Intestinal mucosal barrier integrity is essential for maintaining intestinal health and preventing related diseases. However, in Antibiotic-Associated Colitis (AAC), the antibiotic-induced microecological imbalance not only causes direct damage to the intestinal mucosa, but also impairs the intestinal barrier function, increasing its vulnerability to harmful external stimuli (Xu et al., 2023). In this study, we aim to investigate the function and mechanism of Zinc Gluconate (ZG) on intestinal mucosal barrier damage using a composite model of the dual induction of antibiotics and Lipopolysaccharide (LPS)-induced intestinal mucosal barrier injury. We also examined the effect of ZG on intestinal flora.
Antibiotics significantly impact the microbial population in the gut, reducing the abundance of beneficial bacterial, whereas increasing the abundance of harmful bacteria. The microecological imbalance disrupts the mucosal barrier, as evidenced by increased mucosal barrier permeability, aberrant expression of mucosal barrier proteins, and mucosal barrier cell apoptosis. Furthermore, the disruption lowers the intestinal mucosal barrier resistance to external pathogenic factors, creating a potential pathway for AAC (Kaminsky et al., 2021; Xu et al., 2023). Antibiotics induce a microbial imbalance, which, in turn, disrupts the mechanical barrier, significantly lowering the gut mucosa’s resistance to reinfection by Gram-negative bacteria. Specifically, antibiotic-induced changes in the microbial community severely impaired the biological barrier function, resulting in reduced mucus production and secretion of antimicrobial peptides (Kwon et al., 2022; Li X. et al., 2023). This impact led to an increased susceptibility to reinfection with Gram-negative bacteria, offering novel insights into the mechanisms underlying antibiotic-induced mucosal barrier damage. Furthermore, LPS exposure could cause increased permeability and disruption of the integrity of the gastrointestinal mucosal epithelial Tight Junction (TJ). This process allows endotoxins to infiltrate the gut via paracellular osmosis, causing endotoxin translocation and aberrant cytokine expression, thereby worsening intestinal inflammation and injury (Zhang and Li, 2012; Zhao et al., 2023). Therefore, antibiotic-induced microecological dysregulation may result in Gram-negative bacteria-related infections.
In our previous research, we determined ZG’s median lethal dose (LD50) and evaluated its safety on mice under different Sub-Lethal Doses (SLD) (Wang et al., 2024). Our findings (from the aforementioned previous experiments) provided the basis for selecting different ZG doses in this study. As a supplementary form of zinc, ZG plays a unique role in maintaining intestinal mucosal barrier integrity, regulating intestinal flora, and suppressing inflammatory responses (Lai et al., 2016). According to research, ZG can maintain intestinal mucosal barrier integrity and repair related injuries by promoting intestinal mucosal cell regeneration and regulating the expression of mucosal barrier-associated proteins (He et al., 2019). Additionally, ZG could exert antioxidant and anti-inflammatory effects (Lang et al., 2022). Furthermore, ZG could impact the richness and diversity of intestinal microorganisms, as well as the relative proportion of beneficial and harmful bacteria, via multiple channels, such as immune response regulation and production of antibacterial substances, thereby influencing the repair of intestinal mucosal barrier damage (Li et al., 2021; Samuelson et al., 2022). Studies have shown that zinc repairs intestinal mucosal damage by promoting intestinal tissue repair mechanisms, mitigating inflammation, and restructuring the gut microbiota to regulate mucosal integrity via modulating the NF-κB signaling pathway (Yang et al., 2020; Samuelson et al., 2022).
Previous studies on the repair of intestinal mucosal barrier damage were primarily based on a specific disease model and a single causative factor. In this regard, there are many unanswered questions regarding the mechanism underlying intestinal mucosal barrier damage, as well as the potential impact of treatment strategies such as the dual administration of antibiotics and LPS, which form the basis and motivation of this study. Herein, we simulated the actual disease situation by establishing a composite model of the dual induction of antibiotics and LPS-induced intestinal mucosal barrier damage in mice. Furthermore, we administered different ZG treatments to determine the optimal ZG dose and its efficacy in intestinal mucosal damage repair. In addition to elucidating the mechanism underlying intestinal barrier disorders, this study also provides new ideas and strategies for treating other related illnesses.
2 Materials and methods 2.1 Experimental animalsHealthy Specific Pathogen-Free (SPF)-grade male C57BL/6 J mice (aged = 6–8 weeks; average body weight = 20 ± 2 g) were sourced from the Experimental Animal Center of Guangxi Medical University (License number: SCXK Gui 2020-0003). All animal experimental procedures were approved by the Ethics Committee of Guangxi Medical University. One week before the experiment, the animals were acclimatized and housed in a barrier-level animal room with controlled temperature (22–25°C) and relative humidity (50–70%) and a 12 h/12 h light/dark cycle. During the experiment, the mice were fed an SPF-grade growth and reproduction diet and had ad libitum access to food and water.
2.2 Main reagents and drugsZinc gluconate (analytical grade, purity = 98%) was acquired from Macklin (Shanghai, China). Ampicillin (A9518-25G-9), neomycin sulfate (N6386-5G), metronidazole (M1547-25G), gentamicin sulfate (E003632-1 g), vancomycin (1404-93-9) and LPS (O55:B5, L2880-25MG) were all purchased from Sigma-Aldrich (United States, purity > 95%). The electron microscope fixative solution (G1102), Bovine Serum Albumin (BSA, GC305010), and the 4% paraformaldehyde fixative solution were purchased from Servicebio (Wuhan, China).
2.3 Main experimental instrumentsThe main experimental instruments included a high-speed low-temperature tissue grinder (Beijing, China), an ultra-trace Ultraviolet (UV) spectrophotometer (ThermoFisher Scientific, United States), an enzyme marker (Elx-808, Bio-Tek, United States), an electrophoresis and electrostatic transducer (Bio-Rad, USA), a Transmission Electron Microscope (TEM; HT7800, Hitachi), a dual-color infrared imaging system (ODYSSEY, Licor), an optical microscope (BX53F + DP73, OLYMPUS, Japan), and a 7,500 Real-Time Fluorescence Polymerase Chain Reaction (RT-PCR) Instrument (Applied Biosystems, United States).
2.4 Preparation of the antibiotic mixtureIn our previous study, we successfully constructed an AAC mouse model via the Antibiotic cocktail (ABX) gavage approach (Yang et al., 2022). Herein, the ABX formulation scheme was as previously described (Hill et al., 2010; Liu J. et al., 2023). Specific ingredients included ampicillin (1 mg/mL), metronidazole (1 mg/mL), gentamicin (1 mg/mL), neomycin sulfate (1 mg/mL), and vancomycin hydrochloride (0.5 mg/ mL).
2.5 Preparation of the LPS solutionThe LPS-induced intestinal infection was defined as in previous literature (Ding et al., 2004). The specific preparation approach was as follows. First, 25 mg LPS crystals were dissolved fully in 5 mL sterile physiological saline to obtain a stock solution with a concentration of 5 mg/mL. Subsequently, the solution was stored separately in a −20°C refrigerator in the dark. During the experiment, the appropriate amount of the stock solution was suctioned and diluted to obtain a final concentration of 0.5 mg/mL.
2.6 Preparation of the ZG solutionHerein, the ZG solution was prepared as previously described (Lai et al., 2016; Hsieh et al., 2017). Briefly, ZG was dissolved in ultrapure sterile water to obtain the Zn(C6H1107)2 stock solution (with a concentration of 9.11 mg/mL). The stock solution was then sterilized using a 0.22 μm filter and stored in the dark at 4°C. To ensure mice from each dosage group received the same volume of the ZG solution, experimental concentrations were obtained with an equal-volume dilution of the stock solution. We previously determined the median lethal dose (LD50) of ZG and evaluated its safety in mice (Wang et al., 2024). Herein, 0.57 mg/mL, 1.14 mg/mL, and 3.42 mg/mL were selected as the low, medium, and high dose concentrations of the ZG solution, respectively.
2.7 Experimental grouping and the dosing regimenWe sourced 30 SPF-grade C57BL/6 J male mice and then acclimatized and fed them for 1 week before the experiment. The animals were randomly divided into five groups (N = 6): Normal Control (NC), model control (NS + ABX/LPS), low-dose ZG (ZG(L) + ABX/LPS), medium-dose ZG (ZG(M) + ABX/LPS), and high-dose ZG (ZG(H) + ABX/LPS). The NC group was left untreated throughout the experiment but received a normal diet and sufficient water. The remaining four groups were first subjected to continuous ABX gavage for 7 days (twice/day) to create an AAC mouse model. On day eight, LPS (5 mg/kg) was intraperitoneally injected to further induce the infection and exacerbate intestinal mucosal injury. Following that, on days 9, 11, and 13, the NS + ABX/LPS group received a tail vein injection of 0.9% Normal Saline (NS), while the ZG(L) + ABX/LPS, ZG(M) + ABX/LPS, and ZG(H) + ABX/LPS groups received tail vein injections of low (5.13 mg/kg), medium (10.26 mg/kg), and high (30.78 mg/kg) doses of the ZG solution, respectively.
The injection doses of ZG and NS were both set at 9 mL/kg but were adjusted per body weight changes in experimental mice on each day as the study progressed. Mice in all groups received adequate food and water. The body weights of mice were determined and recorded on days 1, 4, 8, 9, 10, 12, and 14. Furthermore, diarrhea was assessed in mice at fixed time points each day after ABX gavage (days 4 and 8), after LPS administration (day 9), and after ZG treatments (days 10, 12, and 14). Figure 1A shows the study program and design.
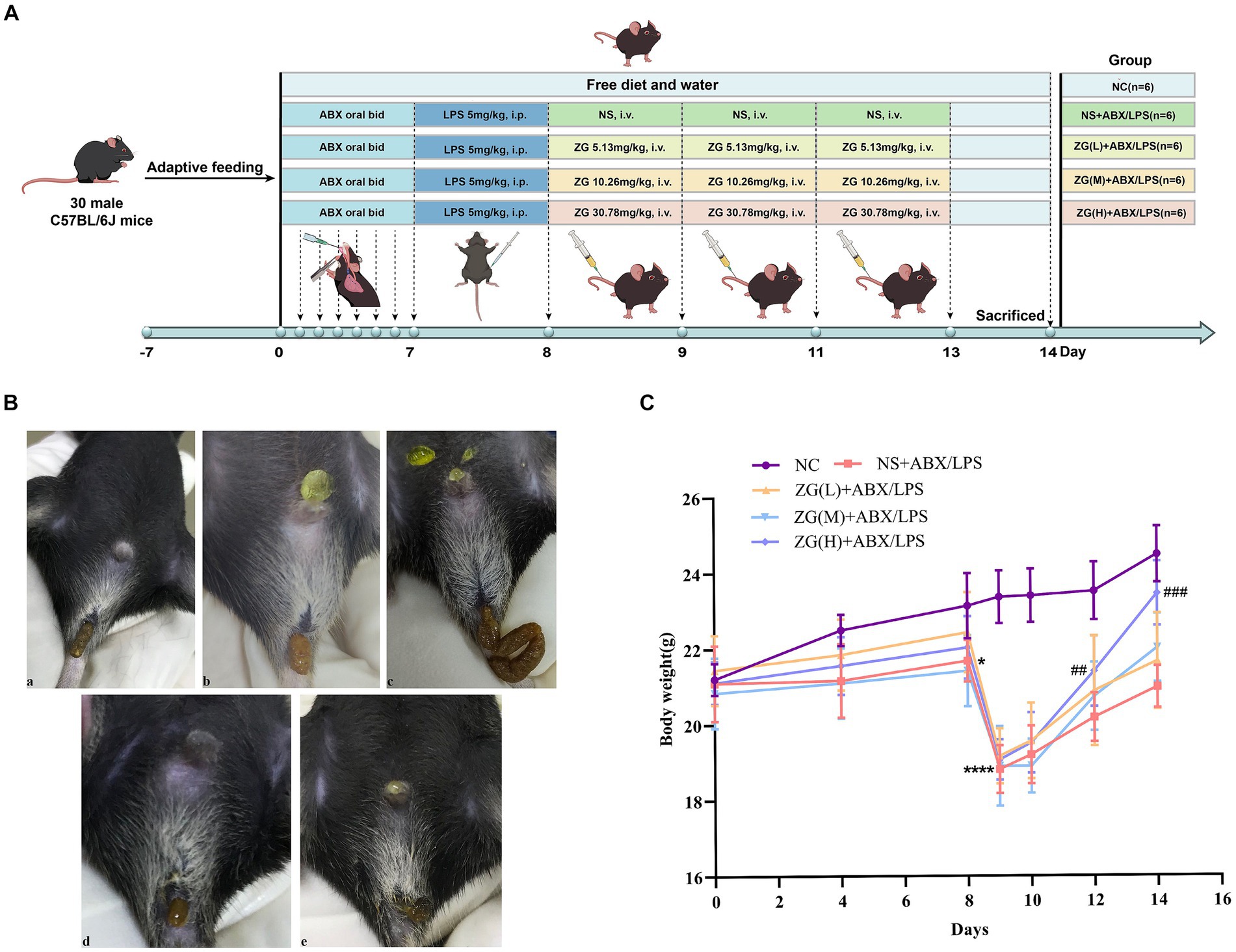
Figure 1. Experimental design protocol and changes in feces and body weight of mice during the experiment. (A) Animal experiment protocol and design. (B) Changes in feces of mice: (a) normal stool (b) Wet stools (c) Pasty stools (d) Semiliquid stools (e) Watery diarrhea. (C) Changes in body weight of mice. Compared with group NC: *p < 0.05, ****p < 0.0001; Compared with group NS + ABX/LPS: ##p < 0.01, ###p < 0.001.
2.8 Sample collectionOn day 14, the animals were anesthetized via inhalation of 1.5% isoflurane. Following that, blood was collected by removing the eyeballs, separating the serum, and storing it at −80°C. Subsequently, the mice were euthanized via cervical dislocation. The intestinal segment 5 cm proximal to the ileocecal junction was dissected and cut into ~1–2 cm sample tissues. All intestinal contents were then gently washed with pre-cooled physiological saline, and a portion of the sample tissue was fixed in a 4% paraformaldehyde solution for Hematoxylin & Eosin (H&E) staining and Immunohistochemistry (IHC) analysis. Another portion was immersed in the electron microscope fixative solution for TEM observation. The remaining sample tissues were labeled and stored in portions at −80°C for Western Blotting (WB) and RT-PCR assays. Furthermore, the contents of the cecum were collected and immersed in liquid nitrogen for gut microbiological testing.
2.9 Ileum histopathologyIleum specimens were obtained, routinely dehydrated, paraffin-embedded, sliced into 5 μm sections, deparaffinized with xylene, dehydrated with gradient alcohol, and subjected to H&E staining. The morphology of the intestinal tissues was then observed and photographed using a light microscope. The height of five intact villi (villus length, V) and the depth of five crypts (crypt depth, C) in each section were measured separately, and then the data were recorded. Following that, the chorionic ratio [Villous to Capillary Ratio (VCR or V/C)] was determined.
2.10 Transmission electron microscopyIleal specimens were obtained, sliced into 1 mm × 1 mm × 1 mm tissue blocks, rinsed and fixed in 0.1 M Phosphate Buffered Saline (PBS; PH7.4), dehydrated in gradient alcohol, osmotically embedded, polymerized, and ultra-thinly sliced (60–80 nm). Following that, the slices were stained with alcohol-saturated 2% uranyl acetate and 2.6% lead citrate solution and then visualized and photographed using TEM.
2.11 ImmunohistochemistryThe paraffinized sections were recovered through heating to 98°C in 10 mM citrate buffer (PH 6. 0) for 10 min. Endogenous peroxidase was blocked with 10% (v/v) H2O2 for 30 min, while nonspecific antigens were blocked with serum at Room Temperature (RT) (20–25°C) for 30 min. The paraffinized sections were then incubated with rabbit anti-JAMA (1:400; GB111265-100; Servicebio, Wuhan, China) and rabbit anti-MLCK (1:400; GB113358-100; Servicebio, Wuhan, China) primary antibodies at 4°C overnight and then treated with Horseradish Peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibodies (1:200; GB23303; Servicebio, Wuhan, China). Photographic images were captured and analyzed using an OLYMPUS microscope and Image-Pro Plus 6.0 software, respectively.
2.12 Real-time quantitative polymerase chain reactionTotal RNA was extracted from ~30 mg of colon tissue using NucleoZol reagent (740404.200, Gene Co. Ltd). Subsequently, RNA was quantified using a spectrophotometer at a wavelength of 260 nm. Complementary DNA (cDNA) was obtained via reverse transcription using the HiScript III RT SuperMix for qPCR (+gDNA wiper) Kit (R323-01, Vazyme, Nanjing, China). The ChamQ Universal SYBR qPCR Master Mix Kit (Q711-03, Vazyme, Nanjing, China) was used to perform qPCR on the ABI 7500 system (Applied Biosystems, CA, United States). Sangon Biotech Co. Ltd. (Shanghai) synthesized the primers used herein (Supplementary Table S1). The RT-QPCR data were analyzed using the 2−ΔΔCT method, with the GAPDH gene expression as the endogenous control.
2.13 Western blottingTotal protein was isolated from the ileum and then quantified using the BCA Protein Assay kit. Protein (20 μg) from different samples was separated using 8 ∼ 12% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) (Supplementary Table S2) and then transferred to Polyvinylidene Difluoride (PVDF) membranes. Supplementary Table S3 details the WB-related gene transfer conditions. The membranes were blocked with 5% skimmed milk for 4 h at RT and then probed overnight at 4°C with primary antibodies, including rabbit anti-ZO-1 (1:800, ab96587, Abcam), rabbit anti-Occludin [1:1,000, Cat #91131, Cell Signaling Technology (CST)], rabbit anti-Claudin-1 (1:1,500, AF0127, Affinity), rabbit anti-TLR4 (1:1,500, AF7017, Affinity), rabbit anti-NF-κB/p65 (1:2,000, Cat #8242, CST), and rabbit anti- β-actin (1:2,500, Cat #4970, CST), which was used as the loading control. Subsequently, the membranes were incubated with a goat anti-rabbit HRP-linked secondary antibody (1:10,000, Cat #bs-0295G-HRP, Bioss antibodies) for 1 h. The membranes were visualized using the Western BrightTM ECL kit (Advansta, CA, United States).
2.14 Enzyme-linked immunosorbent assayThe concentrations of DAO, D-LA, and ET in serum were determined using ELISA kits (FANKEW, Shanghai, China; and Shanghai Kexing Trading Co., Ltd., China) per the manufacturers’ instructions.
2.15 Intestinal flora 16S rRNA gene sequencingGenomic DNA was extracted from the collected samples using the CTAB approach (Yu C. et al., 2023). A Nanodrop 2000 UV–Vis spectrophotometer (Thermofisher Scientific, Wilmington, MA, United States) and 1% agarose gel electrophoresis were used to examine the concentrations and quality of the DNA samples. The forward primer 341F (5′-CCTAYGGGRBGCASCAG-3′) and reverse primer 806R (5′-GGACTACNNGGGTATCTAAT-3′) were designed to amplify the V3 + V4 hypervariable regions of the 16S rDNA gene on a thermocycler PCR system. The PCR products were detected using 2% agarose gel electrophoresis and were gelled and recovered using an AxyPrep DNA gel recovery kit (Axygen Biosciences, Union City, CA, United States). Following quantification and homogenization, the DNA products underwent paired-end sequencing on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, United States). Statistical analyses were performed using R (Version 2.15.3). Mice ileum contents were obtained via 16S rRNA high-throughput sequencing, commissioned by Wekemo Tech Group Co., Ltd. (Shenzhen, China).
2.16 Statistical analysisData were presented as Mean ± Standard Error of the Mean (mean ± SEM). The data were first collated using Excel 2019. One-way ANOVA was performed using SPSS27.0 software, and the Tukey method (for normally distributed data with equal variances) and the Kruskal-Wallis test (Dunn’s method; for non-normally distributed data) were used for multiple pairwise comparisons. The test value was set to p = 0.05, and results or differences with p < 0.05 were considered statistically significant. Drawings were generated using GraphPad Prism 9.0 software.
3 Results 3.1 Body weight changes in miceSupplementary Table S4 and Figure 1C show the changes in body weight in mice during the experiment. No statistically significant difference (F = 0.29, p = 0.884) was found in baseline body weight (day 0) of mice in each group, indicating that they were comparable across all five groups. As the experiment progressed, the weight of mice in the NC group exhibited a continuous growth trend. In contrast, the overall trend of weight changes in the other four groups remained basically the same, although a decrease was noted on day 4, which later gradually increased to the baseline level by day 8. Furthermore, a linear decline was found on day 9, while a gradual increase was noted from day 10 onwards. Notably, the NC group exhibited a higher final body weight (day 14) compared with the other four groups.
On day 4, there was no significant change in body weight in the NS + ABX/LPS group compared with the NC group (p > 0.05). However, on day 8, the NS + ABX/LPS group exhibited a significant decrease in body weight compared with the NC group (p < 0.05), with a more significant reduction found on Day 9 (p < 0.0001). On day 10, no significant increase in the body weight of mice was noted in all the ZG treatment groups compared with the NS + ABX/LPS group (p > 0.05). Furthermore, on days 12 and 14, the ZG(H) + ABX/LPS group exhibited a significant increase in body weight compared with the NS + ABX/LPS group (p < 0.01 or p < 0.001). These results suggested that the concurrent induction of antibiotics and LPS-induced intestinal barrier damage had a substantial negative impact on the body weight of mice, representing an effect that was effectively reversed by ZG treatment.
3.2 Changes in mice fecesFigure 1B and Table 1 illustrate the changes in mice feces and diarrhea scores during the experiment, respectively. The NC group exhibited normal feces throughout the experiment. On the other hand, the NS + ABX/LPS group developed diarrhea from day 4 onwards, which manifested as a significant increase in diarrhea scores as the study progressed (p < 0.05). On the other hand, the ZG treatment group exhibited a significant reduction in diarrhea scores as the study progressed (p < 0.05).
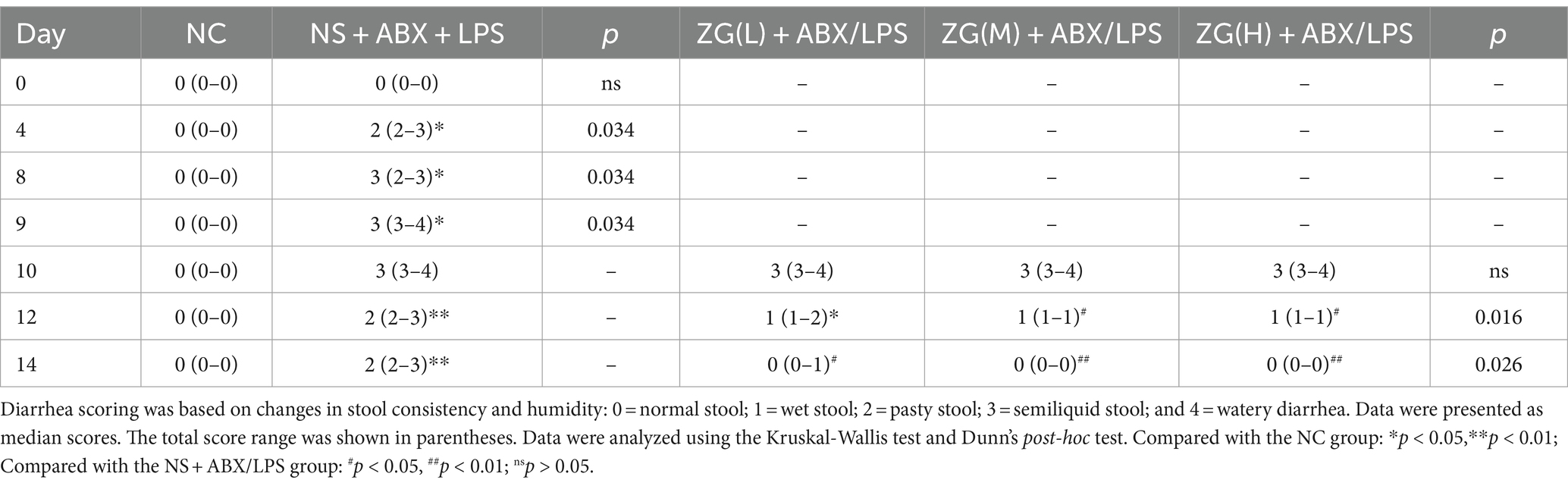
Table 1. ZG reduced diarrhea scores in mice with the dual induction of antibiotics and LPS-induced diarrhea.
On day 10, no significant difference in diarrhea scores was identified between the ZG treatment group and the NS + ABX/LPS group. On day 12, diarrhea scores in the ZG(M) + ABX/LPS and ZG(H) + ABX/LPS groups significantly decreased (p < 0.05). On day 14, ZG treatment group exhibited significantly lower diarrhea scores (p < 0.05), with some demonstrating even greater reductions (p < 0.01). Compared with the NC group, diarrhea scores were significantly higher in the other four groups on day 10 (p < 0.05). On day 12, diarrhea scores were significantly elevated in the NS + ABX/LPS and ZG(L) + ABX/LPS groups (p < 0.05), and they were also significantly escalated in the NS + ABX/LPS group on day 14 (p < 0.05). Notably, the diarrhea scores in the ZG treatment groups were consistent with those in the NC group on day 14. These findings suggested that the dual induction of antibiotics and LPS-induced intestinal dysfunction caused diarrhea in mice, in which ZG treatment significantly ameliorated. However, the amelioration of diarrheal symptoms exhibited variability across different doses of ZG.
3.3 Pathological and morphological results of mouse ileum tissue 3.3.1 The ileal mucosal integrity of mice in each groupThe structural integrity of the mouse ileal mucosa following HE staining was assessed using a light microscope (Figures 2A–D). In the NC group, mice displayed a typical histological appearance of the ileum, characterized by an intact intestinal mucosa, normal glandular and crypt structures, and absence of epithelial cell shedding. Moreover, the intestinal villi exhibited a dense and well-arranged pattern, primarily composed of a single layer of columnar epithelium and goblet cells.
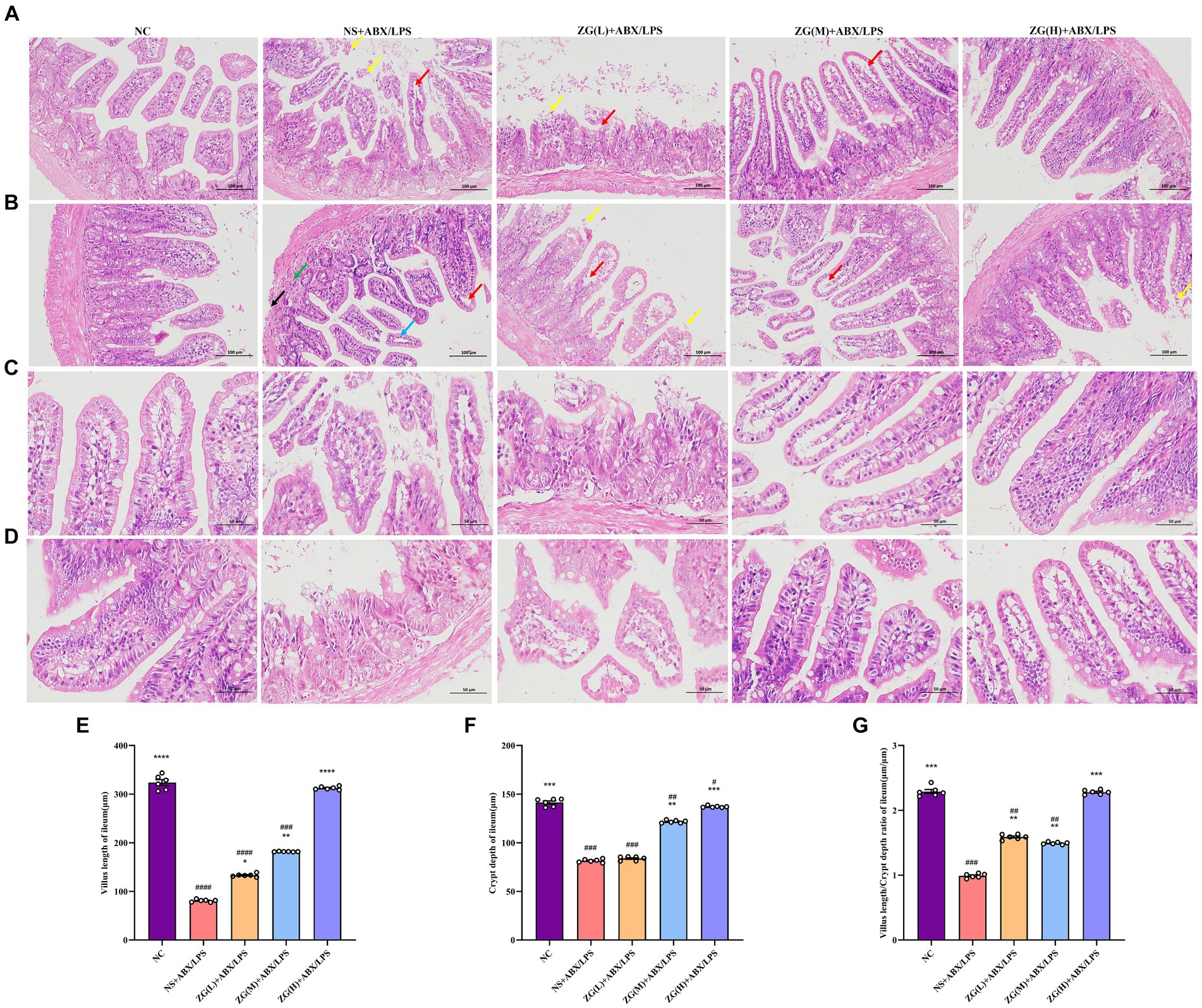
Figure 2. Representative images of histopathological and morphological changes in the ileum of each group of mice (HE staining, A,B: ×200, C,D: ×400). The detachment of intestinal villous epithelium is indicated by yellow arrows, while separation from the lamina propria and widening of the interstitial space are denoted by red arrows. Cytoplasmic laxity of intestinal villous epithelial cells is highlighted by blue arrows, granulocytic infiltration by green arrows, and disruption of the muscularis propria structure by black arrows. (E) Villus length (F) crypt depth (G) VCR. Compared with group NC: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Compared with group NS + ABX/LPS: #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001.
In contrast, NS + ABX/LPS mice showed severe damage to the ileal tissue mucosa, evidenced by significant shedding of intestinal villous epithelial cells (yellow arrow), separation between epithelium and lamina propria with widened gaps (red arrow), and partial loss of crypt structure. Additionally, the intestinal villus epithelial cells displayed a disorganized cytoplasm (blue arrow), accompanied by varying degrees of granulocyte infiltration (green arrow), disruption of the muscle layer structure (black arrow), and depletion of goblet cells. Comparatively, mice in the ZG (L) + ABX/LPS group exhibited a slight improvement in pathological ileum damage, accompanying by persistent separation of epithelium from lamina propria and widened gaps (red arrows). Moreover, partial detachment of intestinal villus epithelial cells (yellow arrows), loss of crypt architecture, and goblet cell depletion were observed. In the ZG(M) + ABX/LPS and ZG(H) + ABX/LPS groups, significant alleviation of pathological ileum damage was noted, which was characterized by the increased height of intestinal villi and depth of crypts. Occasional separation of epithelium from lamina propria (red arrow) and goblet cell proliferation were also evident.
3.3.2 The villus height, crypt depth, and VCR of mice in each groupThe height of the ileal villi, depth of crypts, and VCR values were further determined and analyzed in each group of mice under a light microscope (Figures 2E–G). Compared to the NC group, NS + ABX/LPS mice exhibited a significant decrease in ileal villus length, crypt depth, and VCR (p < 0.001), with certain animals showing even more remarkable reductions (p < 0.0001). Moreover, compared to the NS + ABX/LPS group, mice in the ZG treatment group exhibited significantly elevated ileal villus length, crypt depth, and VCR (p < 0.01), with some demonstrating even greater enhancements (p < 0.0001).
3.4 TEM observations of the mouse ileumUltrastructural changes in the mouse ileum tissue were observed under a TEM (Figure 3). Ileal IECs of NC mice were columnar, with a long, tightly arranged and neatly distributed microvilli morphology. Other notable features encompassed precisely delineated TJ and intermediate junction structures, as well as the presence of bridging granule formations. Furthermore, cellular gaps were tightly packed, with clearly defined epithelial cell membranes and nuclear membranes. The cell nuclei were regular, with homogeneous cytoplasmic distribution and scattered organelles. Additionally, mitochondria, rough endoplasmic reticulum (RER), and lysosomes were visible, with intact membrane structures. Conversely, the IECs of NS + ABX/LPS mice exhibited cone-shaped morphology, with numerous microvilli shedding and disappearing. Additionally, TJs, intermediate junctions, and desmosome structures were absent, accompanied by widened intercellular spaces, partial damage and dissolution of epithelial cell membranes, and decreased cytoplasmic density. Furthermore, the nucleus was sunken (with an abnormal shape), the mitochondria were swollen, the membrane structure was blurred, the matrix was partially dissolved and cavitated, and the cristae were broken and invisible. Additionally, the RER expanded, with ribosomes sparsely distributed on the endoplasmic reticulum (ER), and lysosomes were visible. Compared with the NS + ABX/LPS group, with the increase of the ZG treatment dose, the morphology of IECs was gradually improved, the microvilli were arranged neatly, the ileum structure was gradually restored, and the epithelial cell membrane gradually became intact. Furthermore, organelle structures, such as the nucleus, mitochondria, and RER were gradually improved. These findings demonstrated that ZG exerted a therapeutic effect on the dual induction of antibiotics and LPS-induced intestinal mucosal damage.
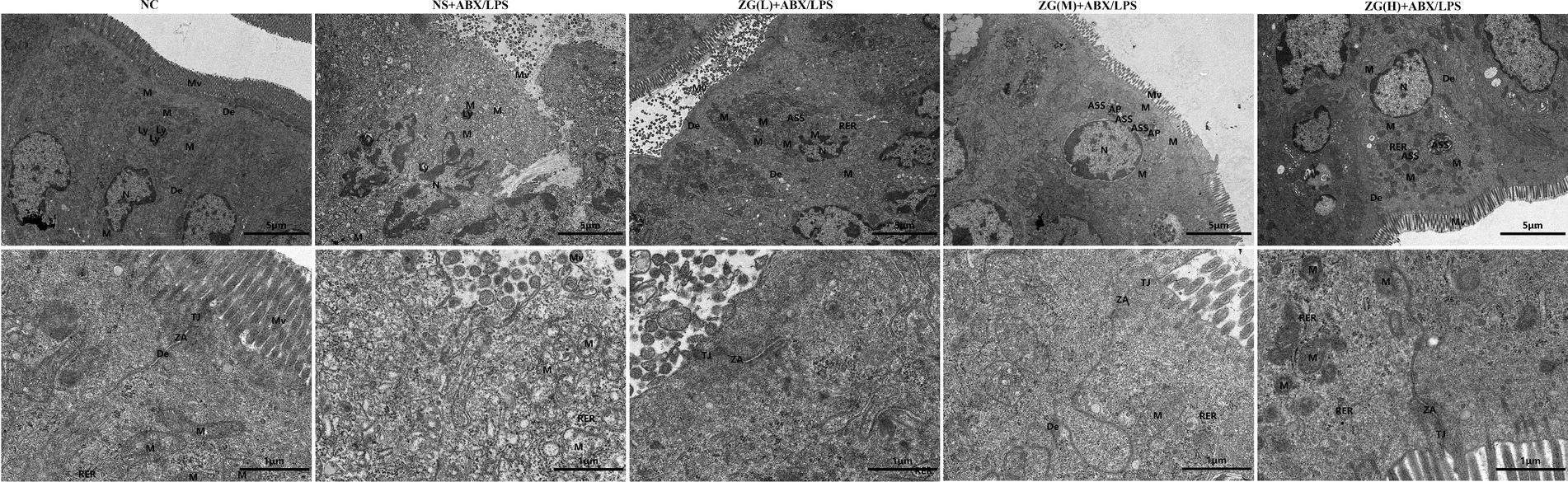
Figure 3. Representative images of ultrastructural changes of ileum in mice of each group (×8,000). Mv, Microvilli; TJ, Tight junction; ZA, Zonula Adherens; De, Desmosome; N, Nuclear; M, Mitochondria; Ly, Lysosome; ASS, Autolysosome; AP, Autophagosome; RER, Rough endoplasmic reticulum.
3.5 The effects of ZG on TJ-related gene and protein expression levels in IECs 3.5.1 ZO-1 gene and protein expression levelsZonula Occludens-1 (ZO-1) is a TJ protein belonging to the MAGUK protein family. It is essential for maintaining and regulating intracellular TJ formation and function (Yu S. et al., 2023). According to the results of RT-PCR (Figure 4B), the ileal mucosa of NS + ABX/LPS mice exhibited a significantly lower ZO-1 mRNA expression level compared with that of NC mice (p < 0.0001). On the other hand, compared with the NS + ABX/LPS group, ZO-1 mRNA expression level was significantly higher in the ZG treatment group (p < 0.01), with some demonstrating even a higher expression level (p < 0.0001).
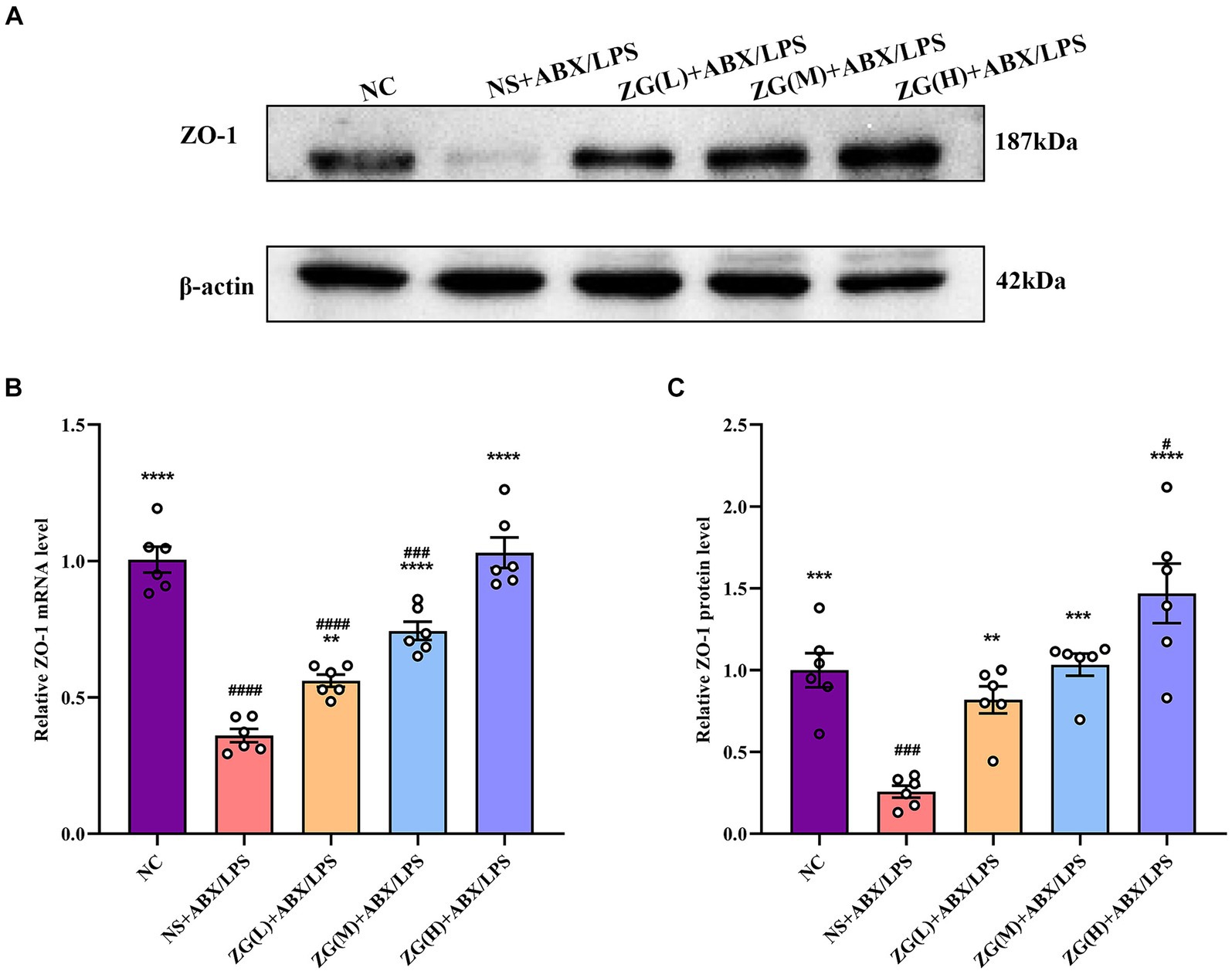
Figure 4. ZO-1 mRNA and protein relative expression levels in the ileum of mice in each group. Compared with group NC: **p < 0.01, ***p < 0.001, ****p < 0.0001; Compared with group NS + ABX/LPS: #p < 0.05, ###p < 0.001,####p < 0.0001. (A-C) The protein expression of tight junction protein (ZO-1) of ileum tissue in each group. (B) The mRNA expression of tight junction protein (ZO-1) of ileum tissues in each group.
According to the results of WB (Figures 4A,C), the ZO-1 protein expression level was significantly lower in the NS + ABX/LPS group than that in the NC group (p < 0.001). Furthermore, compared with the NS + ABX/LPS group, the ZO-1 protein expression level was significantly higher in the ZG treatment group (p < 0.001), with some demonstrating even a higher expression level (p < 0.0001). Notably, ZG(H) + ABX/LPS exerted the greatest promotional effect on ZO-1 expression level. These findings suggested that the dual induction of antibiotics and LPS-induced mouse ileal mucosal injury significantly downregulated the ZO-1 gene expression level, impairing mucosal barrier function. On the other hand, different doses of the ZG treatment could significantly upregulate the ZO-1 gene expression level, especially at high doses (ZG(H) + ABX/LPS), indicating that ZG could exert a dose-dependent positive effect regarding the repair and enhancement of mucosal barrier function.
3.5.2 Occludin gene and protein expression levelsOccludin, a crucial protein in intracellular connections, plays a critical role in their formation and stability. This gene facilitates the formation of TJs by interacting with other intracellular junction proteins, such as Claudin and ZO-1, thereby promoting the close alignment of IECs and minimizing gaps, ultimately enhancing intestinal barrier integrity (Suzuki, 2020; Kuo et al., 2022). According to the results of RT-PCR (Figure 5B), the ileal mucosal Occludin mRNA expression level was significantly lower in the NS + ABX/LPS group than that in the NC group (p < 0.0001). Furthermore, compared with the NS + ABX/LPS group, a significantly higher Occludin mRNA expression level was identified in the ZG(H) + ABX/LPS group (p < 0.01).
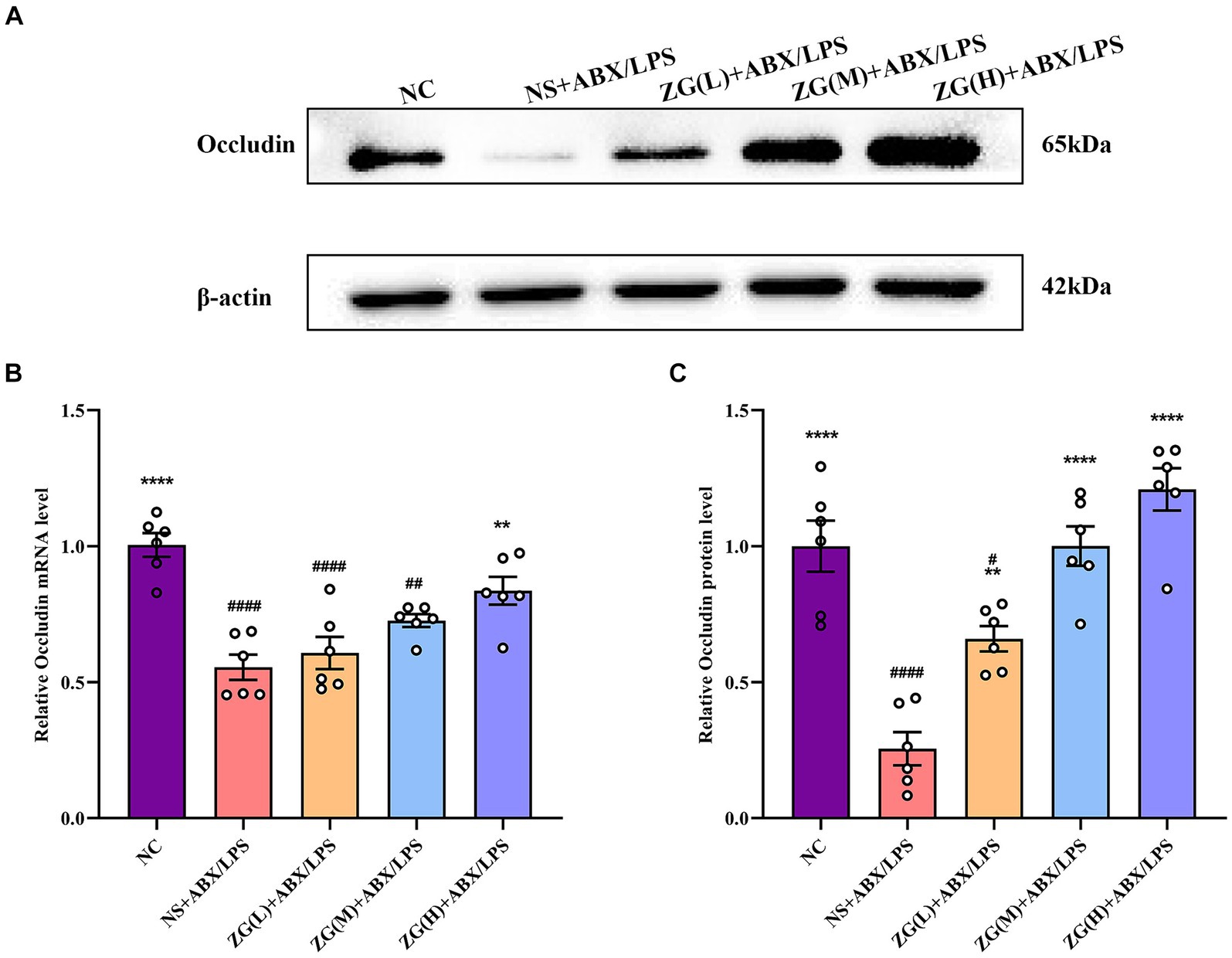
Figure 5. Relative expression levels of Occludin mRNA and protein in the ileum of mice in each experimental group. Compared with group NC: **p < 0.01, ****p < 0.0001; Compared with group NS + ABX/LPS: #p < 0.05, ##p < 0.01,####p < 0.0001. (A-C) The protein expression of tight junction protein (Occludin) of ileum tissue in each group. (B) The mRNA expression of tight junction protein (Occludin) of ileum tissues in each group.
According to WB results (Figures 5A,C), Occludin protein expression level was significantly lower in the NS + ABX/LPS group than that in the NC group (p < 0.0001). Furthermore, compared with the NS + ABX/LPS group, the ZG treatment group exhibited a significantly higher Occludin protein expression level (p < 0.01), suggesting that ZG treatment has a beneficial effect on maintaining or restoring the integrity of the intestinal barrier, with some demonstrating even a higher expression level (p < 0.0001). Higher levels of Occludin indicate improved tight junction integrity, which can help prevent the passage of harmful substances from the gut lumen into the bloodstream, thereby protecting against intestinal barrier dysfunction and associated inflammatory responses. Notably, the ZG(M) + ABX/LPS and ZG(H) + ABX/LPS treatments exerted the greatest promotional effects on Occludin expression level, suggesting a dose-dependent response to ZG treatment. Thus, higher doses of ZG may lead to more pronounced improvements in intestinal barrier function, potentially due to increased bioavailability or enhanced pharmacological effects at higher concentrations. These findings indicate that the dual induction of antibiotics and LPS downregulated Occludin expression level in the ileal mucosa of mice, compromising the intestinal mucosal barrier. On the other hand, ZG treatment (especially the high-dose) significantly upregulated Occludin expression level in the ileal mucosa of mice, potentially contributing to the repair and preservation of the intestinal mucosal barrier.
3.5.3 Claudin-1 gene and protein expression levelsClaudin-1, an important member of the TJ protein family, is abundantly expressed in IECs and is crucially involved in maintaining intestinal barrier integrity (Masterson et al., 2019). According to the RT-PCR results (Figure 6B), ileal mucosal Claudin-1 mRNA expression level was significantly lower in the NS + ABX/LPS group than that in the NC group (p < 0.0001). Furthermore, compared with the NS + ABX/LPS group, the ZG(H) + ABX/LPS group had a significantly higher Claudin-1 mRNA expression level (p < 0.0001). Although the ZG(L) + ABX/LPS and ZG(M) + ABX/LPS groups exhibited a higher Claudin-1 mRNA expression level relative to the NS + ABX/LPS group, no statistically significant difference was noted (p > 0.05).
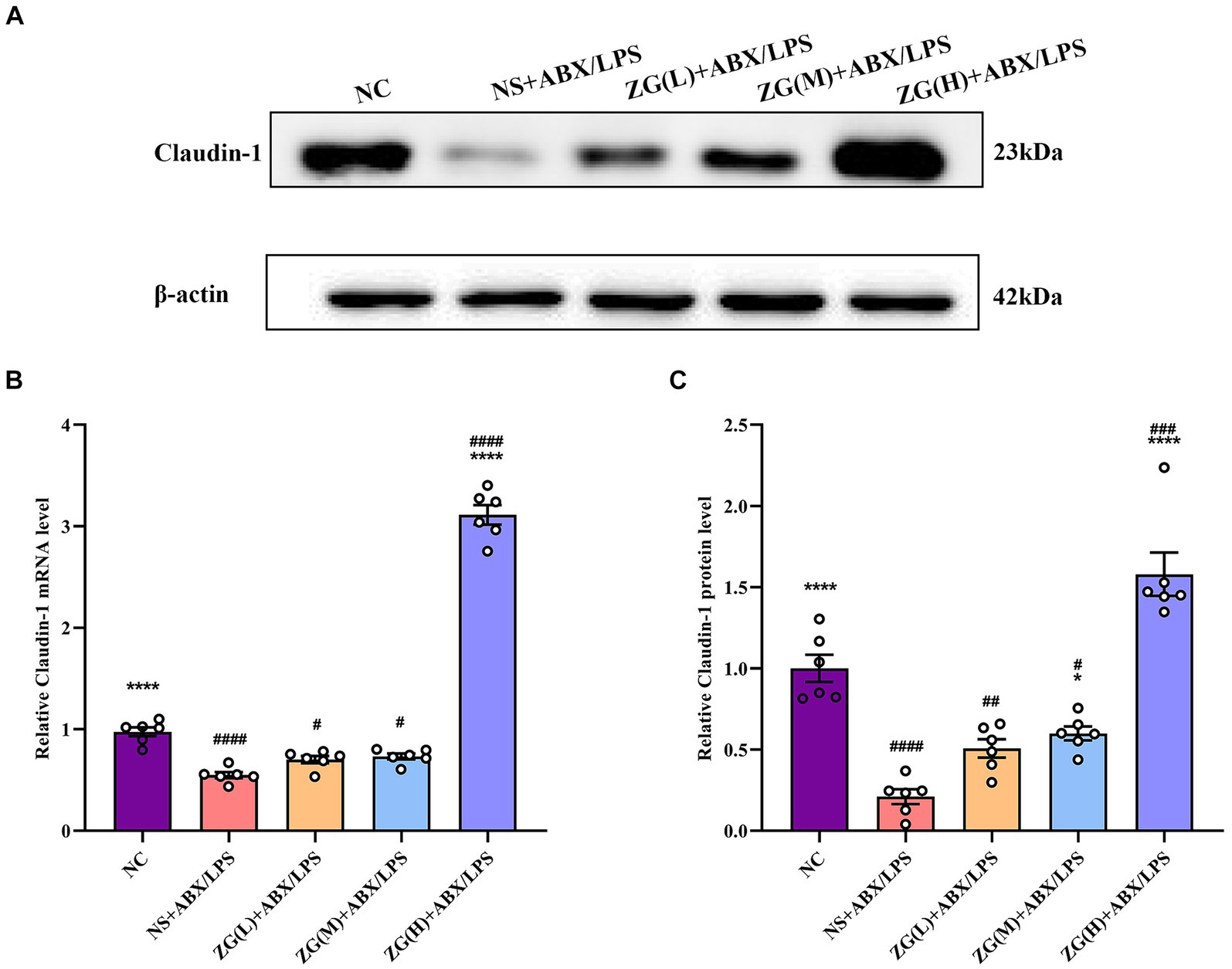
Figure 6. Claudin-1 mRNA and protein relative expression levels in the ileum of mice in each group. Compared with group NC: **p < 0.01, ***p < 0.001, ****p < 0.0001; Compared with group NS + ABX/LPS: #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001.
According to the WB results (Figures 6A,C), Claudin-1 protein expression level was significantly lower in the NS + ABX/LPS group than that in the NC group (p < 0.0001), suggesting impaired tight junction integrity in the intestinal mucosa of mice subjected to dual induction of antibiotics and LPS. Furthermore, compared with the NS + ABX/LPS group, the ZG treatment groups (except ZG(L) + ABX/LPS) exhibited a significantly higher Claudin-1 protein expression level (p < 0.05), with one group demonstrating even a higher expression level (p < 0.0001), highlighting that ZG has a protective effect on tight junction integrity, potentially by promoting the expression level of Claudin-1. Notably, ZG(H) + ABX/LPS group exerted the greatest promotional effect on Claudin-1 mRNA and protein expression levels. The upregulation of Claudin-1 expression by ZG may contribute to the preservation of tight junction structure and function, thereby preventing the leakage of harmful substances across the intestinal epithelium. These findings suggest that the dual induction of antibiotics and LPS severely damaged the structure and function of the intestinal mucosal barrier. However, after ZG treatment, especially with the high-dose (ZG(H) + ABX/LPS), Claudin-1 expression level was significantly upregulated, indicating that ZG promotes the restoration of intestinal barrier function.
3.5.4 Junctional adhesion molecule A assay resultsJAM-A is a transmembrane glycoprotein belonging to the immunoglobulin superfamily that is predominantly found in the TJ of epithelial and endothelial cells. It is critically involved in the assembly and maintenance of the TJ and the establishment of epithelial cell polarity (Czubak-Prowizor et al., 2022). According to the RT-PCR results (Figure 7C), JAM-A mRNA expression level was significantly lower in the ileal mucosa of NS + ABX/LPS mice than that of NC mice (p < 0.01). Furthermore, compared with the NS + ABX/LPS group, ZG treatment groups [except ZG(L) + ABX/LPS] exhibited a significantly higher JAM-A mRNA expression level (p < 0.001), with one group demonstrating even a higher expression level (p < 0.0001).
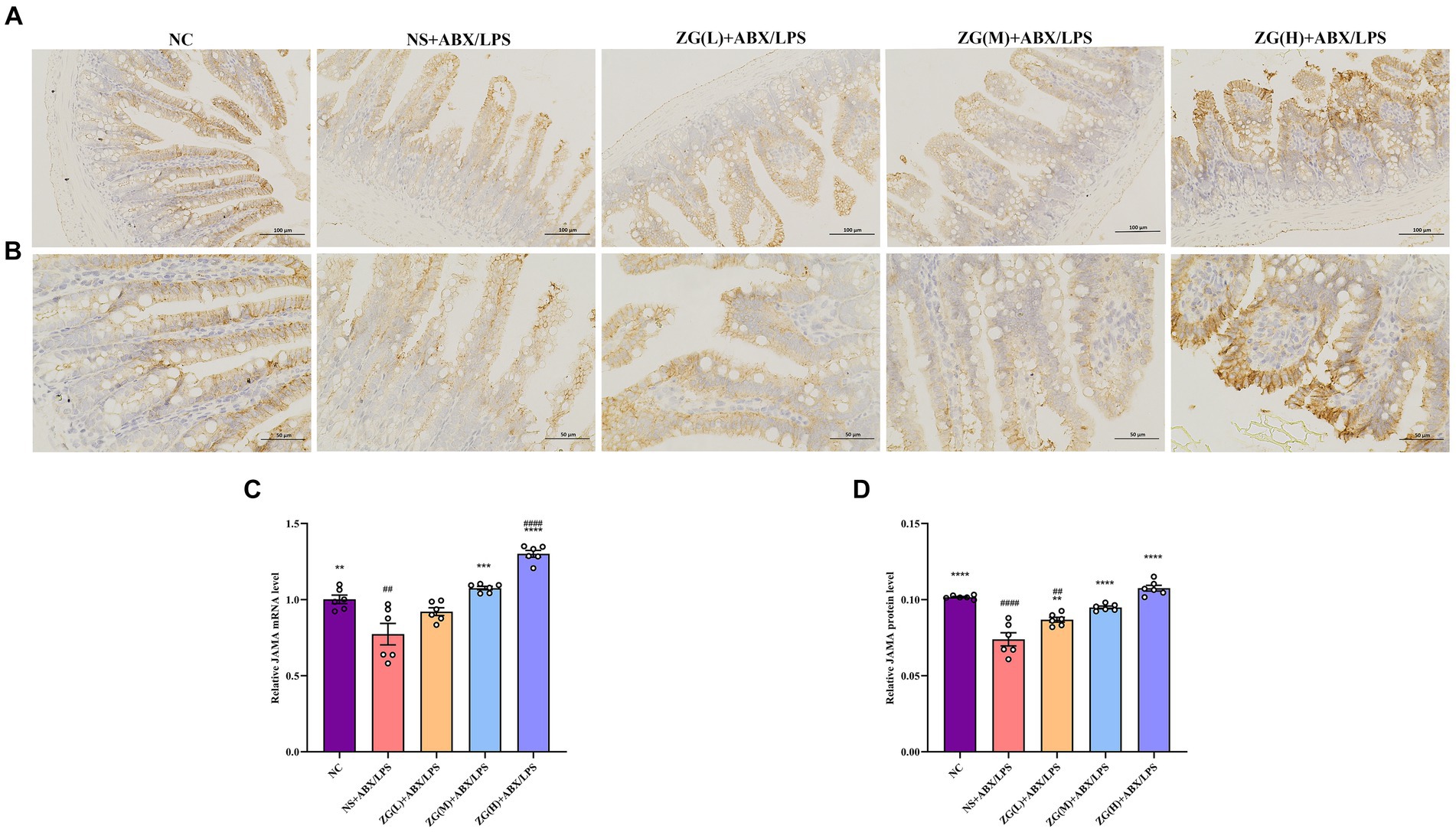
Figure 7. Representative images of JAM-A immunohistochemical staining of mouse ileum tissue (A: ×200, B: ×400). JAM-A mRNA (C) and protein (D) relative expression levels in the ileum of mice in each group. Compared with group NC:**p < 0.01, ***p < 0.001, ****p < 0.0001; Compared with group NS + ABX/LPS: ##p < 0.01, ####p < 0.0001.
Figures 7A,B illustrate that in NC mice, the JAM-A protein level was mainly expressed in the cytoplasm and cell membrane, and the positive cells were brown or brown granular and evenly distributed at the edge of IECs. This distribution pattern is indicative of intact tight junction structure and normal barrier function. Compared with NC mice, the staining of JAM-A in NS + ABX/LPS mice exhibited non-uniform distribution or fading. This alteration in staining pattern suggests disruption or downregulation of JAM-A protein expression, which is consistent with impaired TJ integrity and compromised intestinal barrier function. According to the IHC results (Figure 7D), JAM-A protein expression level was significantly lower in the NS + ABX/LPS group than that in the NC group (p < 0.0001). This downregulation of JAM-A expression unveiled the detrimental effect of dual induction of antibiotics and LPS on TJ proteins and intestinal barrier integrity. Furthermore, compared with the NS + ABX/LPS group, JAM-A protein expression level was significantly higher in ZG treatment groups (p < 0.001), with some demonstrating even a higher expression level (p < 0.0001). This restoration of JAM-A expression suggests that ZG has a protective effect on tight junction integrity, potentially by promoting the expression of JAM-A. These findings suggest that ZG may exert a protective effect against the dual induction of antibiotics and LPS-induced ileal mucosal barrier damage in mice and help maintain or increase JAM-A expression level, promoting the stability and repair of the intestinal mucosal barrier.
3.5.5 Myosin light chain kinase assay resultsMLCK is a protein kinase that regulates actin contraction and relaxation in IECs via actin phosphorylation, affecting cell shape, intercellular junction stability, and membrane permeability. Furthermore, MLCK regulates inflammatory processes (Huang et al., 2020; Chihade et al., 2023). According to the RT-PCR results (Figure 8C), compared with that of NC mice, MLCK mRNA expression level in the ileal mucosa of NS + ABX/LPS mice was significantly higher (p < 0.05). Furthermore, compared with the NS + ABX/LPS group, MLCK mRNA expression level was significantly lower in the ZG treatment groups (p < 0.01), with some groups demonstrating even a lower expression level (p < 0.0001).
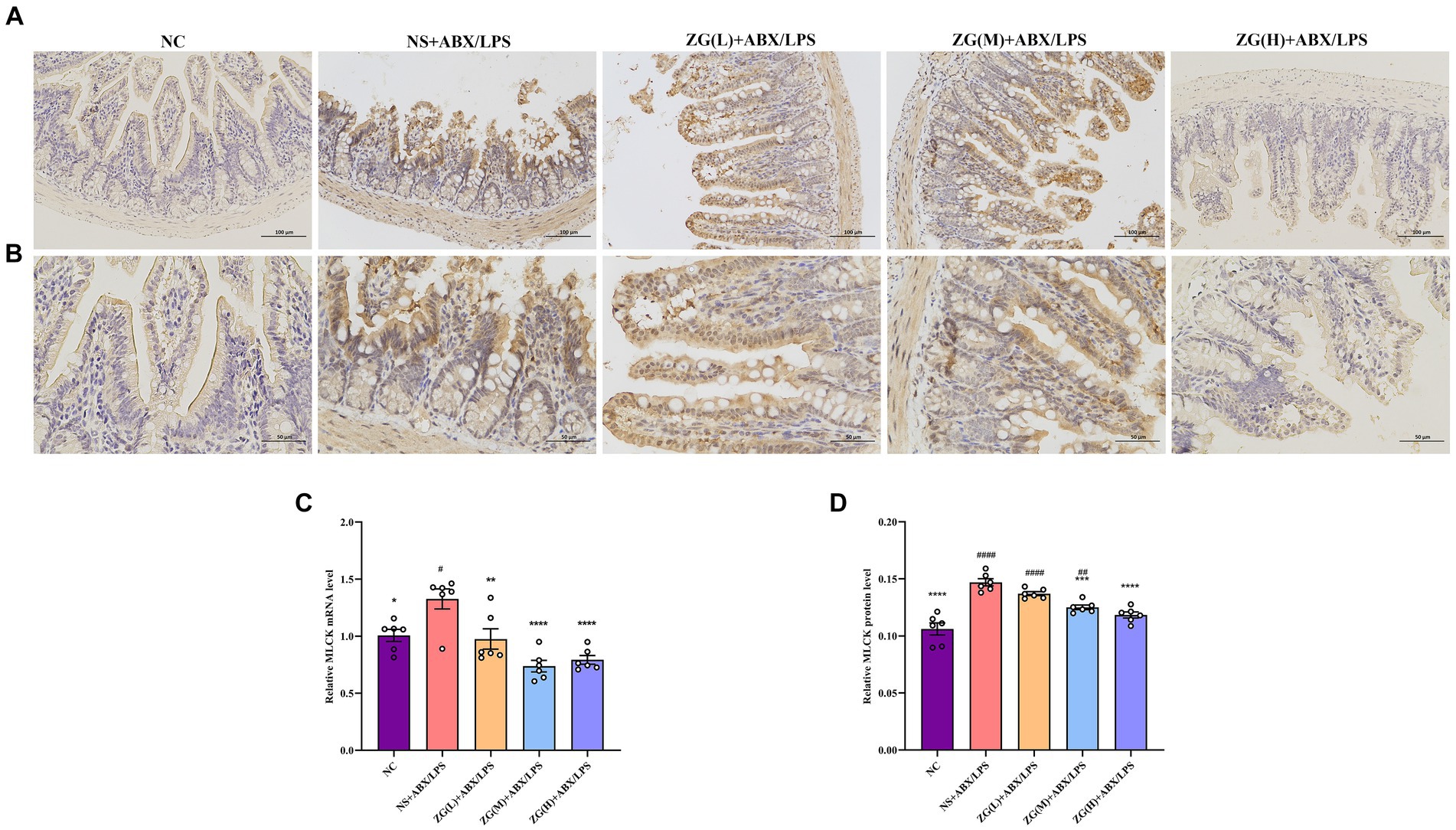
Figure 8. Representative images of MLCK immunohistochemical staining of mouse ileum tissue (A: ×200, B: ×400). MLCK mRNA (C) and protein (D) relative expression levels in the ileum of mice in each group. Compared with group NC: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Compared with group NS + ABX/LPS: #p < 0.05, ##p < 0.01, ####p < 0.0001.
Figures 8A,B illustrate that in NC mice, MLCK protein expression level was mainly in the cytoplasm and cell membrane, and the positive cells were brownish-yellow or brownish granular and uniformly distributed at the edges of IECs. Compared with NC mice, the MLCK staining in NS + ABX/LPS mice displayed irregular distribution or attenuation. According to the IHC results (Figure 8D), MLCK protein expression level was significantly higher in the NS + ABX/LPS group than that in the NC group (p < 0.0001). Furthermore, compared with the NS + ABX/LPS group, MLCK protein expression level was significantly lower in the ZG treatment groups (except ZG(L) + ABX/LPS) (p < 0.001), with one group demonstrating even a lower expression level (p < 0.0001). These findings suggest that the dual induction of antibiotics and LPS-induced intestinal damage could be associated with MLCK overexpression, and ZG could ameliorate the dual induction of antibiotics and LPS-induced intestinal mucosal injury by regulating MLCK expression level.
3.6 Differences in ZG impact on TLR4 and NF-κB/p65 protein expression levels in miceThe TLR4/NF-κB signaling pathway is an essential immune response regulatory system that is involved primarily in the perception and response to external pathogens (e.g., bacterial LPS, viral RNA, etc.) in vivo. In the intestines, this signaling pathway plays a critical role in maintaining intestinal barrier integrity and immune homeostasis (Samuelson et al., 2022).
According to TLR4 WB results (Figures 9A,B), TLR4 protein expression level was significantly higher in the NS + ABX/LPS group than that in the NC group (p < 0.05). Furthermore, compared with the NS + ABX/LPS group, TLR4 protein expression level was significantly lower (p < 0.05) in the ZG treatment groups (except ZG(L) + ABX/LPS), with one group demonstrating even a lower expression level (p < 0.0001). Notably, ZG(H) + ABX/LPS exerted the greatest inhibitory effect on TLR4 protein expression level. According to NF-κB/p65 WB results (Figures 9C,D), NF-κB/p65 protein expression level was significantly higher in the NS + ABX/LPS group than that in the NC group (p < 0.0001). Furthermore, compared with the NS + ABX/LPS group, the ZG treatment groups exhibited a significantly lower NF-κB/p65 protein expression level (p < 0.05), with some demonstrating even a lower expression level (p < 0.0001). Notably, ZG(H) + ABX/LPS exerted the greatest inhibitory effect on NF-κB/p65 protein expression level. These findings suggest that the dual induction of antibiotics and LPS-induced ileal mucosal injury in mice could be associated with the activation of TLR4 and NF-κB/p65 protein levels, and ZG could play an anti-inflammatory and barrier-protective role against antibiotics and LPS-induced intestinal mucosal injury.
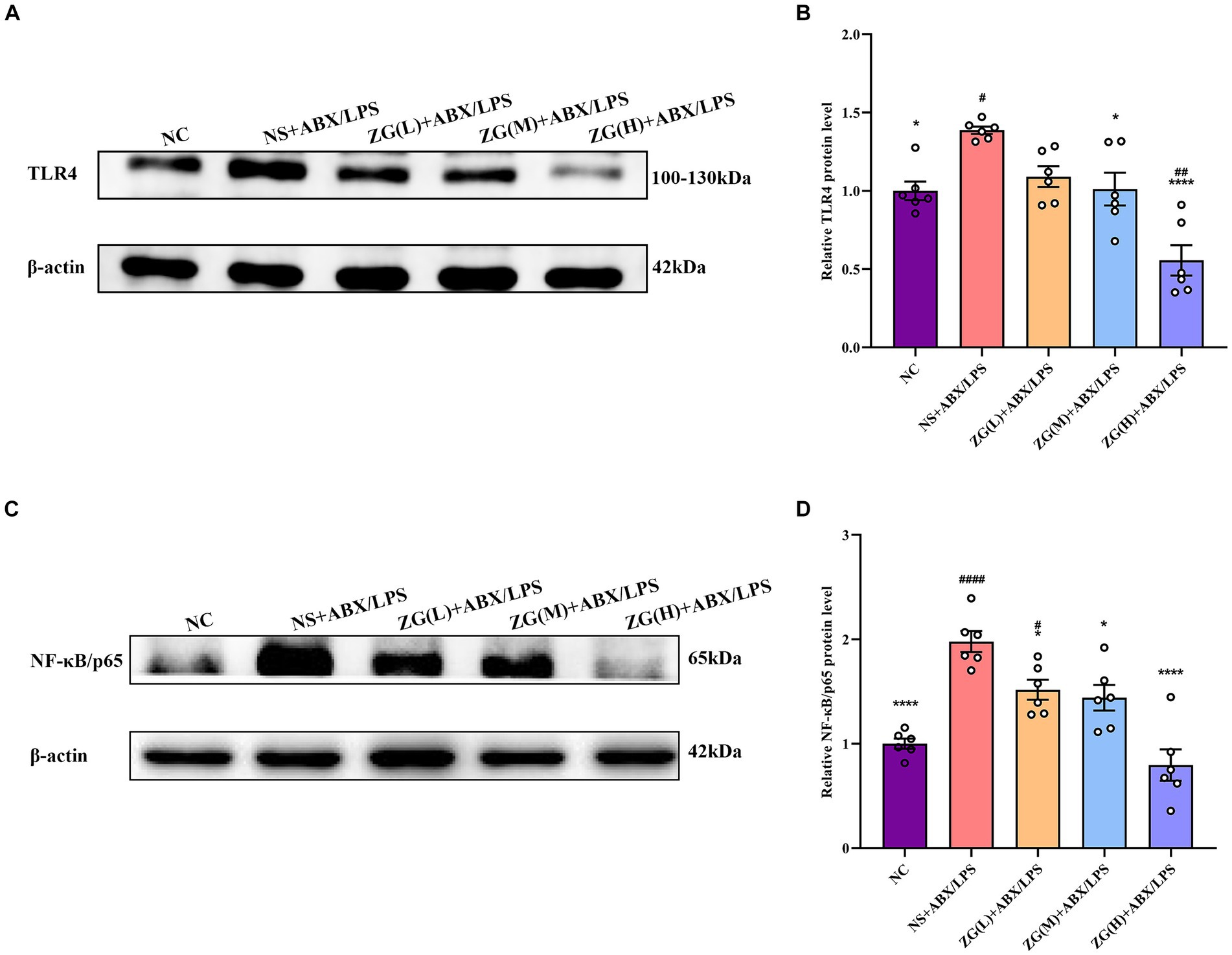
Figure 9. Comparison of variations in the relative expression levels of TLR4 and NF-κB protein in ileum tissues of mice in various groups. Compared with group NC: *p < 0.05, ****p < 0.0001; Compared with group NS + ABX/LPS: #p < 0.05,##p < 0.01,####p < 0.0001. (A-B) The protein expression of TLR4/NF-κB signaling pathway protein (TLR4) of ileum tissue in each group. (C-D) The protein expression of TLR4/NF-κB signaling pathway protein (NF-κB/p65) of ileum tissue in each group.
3.7 Mouse serum levels of DAO, D-LA, and ETMeasurement of intestinal mucosal permeability can reveal the integrity and maturity of the gut. Increased intestinal mucosal barrier permeability may reflect disrupted barrier function. To assess this impairment, the levels of DAO, D-LA, and ET in mouse serum were quantified by ELISA.
Figure 10A indicates that compared with NC mice, the DAO content in the serum of NS + ABX/LPS mice was extremely significantly elevated (p < 0.0001). Compared with NS + ABX/LPS group, the DAO content in the serum of mice in each ZG treatment group was significantly or extremely significantly lower (p < 0.01 or p < 0.0001), reaching the lowest in the ZG(H) + ABX/LPS group. As illustrated in Figure 10B D-LA content in the serum of NS + ABX/LPS mice was extremely significantly higher than that in NC mice (p < 0.0001). Moreover, D-LA content in the serum of mice in each ZG treatment group was significantly or extremely significantly lower compared with the NS + ABX/LPS group (p < 0.01 or p < 0.0001), reaching the lowest in the ZG(H) + ABX/LPS group. Figure 10C showed that compared with NC, the ET content in the serum of NS + ABX/LPS mice was extremely significantly higher (p < 0.0001). The ET content in the serum of mice in each ZG treatment group was extremely significantly lower compared with the NS + ABX/LPS (p < 0.001 or p < 0.0001), being lowest in the ZG(H) + ABX/LPS. These findings indicate that the combined induction of antibiotics and LPS disrupted the intestinal barrier, resulting in heightened permeability of the intestinal mucosal barrier and inflammation. Treatment with ZG may alleviate the rise in intestinal mucosal barrier permeability and alleviate intestinal inflammation.
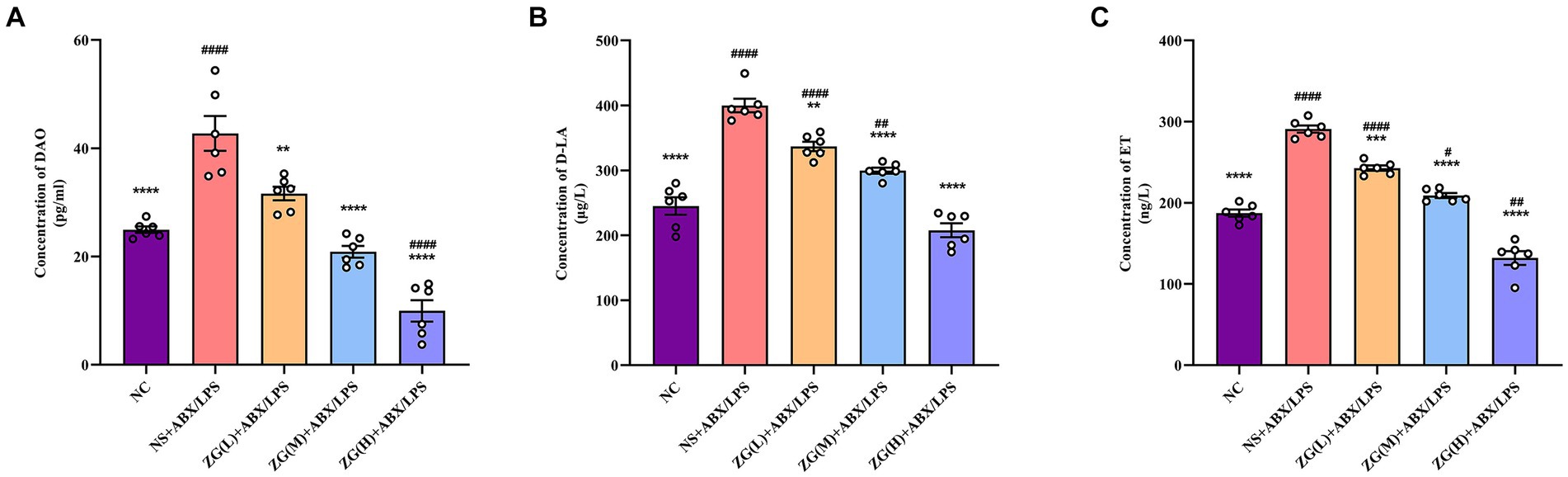
Figure 10. Changes of DAO, D-LA and ET levels in serum of mice in each group. Compared with group NC: **p < 0.01, ***p < 0.001,
留言 (0)