Major depressive disorder (MDD) is a common, heterogenous, episodic, and disabling psychopathological condition characterized by a combination of signs and symptoms that negatively impact patients’ productivity and well-being, including cognitive dysfunction, emotional regulation, motor activity, motivation, and possible suicidal ideation (1, 2). MDD is the most prevalent psychiatric condition, represents approximately 30% of mental disorders, and affects more than 300 million people worldwide, with 5–17% of the world population suffering from the disorder at least once in their lifetime (3). In 2019, MDD was the third cause of years lived with disability in the world’s adult population and the World Health Organization (WHO, Geneva, Switzerland) estimates that, by 2030, depression will have become the leading cause of disability worldwide (4–7). Moreover, MDD is associated with high levels of morbidity and mortality: patients often develop comorbid mental conditions, including posttraumatic stress disorder, anxiety disorders, obsessive-compulsive disorder, substance use disorders, and a lifetime risk of a suicide attempt of 31% (8, 9). The disorder is also associated with non-psychiatric chronic illnesses, including cardiovascular and cerebrovascular diseases, metabolic disorders like type 2 diabetes mellitus and obesity, and cancer (9). In relation to the abovementioned comorbidities, patients’ life expectancy is reduced by 7–13 years (10). In addition to the impact on the people affected, there are economic and social implications, such as the reduction in productivity, higher healthcare costs, and costs incurred by unpaid caregivers (11, 12).
The diagnosis of MDD is based on the assessment of symptoms by the clinician, the self-assessed disease features, and the patient’s lifetime history (13). The International Classification of Diseases (ICD) (from the 6th to the 11th edition) and the Diagnostic and Statistical Manual (DSM) (from I to V edition) provide a set of criteria for diagnosing a major depressive episode (MDE). According to the DSM-5 (14), the simultaneous manifestation of at least five of the nine following groups of symptoms over a two-week period is needed: depressed mood; markedly diminished interest or pleasure in activities; reduced ability to think or concentrate, or indecisiveness; feelings of worthlessness, or excessive or inappropriate guilt; recurrent thoughts of death, or suicidal ideation, or suicide attempts or plans; insomnia or hypersomnia; significant change in appetite or weight; psychomotor agitation or retardation; and fatigue or loss of energy (14). Of these five symptoms, one of the first two must be present (14). A diagnosis of MDD can be made if an MDE is present or occurred in the patient’s history and is not explained by a concomitant primary psychosis or by a known bipolar disorder (14). Indeed, the main differential diagnosis to evaluate is bipolar disorder (BD), with approximately 40% of patients with BD initially misdiagnosed with MDD (15). Therefore, all patients showing symptoms of depression should be screened for BD (16).
The binary approach proposed in the DSM and ICD defines MDD as absent or present. This dichotomic classification has some limitations as it represents a post-hoc construct that reduces information, precision, validity, and reliability of the MDD diagnosis (13). Further characterization of the disorder is needed to overcome the problem of binary diagnosis. This should include an assessment of the current depressive symptoms and suicidal behavior as well as a detailed history of the disorder, including the number, duration, and severity of relapses, and the response to treatments coupled with a good knowledge of the patient’s life history, useful to differentiate between physiological and pathological human stress-related responses (13, 16). The severity of symptoms can be measured with standardized psychometric scales (3) such as the Hamilton Depression Rating Scale (17), Montgomery-Asberg Depression Rating Scale (18), and Beck Depression Inventory (19). Suicidality, in terms of suicidal ideation and attempts, is among the stronger predictors of suicide (20) and should be assessed with validated scales like the Columbia-Suicide Severity Rating Scale (21).
The clinical characterization of MDD is needed not only for diagnostic purposes but also to develop a personalized management plan at a single-patient level (16). This profiling consists of assessing various clinical domains, including clinical subtypes, neurocognition, clinical staging, and functioning and quality of life. MDD is considered a heterogeneous disorder, and many clinical subtypes with specific associations of signs and symptoms were described, including melancholic, atypical, anhedonic, inflammatory, suicidal, anxious, somatic-traits, reactive, psychotic, pseudo-demented, and seasonal subtypes (3, 16). A worse prognosis and a specific treatment indication consisting of antidepressants and antipsychotics were supported by sufficient evidence only for the psychotic subtype that includes delusions and/or hallucinations in the clinical context of a severe MDE (16), suggesting that this specific subtype may be studied as a partial autonomous nosological entity.
Neurocognitive impairment is present in 85–94% of cases during an MDE and 39–44% of cases during remissions. It mainly involves attention, short-term memory, and executive function and accounts for a disproportionately high percentage of patients who have not completely reached the psychosocial functioning they used to have before the onset of the MDE (22–24). A better identification and characterization of patients with MDD with neurocognitive dysfunction might promote the development of new and personalized treatments (25).
Clinical staging refers to two main classifications: the first one is related to the course of the disorder and the second one to the response to treatment (26, 27). The former defines the following five stages: (i) a prodromal phase with (a) non-specific significant or (b) subthreshold depressive symptoms; (ii) a second stage corresponding to the first MDE; (iii) a residual phase characterized by (a) non-specific, (b) residual depressive, or (c) mild chronic depressive (dysthymia) symptoms; (iv) a fourth stage corresponding to (a) a recurrent MDE in a patient that fully recovered from a previous depressive episode or (b) double depression, i.e., an MDE superimposed on dysthymia; and (v) a persistent MDE lasting at least two years without interruption (26). The second classification also defines five stages: (0) no history of treatment failure; (1) failure of one adequate treatment, i.e., 6–8 weeks for antidepressants or 36–52 weeks for psychotherapy; (2) failure of two adequate treatments (12–16 weeks for antidepressants or 36–52 weeks for psychotherapy); (3) failure of three adequate treatments; (4) failure of three or more adequate treatments with at least one involving augmentation or combination strategies (27). Stage 2 corresponds to the most common definition of treatment-resistant depression (TRD) and stage 4 to refractory depression (28).
According to the DSM-5 and ICD-11, an MDE results in a significant impairment in personal, family, social, educational, occupational, or other important areas of functioning (29). In parallel, patients report an important reduction in their self-rated quality of life and satisfaction with life and expect from treatments a restoration of positive emotions, functioning, and meaningfulness of life rather than merely symptom relief (30, 31). The routine assessment of functioning, quality of life, and life satisfaction with standardized instruments like the recovery index promotes a patient-centered perspective, shifting the focus from a symptomatic remission to a functional and personal recovery from MDD (16, 32).
An in-depth clinical characterization can help clinicians in treatment planning and potentially identify more homogenous phenotypes of MDD that may be related to specific biological processes, thus partly overcoming the obstacle of the clinical and biological extreme heterogeneity of this psychopathological condition.
About pathogenesis, it is known that MDD emerges as a result of a complex and mostly obscure interplay between genetic vulnerabilities and environmental factors. There is evidence that female sex, family history, childhood maltreatment, as well as more recent stressors, are risk factors for the development of the disorder. However, a clear understanding of how genetic information interacts with early and recent environmental exposure through epigenomic mechanisms is not available (9). At a genetic level, no single variation was found to be solely responsible for an increased risk of MDD development (33); instead, a combination of variations in over 100 gene loci confer a genetic susceptibility towards disease incidence. Even though MDD hereditability traits can range between 30–50%, social and environmental factors, such as stress and the occurrence of traumatic events, can greatly impact mental health and drive disease pathogenesis (34). As an example, the recent COVID-19 pandemic has led to an increased prevalence of MDD, not only in COVID-19 patients but also in relatives and significant persons, such as close friends (35). Other hypotheses that justify the diversified etiology of MDD have identified dysregulated biochemical pathways, including hormone dysregulation and glucocorticoid increase, monoamine neurotransmitter deficiency (serotonin, dopamine, and norepinephrine), exacerbated neuroinflammation and scarcity of glial cells in the brain (36–38). Postmortem studies of patients with MDD have reported a neuroprogression associated with the disorder consisting of changes in the density and size of neurons and glia in several brain regions combined with a reduced expression of synaptic genes (39).
Despite a wide range of effective treatments, including antidepressants, evidence-based psychotherapies, nonpharmacological somatic treatments, and numerous augmentation strategies, about half of patients remain nonresponsive or poorly responsive to available treatments, thus failing to reach symptomatic remission and functional and personal recovery (9, 40).
Precision medicine, which integrates clinical and biological parameters specific to each individual to stratify treatment groups, may represent a solution to the current standard trial-and-error approach to treatment, moving to a more tailored method that might improve the prognosis of MDD through the allocation of the best treatment while minimizing side effects.
2 Precision medicine: a patient-tailored approachThe multifactorial etiology of MDD, as well as the numerous available treatments, mostly administered on a trial-and-error basis, portray MDD as an interesting target for the application of precision medicine (41). Also described as personalized medicine, this approach aims to combine biologic high-throughput data generated through omic approaches and environmental and lifestyle factors to identify specific individual features predictive of disease susceptibility, prognosis, and treatment response (42). The idea of applying a personalized treatment in psychiatry has been present for more than half a century (43), but only in recent years it became possible to generate and interpret very large datasets, called “big data”, and obtain complete individual profiling able to provide patient-specific details that can be used as diagnostic, prognostic, and treatment response biomarkers or therapeutic targets (44). In fact, constant technological advances led to the shift from basic blotting techniques (Northern, Southern and Western blotting) (45) to advanced genome-wide associating studies (GWAS) (46), single-cell transcriptomics (47) and imaging mass cytometry (48), generating larger amounts of data, which can be nowadays analyzed through artificial intelligence (AI) and machine learning techniques (49).
This opposes the classical approach or “imprecision medicine”, as labeled by Schork, that has led to a huge economic burden associated with MDD: in 2015, the fifth highest-grossing drug in the United States was duloxetine, a serotonin and norepinephrine reuptake inhibitor used to treat depression (50), which was only able to help one in every nine patients (51). Indeed, MDD response heterogeneity remains a critical problem for developing algorithms for implementing recovery rates in clinical practice (52). Inevitably, this represents a significant economic weight for patients and national health systems, incentivizing public and private investment in precision medicine (53).
Various techniques including magnetic resonance imaging (MRI), positron emission tomography (PET) scans and electroencephalography (EEG), have been adopted in the context of MDD, as reviewed in (54); also, as previously introduced, several omic approaches, most commonly genomic (55), transcriptomic (56), proteomic (57), and metabolomic (58) studies have been explored, but a definitive standardized and efficient strategy to accurately categorize patients and apply the appropriate therapy remains elusive.
Genomics aims to find genetic variants associated with disease, response to treatment, or patient prognosis. In this regard, the principal technologies used are whole-genome or whole-exome sequencing, targeted next-generation sequencing (NGS), genotyping and GWAS, which allow for the identification of thousands of genetic variants associated with complex diseases (59, 60).
Epigenomics examines reversible modifications of DNA or DNA-associated proteins, such as DNA methylation or histone acetylation, that can be influenced by both genetic and environmental factors (59, 60). These modifications are frequently detected in pathological conditions, including MDD (61). The technologies used to evaluate epigenetic modifications encompass bisulfite treatment of DNA before routine NGS to evaluate DNA methylation, immunoprecipitation and NGS-based analysis to assess the protein-DNA interaction, and ATAC-seq (Assay for Transposase-Accessible Chromatin using sequencing) to estimate genome-wide chromatin accessibility (59, 60).
Transcriptomics focuses on RNA transcripts analyzing expression levels of both quantity and quality of the transcripts, including messenger RNA (mRNA) and non-coding RNA (ncRNA). Transcriptomics can be performed using both bulk and single-cell methods. Common techniques used in bulk transcriptomics are microarrays and RNA sequencing (RNA-seq); among single cell analysis the gold standards are targeted single-cell transcriptomics (using for example, BD Rhapsody) and scRNA-seq (Smart-Seq2) (59, 60).
Proteomics, on the other hand, focuses on the study of peptide abundance, modification, and interactions. The main technologies used are mass spectrometry (MS)-based, which ensure high-throughput analyses of thousands of proteins in cells or body fluids. Additionally, flow cytometry, including advanced technologies such as FACS (Fluorescence-Activated Cell Sorting) and CyTOF (Cytometry by Time of Flight), and fluorescence imaging techniques are used to evaluate extra- or intracellular proteins, providing information for a large number of cells at a relatively low cost (59, 62).
Metabolomics quantifies various types of small molecules that reflect metabolic functions, in order to measure the response to perturbations like those connected to disease, as well as to observe the intricate relationship between physiology and external events (59). Techniques like liquid chromatography-MS (LC-MS) are appropriate for human cohort discovery investigations since they can accurately monitor tens to hundreds of metabolites simultaneously (63).
Microbiomics involves the study of an entire microbial community, identifying and quantifying the molecules that contribute to its structure, dynamics, and function (59, 64). In particular, metagenomics is the comprehensive analysis of uncultured microorganisms and hosts’ genetic material that applies genomic technologies, like metagenomic NGS (mNGS), in patients’ samples. It represents a significant upgrade from classical molecular assays that target only a restricted number of microbes and give a limited description of the pathogens through the diversity of one gene, using for instance the 16S ribosomal RNA (rRNA) gene. The study of RNA and proteins from microbiota can also be implemented through metatranscriptomics or metaproteomics (65, 66).
In the specific case of brain disorders, connectomics utilizes neuroimaging techniques, mainly MRI, to provide an exhaustive map of the whole neural connections, representing the study of functional and structural brain connectivity (67, 68). These techniques can improve the identification of personalized targets for neuromodulatory treatments (69).
All the above-mentioned omic technologies may help to identify new depression-specific biomarkers, even though this may rely on the lack of integration of such big data by system biomedicine approaches (41, 70). Overall, understanding the complex interplay of genetic, environmental, and physiological factors in MDD is crucial for improving prevention and treatment strategies for this debilitating condition. For that, an integrated multi-omic approach, which not only combines data from the single different omics but also considers the interactions between them, is gaining importance as an innovative strategy for biomarker discovery and validation in MDD (46).
In particular, cytomics is a multi-omic strategy used to determine the molecular phenotype of single cells that links various aforementioned omics sciences with cell and tissue dynamics and function, considering also its modulation by external factors. Naturally, it takes advantage of techniques that are transversal to other omic sciences, such as flow cytometry and fluorescence microscopy, to generate a wide array of data from each single cell of interest (71).
Lastly, immunomics integrates molecular immunology, genomics, proteomics, transcriptomics, cytomics and bioinformatics by studying antigens or epitopes that interact with the host’s immune system with a multi-layer omic approach (72, 73). There has been increasing evidence that MDD is linked to a systemic immunological activation over the last two decades (74), affecting inflammatory markers, immune cell counts, and antibody titers (75, 76).
In this review, the promising role of precision medicine-based approaches as diagnostic, prognostic and therapeutic tools for MDD will be explored, focusing particularly on the potential of biomarkers associated with the immune system as well as the current state of AI and machine learning approaches applied to the psychiatric field (Figure 1).
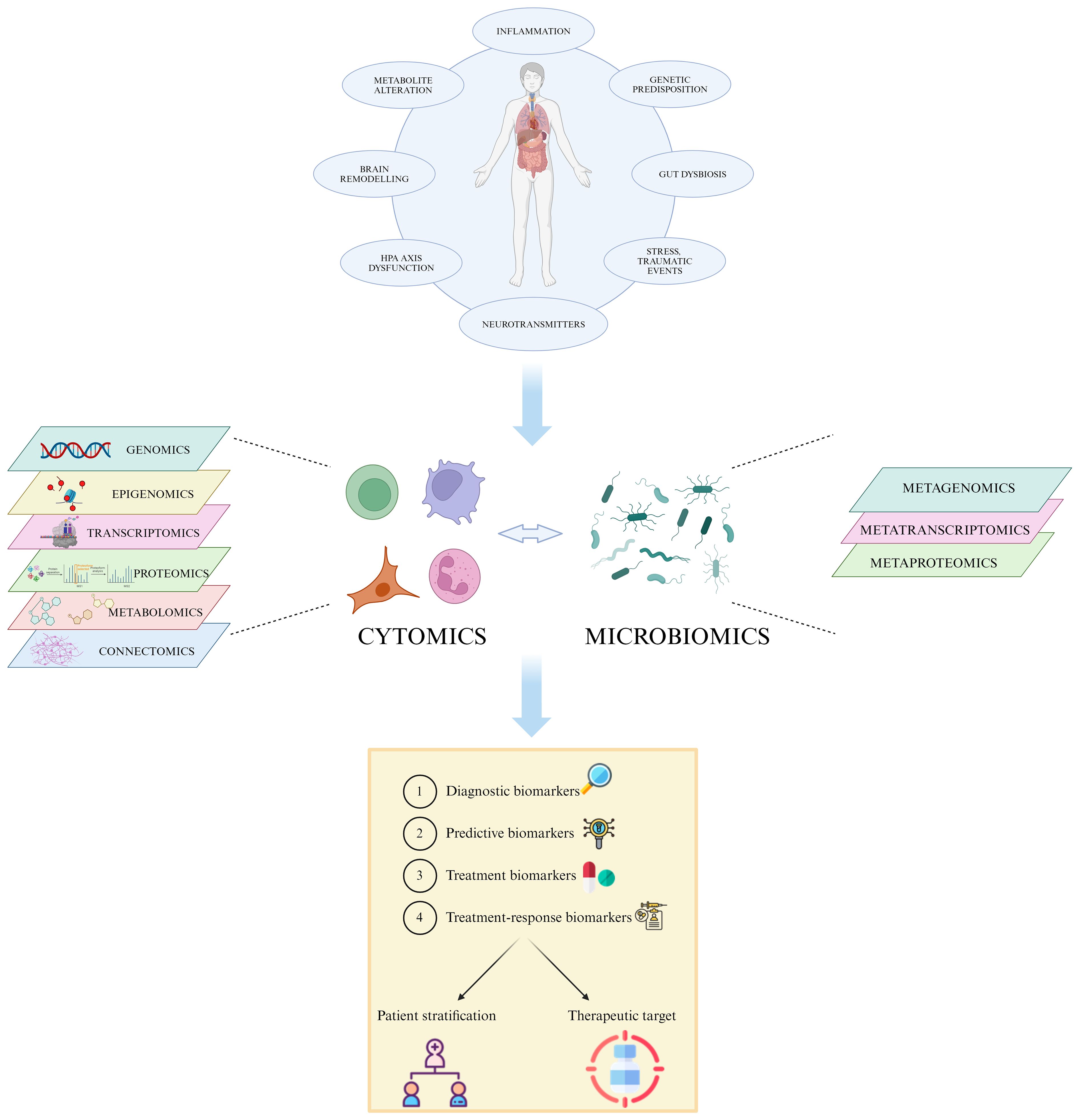
Figure 1 Multi-omic approach in MDD. The heterogeneity of MDD and its diverse etiology hinders the accurate patient stratification and appropriate treatment choice. The integration of high-throughput data generated by single omic technologies, such as genomics, transcriptomics and proteomics, into a multi-omic system permits the identification of specific individual features able to predict disease susceptibility, prognosis, and treatment response, leading to a more effective personalized care. Created with BioRender.com.
3 Application of precision medicine in MDDGiven the challenges in accurately diagnosing and treating MDD, which has a misdiagnosis rate of approximately 66% (77) and a treatment-resistant prevalence of 31% (78), finding more effective alternative approaches has become essential. By integrating both biological and non-biological characteristics of individuals, precision medicine facilitates the clustering of patients, thereby enabling more tailored and effective treatment strategies to improve diagnosis, treatment, and prevention efficacy (77, 79).
Precision medicine, with its multi-omic profiling that integrates genetic and epigenetic data, biomarkers, clinical characterization, and environmental exposures, holds revolutionary potential in psychiatry, aiming to enhance psychiatric diagnoses and treatment efficacy (77, 79). Through the analysis of multi-omic signatures, researchers can gain a deeper understanding of the complex interplay between genetic predispositions, metabolic pathways, and environmental influences in the onset and progression of depression, ultimately leading to more effective personalized treatment strategies for MDD (46, 77, 79). Also, another perceived challenge to adopt the biomarker approach in depression and other psychiatric disorders has been the difficulty in accessing tissue samples, a hurdle that has been surmounted in diseases like cancer, where most advances in precision medicine have occurred (80). However, the recent use of circulating cancer biomarkers, such as microRNAs (miRNAs), to detect early metastatic spread has validated the potential of liquid biopsy. This is highly relevant to molecular studies in MDD, where changes in circulating miRNAs, regulatory enzymes, and inflammatory cytokines have been linked to treatment outcomes (81).
Biomarkers are defined as “indicators of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention that can be measured and evaluated objectively” (82). In clinical practice they can be distinguished in: i) diagnostic biomarkers, if they are able to discriminate between the presence or absence of a specific disease, for example supporting the differential diagnosis between MDD and BD in patients with a first MDE; ii) predictive biomarkers, if they can predict the disease onset; iii) treatment biomarkers, if they can predict optimal treatment options and iv) treatment-response biomarkers, if they can measure the effectiveness of the treatment (83).
Given that MDD is linked not only with changes in brain structure and function, but also with gastrointestinal factors, the immune system, the endocrine system, neurotrophic factors, hormones, and oxidative stress, there are a plethora of biomarkers associated with these domains. Unfortunately, due to the heterogeneity of the disease, no specific biomarker has been found for MDD yet (84).
3.1 Soluble biomarkers – proteomics and metabolomics in MDDCurrently, a wide range of soluble biomarkers are used in the context of MDD, including endocrine hormones, monoamine neurotransmitters, neuropeptides, immune and inflammatory products, growth factors, and metabolic substances (such as adipokines and lipidemic factors). Among them, those linked to the enhanced activity of the Hypothalamic–pituitary–adrenal (HPA) axis, such as cortisol, serotonin, and brain-derived neurotrophic factor (BDNF), are the most studied for MDD (85). However, finding a more specific biomarker or a panel of biomarkers able to reflect the patients’ neuroinflammatory state and response to therapy has become a pressing need. In this context, immune and inflammatory markers such as cytokines and oxidative stress elements have been extensively studied in association with depression. Indeed, several studies and meta-analyses highlighted a strong association between inflammation and MDD, revealing elevated concentrations of C-reactive protein (CRP), interleukin-6 (IL-6), IL-12, tumor necrosis factor-α (TNF-α), and IL-1β in the serum of MDD patients (81, 86). Interestingly, the increased levels of some of these cytokines (i.e. IL-6, TNF-α, CRP and IL-1β) have been reported to correlate with lower response to pharmacological treatment (87). These findings are in line with a recent work by Xu et al., showing that specific sets of peripheral cytokines reached a good accuracy in discriminating non-responders from responders with sensitivity and specificity over 80%, revealing them as promising biomarkers for MDD diagnosis and antidepressant response (88). Along with inflammation, a recent study by Ait Tayeb et al. suggests that also oxidative stress, measured on various matrices such as serum, plasma, or erythrocytes, has a critical role in MDD according to disease stage and clinical features (89).
In recent years, approaches that integrate multi-omic platforms have been explored with the purpose of accelerating clinical biomarker discovery and deeply investigating the pathophysiological mechanisms in psychiatric disorders. Among them, MS-based proteomic techniques can analyze the whole proteome of an individual in an unbiased manner, overcoming the limitations encountered with more traditional methods such as western blotting or enzyme-linked immunosorbent assay (ELISA), allowing for the discovery of novel disease biomarkers. In a work by Silva-Costa et al., an untargeted MS proteomic approach unveiled a first potential biomolecular signature for late life depression and the biological pathways related to this condition, which could be suitable targets for the development of novel strategies for its prevention, early diagnosis, and treatment (90). In another work by Schubert and colleagues, a workflow was developed starting from the phenotype of cognitive impairment in remitted MDD to illustrate an application of systems biology approach; using a weighted gene co-expression network analysis (WGCNA) in the discovery phase, followed by a targeted proteomic analysis of the results, the authors were able to identify cellular mechanisms and candidate biomarkers for cognitive dysfunction in MDD (91).
On the other hand, metabolomics can provide a functional readout of the inner cell state and help identify biochemical signatures or biomarkers specific to different disorders, including MDD (92). In particular, MS-based analytical metabolomic approaches can be used in the study of psychiatric conditions due to their high-throughput capacity and ability to resolve a large number of metabolites with little to no need for purification (93). In a work conducted by Pan et al., using a GC-MS coupled with LC-MS/MS-based targeted metabolomics approach in the early stage of MDD, the authors identified a plasma neuro-metabolite signature able to discriminate first-episode, antidepressant drug-naïve depressed patients from healthy controls with high accuracy. Notably, their results indicate that the identified biomarker panel – composed by gamma-aminobutyric acid (GABA), dopamine, tyramine and kynurenine – was able to accurately diagnose blinded samples with both high sensitivity and high specificity (94). MacDonald et al. consider metabolomics a cost-effective and non-invasive technique to screen MDD individuals, monitoring their response to treatment and guiding their management. Additionally, combined with the screening of online drug registries, metabolomics can be a helpful tool to accelerate the discovery of novel druggable targets (92).
3.2 Genetic biomarkers – genomics and transcriptomics in MDDGenomics, by finding genes that contribute in predicting MDD susceptibility and therapy response, has played a significant role in precision medicine for MDD, expanding the field of pharmacogenetics and increasing clinical outcomes through an accurate diagnosis (95–97). Genetic predisposition in MDD relies on a polygenic trait: approximately 40% to 70% of MDD patients have susceptibility loci mainly located in genes involved in the serotonergic system and in the HPA axis, such as polymorphisms in 5-hydroxytryptamine (5HT; serotonin) transporter (5-HTT) and in tryptophan hydroxylase (TPH) genes (TPH1 and TPH2) (95). However, other genes have been studied in association with antidepressant response and thus they could be considered as treatment-response genetic biomarkers for MDD. This group includes catechol-O-methyltransferase (COMT) which plays a central role in noradrenaline degradation (98, 99), monoamino oxydase A (MAOA), that contributes to monoamines degradation (100–102), multi-drug resistance 1 (MDR1/ABCB1) genes encoding P-glycoprotein (103–105), glutamate receptor ionotropic kainate 4 (GRIK4) (106, 107), Potassium Two Pore Domain Channel Subfamily K Member 2 (KCNK2/TREK1), which encodes a neuronal potassium channel, Phosphodiesterase 2A (PDE2A) encoding for the enzyme which metabolizes cyclic AMP, transcription factor cyclic adenosine monophosphate response element binding protein (CREB1), BCL2 and many other candidate genes (108, 109).
In order to advance the understanding of the complex genetic architecture of depression and provide new tools for further investigating the disease etiology, several studies of genome-wide meta-analysis have been performed. Among them, a large-scale genome-wide association study conducted by Howard and colleagues identified 102 independent variants, 269 genes, and 15 gene sets associated with depression, including both genes and gene pathways linked to synaptic structure and neurotransmission (55). Interestingly, among the identified genes, the ones implicated in immune dysregulation have been extensively discussed in (110). In particular, Tubbs and coworkers grouped these 34 immune-related genes in 5 main categories based on their functions. Into the first category falls the majority of these genes, which contributes to general lymphoid development, implicating multiple white blood cell types. In contrast, other genes exhibit cell-type specific functions, for instance, some genes are involved in T-cell activation and development, other are crucial regulators of B cells, some of them have been associated to antigen presentation or are cytokine-related genes. Finally, some played a role in neuroinflammation, highlighting again the close link between immune system and brain functions (110).
Of note, among the predictive markers of treatment-response, in the context of personalized medicine, one of the most interesting categories is represented by the drug-metabolizer enzymes belonging to cytochrome P450 (CYP) family, which comprise more than 200 isoenzymes responsible for antidepressant drugs’ metabolism (111). Indeed, several single nucleotide polymorphisms (SNPs) in CYP family genes may significantly impact the metabolism of these drugs, resulting in different categories of patient metabolizers: poor metabolizers, intermediate metabolizers, extensive metabolizers, and ultra-rapid metabolizers. This is crucial when considering the potential for adverse effects or therapy inefficacy (112–114). However, the CYP genotyping is still not used in clinical application to predict MDD treatment response. Thus, integrating pharmacogenetic testing for CYP enzymes into MDD clinical management could allow healthcare providers to establish a personalized antidepressant treatment, which would minimize the risk of adverse drug reactions and improve the effectiveness of pharmacotherapy for depression (112–114).
In recent years, also miRNAs have emerged as attractive clinical biomarkers for diagnosing depression and other psychiatric disorders. Indeed, miRNA levels have been found impaired in schizophrenia, bipolar disorder, and depression (115). In particular, it has been demonstrated that polymorphisms in the let7 miRNA family and a variant of the miR-30 family (specifically miR-30e) lead to increased MDD susceptibility (116, 117). More specifically, let-7c and let-7b miRNA expression have been found significantly lower in treatment-resistant patients (118). Interestingly, a bioinformatic analysis revealed that those miRNAs are involved in the regulation of PI3K-AKT-mTOR signaling pathway, which has been previously reported to be dysfunctional in depression (119). Circulating miR-134 is another example of biomarker for neuropsychiatric disorders, reaching 79% sensitivity and 84% specificity in MDD patients. Furthermore, reduced levels of miR-200 have been found in depressed individuals, while the miR-144 and miR-146a have an inverse relationship with depressive symptoms (85). However, the interpretation of differential gene expression in brain tissue homogenates remains challenging due to the heterogeneous cellular composition of the sample. Thus, in the last years, there has been a rising interest in using single-cell sequencing approaches, which have revealed that gene expression patterns in the brain are cell-type specific. A work by Nagy et al. uses a single-nucleus transcriptomic technique in cells from the dorsolateral prefrontal cortex of patients with MDD that committed suicide, revealing the dysregulation of gene expression in almost 60% of the cell types identified, with a total of 96 differentially expressed genes. Given the complexity of psychiatric disorders such as MDD, investigating the role of each cell subtype in the brain could be relevant and requires single-cell resolution techniques (120). A similar approach has been used in non-human primates to integrate single-nucleus RNA-sequencing (snRNA-seq) and spatial transcriptomics: the snRNA-seq data resulted in the identification of six enriched gene modules linked to depressive-like behaviors, which were resolved by spatial transcriptomics. Findings indicate cell-type and cortical layer-specific gene expression changes and identify one microglia subpopulation associated with depressive-like behaviors in female non-human primates (121). This evidence indicates that combining these two approaches of snRNA-seq and spatial transcriptomics allows to improve spatial resolution of depression traits, highlighting the role of microglia in stress-inflammation.
3.3 Immune system – cytomics and immunomics in MDDThere has been increasing evidence that MDD is linked to a systemic immunological activation over the last two decades (74), affecting inflammatory markers, immune cell counts, and antibody titers (75, 76). Therefore, inflammation is a crucial factor to consider when studying MDD (122): it has already been reported that inflammatory diseases, e.g. rheumatoid arthritis, can increase the risk of developing depressive symptoms (123); not only that, levels of pro-inflammatory molecules are often augmented in MDD patients (124), and the administration of antidepressants together with anti-inflammatory drugs, such as celecoxib, are able to counteract more quickly depressive episodes (125). However, this strategy should be considered with caution, considering that anti-inflammatory treatment is highly unspecific and only half of MDD patients have a notable inflammatory profile (126).
The immune dysregulation extends beyond peripheral organs, also impacting the brain (127). Interestingly, similar immune system changes have been found in other mental health conditions like bipolar disorder and schizophrenia. This suggests that there might be common pathways in all these conditions (87). For example, after persistent inflammatory stimuli, many cell types of the brain undergo morphological, functional, and quantitative changes, in particular microglia and astrocytes (128). Unexpectedly, evidence also shows the opposite: some patients are affected not only by immune activation but also by immune suppression (129). This further complicates the immunological picture of MDD, which may thus reflect the effects of an infection, an autoimmune disorder, or genetic sensitivity that can exacerbate the innate and adaptive immune systems’ response to stress. For instance, autoimmune disorders and infections can influence CD4+ T helper (Th) cell function, cytokine, and antibody production (74).
In the central nervous system (CNS), cytokines are produced and released by immune (such as microglia), endothelial or neuronal cells, being key regulators of inflammation and cellular activities, and playing a crucial role in MDD pathology (130). There are primarily two types of cytokines: pro-inflammatory cytokines, which promote inflammation, and anti-inflammatory cytokines, which mitigate it (131). In the brain, cytokines are involved in organ development, influencing neuronal integrity, neurogenesis, and synaptic remodeling (132). However, psychological and physical stressors may prolong the activation of pro-inflammatory cytokines to a pathological level, disturbing multiple neuronal functions, like neurotransmitter impairment, apoptosis, and reduced neurogenesis (133, 134), thus supporting MDD-related neuroprogression (135).
Circulatory cytokines, instead, can impact brain inflammation through various pathways, including humoral (through leaky regions of the blood-brain barrier (BBB)), neural (through signals via afferent nerve fibers, in particular vagus nerve), and cellular routes (through stimulation of microglia in order to attract monocytes in the brain), crossing the BBB (136, 137). In particular, several pathways can lead to immune/cytokine dysfunction that contribute to the pathogenesis of depression: first, an activation of HPA due to environmental stress, as well as elevated systemic pro-inflammatory cytokines, leads to an increase of cortisol production. Even though cortisol is typically immunosuppressive, emerging theories suggest that elevated levels may lead to glucocorticoid resistance in immune cells, interrupting the inhibitory feedback mechanism. Additionally, cortisol is proposed to have a pro-inflammatory activity during stress by stimulating the extrahepatic enzyme 2,3-indolinime dioxygenase (IDO), which it is found in different types of cells including brain and immune cells (138, 139), leading to serotonin depletion. Specifically, IDO breaks tryptophan, a precursor of serotonin, into kynurenine, leading again to a serotonin depletion (140). Kynurenic acid, then, is not neurotoxic itself, but when it is metabolized to quinolinic acid (141) it modulates the synthesis of IL-1β and IL-6. These cytokines inhibit the glutamate re-uptake, therefore impairing the production of trophic factors while decreasing the brain plasticity and increasing the oxidative stress damage (142). Consequently, significant tissue damage occurs in many brain regions implicated in mood regulation (143) which further triggers the inflammatory response (144). This explains the detection of neuroactive substances such as quinolinic acid in the plasma and cerebrospinal fluid of patients with MDD (132).
The inflammatory process in MDD is influenced by stress-induced danger/damage-associated molecular patterns (DAMPs, also known as alarmins) involving nuclear factor kappa B (NF-κB) and the inflammasome pathway (74). It is important to distinguish between inflammation and para-inflammation, as both conditions coexist in MDD. The former is triggered by pathogens via pathogen-associated molecular patterns (PAMPs), while the latter is induced by psychological stress or DAMPs, and therefore it is defined as “sterile inflammation”. DAMPs, produced in response to stress, activate innate immune cells via the toll-like receptor (TLR) pathway, in particular TLR4, stimulating pro-inflammatory cytokine production, such as IL-1β, TNF-α, and IL-18. These cytokines have been found with a higher expression in patients with MDD, suggesting their involvement in the disease (145, 146). Notably, as evidence of the significance of TLR4 involvement in MDD, its blood level decreases in patients following a successful treatment (147).
Given the relevance of inflammation and the abundance of pro-inflammatory biomarkers in MDD, understanding the role of immune cells becomes of paramount importance, as these are able to produce but also get modulated by cytokines (148). In a physiological state, an effective network of mononuclear phagocytes is present in the CNS. The most prevalent cells in this network, around 10% of cells in the brain, are called microglia and they are considered the CNS’s innate immune system. They are produced as early myeloid progenitors in the embryonic yolk sac and move to populate the entire parenchyma (149). Peripheral immune cells, on the other hand, cannot enter the parenchyma, being only present in the meningeal borders of the CNS, as the presence of adaptive immunity within the brain parenchyma is symptomatic of chronic brain infection, inflammation or autoimmunity (150, 151).
The high interaction between microglia and neurons plays a fundamental role in shaping brain circuits by influencing the intensity of synaptic transmissions and modelling neuronal synapses. These interactions have additional tasks, such as phagocytosing and removing bacteria, dead cells, protein aggregates, and other particulate and soluble antigens that could harm the CNS after injury (149, 152). They contribute to various aspects of immune responses and tissue repair in the CNS, comprising the secretion of soluble factors, such as cytokines or neurotropic factors (153). Microglia continuously monitor their surrounding with motile multiple branches and processes to survey any disturbance. When they become amoeboid-shaped it indicates a highly activated state linked to pro-inflammatory processes, mirroring their reaction to a wide range of stimuli (154).
Their activation is categorized as classical (M1) or alternative (M2). M1 activation leads to a pro-inflammatory and neurotoxic state; M2 activation, on the contrary, promotes the release of anti-inflammatory cytokines (155). Disrupting the balance between these two activation states through neuroinflammation can lead to impaired microglial functions, which in turn have the potential to promote acute or chronic pathologic processes in the CNS (149). For example, abnormal activation of microglial cells was noticed in patients with MDD, along with a decrease in neurogenesis and an increase in glutamate toxicity (156–158).
Several studies have evaluated the immunophenotypic changes in peripheral blood immune cells, recently summarized and meta-analyzed in two systematic reviews (148, 159). Although in both cases the authors conclude that immune cell alterations can be found in MDD patients when compared to controls, representing possible biomarkers of the disease, their outcomes do not provide a clear answer on which cell subset(s) drive depression. Sørensen et al. described twelve populations upregulated in MDD patients, compared to healthy controls: these include total leukocyte, granulocyte, basophil, neutrophil and monocyte absolute counts, but also more specific subsets such as natural killer (NK) cells, B cells, intermediate monocytes, both naïve and memory CD4+ Th cells, immature double positive T cells and CD25+ and HLA-DR+ activated T cells. Additionally, the ratios between CD4+/CD8+ cells, neutrophils/lymphocytes and Th17/T regulatory (Tregs) cells were also higher in MDD patients than in controls. On the other hand, a downregulation of CD16+ NK cells and NK T cells (NKT) was observed in MDD (159). On the opposite, Foley et al., showed that percentages of lymphocytes and both Th1 and Th2 subsets were downregulated in MDD patients, while total CD4+ Th cell counts were increased, compared with healthy controls (148). The discrepancies between these two studies might be due to the methodology, the inclusion criteria, and consequently the number of records included in the meta-analysis (104 vs 27). Of note, not all original research articles converge in the same conclusion: the results hereby reported refer to the pooled standardized mean difference found between MDD patients and healthy controls, in which for the same specific subset each article may report divergent data, which are then grouped and given a different weight, based on sample size. Also, regarding the chosen methodology, while Sørensen et al. used both random- and fixed-effect models, Foley et al. opted only for the fixed-effect meta-analysis, which can contribute to their inconclusive results (148, 159).
Due to the heterogeneity of MDD, a general immune profiling of the disease might not be relevant for treatment choice. As previously introduced, not all MDD patients exhibit a strong immune dysregulation. Interestingly, the culprit subsets can vary even among subjects that demonstrate an exacerbated inflammatory profile. Initially, Lynall et al. showed that a combined analysis of the absolute counts of CD4+ Th cells, neutrophils, and eosinophils can accurately distinguish MDD patients from controls; subsequently, within the MDD group, a simultaneous assessment of neutrophils with NKT, and B cells seemed to be the most reliable predictor of disease severity (160). Attempting to categorize the data in an unbiased manner, the authors applied a Gaussian finite multivariate mixture model with a consensus clustering algorithm: this approach revealed four distinct subgroups of patients according to their differential frequency of immune cell populations. While one of the subgroups represented the already described “uninflamed MDD” (58 out of 206 patients), exhibiting low immune cell counts and pro-inflammatory cytokines expression (CRP and IL-6), the other three subgroups represent different inflammatory phenotypes. The rarest profile (10 patients) was enriched in non-classical and intermediate monocytes, eosinophils, B cells, and CD16high NK cells; in contrast, the most common phenotype (100 patients) showed increased counts of lymphoid cells, particularly CD4+ and CD8+ T lymphocytes and B cells; lastly, the third subgroup (38 patients) displays higher counts of granulocytes and myeloid cells, with the exception of non-classical monocytes (160). Although the authors could not find a correlation between the subgroups and clinical/demographic features, it is clear how important the immune system is in MDD. Therefore, an integrative immunological approach could provide a crucial immunomics overview. This approach utilizes immunophenotyping through flow cytometry, combined with other immunological techniques such as MHC tetramers, ELISpot assay, and HLA-binding assay, to aid in the diagnosis, stratification, and treatment choice for MDD (Figure 2) (72, 73, 161).
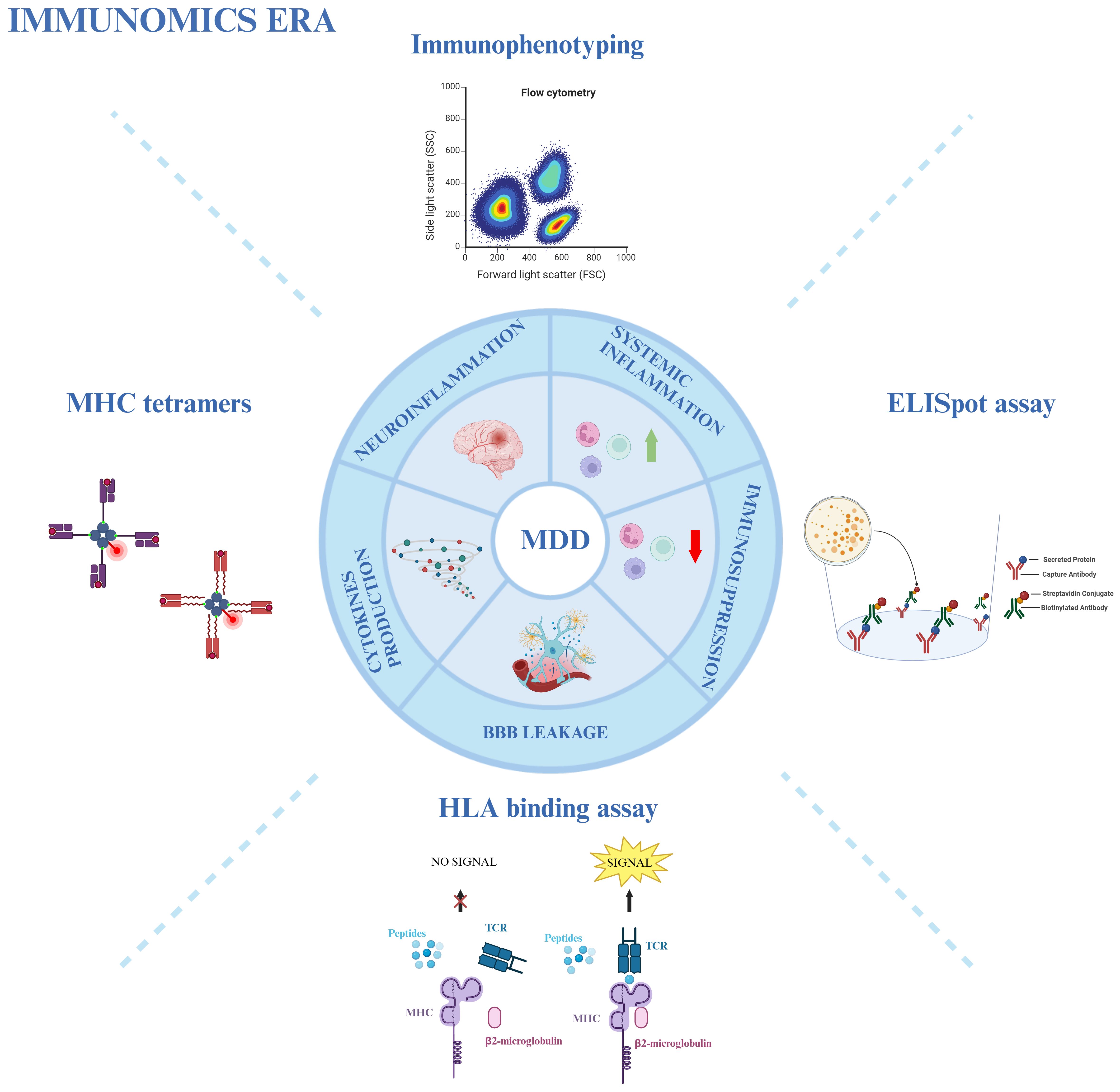
Figure 2 Immunomics approach in MDD. Various immunological techniques provide an overview of the involvement of the immune system in MDD. Immunophenotyping through flow cytometry, MHC tetramers, HLA-binding assays, and ELISpot assays are used to study neuro- and systemic inflammation, immunosuppression, blood-brain barrier (BBB) leakage, and cytokine production in MDD patients. Created with BioRender.com.
3.4 Microbiota and gut-brain axis - metagenomics in MDDThe connection between the gastrointestinal tract, including gut-associated lymphoid tissue (GALT), the activation of a low-grade inflammatory response, an increased intestinal permeability, and psychiatric symptoms has become more and more clear in recent years (162). The gastrointestinal tract is the interaction site of the immune system with an extensive number of intestinal microbiota, so it may be seen as the “immune gate” of many pathological conditions, including mental disorders like MDD (163).
Gut and brain are able to cross-regulate each other: gut affects brain function, particularly in regions related to stress regulation. In turn, the brain controls motor, sensory, and secretory modalities of the gastrointestinal tract (164). This mutual cross-talk is the so-called gut-brain axis (GBA). The permeability of the gut may be compromised by the diet, antibiotic treatment, stress, or infections (165), allowing antigenic material, such as food-derived antigens or lipopolysaccharides (LPS), to cross to the periphery. This can trigger immune-inflammatory responses, including neuroinflammation through TLRs, which increase the production of pro-inflammatory cytokines like IL-6, IFN-γ, CRP, and TNF-α (166). For example, LPS has been linked to cytokine-mediated illness behavior (described as the coordinated set of behavioral alterations as a result of an infection, caused by pro-inflammatory cytokines (167) and partly superimposable with MDE symptoms), microglial activation, neuronal cell death, and cognitive decline (168). A theory has been proposed defending that exposure to stress through stress hormones, inflammation and autonomic changes (169) modifies brain function by altering the gut microbiome, which in turn alters NLR Family Pyrin Domain Containing 3 (NLRP3) and IL-1β-driven pathways (170). In this vision, microbiome modifications can happen early in the disease and may even be a factor in the initiation of MDD, creating a pathological vicious cycle in which pathogenic changes in MDD over time further contribute to dysbiosis (171).
The interconnection between microbiota and brain can affect MDD patients primarily through the HPA axis, tryptophan metabolites, and microbial products, such as short-chain fatty acids (SCFAs) (like acetate, butyrate, and propionate), indoles (tryptophan metabolites) or through neurotransmitters. Microbiota disbalance increases HPA axis activity in response to stress (172, 173). It can influence serotoninergic, dopaminergic, glutaminergic, noradrenergic, and GABA neurotransmission or, in some cases, bacteria can produce these neurotransmitters by themselves (174). In particular, Streptococcus, Escherichia, and Enterococcus produce serotonin; Escherichia, Bacillus and Saccharomyces produce norepinephrine/dopamine; Lactobacillus and Bifidobacterium secrete GABA (175). Even if it is unlikely that neurotransmitters produced by microbiota can reach the brain, with the exception of GABA, they can indirectly influence the brain activity through the enteric nervous system (176). Also, tryptophan metabolism pathway can be controlled by products generated by some bacteria, for example Bifidobacterium infantis (177, 178). Specifically, SCFAs are not only involved in the BBB integrity, but they can also increase the tryptophan conversion rate into serotonin, indirectly influencing its amount in the brain (179); indeed, SCFAs resulted in being depleted in patients with MDD (180). Moreover, products of tryptophan called indole have been described as neuroactive signaling molecules able to regulate emotional behavior. Indeed, increased tryptophan catabolism into indoles is associated with reduced serotonin availability and increased neuroinflammation. Moreover, several pathways may include the direct effects of indole on central receptors or the activation of the vagus nerve through the influence of gut bacteria stimulating the neuroinflammatory state (173).
In addition, the gut microbiota can modulate distinct subsets of CD4+ Th cells through the stimulation of immunological signaling pathways. For example, different studies showed that Tregs and CD4+ Th effector cells were decreased in animals receiving long-acting antibiotic treatment. Foxp3+ Tregs are defective in germ-free mice, and SFCAs can induce their proliferation instead. Furthermore, Bacteroides fragilis stimulate Th1 cell growth (180). Also, segmented filamentous bacteria have been shown to enhance depression susceptibility through the induction of Th17 cells releasing IL-17A (181, 182).
Given all of that, it is becoming evident that it is fundamental to learn more about the landscapes of altered bacteria, bacteriophages, and fecal metabolites as well as their reciprocal interaction in the gut ecosystem of MDD patients, in order to better understand the pathophysiology/pathogenesis and to identify diagnostic and prognostic markers for clinical applications. Combination of metagenomic and metabolomic analyses is the ultimate strategy to explore the taxonomic and functional features of the microbiome (183).
Several studies have shown that patients with depression display abundance in some bacterial species and deficiency in others, compared to healthy individuals (184, 185). Some of them have focused on identifying differential bacteria with a phylogenetic resolution to the genus or family level. Results showed that the majority of the upregulated species in MDD belonged to the phylum Bacteroidetes and Proteobacteria, whereas the major downregulated species belonged to the phylum Firmicutes (186, 187). Bacteroides species are particularly important in the interactions with the immune system since they can induce the production of cytokines. On the other hand, beneficial anti-inflammatory effects can be mediated by Blautia species, decreased in MDD patients (188).
Furthermore, Zheng and colleagues discovered a unique microbe-based panel that was capable of differentiating between unipolar and bipolar depression, suggesting a specific alteration of the Bacteroidaceae family in patients with MDD as compared to those with BD (189).
In addition to metagenomics, also metabolomics plays an important role in the MDD interaction with the microbiota. The altered metabolites involved in MDD are mainly implicated in amino acid, nucleotide, carbohydrate, and lipid metabolism. In fact, MDD has been linked to significant disruptions in the metabolism of amino acid neurotransmitters, including dopamine, glutamate, and GABA. Moreover, Bacteroides and Blautia species were found to be altered at genus levels and correlated with amino acid and lipid metabolism (188).
As already mentioned, all these processes lead to depressive- and anxiety-like behavior (170). For this reason, the use of probiotics (living non-pathogenic organisms beneficial for the host), prebiotics (fibers that feed probiotics), and symbiotic (probiotics and prebiotics together) could exhibit beneficial effects in the regulation of depression pathogenesis. A meta-analysis of six trials about the use of probiotics with MDD patients shows a beneficial effect associated with its use in combination with antidepressants (190). In particular, the action of probiotics seems to promote the downregulation of HPA axis, which is overactive in MDD patients (191); in parallel, probiotic administration increases the biosynthesis of GABA (192), as well as upregulates tryptophan production and consequently serotonin availability (178). Some research also indicates that fecal transplantation of microbiota may reduce depressive symptoms and improve quality of life in different clinical samples, including patients with MDD (193–195).
Due to the strength of these techniques, it is highly relevant to link metagenomics and metabolomics with other multi-omic techniques, to obtain a complete overview of multifactorial diseases, as is the case of MDD (196).
3.5 Neuroimaging biomarkers – image-transcriptome analysis in MDDNeuroimaging biomarkers are non-invasive intuitive tools that permit the visualization of brain structures and functions in vivo, allowing for a better understanding of psychiatric disorders and their treatment response. Moreover, neuroimaging techniques have the advantage of allowing the structural and functional study of the CNS in vivo, overcoming the limitation of the post-mortem studies. Various neuroimaging methods used to investigate MDD features include functional MRI (fMRI), magnetic resonance spectroscopy (MRS), PET scan, single photon emission computed tomography (SPECT), EEG, and near-infrared spectroscopy (NIRS) (54, 85). Among these techniques, fMRI is commonly used in both classical neuroimaging and neuroimaging genetics, thus representing an essential tool in personalized medicine in psychiatry (95). By linking genetic variations with protein function, brain structure, structural and functional brain connectivity, and mental manifestations, neuroimaging genetics aims to integrate genetics, psychiat
留言 (0)