- High predictive accuracy for AIS prognosis.
- Integrates MRI radiomics with clinical factors.
- Utilizes advanced machine learning techniques.
- Provides a validated clinical-radiomics nomogram.
- Facilitates personalized AIS management.
1 IntroductionIschemic stroke remains a formidable public health concern due to its high incidence, mortality, and morbidity rates, exerting a profound impact on society, families, and the affected individuals (1). Despite concerted efforts in recent years toward the management, treatment, and prevention of ischemic stroke, a significant proportion of patients fail to receive timely and effective intervention. This failure is often attributed to delayed recognition of symptoms, a lack of awareness regarding the urgency of medical care, and the unavailability of adequate facilities in primary healthcare settings, leading to varying extents of neurological deficits. The cornerstone of acute ischemic stroke treatment in the acute phase includes intravenous alteplase thrombolysis (2, 3) and mechanical thrombectomy (4). However, the application of intravenous thrombolysis is constrained by a narrow therapeutic time window, and stringent inclusion and exclusion criteria limit mechanical thrombectomy. Recent observations suggest a shift toward an increasing incidence of stroke among younger populations, a trend linked to improved living standards and heightened work-related stress (5, 6). Factors such as the timing of intervention, location and volume of the infarct, and post-stroke treatment and rehabilitation efforts are pivotal in determining patient outcomes and survival rates (7). Consequently, the accurate prediction of acute ischemic stroke prognosis becomes essential for evaluating the severity, identifying potential adverse outcomes, gauging rehabilitation prospects, and enhancing doctor-patient communication and clinical decision-making processes.
When cerebrovascular diseases are suspected or need exclusion, Computed Tomography Angiograms (CTA) and Magnetic Resonance Angiograms (MRA) have demonstrated high specificity and sensitivity. However, Digital Subtraction Angiograms (DSA) remains the definitive gold standard, providing unparalleled diagnostic insight. Despite its utility in detailing intravascular conditions, DSA’s invasiveness and radiation exposure constrain its widespread clinical application.
Developing non-invasive methodologies with minimal radiation exposure is imperative in clinical practice to mitigate these limitations. Such advancements aim to improve the evaluation of treatment outcomes and prognostic accuracy in acute cerebral infarction.
Predictive models that amalgamate clinical observations, imaging findings, laboratory data, and other variables are instrumental in predictive assessment. These models enable comprehensive evaluations of rehabilitation prospects, survival rates, and disease incidence through mathematical and statistical approaches. A pioneering effort in this domain was conducted by Karen C. Johnston’s team in 2000 (8), utilizing the NIH Stroke Scale (NIHSS), Barthel Index (BI), and Glasgow Coma Scale (GCS) to gauge acute ischemic stroke prognosis with promising results. Furthering this initiative, they integrated NIHSS scores and CT infarct volumes to adeptly predict patient outcomes at 3 months. Zhao et al. (9) further explored 30-day survival prediction in acute ischemic stroke patients by analyzing post-stroke blood routine and biochemical markers, including Neutrophil-to-Lymphocyte Ratio (NLR), Prognostic Nutritional Index (PNI), Systemic Immune-Inflammation Index (SII), and Risk Assessment (RA).
Radiomics, employing sophisticated image processing to extract detailed features from imaging studies of acute ischemic stroke patients, unveils in-depth insights into pathophysiological alterations. This technique enhances early diagnosis, disease type determination, precise lesion localization and quantification, and fosters accurate prognosis evaluation and treatment outcome assessment (10–13).
Clinical radiomics models, leveraging features derived from diffusion-weighted imaging (DWI), fluid-attenuated inversion recovery (FLAIR), and apparent diffusion coefficient (ADC) scans, have shown commendable efficacy in prognosticating outcomes for patients with acute ischemic stroke (14, 15). Despite these advancements, the utilization of imaging attributes and clinical data in appraising treatment effectiveness and forecasting the prognosis of acute ischemic stroke remains underexploited. Notably, a blend of T1-weighted images (T1w), T2-weighted images (T2w), and DWI is prevalently employed for assessing patients post-onset. This fact highlights the critical need for an exhaustive amalgamation of various imaging techniques to refine the precision and utility of predictive models in determining acute ischemic stroke outcomes. The potential to enhance predictive accuracy and clinical decision-making through such integrated models is vast yet underleveraged.
The objective of this research is to develop a model that effectively combines T1-weighted (T1w), T2-weighted (T2w), and diffusion-weighted imaging (DWI) features with pertinent clinical parameters. This model explores the associations between imaging characteristics and crucial clinical information, enhancing our understanding of acute ischemic stroke. The primary goal is to create an accurate and individualized decision support system that enriches the treatment process for patients experiencing acute ischemic stroke. We anticipate facilitating significantly improved patient outcomes by achieving this integration, ultimately benefiting those impacted by this condition.
2 Materials and methods 2.1 SubjectsThis retrospective study recruited participants from two healthcare institutions, Xi’an Central Hospital (Center 1) and Tongchuan Mining Bureau Central Hospital (Center 2), with ethical approval from the respective hospitals’ Ethics Committees by the Declaration of Helsinki. A collective cohort of 506 patients diagnosed with Acute Ischemic Stroke (AIS) was retrospectively analyzed across both centers during the period from January to December 2021. Eligibility criteria for inclusion comprised admission within 24 h following symptom onset, an initial assessment using the National Institutes of Health Stroke Scale (NIHSS) upon admission, and undergoing diffusion-weighted imaging (DWI) within the first 72 h post-symptom onset.
Exclusion criteria were defined to omit patients who underwent reperfusion therapies for AIS, including intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA), urokinase (UK), and tenecteplase (TNK-tPA), as well as those who received bridging therapy (mechanical thrombectomy) or endovascular treatments (Center 1: n = 17, Center 2: n = 31). Given the unavailability of UK and TNK-tPA at the study sites, these treatments were not considered. Further exclusions applied to patients with hemorrhagic stroke, traumatic brain injury, subarachnoid hemorrhage, and hemorrhagic infarction (Center 1: n = 1, Center 2: n = 2), those presenting severe MRI artifacts (Center 1: n = 1, Center 2: n = 5), diagnosed with malignancies (Center 1: n = 0, Center 2: n = 1), or lost to follow-up (Center 1: n = 10, Center 2: n = 41; Figure 1).
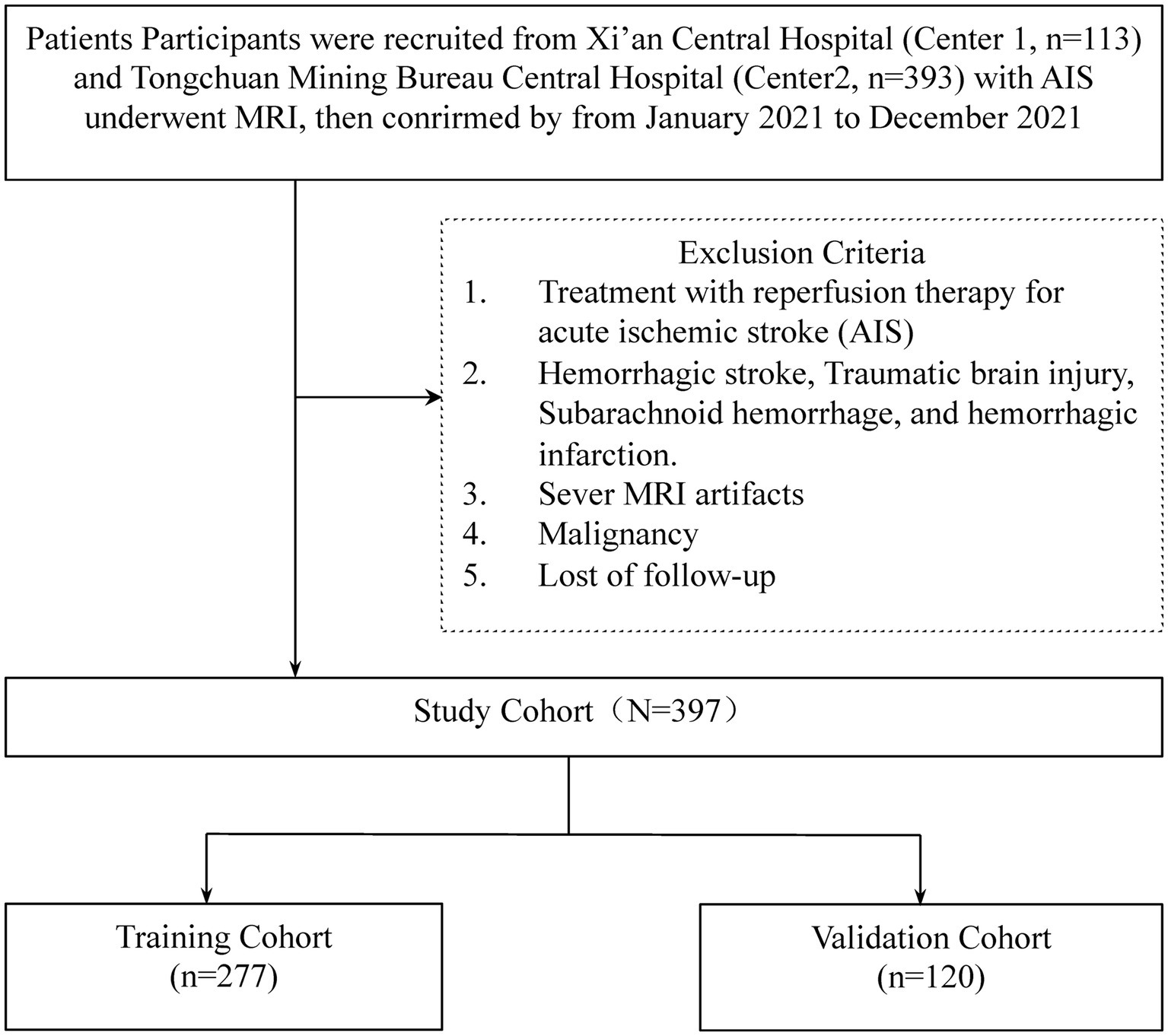
Figure 1. The workflow of the patient selection.
Baseline clinical data, encompassing demographics, medical history (e.g., hypertension, hyperlipidemia, hyperuricemia, hypoproteinemia, hyperhomocysteinemia, diabetes, smoking, drinking, prior stroke, atrial fibrillation, coronary artery disease, chronic heart failure, arthrosis, hemadostenosis), TOAST classification, along with an extensive set of laboratory parameters and imaging features, were meticulously extracted from medical records. Two experienced neurologists, each with a decade of practice and blind to clinical and imaging data, conducted structured telephone interviews to determine patients’ modified Rankin Scale (mRS) scores 6 months after hospital discharge, categorizing prognosis into favorable (mRS ≤ 2) and adverse (mRS > 2) outcomes. Although pre-stroke mRS scores are insightful for assessing baseline functionality, their exclusion aims to assure data integrity and minimize bias. The study’s emphasis on uniformly assessed, quantifiable factors across all participants enhances the predictive model’s validity, striving to eliminate confounding influences and bolster the reliability of the findings.
2.2 Image data acquisitionMRI scans were performed on all participants within 72 h post-symptom onset using the EXCITE HD 1.5 T MRI system by GE Healthcare, Milwaukee, WI, United States. Transverse T1-weighted fast spin echo (FSE) imaging was executed with specific parameters: TR/TE = 2,259/25.4 ms, slice thickness/gap = 5/1.5 mm, bandwidth = 244 Hz/Px, FOV = 240 × 240 mm2, and acceleration factor (R) = 2. For T2-weighted FSE imaging, the settings were TR/TE = 5,582/111 ms, slice thickness/gap = 5/1.5 mm, bandwidth = 244 Hz/Px, FOV = 240 × 240 mm2, and R = 3. Diffusion-weighted imaging (DWI) utilized single-shot echo planar imaging (SS-EPI) with TR/TE = 3,203/83.9 ms, slice thickness/gap = 5/1.5 mm, bandwidth = 3,906 Hz/Px, FOV = 240 × 240 mm2, R = 2, and b-values of 0 and 1,000 s/mm2 (Table 1).
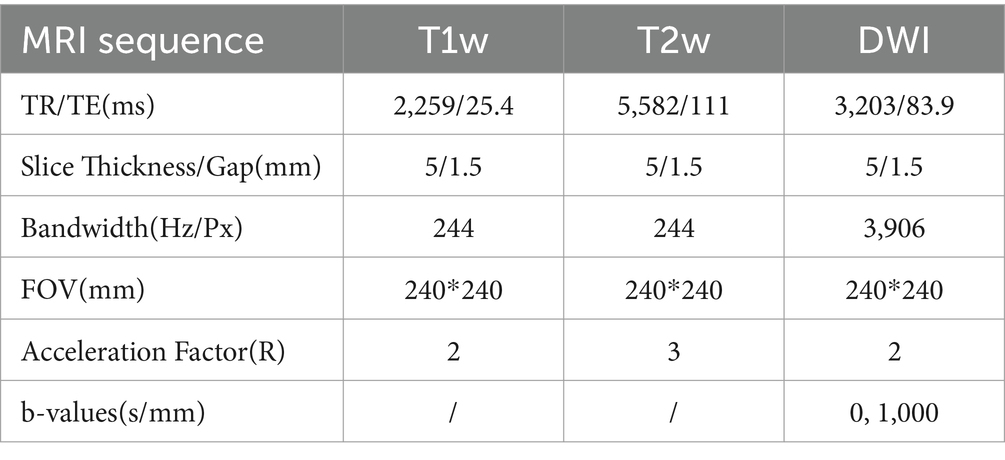
Table 1. Comparison of MRI sequence parameters.
2.3 VOI delineated and radiomics feature extractionVolumes of Interest (VOIs) were meticulously delineated using 3D-Slicer Software, while Pyradiomics software (version 3.0.1) facilitated the computation of radiomics features, adhering to the Image Biomarker Standardization Initiative’s guidelines. A radiologist, blind to the patient’s clinical information, performed the initial segmentation of MRI images. These segmentations were then reviewed and refined by a senior neuroradiologist with extensive experience. The segmentation process targeted the entire infarct region. Utilizing PyRadiomics, various radiomics features were extracted from these VOIs, including shape-based, first-order statistical, and several gray-level matrix features, from T1-weighted, T2-weighted, and diffusion-weighted images, totaling 4,682 features. This methodical extraction process ensures a comprehensive analysis of imaging data, which is crucial for evaluating acute ischemic stroke prognosis (Figure 2).
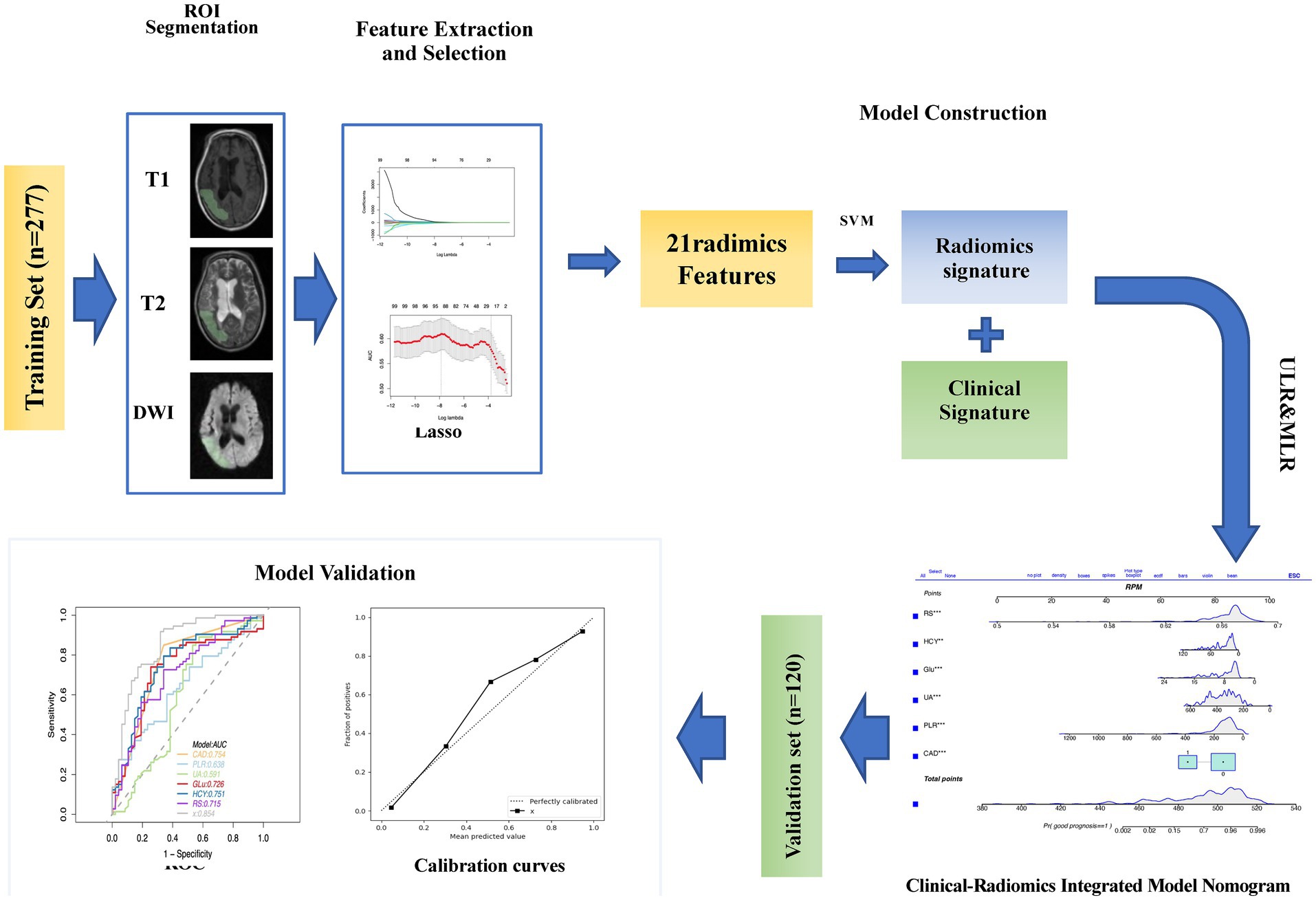
Figure 2. The workflow of the study is as follows: ROI (Region of Interest) segmentation was performed using 3D Slicer. The images were preprocessed for feature extraction. After feature evaluation and model construction, clinical radiomic signatures and imaging radiomic signatures were generated and used to construct a clinical-radiomic model. The performance of this model in predicting the prognosis of acute ischemic stroke (AIS) was validated in the validation set.
2.4 Features selectionGiven the high-dimensional nature of radiomics features in acute ischemic stroke (AIS) analysis, the study aimed to pinpoint features significantly correlated with outcome predictions in the training cohort. Initial feature selection was performed using a U-test, setting a p-value threshold 0.05 to filter out non-significant and redundant features. Further refinement involved a correlation analysis to eliminate features with a correlation coefficient above 0.9. The Least Absolute Shrinkage and Selection Operator (LASSO) algorithm was then utilized to finalize feature selection, identifying the most predictive features through fivefold cross-validation.
2.5 Prediction development and diagnostic validationUnivariable logistic regression identified potential clinical predictors of AIS outcomes within the training cohort. Subsequently, significant predictors were analyzed through multivariable logistic regression, employing backward stepdown selection to isolate independent clinical predictors. These findings were presented as odds ratios with corresponding 95% confidence intervals, forming the basis of a multivariable clinical prediction model. The model combined these independent clinical predictors with the radiomics signature to create a comprehensive clinical-radiomics model, which underwent rigorous evaluation through ROC curve analysis and other statistical measures to assess its discriminative performance.
2.6 Clinical usefulness and calibration curves of the clinical-radiomics modelThe clinical-radiomics model’s calibration was examined using calibration curves alongside the Hosmer-Lemeshow test to determine the model’s fit accuracy.
2.7 Statistics analysisThe study used statistical methods to analyze demographic and clinical data, including independent t-tests for normally distributed data, Mann–Whitney U tests for non-normally distributed data, and chi-square tests for categorical variables. The predictive performance of clinical, radiomics, and clinical-radiomics models was evaluated through Receiver Operating Characteristic (ROC) curves. Model comparisons were conducted using the Delong test. Calibration of the clinical-radionics model was assessed with calibration curves and the Hosmer-Lemeshow test. Statistical analyses were conducted in R software, with significance determined by a two-tailed p-value of <0.05.
3 Results 3.1 Baseline characteristics of patientsThe baseline characteristics of patients within both the training and validation groups are detailed in Table 2. Analysis revealed no statistically significant disparities between the cohorts (p > 0.05), indicating comparable baseline conditions. Within the training cohort, patients experiencing unfavorable outcomes (modified Rankin Scale, mRS, > 2) accounted for 66.78% (182 out of 277), while in the validation group, this proportion stood at 60.83% (73 out of 120).
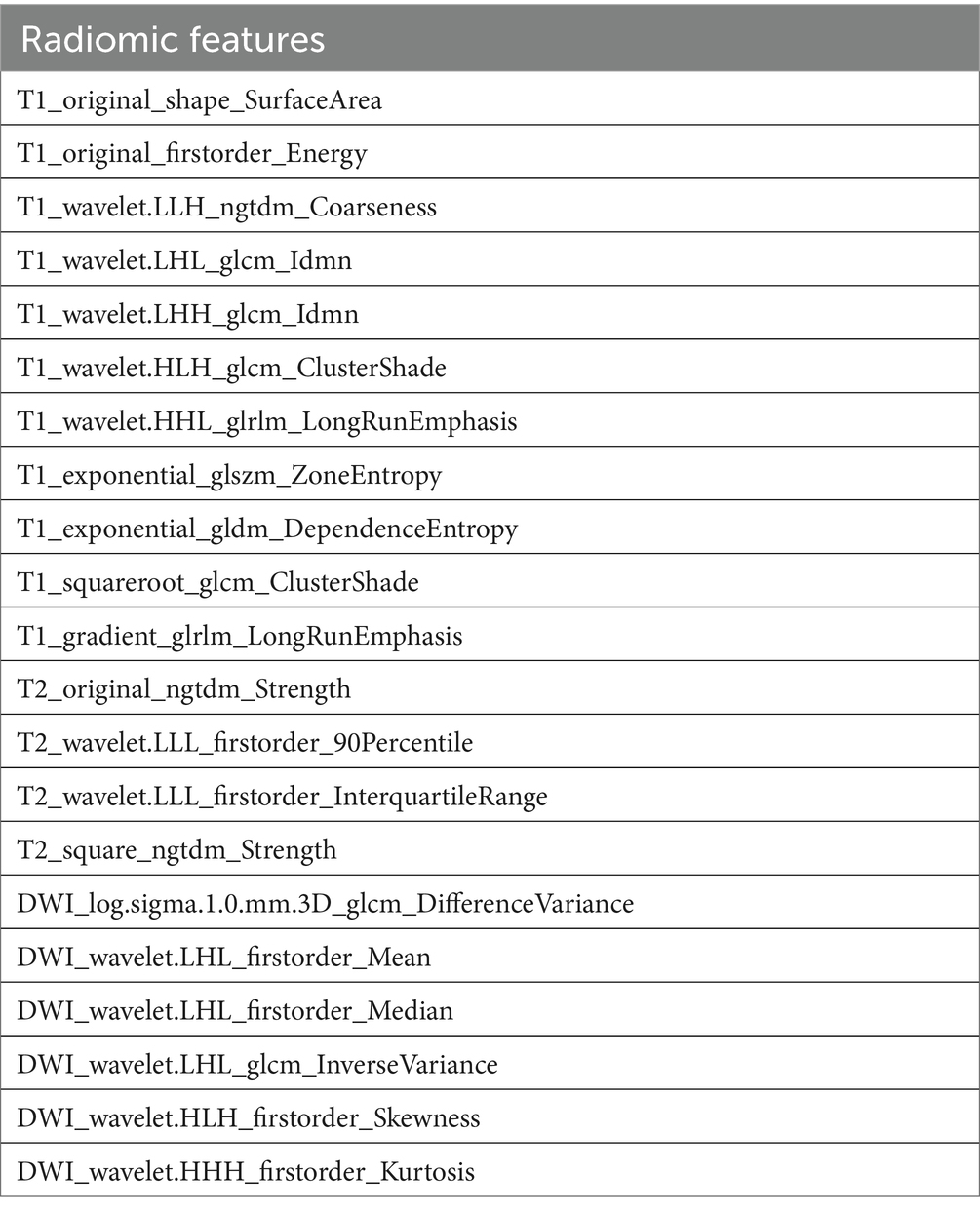
Table 2. The characteristic of 21 final feature for radiomics assessment.
3.2 Radiomics feature selection and LASSO logistic regression findingsIn refining our dataset, redundant features were eliminated through a U-test and Spearman correlation analysis, narrowing down the selection from an initial pool of 4,682 image features extracted from the Volumes of Interest (VOIs) to 791 radiomic features. This step was essential for enhancing the dataset’s manageability and relevance. Following this initial reduction, the Least Absolute Shrinkage and Selection Operator (LASSO) logistic regression method was applied to further distill these features, focusing on identifying those of optimal predictive value. Determining the most appropriate lambda value was crucial in this context; to this end, five-fold cross-validation was utilized, selecting a lambda value within one standard error of the minimum. Through meticulous selection, a final set of 21 radiomic features was identified (as detailed in Table 3). This rigorous methodology underscores the accuracy and efficacy of the resulting predictive model (illustrated in Figure 3).
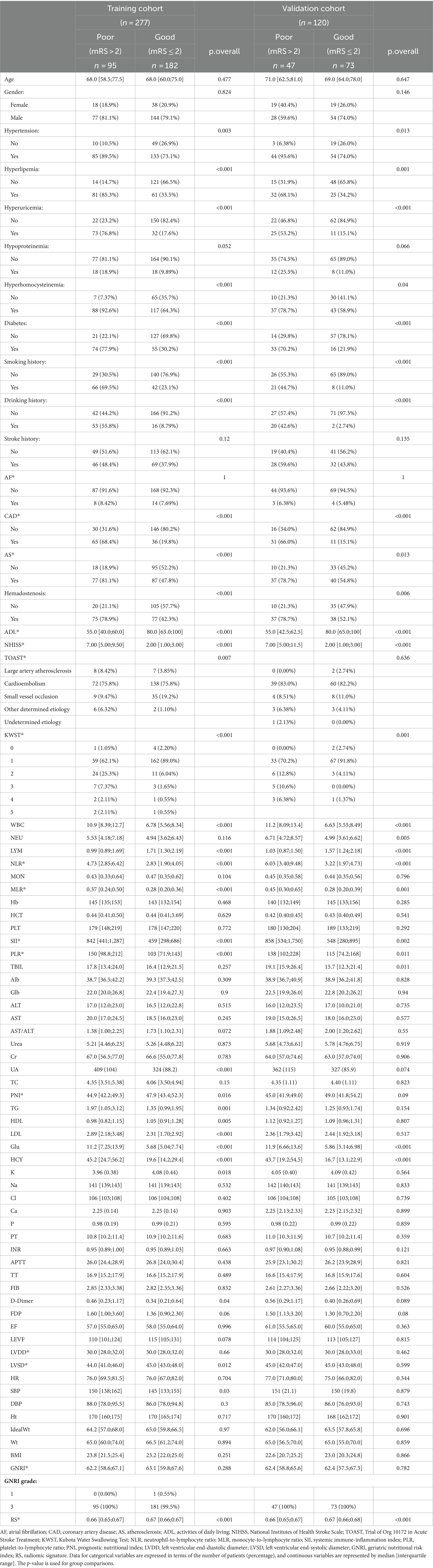
Table 3. Baseline characteristics of patients in the training and validation cohorts.
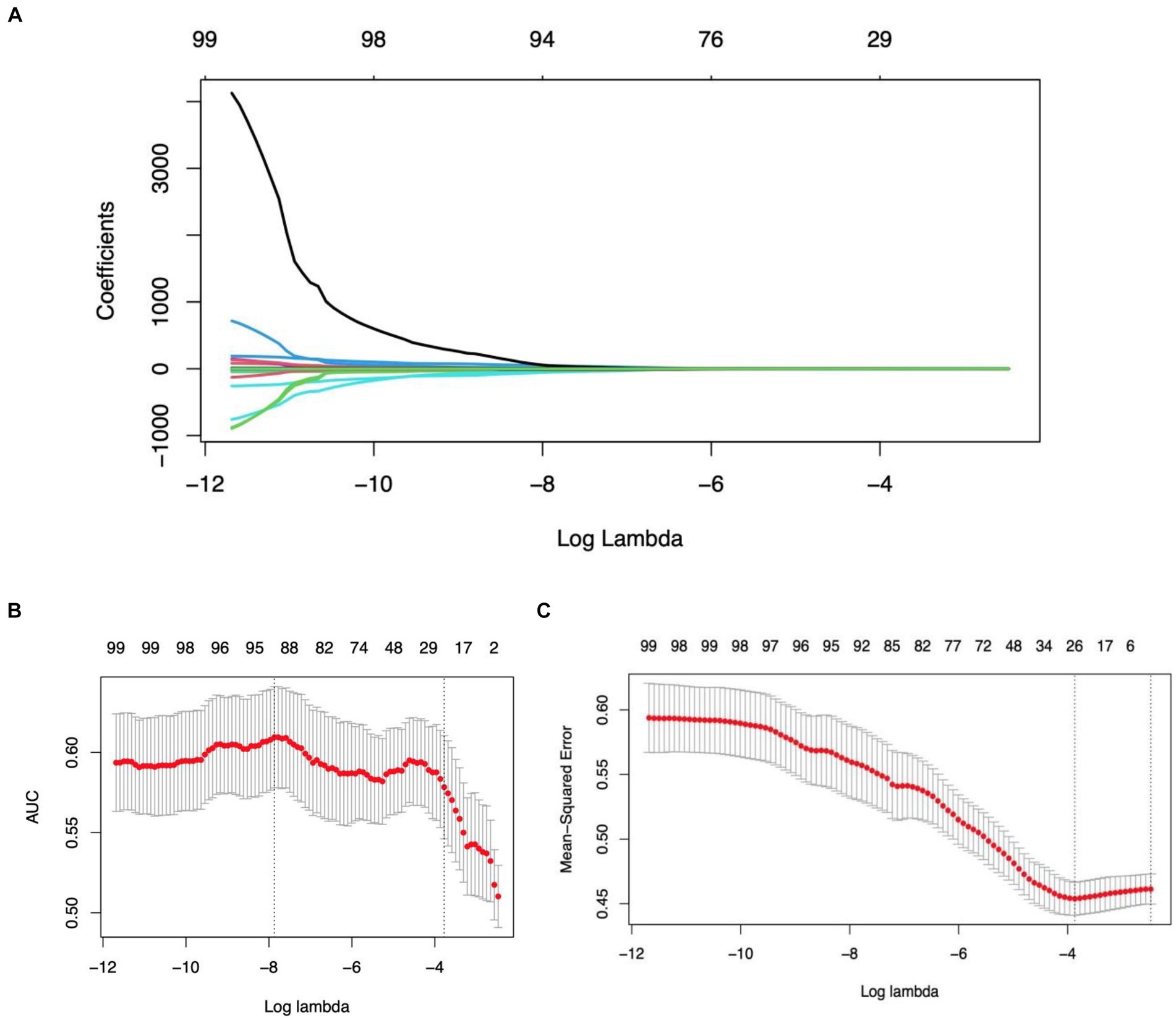
Figure 3. Illustration of the LASSO model’s application, employing a tuning parameter (lambda) and utilizing five-fold cross-validation based on both the minimum criteria and one standard error (1se), facilitating the selection of radiomics features during the feature selection phase.
3.3 Establishment and performance of the clinical featuresTable 4 presents the results of the multivariate logistic regression analysis, identifying significant variables (p < 0.05) such as Coronary Artery Disease (CAD), White Blood Cell count (WBC), Platelet-to-Lymphocyte Ratio (PLR), Uric Acid (UA), Glucose (Glu), Homocysteine (HCY), and the Radiomics Score (RS). These variables have been pinpointed as independent predictors for clinical functional outcomes. Utilizing these determinants, we constructed a radiomics nomogram within the training set, creating a clinical-radiomics comprehensive prediction model (illustrated in Figure 4).
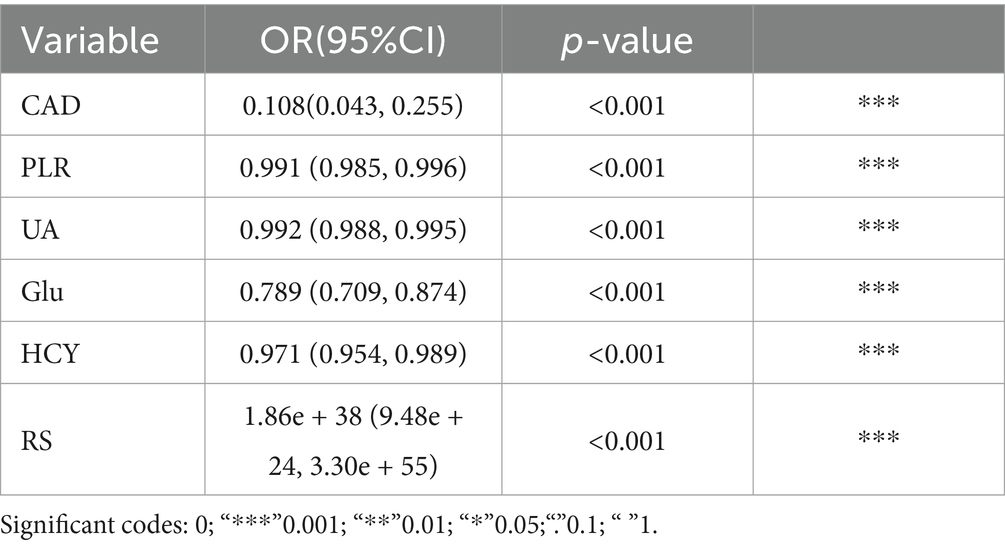
Table 4. Multivariate logistic regression.
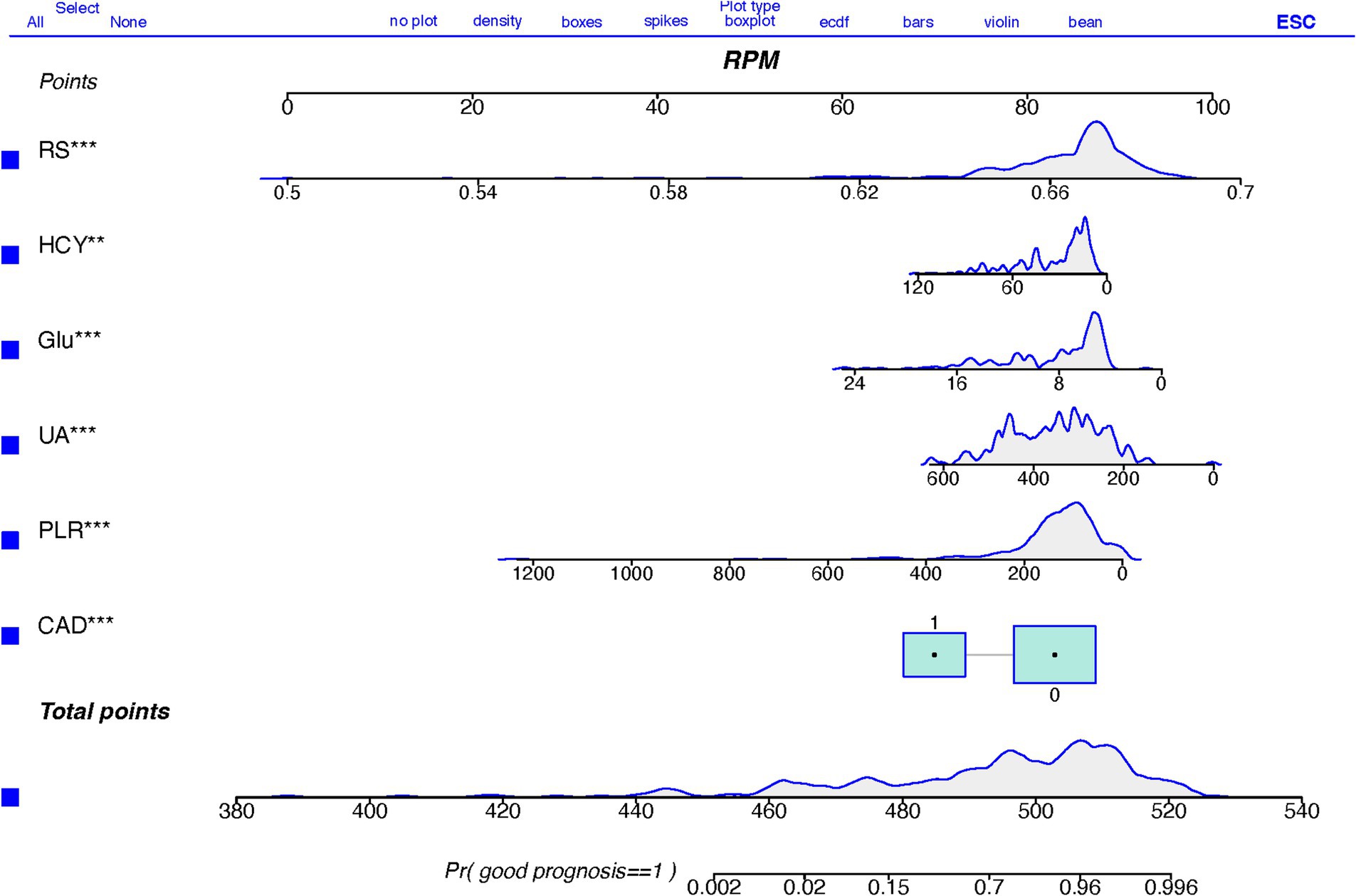
Figure 4. A nomogram based on clinical-radiomics models for predicting AIS outcomes.
The nomogram is meticulously designed, highlighting the relative importance of variables identified through multivariate regression analysis. A scale is displayed at the top of the column chart, with predictive model variables listed on the left. Each independent variable is assigned a score, correlating to specific values, with the length of each segment indicating its contributory weight to the outcome event. Hence, longer segments underscore a variable’s heightened significance. By aggregating scores for individual variables, a total score is achievable. This cumulative score facilitates the determination of the linear predictor and predicted probability for Acute Ischemic Stroke (AIS) prognosis through the scale provided below. A prediction value of 1 suggests a favorable prognosis, whereas 0 implies a less favorable prognosis.
3.4 Performance of the combined clinical-radiomics modelThe performance of the combined clinical-radiomics model was meticulously evaluated using Receiver Operating Characteristic (ROC) curves. This comprehensive model exhibited impressive predictive accuracy for Acute Ischemic Stroke (AIS) outcomes, as evidenced by Area Under the Curve (AUC) scores of 0.940 (95% Confidence Interval [CI]: 0.912–0.969) in the training cohort and 0.853 (95% CI: 0.781–0.926) in the validation cohort. Notably, in the validation cohort, the clinical-radiomics model achieved high sensitivity (91.8%) and moderate specificity (68.1%), indicating its robust ability to accurately predict patient outcomes. The Positive Predictive Value (PPV) of the clinical-radiomics approach in the validation set was notably high, at approximately 81.7%. The Radiomics Score (RS) and the clinical-radiomics models yielded AUC values of 0.715 (95% CI: 0.619–0.810) and 0.854 (95% CI: 0.781–0.926), respectively, as illustrated in Figure 5.
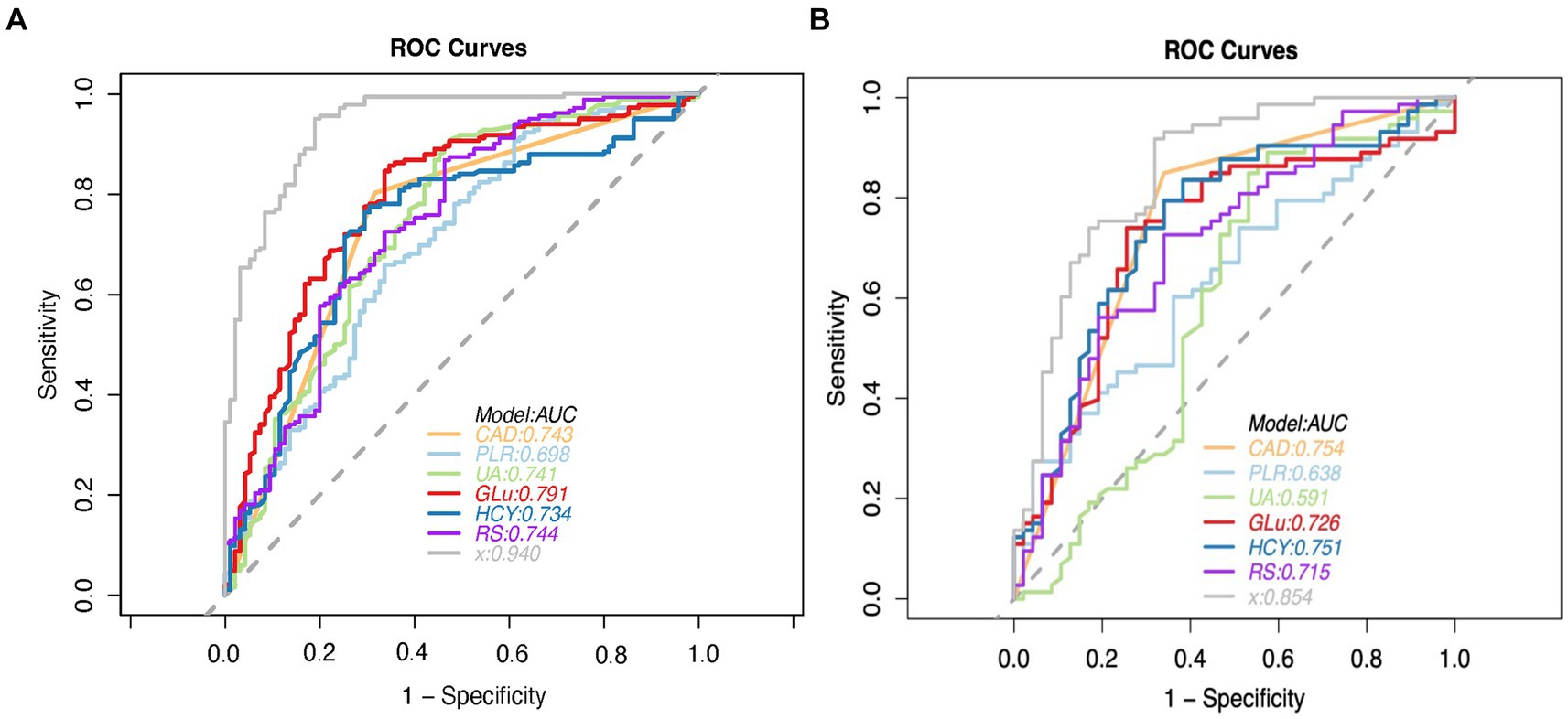
Figure 5. ROC curve analysis of predictive models for modified rankin scale (MRS) outcomes in the training (A) and validation (B) cohorts, with the clinical-radiomics model highlighted. CAD = coronary artery disease, PLR = platelet-lymphocyte ratio, UA = serum uric acid level, GLU = fasting plasma glucose, HCY = homocysteine, RS = Radiomics Signature, x = Clinical-Radiomics model.
Clinical variables such as Coronary Artery Disease (CAD), Platelet-to-Lymphocyte Ratio (PLR), Uric Acid (UA), Glucose (Glu), and Homocysteine (HCY) demonstrated significant predictive utility, as detailed in Table 5. The DeLong test revealed no significant differences in the performance of these three individual models (all p > 0.05), underscoring the consistency of the predictive capability across the models.
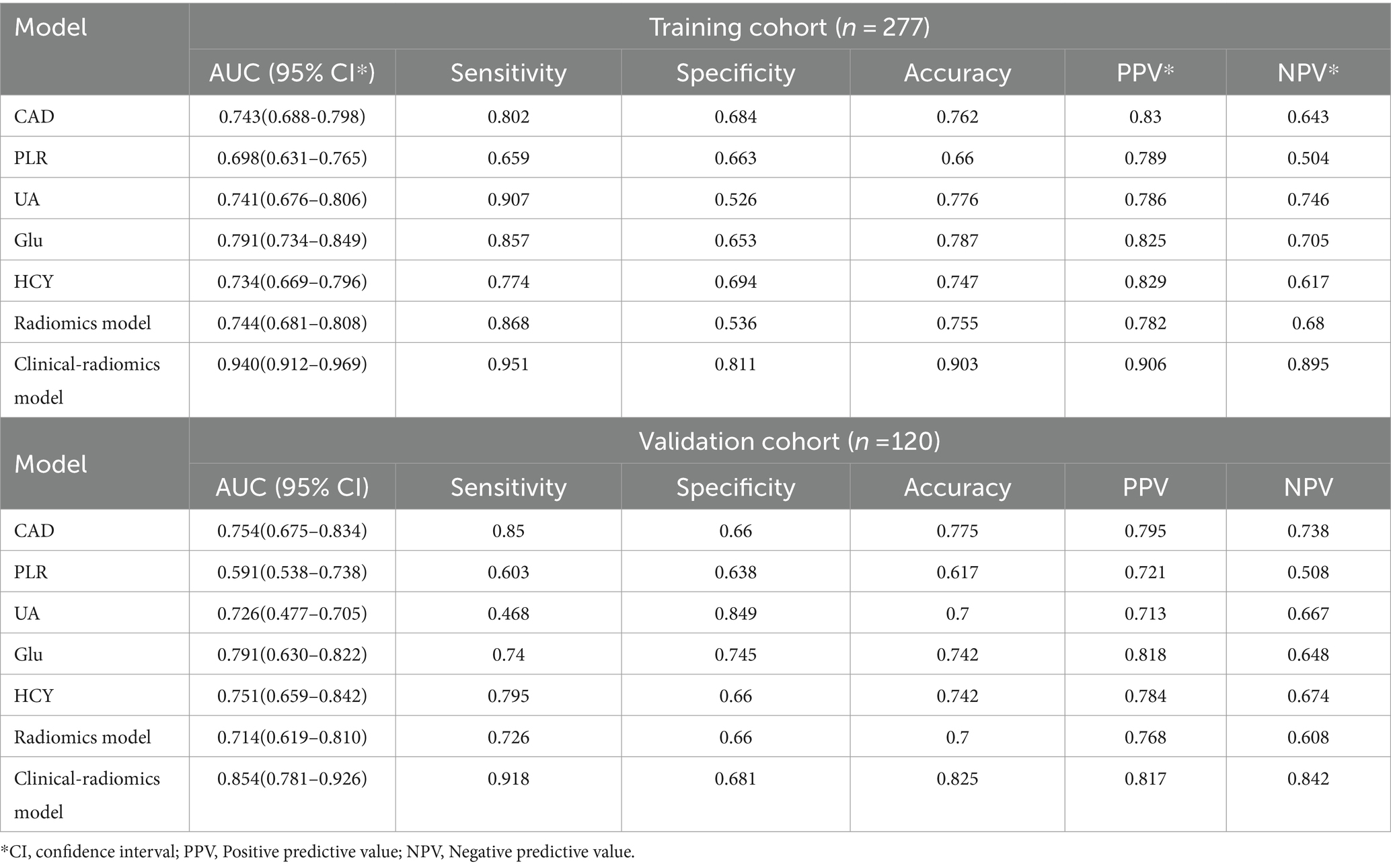
Table 5. Predictive performance of three models in the training and validation cohorts.
3.5 Clinical usefulness and calibration curves for the clinical-radiomicsWhen contrasted with single-scale prediction models, the clinical-radiomics model showcased a superior capability in discriminating performance evaluation. As depicted in Figure 6, calibration plots demonstrated a commendable congruence between the model’s predictions and the actual clinical outcomes for Acute Ischemic Stroke (AIS). This alignment indicates that the clinical-radiomics model provides a significantly enhanced net benefit over traditional single-scale models, underscoring its greater clinical utility.
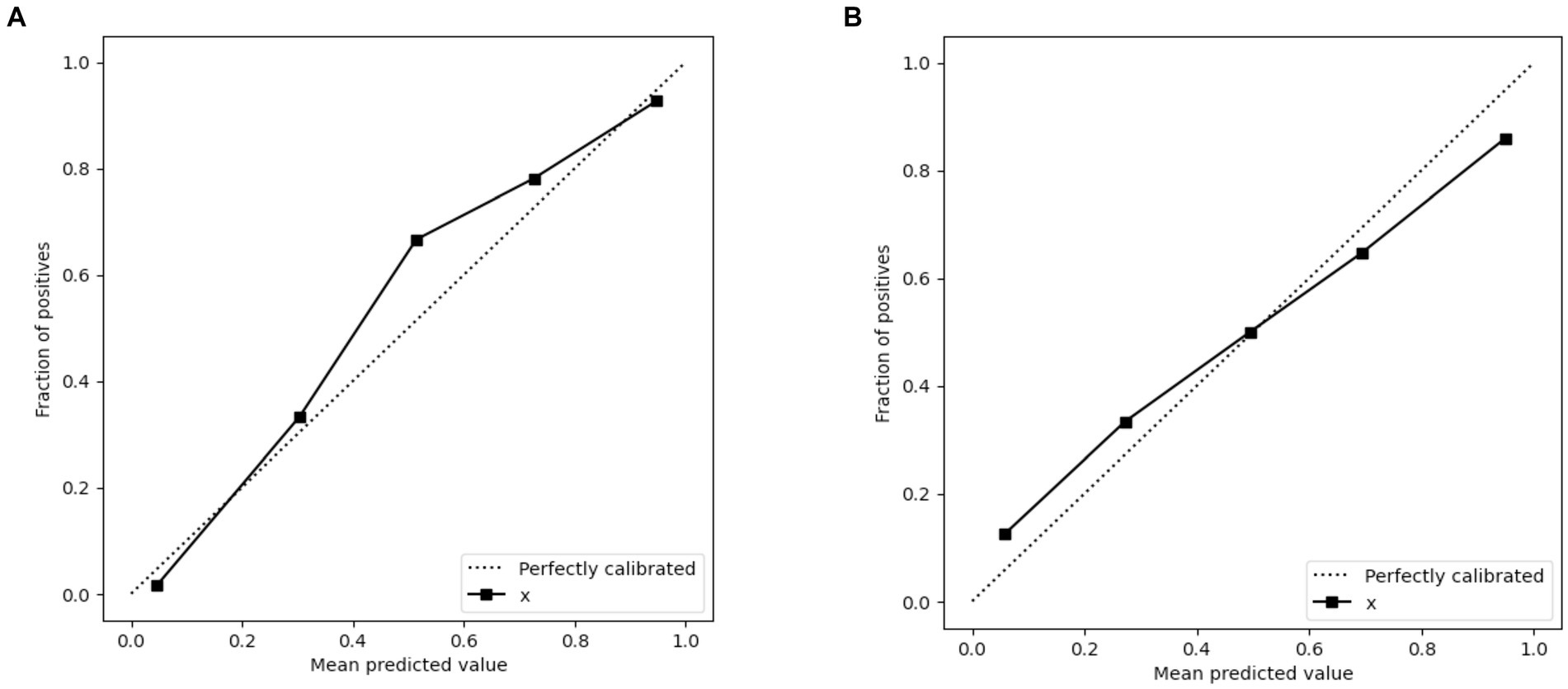
Figure 6. Calibration curves of the clinical-radiomics model in the training set (A) and validation set (B) for predicting AIS outcomes. x = Clinical-Radiomics model.
4 DiscussionClinical predictive models are increasingly utilized in acute ischemic stroke management, serving as a critical role in prognosis and diagnostic support. Such as the widely recognized modified Rankin Scale (mRS), which could provide prognostic insights based on thorough patient evaluations, have gained broad acceptance in clinical settings. Historically, studies on ischemic stroke prognosis have predominantly utilized non-imaging data, employing various statistical models and machine learning algorithms such as linear regression, support vector machine (SVM), k-nearest neighbors (KNN), logistic regression, decision trees, k-means clustering, random forests (RF), naive Bayes, dimensionality reduction techniques, and gradient boosting. However, these methods have limitations, particularly their dependence on basic clinical data without incorporating detailed biomarkers or complex imaging findings. Such limitations may impede a comprehensive understanding of the multifaceted nature of the disease. Moreover, despite the importance of these thorough assessments, they may not adequately account for individual variability and specific lesion characteristics inherent in the nonlinear dynamics and subjective assessments of stroke prognosis evaluation systems, posing challenges in reflecting the dynamic progression of the condition (16, 17). The advancement of precision medicine calls for more sophisticated and precise methods capable of navigating the intricacies of ischemic stroke prognosis. In this context, artificial intelligence (AI) models emerge as potent tools, offering the potential for more tailored and accurate prognostic predictions.
The progression of precision medicine necessitates more sophisticated and precise methodologies capable of addressing the intricate nature of ischemic stroke prognosis. As a result, there is a growing focus on integrating diverse data sources, such as clinical observations, radiomics features, and biomarkers, to construct predictive models that provide improved accuracy, comprehensiveness, and personalization. Technological advancements have facilitated an increase in studies integrating imaging data (e.g., CT and MRI scans) with state-of-the-art artificial intelligence machine learning techniques to enhance the accuracy and sensitivity of stroke prognosis prediction models. This evolving paradigm highlights the comprehensive utilization of diverse data types, especially imaging data, creating new opportunities for advancing stroke prognosis research and achieving more detailed and precise assessments of patient prognoses in the medical domain (18).
Radiomics and artificial intelligence hold great promise in the management of acute stroke. Cranial CT is the standard screening tool post-stroke, with the Alberta Stroke Program Early CT score (ASPECTS) being a rapid, straightforward, and reliable method for assessing early ischemic changes in patients with ischemic stroke, which is crucial for predicting treatment outcomes and prognosis (19). Nicolae et al. (20) retrospectively analyzed 340 patients revealed that ASPECTS effectively predicts the prognosis of patients with acute ischemic stroke (AIS), with lower scores indicating a larger infarct volume, particularly in diabetic and elderly patients. Chen et al. (21) retrospectively analyzed the CT images of 276 AIS patients, confirming the reliability and accuracy of automated ASPECTS scoring software. Hulin Kuang et al. (22) introduced EIS-Net, a novel multi-task learning network capable of simultaneously segmenting early infarcts and scoring ASPECTS on non-contrast CT images, offering performance comparable to expert assessments in a rapid manner. Masaki et al. (23) developed a deep learning-based automated ASPECTS calculation software utilizing the 3D-BHCA algorithm, demonstrating higher accuracy and efficiency than traditional methods, which could assist physicians in formulating superior treatment plans. Additionally, Qi et al. (24) explored apparent diffusion coefficient (ADC) image signal changes and their quantitative assessments across 207 acute ischemic stroke patients. Their findings suggested these analyses could act as crucial references for estimating acute ischemic stroke volume, thus offering valuable diagnostic insights for clinical practice. Ma’s study (25) corroborated these observations. Future research may integrate multimodal imaging with clinical factors, offering more possibilities for precision diagnosis.
Cerebrovascular malformations, commonly arising from the abnormal development of intracranial vessels, can precipitate severe events such as stroke. Moyamoya disease (MMD), predominantly affecting children and adults, is marked by arterial stenosis and occlusion, with the potential to cause conditions like epilepsy and cognitive delays in pediatric patients. Despite advancements in modern imaging facilitating diagnosis, therapeutic options for MMD are limited, underscoring the critical importance of early detection and intervention to improve patient outcomes (26). The emergence of artificial intelligence (AI), particularly in the realms of deep learning and machine learning, has introduced novel approaches to MMD diagnosis.
In Kim’s study (27), they utilized deep learning and convolutional neural network (CNN) techniques to analyze cranial images from 345 diagnosed MMD patients and 408 control subjects for the detection of MMD. CNN for the analysis of images from MMD patients and controls demonstrated impressive levels of accuracy, sensitivity, and specificity, underscoring the potential of AI in medical imaging.
Qin et al. (28) utilized machine learning models to analyze DSA images and predict mean transit time in MMD or Moyamoya syndrome (MMS) patients, achieving high accuracy in specific brain regions, which could offer significant insights for clinical diagnosis and treatment. Collectively, these studies suggest a promising role for AI in refining MMD diagnosis and treatment strategies.
Multimodal MRI, incorporating a variety of imaging sequences such as DWI, FLAIR, susceptibility-weighted imaging (SWI), and T1w and T2w, offers a rich source of biological insights. This comprehensive imaging approach enables physicians to thoroughly understand the characteristics and extent of lesions, thereby enhancing the precision of disease diagnoses. Additionally, employing radiomics and machine learning techniques, multimodal MRI facilitates the automated extraction and analysis of extensive imaging features. By amalgamating data from different MRI sequences, this strategy supports the development of predictive models capable of accurately determining patient outcomes. The confluence of multiple imaging modalities with sophisticated analytical methods promises more accurate and personalized medical evaluations.
In another significant effort, Quan et al. (14) derived radiomics features from fluid-attenuated inversion recovery (FLAIR) and ADC images across 753 cases, demonstrating their utility in forecasting clinical outcomes in acute ischemic stroke (AIS) patients. External validation showed promising area-under-the-curve (AUC) values across various models, with the combined ADC and FLAIR radiomics model significantly enhancing predictive accuracy for adverse outcomes.
A meta-analysis by Hanna Maria Dragoş et al. (29), reviewing 150 articles, suggests models that merge clinical and imaging features more effectively predict disability outcomes in stroke patients at 3 and 6 months post-event. Wang’s study (30) established three radiomics models and a comprehensive nomogram that integrated clinical features and radiomic signatures, showing robust predictive performance for post-thrombolytic ischemic stroke prognosis.
To predict ischemic stroke outcomes, Yu et al. (31) utilized a machine learning model integrating multimodal images, including DWI, ADC, FLAIR, SWI, and T1w. This model achieved notable performance metrics, with an accuracy of 0.831, sensitivity of 0.739, specificity of 0.902, an F1 score of 0.788, and an AUC of 0.902. Radiomic features extracted using the LightGBM model from multimodal MRI effectively forecast stroke prognosis, demonstrating the model’s high predictive value for clinical outcomes in acute stroke patients. This underscores the potential of multimodal imaging in making precise prognosis predictions for ischemic stroke.
While the feasibility of using machine learning based on radiomics alongside clinical factors for predicting acute ischemic stroke outcomes is recognized, it is crucial to be mindful of possible biases in participant selection across studies. Zhou et al. (32) predicted acute ischemic stroke outcomes by blending radiomic features from multimodal imaging with clinical factors. Their clinical-radiomic nomogram showed superior ROC AUCs in both training and validation groups, achieving 0.868 and 0.890, respectively, surpassing models based solely on clinical or radiomic data. Despite its utility, this study’s single-center nature and restriction to DWI and ADC sequences without considering other modalities may introduce biases.
Future research should aim for broader and more diverse datasets, implement stringent study methodologies, and address potential biases to solidify the reliability and applicability of predictive models. Including data from multiple centers and varied patient demographics will strengthen the external validity of research outcomes, paving the way for advancements in personalized stroke management.
Diverging from prior research, our study utilized extensive datasets and integrated radiomic features from multimodal MRI (T1w, T2w, DWI) with clinical risk factors to develop a clinical-radiomic model to forecast the prognosis of acute ischemic stroke. This model exhibited enhanced performance in the validation set, outperforming individual imaging features or clinical factors in discriminative capacity, calibration, and clinical applicability. The model’s performance metrics in the training set were noteworthy: sensitivity reached 0.951, specificity 0.811, and accuracy 0.903. Furthermore, in the validation set, sensitivity was 0.918, specificity 0.681, and accuracy 0.825. Despite some variability in these metrics, our model consistently offers valuable insights for clinicians in making informed decisions regarding the treatment and prognosis of acute ischemic stroke.
This research leveraged a broad spectrum of imaging modalities for feature extraction, including T1w, T2w, and both raw and processed DWI images. Twenty-one radiomic features were extracted, covering dimensions such as shape, energy, texture, and various gray-level matrices (GLCM, GLRLM, GLSZM, GLDM). These features provide a comprehensive portrayal of ischemic stroke heterogeneity, offering a sophisticated understanding of stroke pathology. Notably, elevated values in these feature analyses correlate with poorer patient outcomes, enabling precise and quantifiable assessments of imaging characteristics linked to ischemic stroke prognosis.
It’s worth noting that our study revealed multivariate logistic regression analysis revealed coronary heart disease, uric acid levels, blood glucose levels, homocysteine, and the platelet-to-lymphocyte ratio (PLR) as independent risk factors affecting stroke prognosis, which align with clinical realities.
Extensive research supports that high blood glucose levels are an independent risk factor for stroke, increasing susceptibility to ischemic stroke by 2–4 times (33). The strong correlation between type 2 diabetes and stroke risk is well-documented across various studies (33, 34). Effective blood glucose management is crucial for reducing the risk of diabetes and its complications, notably the increased risk of stroke.
Coronary heart disease and ischemic stroke often overlap in their underlying mechanisms, with individuals with coronary heart disease at higher risk of cardiovascular events after a stroke. This interaction can lead to complex multi-organ impairment and hinder optimal recovery. A comprehensive treatment approach is essential to manage these interconnected conditions and improve patient outcomes (35).
Uric acid, a product of purine breakdown, has dual roles: it scavenges free radicals and promotes neuronal glutathione synthesis, offering neuroprotection. Elevated uric acid levels are linked to stroke risk due to its oxidative properties, though it also has neuroprotective effects. Maintaining the balance of uric acid is crucial to managing stroke risk (36).
Elevated homocysteine levels, often associated with deficiencies in folate, vitamin B6, and B12, significantly increase stroke risk. For every 5 umol/L rise in homocysteine levels, the risk of stroke escalates by 95%. Higher levels are also linked to early neurological deterioration and increased risk of stroke recurrence and mortality. Monitoring homocysteine levels is crucial in mitigating stroke risk (37).
The platelet-to-lymphocyte ratio (PLR), derived from platelet and lymphocyte counts, serves as an inflammation marker and prognostic indicator for disease progression (38). PLR has predictive value in various conditions, including cancer. In acute ischemic stroke (AIS), heightened PLR levels correlate with larger infarcts and poorer prognosis. PLR also predicts clinical outcomes at 90 days, making it a valuable prognostic biomarker in AIS scenarios (39).
5 LimitationWhile this research offers promising insights, it is imperative to recognize its limitations. The study’s retrospective design introduces the potential for selection bias and the influence of confounding variables. Furthermore, the study is constrained by a relatively small participant pool and a limited scope for external validation. Future research would benefit from prospective, multicenter studies encompassing larger cohorts and broader validation efforts to bolster the findings’ robustness. Additionally, this investigation’s treatment of ischemic stroke etiology, especially concerning cerebral infarction locations, lacks granularity. The reliance on manual delineation for regions of interest (ROI) could introduce subjectivity; thus, subsequent studies might enhance accuracy by adopting advanced software solutions, refining training protocols, and minimizing subjective bias. The inconsistency in standard head MRI sequences across various institutions and divergent institutional protocols posed challenges in evaluating a comprehensive range of MRI sequences. Future endeavors should focus on improving patient engagement, augmenting the collection of multimodal MRI data (e.g., FLAIR, ADC, SWAN), and constructing sophisticated models to overcome these limitations. Importantly, the initial study cohort was composed exclusively of patients who did not receive ischemic reperfusion therapy. Comparative analyses of patients undergoing ischemic reperfusion therapy vs. those who do not could provide valuable insights into the predictive utility of radiological markers and clinical indicators in treated acute ischemic stroke patients.
6 ConclusionIn conclusion, this study demonstrates that the incorporation of radiomic features from T1-weighted (T1w), T2-weighted (T2w), and diffusion-weighted imaging (DWI) with clinical parameters into a cohesive clinical-radiomics model significantly improves the predictive accuracy for the prognosis and treatment responses in ischemic stroke. This advancement fosters a deeper comprehension of therapeutic impacts, prognostic evaluations, and clinical assessments, offering invaluable insights for medical professionals in diagnosis, therapeutic intervention, and rehabilitation. The synergy between radiomic attributes and clinical information heralds a promising avenue for enhancing personalized medicine strategies and elevating patient care outcomes in ischemic stroke.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving human participants were reviewed and approved by the Institutional Review Board at Xi’an Central Hospital and Tongchuan Mining Bureau Hospital. Written informed consent was not required due to the retrospective nature of the study.
Author contributionsKG: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Project administration, Resources, Software, Validation, Visualization. BZ: Data curation, Writing – review & editing. RL: Data curation, Investigation, Writing – review & editing. JX: Data curation, Investigation, Writing – review & editing. QW: Methodology, Software, Visualization, Writing – review & editing. KC: Data curation, Investigation, Writing – review & editing. YS: Data curation, Investigation, Writing – review & editing. JL: Investigation, Writing – review & editing, Project administration, Supervision. WC: Supervision, Writing – review & editing, Project administration. ZL: Project administration, Supervision, Writing – review & editing. ZD: Writing – review & editing, Funding acquisition. NG: Writing – original draft, Writing – review & editing, Funding acquisition.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received financial support from the Natural Science Foundation of Shaanxi Province (Project Code: 2021JM562), the Shaanxi Provincial Science and Technology Plan Project (2022SF-418), the Science and Technology Planning Project of Xi’an City (Project Code: 2023YXYJ0036), and the Xi’an Central Hospital Scientific Research Fund (2024YB04).
AcknowledgmentsThe authors express our gratitude to all individuals involved in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be perceived as potential conflicts of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
Crossref Full Text | Google Scholar
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 333:1581–7. doi: 10.1056/NEJM199512143332401
Crossref Full Text | Google Scholar
3. Hacke, W, Kaste, M, Bluhmki, E, Brozman, M, Dávalos, A, Guidetti, D, et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med Overseas Ed. 359:1317–29. doi: 10.1056/NEJMoa0804656
Crossref Full Text | Google Scholar
4. Bracard, S, Ducrocq, X, Mas, JL, Soudant, M, Oppenheim, C, Moulin, T, et al. Investigators T: mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. (2016) 15:1138–47. doi: 10.1016/S1474-4422(16)30177-6
PubMed Abstract | Crossref Full Text | Google Scholar
5. Yao, M, Ren, Y, Jia, Y, Xu, J, Wang, Y, Zou, K, et al. Projected burden of stroke in China through 2050. Chin Med J. (2023) 136:1598–605. doi: 10.1097/CM9.0000000000002060
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wang, YJ, Li, ZX, Gu, HQ, Zhai, Y, Jiang, Y, Zhao, XQ, et al. China stroke statistics 2019: a report from the National Center for healthcare quality Management in Neurological Diseases, China National Clinical Research Center for neurological Diseases, the Chinese Stroke Association, National Center for chronic and non-communicable disease control and prevention, Chinese Center for Disease Control and Prevention and Institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol. (2020) 5:211–39. doi: 10.1136/svn-2020-000457
PubMed Abstract | Crossref Full Text | Google Scholar
7. Eslami, V, Tahsili-Fahadan, P, Rivera-Lara, L, Gandhi, D, Ali, H, Parry-Jones, A, et al. Influence of intracerebral hemorrhage location on outcomes in patients with severe intraventricular hemorrhage. Stroke. (2019) 50:1688–95. doi: 10.1161/STROKEAHA.118.024187
留言 (0)