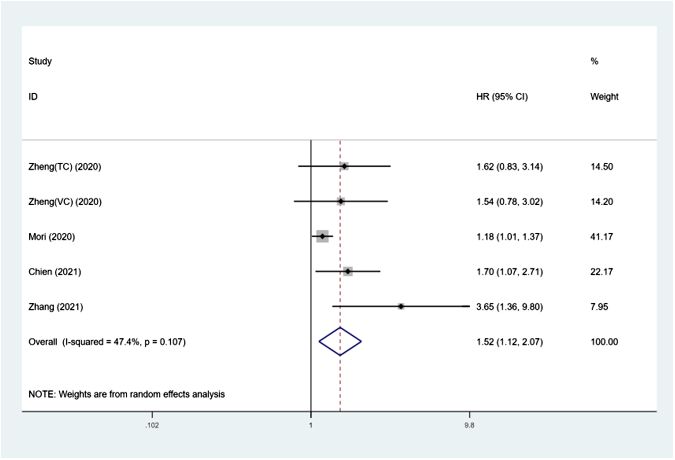
Figure 4 Forest plot of the association between SII and RFS in patients with UTUC.
Correlation of SII with clinicopathological characteristics in UTUCThe findings of additional analysis on the correlation between SII and typical clinicopathological characteristics are displayed in Table 4 and Figure 5. Indicators included in the study are age (old vs young), gender (male vs female), histological grade (high vs low), LVI (present vs absent), pT stage (pT≥3 vs < 3), pN stage (N+ vs N0), multifocality (present vs absence), tumor location (ureter vs pelvis), hydronephrosis (present vs absent), and bladder cancer history (present vs absent). The results showed that higher SII may be related to LVI (present vs absent) (OR=0.83, 95% CI=0.71-0.97, p=0.018), pT stage (pT≥3 vs < 3) (OR=1.82, 95% CI=1.21-2.72, p=0.004), pN stage (N+ vs N0) (OR=3.27, 95% CI=1.60-6.71, p=0.001). Hydronephrosis and a history of bladder cancer are viewed as protective factors in cases of Higher SII, with odds ratios of 0.74 (95% CI=0.58-0.94, p=0.013) and 0.83 (95% CI=0.71-0.97, p=0.018) respectively (Table 4 and Figure 5).
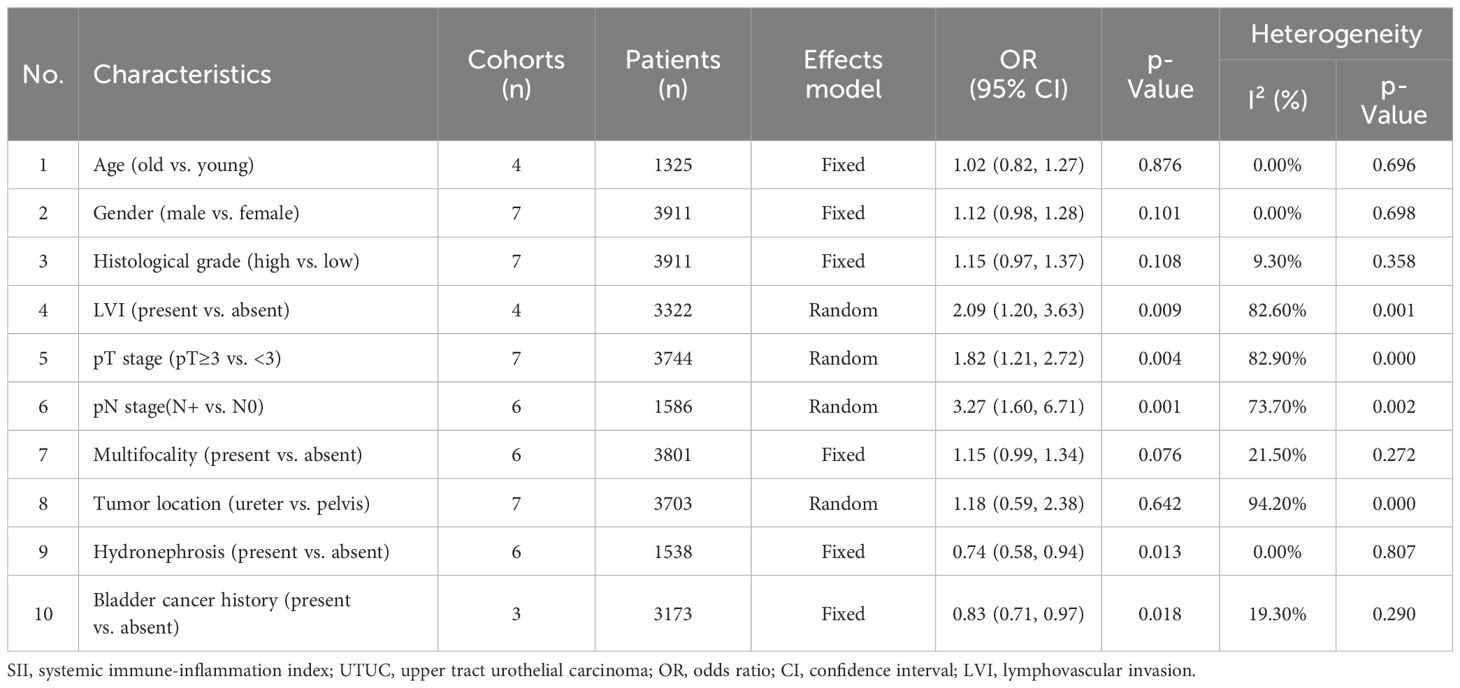
Table 4 Correlations between SII and clinicopathological characteristics in UTUC.
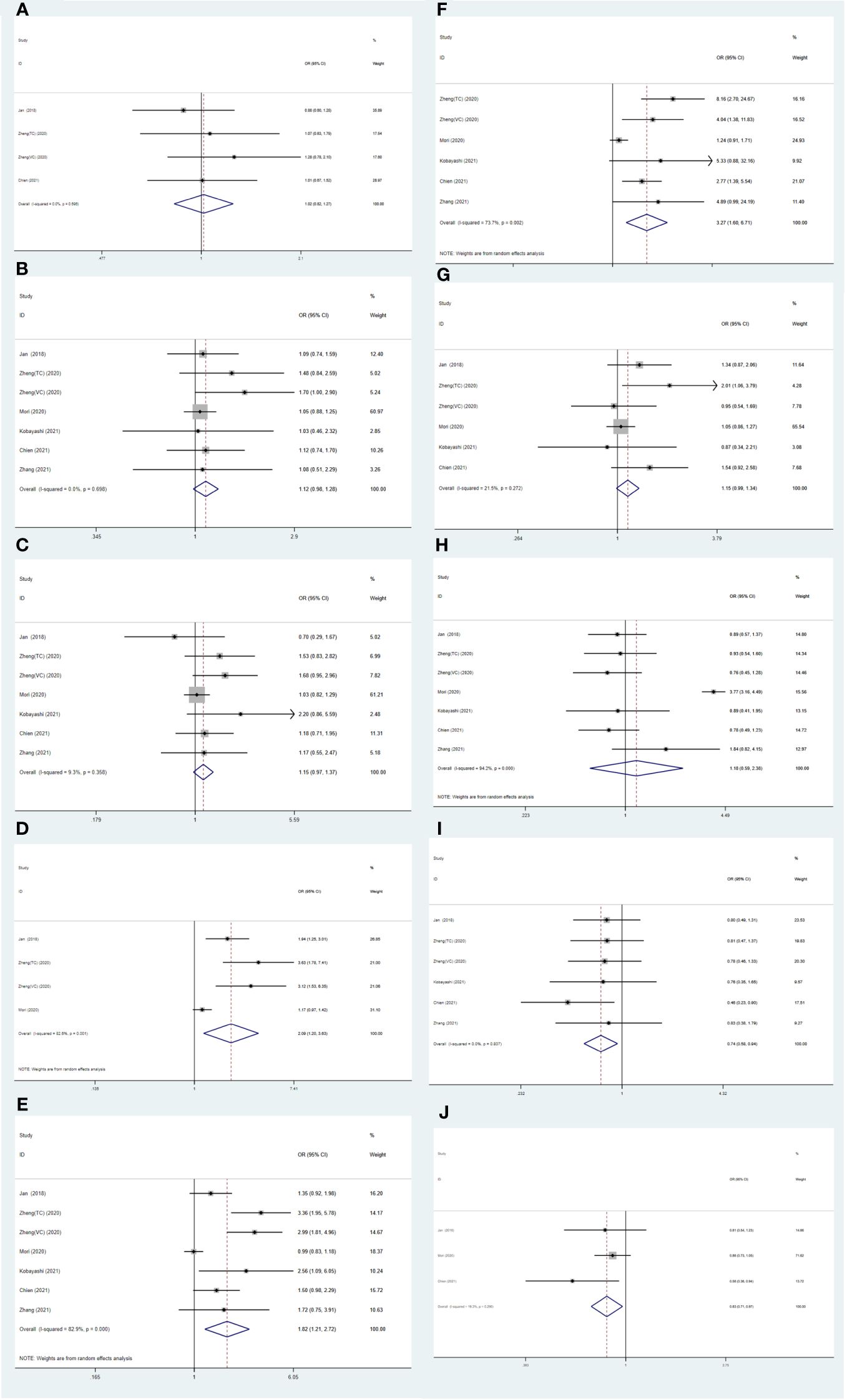
Figure 5 Forest plots of the association between SII and clinicopathological characteristics in UTUC: (A) Age (old vs. young); (B) Gender (male vs. female); (C) Histological grade (high vs. low); (D) LVI (present vs. absent); (E) pT stage (pT ≥ 3 vs. < 3); (F) pN stage (N+ vs. N0); (G) Multifocality (present vs. absent); (H) Tumor location (ureter vs. pelvis); (I) Hydronephrosis (present vs. absent); (J) Bladder cancer history (present vs. absent).
Publication biasSupplementary Figure S1 displays the utilization of Begg’s tests in assessing the publication bias within the literature that was included. The findings indicated the absence of any apparent bias in publication for OS, CSS, and RFS with p-values greater than 0.05 according to Begg’s test results (OS p=0.221, CSS p=0.060, RFS p=0.462).
Sensitivity analysisThe reliability of the combined HRs was assessed by conducting a sensitivity analysis using the leave-one-out test on the three outcome measures of OS, CSS, and RFS. The findings indicated that the conclusion was not influenced by any individual study, demonstrating that the collective impact of this meta-analysis was consistent and trustworthy (Supplementary Figure S2).
DiscussionCarcinogenesis is increasingly regarded as a function of the interaction between the tumor and the surrounding tumor microenvironment (TME) (19, 20). Increasing evidence suggests that the development of a tumor frequently signifies an imbalance in the body’s inflammatory immune response, with inflammation being intricately linked to the tumor. The presence of immune cells is crucial for speeding up the growth of tumors, the formation of new blood vessels, and the spread of cancer to other parts of the body through the stimulation of different cytokines. There has been emerging evidence that neutrophils are involved in cancer-related inflammation. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated by neutrophil toxicity can lead to DNA damage and genetic instability (21), while neutrophils can further advance tumor growth through enzyme release (22–24). Much literature has shown that platelets promote tumor progression and metastasis (25–27). By identifying tumor-associated antigens, T lymphocytes that infiltrate tumors can initiate immune responses against the tumor (28). Research has demonstrated that tumors with T cell inflammation are more responsive to immunotherapy (29).
The SII provides a more comprehensive measure of inflammation in the body than the previous indexes like PLR, NLR, and LMR. The body’s red blood cell, platelet, and lymphocyte levels are included in it. In the research on hepatocellular carcinoma, SII has been identified as a valuable prognostic marker for the first time (30). Subsequently, it has been verified that it has prognostic value in various tumors, and it is a novel marker with the advantages of simplicity and low price. Numerous past meta-analyses have indicated a correlation between SII and the outcome of cancerous growths, including tumors in the digestive system, urogenital system, lung, pancreas, breasts, and more (31–36). The initial meta-analysis of SII in individuals with UTUC examines its predictive value by pooling information from six studies involving 3911 participants. Despite the limited number of studies included, it is considered adequate considering the rare occurrence of UTUC. Furthermore, all the specimens analyzed in this research consisted of over 100 instances in order to minimize any potential disruptions. Our analysis systematically assessed the potential usefulness of SII in predicting clinicopathological characteristics and prognosis in patients with urinary cancer undergoing treatment with RUN. Our statistical analysis indicated that elevated levels of SII before surgery were more reliable indicators of worse survival results, such as reduced OS, CSS, and RFS, along with negative pathological characteristics like LVI (present vs absent), pT stage (pT≥3 vs < 3), and pN stage (N+ vs N0). Previous articles of the same type have reached similar conclusions (37).
According to the 2020 EAU guidelines, patients with UTUC should be divided into low-risk and high-risk groups according to whether the tumor is solitary, tumor size, tumor pathological grade, and whether there is metastasis on preoperative examination (2). In cases of high-risk UTUC, RNU with bladder excision remains the standard treatment (38). There are still some controversial problems in the clinical diagnosis and treatment of UTUC. Clinically, we usually perform lymph node dissection (LND) for patients with possible lymph node metastasis suggested by preoperative imaging or suspected lymph node metastasis found during surgery. Due to the lack of solid evidence and prospective randomized controlled studies, LND is still controversial. Some studies have shown that LND improves survival, even in patients with clinicopathological negative lymph nodes (39, 40). Some relevant studies have proved that ureteroscopic biopsy before radical nephroureterectomy may increase the risk of postoperative bladder recurrence (41–43). Due to this, computed tomography urography (CTU) has become a first-line examination method to diagnose UTUC, due to its high sensitivity and specificity (44, 45). However, for some atypical advanced UTUC, accurate preoperative diagnosis and clinical staging still face challenges.
Preoperative neoadjuvant chemotherapy and postoperative adjuvant therapy are hot topics in this field. Adjuvant chemotherapy (AC) is chemotherapeutic therapy commonly used to reduce tumor recurrence or metastasis after surgery in patients with malignant tumors. Neoadjuvant chemotherapy (NAC) is systemic chemotherapy before local treatment, such as surgery or radiation. For high-risk upper tract urothelial carcinoma, a growing body of data shows that patients receiving neoadjuvant chemotherapy (NAC) prior to RNU may benefit more (46, 47). A study based on National Cancer Database (NCDB) data from the United States shows that preoperative determination of the depth of a primary upper urinary tract tumor remains to be addressed and is a key factor in determining whether to implement NAC in patients with high-grade UTUC (48). Based on a meta-analysis, NAC was associated with statistically significant OS and CSS in all patients and in patients with locally advanced UTUC. AC was associated with improved metastasis-free survival and CSS patients with locally advanced UTUC. In contrast, the association between AC and OS was significant only patients with locally advanced UTUC (49).
In particular, this study further examined the relationship between SII and clinicopathological characteristics, and found that SII was associated with LVI (present vs absent), pT stage (pT ≥ 3 vs<3) and pN stage (N+ vs N0), indicating the potential value of SII in determining preoperative clinical staging and risk stratification. SII can be included in the prognostic model to increase the accuracy and provide more accurate clinical evidence support for NAC and AC treatment. We advocate that SII can be used as a clinical decision aid until a patient’s treatment is determined. Asian patients had more severe and graded diseases than other ethnic groups (49, 50). However, subgroup analysis in this study showed that race was not yet an independent predictor of survival.
This article still has the following limitations. First, this study included a small number of articles, only 7 cohorts of 6 studies, including 3911 patients. Second, among the six included studies, only one was from Europe and the United States, while the remaining five were from Asia, including Japan, China and Taiwan. Additionally, all included studies were retrospective. The above factors suggest that there may have been publication bias in this meta-analysis. We look forward to further improving the meta-analysis in more large-scale clinical studies.
ConclusionAccording to a comprehensive analysis of all included articles, higher preoperative SII was independently associated with poorer survival outcomes and pathological changes. To some extent, SII can be used as a convenient, cheap and reliable prognostic marker for UTUC patients.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributionsZY: Writing – original draft, Writing – review & editing. ZX: Writing – original draft, Writing – review & editing. JM: Writing – original draft, Writing – review & editing. PD: Funding acquisition, Project administration, Resources, Writing – review & editing. SW: Writing – review & editing. JL: Validation, Investigation, Writing – review & editing. YC: Writing – review & editing. YY: Conceptualization, Resources, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Capital’s Funds for Health Improvement and Research (2022-1G-102).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1342996/full#supplementary-material
References1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: A Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
CrossRef Full Text | Google Scholar
2. Roupret M, Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. (2021) 79:62–79. doi: 10.1016/j.eururo.2020.05.042
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Fang D, Li XS. Interpretation of Chinese expert consensus for diagnosis and treatment of upper urinary tract urinary carcinoma. Med J West China. (2019) 31:990–3. doi: 10.3760/cma.j.issn.1000-6702.2018.07.002
CrossRef Full Text | Google Scholar
4. Rouprêt M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. (2013) 189:1662–9. doi: 10.1016/j.juro.2012.10.057
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Soria F, Shariat SF, Lerner SP, Fritsche HM, Rink M, Kassouf W, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. (2017) 35:379–87. doi: 10.1007/s00345-016-1928-x
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Gu L, Ma X, Li H, Chen L, Xie Y, Zhao C, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Sci Rep. (2016) 6:23846. doi: 10.1038/srep23846
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Cantiello F, Russo GI, Vartolomei MD, Farhan ARA, Terracciano D, Musi G, et al. Systemic inflammatory markers and oncologic outcomes in patients with high-risk non-muscle-invasive urothelial bladder cancer. Eur Urol Oncol. (2018) 1:403–10. doi: 10.1016/S1569-9056(18)33378-5
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Bauckneht M, Rebuzzi SE, Signori A, Frantellizzi V, Murianni V, Lodi Rizzini E, et al. The prognostic power of inflammatory indices and clinical factors in metastatic castration-resistant prostate cancer patients treated with radium-223 (BIO-Ra study). Eur J Nucl Med Mol Imaging. (2022) 49:1063–74. doi: 10.1007/s00259-021-05550-6
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Bumbasirevic U, Bojanic N, Simic T, Milojevic B, Zivkovic M, Kosanovic T, et al. Interplay between comprehensive inflammation indices and redox biomarkers in testicular germ-cell tumors. J Pers Med. (2022) 12:3–6. doi: 10.3390/jpm12050833
CrossRef Full Text | Google Scholar
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Jan HC, Yang WH, Ou CH. Combination of the preoperative systemic immune-inflammation index and monocyte-lymphocyte ratio as a novel prognostic factor in patients with upper-tract urothelial carcinoma. Ann Surg Oncol. (2019) 26:669–84. doi: 10.1245/s10434-018-6942-3
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Zheng Y, Yu D, Yu Z, Zhao D, Chen Y, Chen W, et al. Association of preoperative systemic Immune-inflammation Index and Prognostic Nutritional Index with survival in patients with Upper Tract Urothelial Carcinoma. J Cancer. (2020) 11:5665–77. doi: 10.7150/jca.44915
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Mori K, Resch I, Miura N, Laukhtina E, Schuettfort VM, Pradere B, et al. Prognostic role of the systemic immune-inflammation index in upper tract urothelial carcinoma treated with radical nephroureterectomy: results from a large multicenter international collaboration. Cancer Immunol Immunother. (2021) 70:2641–50. doi: 10.1007/s00262-021-02884-w
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Kobayashi S, Ito M, Takemura K, Suzuki H, Yonese I, Koga F. Preoperative models incorporating the systemic immune-inflammation index for predicting prognosis and muscle invasion in patients with non-metastatic upper tract urothelial carcinoma. Int J Clin Oncol. (2022) 27:574–84. doi: 10.1007/s10147-021-02088-3
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Chien T-M, Li C-C, Lu Y-M, Chou Y-H, Chang H-W, Wu W-J. The predictive value of systemic immune-inflammation index on bladder recurrence on upper tract urothelial carcinoma outcomes after radical nephroureterectomy. J Clin Med. (2021) 10:4–9. doi: 10.21203/rs.3.rs-829449/v1
CrossRef Full Text | Google Scholar
18. Zhang X, Gao Y, Xu H, Liu Y, Xiaozhong H, Guanjun L. Preoperative systemic immune-inflammation index to evaluate intravesical recurrence of upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. J Modern Urol. (2022) 27:30–4. doi: 10.3969/j.issn.1009-8291.2022.01.006
CrossRef Full Text | Google Scholar
19. Roth GS, Decaens T. Liver immunotolerance and hepatocellular carcinoma: Patho-physiological mechanisms and therapeutic perspectives. Eur J Cancer. (2017) 87:101–12. doi: 10.1016/j.ejca.2017.10.010
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Güngör N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. (2010) 25:149–54. doi: 10.1093/mutage/gep053
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. (2007) 104:20262–7. doi: 10.1073/pnas.0706438104
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Das A, Monteiro M, Barai A, Kumar S, Sen S. MMP proteolytic activity regulates cancer invasiveness by modulating integrins. Sci Rep. (2017) 7:14219. doi: 10.1038/s41598-017-14340-w
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. (2010) 16:219–23. doi: 10.1038/nm.2084
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Zhang Y, Cedervall J, Hamidi A, Herre M, Viitaniemi K, D'Amico G, et al. Platelet-specific PDGFB ablation impairs tumor vessel integrity and promotes metastasis. Cancer Res. (2020) 80:3345–58. doi: 10.1158/0008-5472.CAN-19-3533
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Liu Y, Zhang Y, Ding Y, Zhuang R. Platelet-mediated tumor metastasis mechanism and the role of cell adhesion molecules. Crit Rev Oncol Hematol. (2021) 167:103502. doi: 10.1016/j.critrevonc.2021.103502
PubMed Abstract | CrossRef Full Text | Google Scholar
29. van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM. Migrating into the tumor: a roadmap for T cells. Trends Cancer. (2017) 3:797–808. doi: 10.1016/j.trecan.2017.09.006
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Zhang Y, Chen B, Wang L, Wang R, Yang X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: A meta-analysis. Med (Baltimore). (2019) 98:e13788. doi: 10.1097/MD.0000000000013788
留言 (0)