A tumor is a medical issue that poses a significant threat to human life. Tumors exhibit significant variability and genetic instability and can have a multifaceted etiology. Tumor treatment approaches encompass surgery, chemotherapy, radiation, targeted medication therapy, gene therapy, immunotherapy, and more (1). Differentiation-inducing drugs are commonly used to treat precancerous lesions and effectively prevent tumor formation. This therapy is only appropriate for patients in the early stages of malignant transformation. The process of preparing the differentiation-inducing chemical is challenging; thus, the therapy’s stability and safety require more confirmation. It is challenging to combat the intricate and fluctuating tumor environment with a single treatment; thus, the creation of integrated anti-tumor medicines is unavoidable. Tumor surgery has extended the lives of some patients and improved their quality of life to some degree, but it still has significant limitations.
Nanoparticles (NPs) are widely used in medicine and pharmacy due to their ability to effectively bypass biological defense systems and circulatory barriers. The applications encompass drug and gene delivery (2), growth and differentiation factor delivery in regenerative medicine (3), vaccination (4), fluorescent biological labelling (5), detection of proteins and pathogens, probing the DNA structure (6), separation and purification of biological molecules and cells (7), contrast agents in imaging, and phagokinetic studies (8).
Nanomaterials have diverse applications and can interact with biological systems due to their small size. They have the ability to bind with many functional units like diagnostic, targeted, and therapeutic molecules and can be directed to almost any physiological area (9). An example is a nanomaterial that is specifically engineered to extend the duration of blood circulation, avoid being taken up by macrophages, and ultimately reach the desired tissue (10). Upon entering a biological environment, a nanomaterial’s surface will be coated by layers of protein to create the protein corona (11). Protein corona formation is affected by factors such as the size, charge, surface modification of nanomaterials, exposure time, and protein components (12). The protein corona changes the physical and chemical properties of the nanomaterials, giving them a biological trait that differs from their original nature (13).
Biofouling is the unintentional and nonspecific attachment of biomolecules, cells, or microorganisms to material surfaces (14). Serum proteins in the blood play a key role in biosorption, leading to the phagocytosis and digestion of foreign substances by reticuloendothelial systems (RES) (15). Nanomedicines may experience a significant reduction in their circulation time in the body, leading to an inadequate therapeutic outcome (16). Protein adhesion occurs through electrostatic and hydrophobic interactions between charged segments and non-hydrophilic pockets in proteins (17). Several methods have been explored to enhance antifouling properties. The key principle in designing antifouling materials is to prevent electrostatic and hydrophobic interactions with biomolecules. This typically involves maintaining a neutral surface charge, high hydrophilicity, and having hydrogen bond acceptors but no hydrogen bond donors (18).
In this review, we introduce the most frequently used anti-fouling materials, describing their principles and giving examples. Moreover, an outlook on the future of anti-fouling targeted diagnostics and therapy for cancers is also presented.
2 Antifouling materialsScientists prioritize modifying nanoparticles to mimic the body’s own cells in order to prevent them from being recognized as alien materials and eliminated by the immune system. Various methods have been and are now being implemented to transform nanoparticles into biocompatible substances. Efforts have been made to cover nanoparticles with non-reactive polymeric materials to prevent interaction with the host’s immune cells, creating a stealth effect that helps the nanoparticles avoid triggering the host’s immune response (19). Nanomaterial cloaking can use either natural or semisynthetic coverings. Natural polymers commonly used include dextran, polysialic acid, hyaluronic acid, chitosan, and heparin. Synthetic polymers, on the other hand, are man-made and include polyvinyl pyrrolidone, polyacrylamide, polyethylene glycol, and PEG-based copolymers like poloxamers, poloxamines, and polysorbates.
PEGylated nanoparticles are vulnerable to oxidative damage and reactive to transition metal ions in biological environments, leading to adverse consequences such as nonspecific protein binding and nanoparticle instability (20). Zwitterionic polymers offer advantageous features such as high hydrophilicity, strong nonfouling ability, biomimetic capabilities, and outstanding stealth characteristics as substitutes for PEGs in NP formulations (21). Several zwitterionic polymers have been created, such as poly (sulfobetaine ethacrylate), poly (2-methacryloyloxyethyl phosphorylcholine), poly (carboxybetaine methacrylate), and their related forms (22, 23).
2.1 PEGPolyethylene glycol (PEG) is a non-toxic and biocompatible polymer. Polyethylene glycol (PEG) and its derivatives have been commonly used as anti-fouling materials since the 1970s (24). Poly (ethylene glycol) (PEG) modification is a commonly used method to decrease the nonspecific adsorption of proteins and cells. Molecules and nanoparticles modified with PEG exhibit extended blood circulation time and reduced nonspecific cellular uptake compared to unmodified materials, which is essential for specific targeting (25). PEG modification is a common technique used to minimize the binding of biofouling to surfaces (26). The feature, commonly referred to as the ‘stealth’ effect, is typically attributed to its low interfacial energy, the high degree of hydration of the hydrophilic polyether backbone, and the mobility and flexibility of the PEG chains (27).
Uniformly sized magnetite (Fe3O4) nanoparticles measuring 10, 20, and 31 nm were synthesized using the thermal breakdown of Fe (III) oleate or mandelate in a high-boiling point solvent (>320°C). The particles were coated with a PEG-containing bisphosphonate anchoring group to give them hydrophilic and antifouling characteristics. The PEGylated particles were analyzed using several physicochemical techniques such as dynamic light scattering, transmission electron microscopy, thermogravimetric analysis, Fourier transform infrared spectroscopy, and magnetization tests. Increasing the particle size from 10 to 31 nm resulted in a drop in the PEG coating quantity from 28.5 to 9 wt%. The PEG created a compact, brush-like layer on the particle’s surface, preventing particle aggregation in water and PBS (pH 7.4) and enhancing circulation time in vivo. Magnetic resonance relaxometry verified that the PEG-modified Fe3O4 nanoparticles exhibited strong relaxivity, which rose as the particle size grew. The particles caused noticeable contrast enhancement in the magnetic resonance imaging during in vivo investigations using a mouse model. Approximately 70% of the administered 20-nm magnetic nanoparticles remained in the bloodstream after four hours. However, their accumulation in the tumor was minimal, possibly because of the anti-fouling characteristics of PEG (28).
Yuan Sui and colleagues describe the creation and analysis of antifouling Gadolinium oxide (Gd2O3) nanoparticles (NPs) that have been altered with PEG to enhance biocompatibility for MR imaging. This article discusses the solvothermal breakdown of gadolinium (III) in the presence of Na3cit, observed through surface modification using PEG and L-Cys. The nanoparticles were verified using transmission electron microscopy (TEM), dynamic light scattering (DLS), and UV-visible spectroscopy. The morphological analysis indicates that the perfect Gd2O3-PEG-Cys-NPs have a consistent distance of 7.9 ± 0.4 nm, with minimal variation in size. This suggests that the surface modification does not significantly change the core size of the Gd2O3-NPs compared to the pristine sodium citrate-stabilized Gd2O3-NPs. The Gd2O3-PEG-L-Cys-NPs exhibit great stability at room temperature, are water dispersible, and demonstrate reduced cytotoxicity at high concentrations. The T1-weighted MR images clearly showed that the PEG-coated Gd2O3-PEG and Gd2O3-PEG-Cys-NPs, with or without Cys, had the potential to be used for T1-weighted MR imaging. The nanoparticles show no toxicity towards human blood, indicating their biocompatibility for medical uses in humans. The Gd2O3-PEG-Cys-NPs exhibit excellent r1 values, good cytocompatibility, target particular cancer cells, and enable dual-mode MR imaging of lung metastasis cancer models in vitro (Figure 1A) (29).
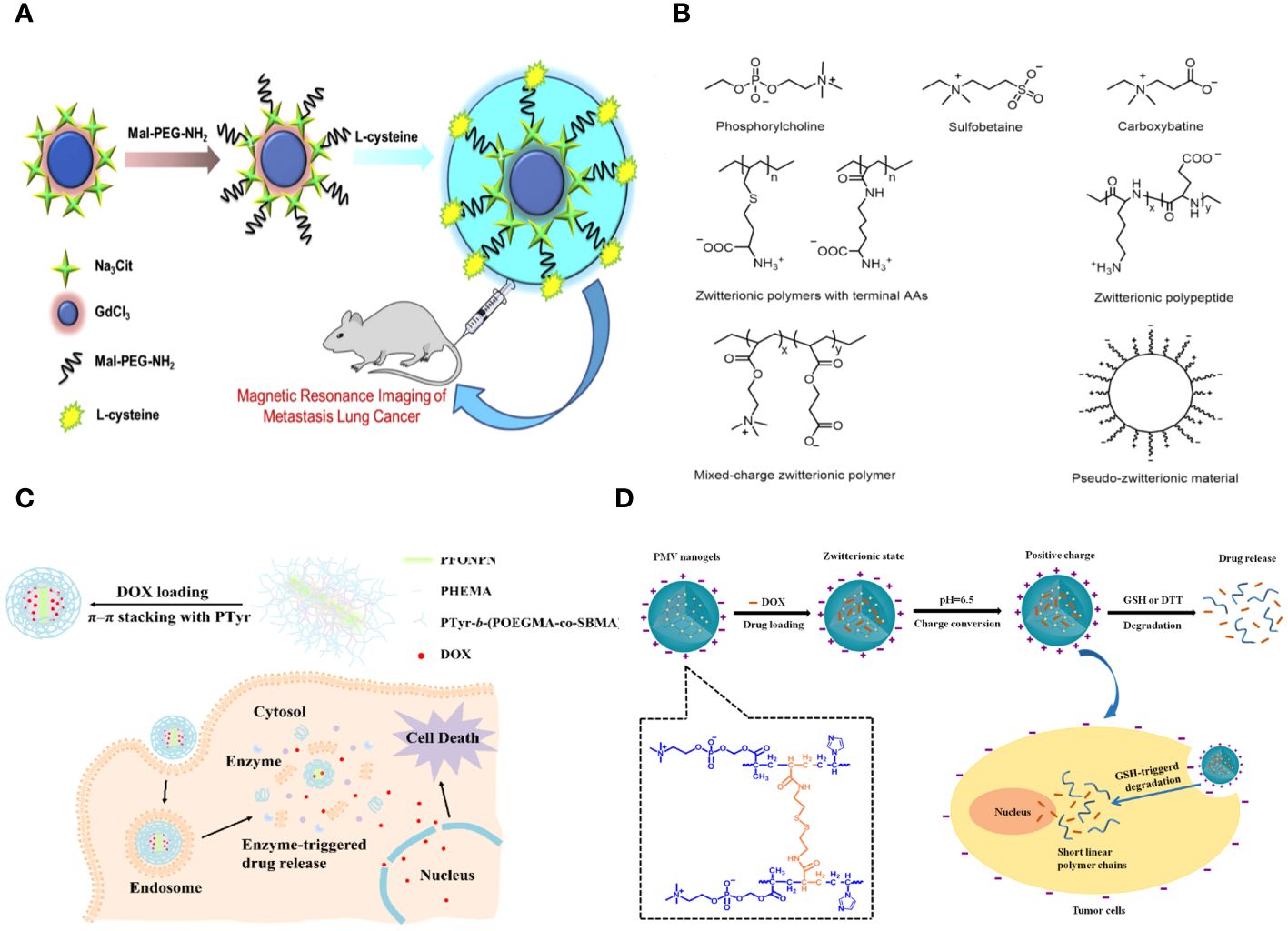
Figure 1 (A) Schematic representation of the synthesis of the Gd2O3- PEG-L-Cys-NPs. The Gd2O3- PEG-L-Cys-NPs in vitro application of MRI of metastasis lung cancer (29). (B) Models and structures of zwitterionic materials. (A) Chemical structures of the common zwitterionic groups. (B) Zwitterionic poly (amino acids) and polypeptide. (C) Mixed-charge zwitterionic polymers that have balanced cationic and anionic groups in different monomer units, and pseudo-zwitterionic materials with equimolar negative and positive charge binding to the same medium. (C) Schematic representation of the synthesis, drug loading, cellular uptake, and rapid enzyme-responsive drug release of the zwitterionic conjugated bottlebrush copolymers (30). (D) Illustration of the preparation, charge-conversion ability and biodegradable behavior of poly (2-methacryloyloxyethyl phosphorylcholine-s-s-vinylimidazole) (PMV) nanogels (31).
Reportedly, PEGylations lose their protein repellent ability at temperatures over 35°C (32). PEG is vulnerable to oxidation when exposed to oxygen or transition metal ions, causing damage to its non-ionic structure and leading to loss of function in many biochemically significant solutions (33). Moreover, PEG has been shown to be challenging to naturally metabolize, and multiple injections of PEGylated formulations can decrease the bioactivity of enclosed biomolecules because of antibodies generated by the immune system (34). The gene entrapment efficiency of PEGylated cationic liposomes would decrease because of the reduction in positive charges (35). Therefore, it is essential to identify alternative materials that can withstand biosorption during blood circulation, remain stable in various environments, be metabolized through biodegradation, and have minimal immunogenicity apart from PEG.
2.2 ZwitterionsZwitterions, which have both positive and negative charged groups, create a neutral charge. They are thought to be better at keeping things from sticking to nanomaterial surfaces for cancer diagnosis than PEG or OEG. Nanomaterials that have been changed zwitterionically have a surface with an equal number of negative and positive groups. This lets a lot of water molecules stick to the surface as a hydration layer through hydrogen bonding. This results in a highly hydrophilic surface that protects the materials from nonspecific protein pollution (36).
Polyzwitterions can be fabricated into various structural forms such as brushes (37), films (38), hydrogels (39), particles (40), membranes (41), and coatings (42), each serving different functions like antifouling (43), stimuli responsiveness (44), lubrication (45), self-healing (46), antibacterial properties (43), and biosensing capabilities (47). Because of their high tolerance to extremely salty conditions, these materials have the potential to be used in various applications such as ionomers (48), fibers (49), rheology modifiers (50), drug/gene delivery vehicles (51), analogues of biological structures (52), and anti-fouling materials (53). Zwitterionic materials not only have excellent antifouling properties but also improve biocompatibility, decrease immunological response, facilitate cellular uptake of chemical drugs and genes, and extend circulation time (54). Zwitterionic alterations can offer unique capabilities as drug transporters, including responsiveness to stimuli and targeting of tumors (55).
Zwitterions can be categorized as betaine-like zwitterions or mixed-charge zwitterionic materials based on the location of the cationic and anionic groups on the same unit (Figure 1B). Zwitterionic polymers predominantly contain quaternary ammonium as cations, forming phosphorylcholine (PC), sulfobetaine (SB), and carboxybetaine (CB) with phosphonates (PO3-), sulfonates (SO3-), and carboxylates (COO-) accordingly. Aside from zwitterionic polymers with charged segments on the same side chains, there are mixed-charge materials with equal positive and negative charged components in separate monomer units or attached to the same medium (such as mesoporous silica nanoparticles) to ensure overall electrical neutrality. These ‘spurious’ zwitterionic materials possess comparable antifouling capabilities due to their similar architectures. Furthermore, researchers have successfully incorporated amino acids or peptides to create zwitterionic carriers, which are a type of natural zwitterions, demonstrating excellent resistance to nonspecific absorption and possessing distinctive features.
Fangjun Liu et al. created enzyme-responsive theranostic zwitterionic bottlebrush copolymers with brush-on-brush architecture. The copolymers include a fluorescent PFONPN backbone that interacts with DOX through fluorescence resonance energy transfer, primary PHEMA brushes, and secondary graft brushes with enzyme-degradable PTyr side chains and zwitterionic P(OEGMA-co-SBMA) side chains. They achieved this by combining Suzuki coupling, NCA ROP, and ATRP techniques. The brush-on-brush copolymer created has a particular response to the tumor microenvironment. It can create individual micelles in water with a high drug capacity due to the extremely hydrophilic zwitterionic brushes. This copolymer serves as an innovative nanoplatform for cancer treatment and diagnosis (Figure 1C) (30).
Biodegradable nanogels made of poly (2-methacryloyloxyethyl phosphorylcholine-s-vinylimidazole) (PMV) were created by a single-step reflux precipitation polymerization process, resulting in homogeneous spherical shapes. The method was both clean and efficient. The PMV nanogels maintained a zwitterionic state at pH 7.4 and transitioned quickly to a positively charged state at pH 6.5 in the tumor extracellular environment. The charge-conversion capacity of PMV nanogels was demonstrated by proton nuclear magnetic resonance spectra and an acid-base titration experiment, which showed that the imidazole ring becomes protonated in an acidic environment. The protein stability experiment demonstrated that PMV nanogels showed resistance to protein adsorption at pH 7.4 for up to 7 days but readily adsorbed protein at pH 6.5. Also, PMV nanogels had a property called “reductibility,” which meant they could break down into shorter linear polymer chains when they were exposed to reducing agents. Hence, the doxorubicin (DOX) release was precisely regulated, with minimal leakage under normal circumstances (7.8% in 48 hours) and rapid release in 10 mM glutathione at pH 7.4 (78.9% in 48 hours). The PMV nanogels demonstrated increased cellular absorption by tumor cells at pH 6.5 compared to pH 7.4, leading to a strong cytotoxic effect of DOX-loaded PMV nanogels against tumor cells, as observed using confocal laser scanning microscopy and flow cytometry (Figure 1D) (31).
2.3 Proteins and peptidesAlbumin is often used as a blocking agent to keep background signals from messing up different types of experiments, such as immunocytochemistry and Western blot. The antifouling capability is due to the distinctive composition of amino acids in the albumin structure, resulting in a well-balanced charge distribution (56).
Functionalized anti-fouling peptides are popular as an anti-fouling material due to their natural biocompatibility and are commonly used in an electrochemical assay for tumor markers. Peptides have significant hydration properties due to their polar functional groups and zwitterionic charges, which contribute to their anti-fouling effect. Peptides that are hydrophilic and amphiphilic but lack charge exhibit anti-fouling qualities. An example is the anti-fouling portion of peptides containing the EK motif, which was engineered to be electrically neutral (57). Neutral or hydrophilic anti-fouling peptides with certain functional groups can be created. Peptides provide numerous benefits, making them an excellent choice for biodegradable anti-fouling compounds (58).
Peptides can form precise nanostructures that are beneficial for targeting in biological systems, although they exhibit low bioavailability, possible immunogenicity, and inadequate metabolic stability. Peptidomimetic self-assembled nanoparticles can contain biological recognition patterns while also offering certain technical characteristics. Inorganic nanoparticles, covered with self-assembled macromolecules to enhance stability and prevent fouling and linked with ligands unique to a target, are improving imaging resolution from anatomical to molecular levels. Nanoparticles conjugated with ligands are appealing for delivering drugs selectively to cells in tumors due to their high transport capacity and cell selectivity based on the ligand. Peptidomimetic nanoparticles can enhance binding to surface receptors on cancer cells, leading to increased uptake and decreased drug resistance. Self-assembled nanoparticles linked with peptidomimetic antigens are optimal for prolonged display of vaccination antigens to dendritic cells, leading to activation of the T-cell-mediated adaptive immune response. Self-assembled nanoparticles are a feasible substitute for encapsulation in providing prolonged release of proteins in tissue engineering. Cell-penetrating peptides attached to nanoparticles are used as vectors for intracellular delivery of genes and as agents for transferring plasmids (59).
Angela Maria Cusano and colleagues discuss a universal method for directly detecting a particular tumor biomarker in serum. Detection is enabled via a protein-binding peptide chosen through an enhanced phage display method and then attached to modified microparticles (MPs). Protein biomarkers provide abundant data for non-invasive diagnostic and prognostic studies. MP-based assays are increasingly used for handling soluble biomarkers; however, their application in serum is hindered by the intricate biomolecular surroundings. Their method surpasses the existing constraints by creating a selective MP with an anti-fouling layer that effectively captures the target protein while remaining unaffected by other substances in the background. Their technique effectively isolates human tumor necrosis factor alpha from serum with a high level of selectivity (Figure 2A) (60).
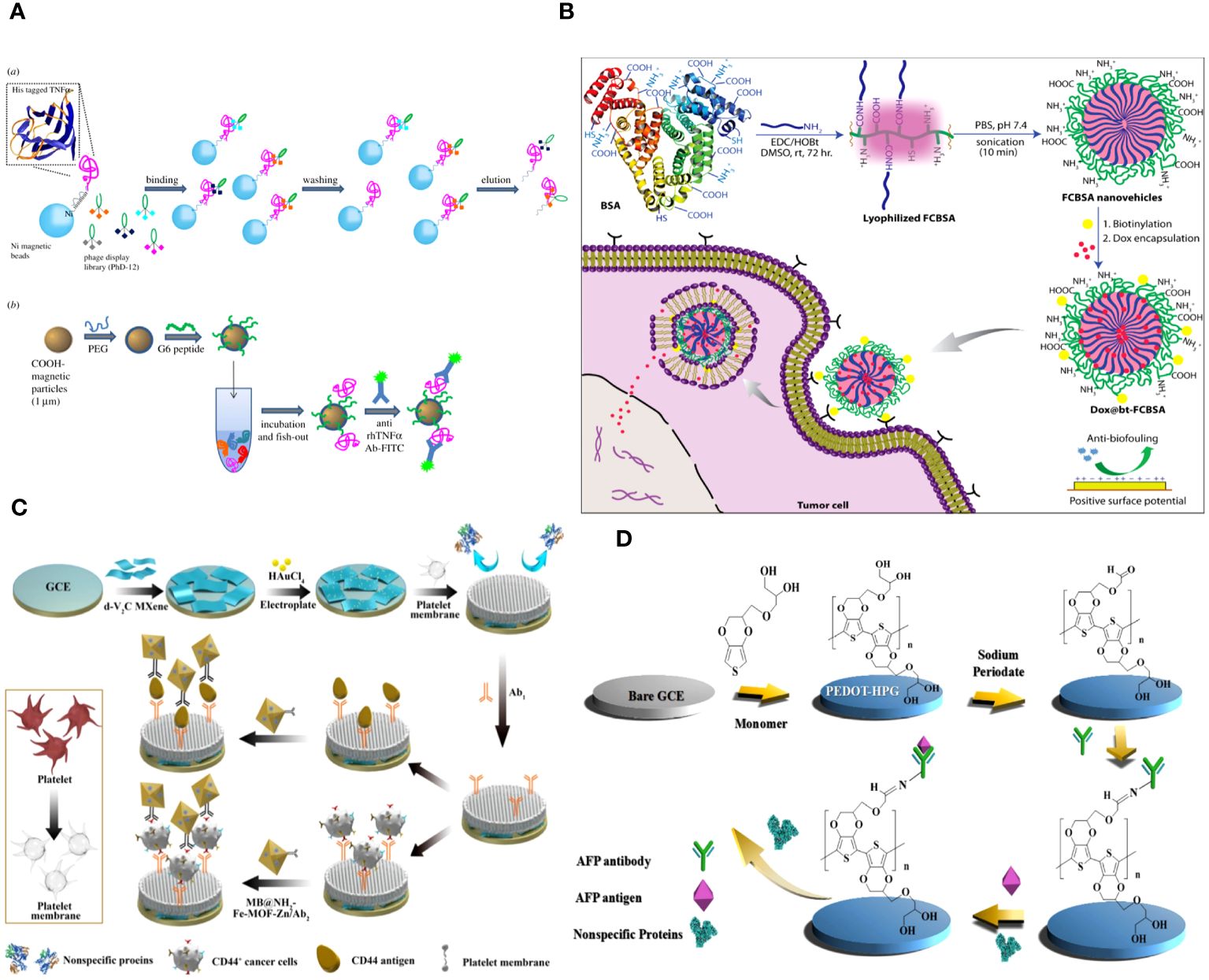
Figure 2 (A) Graphical scheme for the design and development of MP-based bioassay for fishing-out of soluble biomarkers. (a) Experimental scheme for selection of phage-displayed peptides with high affinity and specificity for rhTNFa performed on magnetic nickel-coated beads. (b) Graphical representation of integrated system for detection or fishing-out of any soluble biomarker in complex biological medium. This MP-based bioassay consists of selective capturing of target protein due to the presence of a specific binding peptide (previously selected by modified phage display procedure). The binding event is then detected by immunofluorescence measurements (60). (B) Schematic representation of the synthesis of fatty-amine-conjugated cationic BSA nanovehicles formulation, its surface modification with biotin, the capacity for antibiofouling, and successful encapsulation and delivery of anticancer drug Dox to biotin-receptor-positive cancer cells (61). (C) The fabrication process of the electrochemical immunosensor with anti-fouling capability for detection of CD44 (62). (D) Schematic illustration of the fabrication process of the AFP biosensor with PEDOT-HPG (63).
Abhishek Saha and colleagues describe a straightforward method for creating fatty-amine-conjugated cationic BSA (FCBSA) nanoparticles by attaching laurylamines to the BSA protein. When glutamic acid or aspartic acid residues partially neutralize by forming an amide bond with laurylamines, cationic nanoparticles are produced under physiological conditions. These nanoparticles have an isoelectric point of 7.7 and a zeta potential of +7 mV at pH 7.2. The NPs demonstrate strong resistance to heat, pH, and proteolytic enzyme stressors. The NPs showed outstanding biocompatibility with normal and cancer cell types. The protein NPs effectively trap the hydrophobic anticancer medication doxorubicin (Dox) and exhibit controlled release characteristics (about 40% release after 3 days), stability in human blood serum, resistance to fouling, and a stronger attraction to anionic membranes. The biotin-labelled cationic FCBSA (bt-FCBSA) exhibited nearly identical biophysical characteristics as FCBSA. Additionally, cellular investigations demonstrated that bt-FCBSA effectively transports Dox to biotin receptor-positive HeLa cells, resulting in substantial cell mortality. An in vivo study showed that Dox-encapsulated bt-FCBSA significantly inhibited tumor growth in female Swiss albino mice with Ehrlich ascites carcinoma cells (Figure 2B) (61).
2.4 Cell membraneThe cell membrane, consisting of phospholipid bilayers and proteins, acts as a semipermeable barrier to prevent large biomolecules from entering the cell (64). Many biomimetic platforms were created by coating materials with cell membranes to replicate this characteristic. These materials, camouflaged with cell membrane, possess biocompatibility, low immunogenicity, extended blood circulation time, and antifouling properties due to inheriting the natural qualities of the cell membrane (65). Cell membrane-coated materials have demonstrated potential uses in medication delivery, imaging, and cancer diagnostics.
The cell membrane demonstrates excellent biocompatibility. The hydrophilic phospholipid head was exposed to the sample because of the topology of the phospholipid bilayer. Meanwhile, the head of the phospholipid is composed of a negatively charged phosphate group and a positively charged quaternary ammonium group, resulting in overall surface electroneutrality (36, 66). The antibacterial fouling ability of the PM/AuNPs/d-V2C-modified electrode relies on the steric hindrance of glycoproteins, the presence of zwitterionic headgroups in phospholipid bilayers, and the high hydrophilicity of the interface (Figure 2C) (62).
Red blood cells are the most prevalent type of blood cell in the circulatory system. RBC membrane-based materials have significant potential in clinical applications such as drug delivery, immunological evasion, tumor imaging, and cancer diagnostics due to their biocompatibility, biodegradability, long circulation half-life, and anti-nonspecific adsorption ability (67).
White blood cells (WBCs) play a crucial role in various serious illnesses, including infections, cancer, and inflammatory disorders. They also aid in immunological functions and build up in different diseased regions. Given that isogenous white blood cells (WBCs) do not participate in the cluster reaction within the same environment, the utilization of nanoparticles based on the membrane camouflage of WBCs, such as neutrophils, macrophages, and T cells, can effectively mitigate nonspecific leukocyte binding. This is achieved by coating the membrane of WBCs with nanoparticles. Consequently, the background of white blood cells (WBCs) might be reduced, leading to an enhancement in the purity of CTC capture. Consequently, multiple platforms containing membrane-coated nanoparticles for white blood cells (WBCs) have been created to isolate cancer stem cells (CTCs).
In addition to red blood cells (RBCs), many types of cells have been considered based on the specific therapeutic purposes required. These include RBCs, white blood cells (WBCs), platelets, stem cells, cancer cells, and other non-traditional sources. The material cores considered in this study include biodegradable polyester nanoparticles (PLA, PLGA, and PCL), mesoporous silica nanoparticles, magnetite nanoparticles, gelatin, and ultra-caprolactone nanoparticles (UCNPs). The encapsulation of cancer cells within PLGA nanoparticle cores has been employed for the purpose of targeting melanoma (68). Additionally, stem cell-coated PLGA nanoparticles have been utilized for the effective targeting of orthotropic breast cancer (69). Furthermore, macrophage-coated mesoporous silica nanoparticles have been employed as a biomimetic platform (70). Neutrophil-coated nanoparticles (NPs) have been developed as a means of delivering chemotherapeutic medicines for the treatment of recurrent glioblastoma. Additionally, these nanoparticles have shown potential for suppressing inflammation of the synovial membrane and mitigating joint damage in individuals with inflammatory arthritis (71). The toxins secreted from staphylococcus, specifically alpha-hemolysin, were absorbed by nanoparticles coated with RBC membranes (72). In addition to the aforementioned, various other NP cores can be utilized for extensive therapeutic applications. In their study, Yang et al. (2019) developed a cancer cell membrane shell and a core composed of silica nanoparticles. These components were designed to encapsulate a photodynamic agent, chlorin e6, with the aim of establishing an efficient photodynamic therapy (73). Gold nanoparticles coated with lipids have been utilized in many applications, such as drug transport, diagnostics, and sensing (74).
Hu et al. did a study on how to use PD-1 receptor-presenting membrane-coated paclitaxel dimers nanoparticles (PD-1@PTX2 NPs) to make treatment work better. Shrouded on the PD-1 cell membrane, the PTX dimer demonstrated efficient cellular absorption and enhanced cytotoxicity against cancer cells. PD-1@PTX2 nanoparticles have the ability to specifically attach to PD-L1 ligands that are present on breast cancer cells. The nanoparticles demonstrate a notable rate of tumor growth reduction, namely 71.3%, in mice that hold 4T1 xenografts. Additionally, these nanoparticles greatly extend the survival period in animal models of breast cancer. Furthermore, our nanoparticles made it possible for 3.2 times more CD8+ T cells to enter tumors and a 73.7% drop in the number of regulatory T cells (Tregs) to be present, which strengthened the immune response against tumors. The results underscore the potential application of PTX nanoparticles on immune checkpoint receptors to enhance the efficacy of chemoimmunotherapy. This offers an additional method for enhancing cancer treatment (75).
2.5 Other anti-fouling materialsHydrogels are interconnected structures made of hydrophilic polymers that include many hydrogen bonds. Hydrogels have a high specific surface area and a distinctive three-dimensional network structure, allowing them to hold a variety of modified materials for electrode modification. It is possible for hydrogels to hold many molecules with different functions inside them, giving them high conductivity, strong electrochemical signals, and great catalytic performance. Simultaneously, the microenvironment within hydrogels can enhance the stability and biological function of biomolecules. Moreover, the high permeability of hydrogels can expedite the movement of tiny molecules and ions, as well as the quick transfer of electrons. Hydrogels have unique features that make them highly promising for constructing electrochemical immunosensing surfaces (76).
A new redox hydrogel called PANI-PThi gel was created for very sensitive and protein-repellent amperometric immunosensing of carcinoma antigen-125 (CA12–5). The PANI-PThi gel-modified electrode underwent an anti-fouling performance test by being submerged in a PBS solution containing various serum concentrations for 12 hours. The current fluctuation range did not exceed 4 µA, demonstrating the PANI-PThi gel’s ability to notably decrease the non-specific adsorption of proteins in human serum. The SWV curves showed that the suggested hydrogel has a substantial and consistent signal, maintaining 94.6% even after a month. The response of PANI-PThi gels is enhanced by the presence of H2O2, leading to an extended linear range for the immunosensor. The biosensors’ results aligned closely with ELISA when detecting clinical serum samples (77).
The following studies examined how branching impacts the blood circulation and tumor targeting of polymer nano vehicles in living organisms. Star-branched copolymers of poly (lactic acid) and poly (2-methacryloyloxyethyl phosphorylcholine) (PLA-PMPC) were synthesized with umbrella-type AB3, (AB3)2, and (AB3)3 architecture by branching at the PLA core for the intended application. Micelles formed from these copolymers were used to study the impact of core branching on blood circulation and tumor targeting. Branching altered the polymeric self-assembly in solution, leading to modifications in the size and surface anti-fouling properties of the polymeric micelles, as indicated by the results. Star-branched copolymer micelles with increased branching degree improved the persistence of their payload in blood, extending the half-time from 7.1 and 8.6 hours to 13.8 hours and resulting in 1.72 times more content at the tumor site. The research indicates that increasing the branching degree of amphiphilic copolymers could be a beneficial approach for creating carriers with improved circulation and targeting abilities in living organisms (78).
Li et al. developed a new antifouling platform named Fe3O4@SiO2@PTMAO@Aptamer by attaching polymeric trimethylamine N-oxide (PTMAO) onto Fe3O4@SiO2 nanoparticles and then linking it with two aptamers for capturing circulating tumor cells (CTCs) (79).
Hyperbranched polyglycerol (HPG) exhibits high hydrophilicity due to its dense, spherical structure containing several hydroxyl groups. PEDOT was polymerized with HPG using electrochemical polymerization to enhance conductivity. The hydration layers generated on the surface of PEDOT-HPG are a result of the many hydroxyl groups present, which prevent protein adsorption and discourage non-specific cell adhesion. The study demonstrated that the biosensor exhibited strong resistance to fouling and high sensitivity, with a detection limit of 0.035 pg mL-1 (Figure 2D) (63).
3 Application of antifouling materials in oncology3.1 Improvement of tumor diagnosisCancer is still regarded as one of the most lethal diseases. Prompt diagnosis and therapy are now the preferred methods to enhance the survival rate of cancer patients. Accurate medical diagnosis of tumor characteristics, such as its location, boundaries, and spread to other areas, is crucial for determining appropriate treatment. Several diagnostic methods rely on contrast agents to improve imaging efficiency, resolution, and precision, including fluorescence imaging, magnetic resonance imaging (MRI), computed tomography (CT), and radioisotope-based nuclear imaging techniques. Nanomaterials have gained attention due to the limitations of traditional contrast agents with low molecular weights, such as high concentration-induced renal toxicity, low imaging efficiency, rapid metabolic pathway-induced short imaging time window, and lack of specificity. Examples include Omnipaque for CT imaging and Magnevist for MR imaging. This is mostly due to their inherent features that can be utilized for specific surface modifications to produce adjustable pharmacokinetics and as bases to incorporate imaging agents or pharmaceuticals for diagnostic and therapeutic purposes (80).
Nanomaterials are commonly used as platforms to carry imaging agents for cancer detection. Typically, most nanomaterials tend to accumulate in organs related to the reticuloendothelial system (RES), like the liver, spleen, and lungs, following systemic injection. While the method of modifying nanomaterials using polyethylene glycol (PEG) has somewhat alleviated the issue, obstacles persist for additional therapeutic uses. Nanomaterials modified with zwitterionic surfaces have demonstrated superior antifouling properties compared to those modified with PEGylation.
3.1.1 Antifouling materials in tumor imagingMolecular imaging technologies like optical (81) computed tomography (82), magnetic resonance (MR) (83), positron emission tomography (PET) (84), and single-photon emission computed tomography (SPECT) (85) are used for disease diagnosis because of their inherent benefits (86). Magnetic resonance imaging (MRI) is widely used in clinical diagnosis for its exceptional resolution and tomographic abilities (87). Nanomaterial-based contrast agents are commonly used in MR imaging applications to enhance imaging sensitivity and reliability.
Over the last ten years, nanotechnology has rapidly advanced in creating and producing several types of nanoparticles for magnetic resonance imaging purposes. Gd (III)-loaded nanocarriers, such as dendrimers (88), liposomes (89), micelles (90), and inorganic NPs (91), provide significantly improved image contrast compared to tiny-molecule Gd (III) complexes. Furthermore, due to the extended blood circulation time and easy surface modification of NPs, NP systems can achieve increased imaging duration, desirable compatibility with living organisms, and enhanced imaging accuracy. Carbon nanotubes (CNTs) (92) are considered highly appealing because of their exceptional characteristics, including ultrahigh surface area, ultralight weight, outstanding chemical and thermal stability (93), and extended circulation duration, which make them stand out among other nanomaterials (94). Ultra-short single-walled carbon nanotubes (SWNTs) have been utilized as a high-performance T2-weighted magnetic resonance imaging (MRI) contrast agent in MR imaging applications. This is attributed to the paramagnetic properties of the SWNTs and the presence of iron catalyst nanoparticles.
Zhijuan Xiong and colleagues describe the creation and analysis of antifouling zwitterion carboxybetaine acrylamide (CBAA)-modified dendrimer-entrapped gold nanoparticles (Au DENPs) for improved CT imaging purposes. The CBAA-modified nanodevice demonstrates superior protein resistance, reduced macrophage uptake and liver accumulation, and prolonged blood half-life compared to the PEGylated material, resulting in improved CT imaging of the blood pool, lymph nodes, and tumors (Figure 3A) (95).
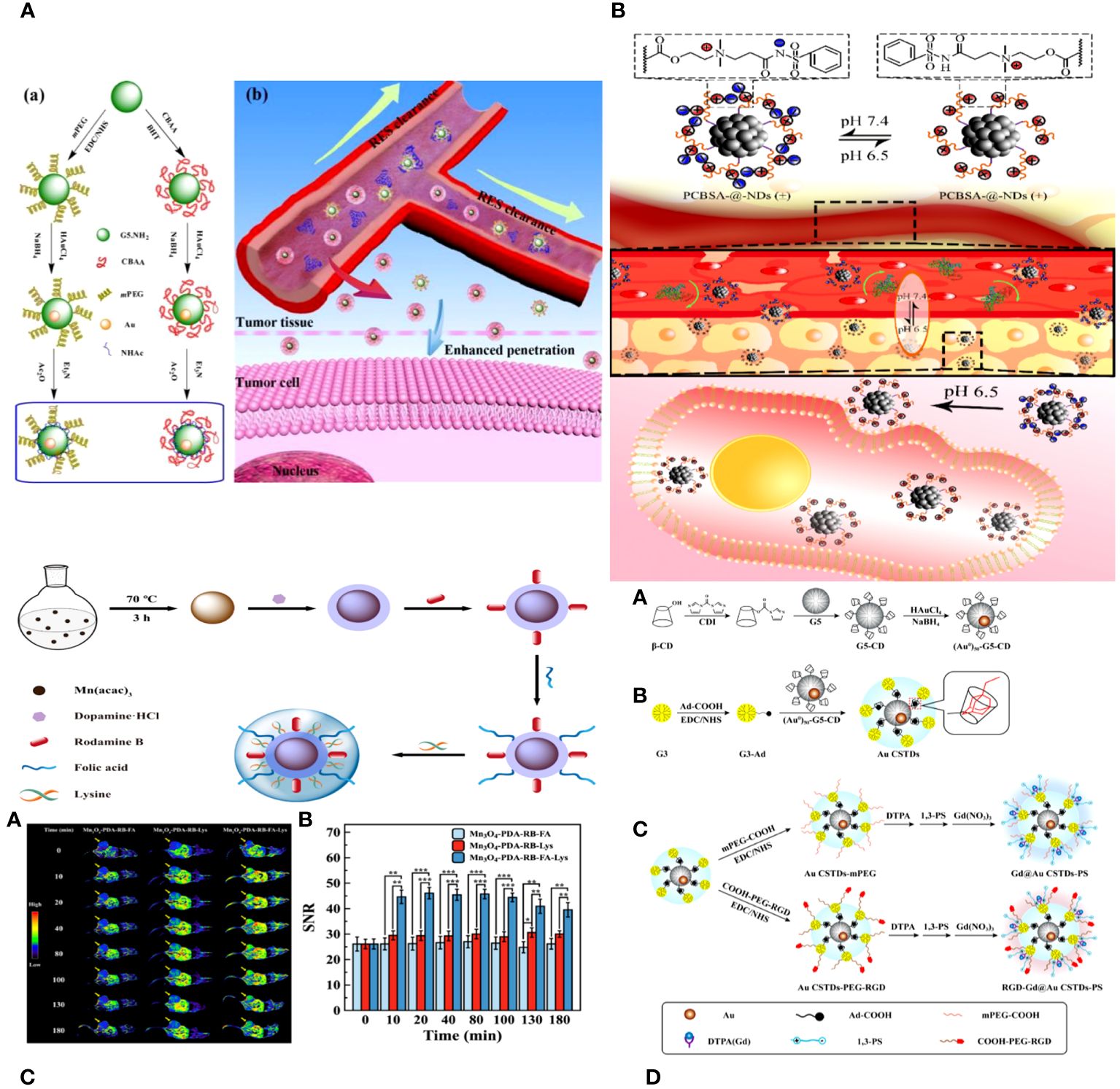
Figure 3 (A) Schematic illustration of the synthesis of CBAA- or PEG-modified Au DENPs (a) and the good antifouling property of CBAA-modified Au DENPs in blood vessels for imaging applications (b) (95). (B) The surface charge-conversional performance of PCBSA-@-NDs and tumor cell uptake under tumor pHe (96). (C) Schematic presentation of the preparation of Mn3O4−PDA−RB−FA−Lys NPs (97). (D) Schematic illustration of the synthesis of RGD-Gd@Au CSTDs-PS (98).
Biyu ZHOU et al. developed a zwitterionic polymer coating on nano diamonds (ND) with pH-responsive properties for improved imaging of tumor cells using commercial NDs. The process began by grafting poly (carboxybetaine methacrylate) onto the virgin NDs using surface-initiated reversible addition-fragmentation chain transfer (RAFT) polymerization. PCBMA-@-NDs were replaced with benzene sulfonamide (PCBSA-@-NDs) using one-step carbodiimide chemistry to make them pH-responsive and enhance their interaction with tumor cells. The surface modification of the polymer was analyzed using FTIR, 1H NMR, and TGA. PCBMA-@-NDs and PCBSA-@-NDs exhibited improved dispersibility, increased fluorescence intensity, and superior antifouling properties compared to pristine NDs. Furthermore, PCBSA-@-NDs may reversibly convert its zwitterionic surface (at pH 7.4) to a positive charge (at pH 6.5) by protonating or deprotonating acylsulfonamide. PCBSA-@-NDs exhibited superior cell affinity and imaging performance compared to zwitterionic NDs in a slightly acidic tumor environment, as confirmed by fluorescence microscopy and flow cytometry (Figure 3B) (96).
Peng Wang and colleagues introduce the development of antifouling zwitterion-functionalized manganese oxide (Mn3O4) nanoparticles (NPs) that are coated with folic acid (FA) for precise imaging of tumors using magnetic resonance (MR) technology. Diethylene glycol-stabilized Mn3O4 nanoparticles were synthesized using a solvothermal method. They were then coated with polydopamine, labelled with rhodamine B for fluorescence, conjugated with folic acid through amide bond formation, and lastly coated with L-lysine zwitterions. The Mn3O4 NPs created in this manner exhibit superb water dispersibility and colloidal stability, effective protein resistance, and favorable cytocompatibility. Because of the PDA and Lys changes, the multifunctional Mn3O4 NPs have an extremely high r1 relaxivity of 89.30 mM−1 s−1. They also facilitate targeted tumor MR imaging by including FA ligands. The zwitterion-functionalized Mn3O4 nanoparticles can be used as a high-quality contrast agent for targeted magnetic resonance imaging of many biological systems (Figure 3C) (97).
Combining various imaging technologies is frequently utilized in clinical settings to enhance the accuracy of cancer diagnosis by overcoming the limitations of individual imaging modalities. Computed tomography (CT) is a commonly used imaging tool that provides detailed 3-dimensional images with high spatial and density resolution for anatomical structure and functional information (99). On the other hand, magnetic resonance (MR) imaging offers high sensitivity and excellent resolution for soft tissue (100). Thus, creating new CT/MR dual-mode imaging contrast agents could combine the benefits of both imaging techniques and enhance the precision and sensitivity of cancer detection (101).
Renna Liu and colleagues developed multifunctional core-shell tecto-dendrimers (CSTDs) containing gold nanoparticles (Au NPs) for dual-mode imaging of tumors using computed tomography (CT) and magnetic resonance (MR). β-cyclodextrin (CD)-modified generation 5 poly(amidoamine) (PAMAM) dendrimers were synthesized and encapsulated with Au NPs at the core in this study. Third-generation PAMAM dendrimers that were changed with adamantine worked as a protective layer to make Au CSTDs, which are threaded structures made of cyclodextrin and gold nanoparticles. This was done by adamantine and cyclodextrin interacting with each other on a supramolecular level. The Au CSTDs was replaced with RGD peptides that had PEG between them, along with a Gd chelator and 1,3-propane sultone. Gd (III) ions were then chelated. The synthesized Au CSTDs are multifunctional nanoparticles with a mean size of 11.61 nm. They are very stable as colloids, block X-rays well, have a high r1 relaxivity (9.414 mM−1s−1), are good at preventing fouling, and are compatible with cells. The multifunctional Au CSTDs makes it possible to use targeted CT/MR imaging of a breast cancer model in living organisms. This is possible because RGD helps target αvβ3 integrin-overexpressing cancer cells. They can be removed from the body through a metabolization pathway with an excellent biosafety profile. The advanced Closed System Transfer Devices (CSTDs) can be used as a precise imaging tool in both CT and MR modes to find different types of cancer that have high levels of the αvβ3 integrin (Figure 3D) (98).
3.1.2 Application of antifouling materials in biosensorsBiosensors have been extensively utilized in several fields such as biotechnology, food inspection, medical diagnostics, and environmental monitoring since their inception by Lyons and Clark in the 1960s (102). Electrochemical biosensors are a very sensitive detection technology that offers the benefits of easy operation and cost-effectiveness (103). Currently, the extended implementation of electrochemical biosensors encounters two significant obstacles. One must determine how to implement it in clinical settings when faced with resistance to interference. The second issue is how to sustain high detection efficiency following the application of antifouling compounds. Utilizing electrochemical biosensors in clinical practice is currently a significant challenge due to the potential issue of nonspecific protein adsorption in complex biological samples like human whole blood. This can lead to decreased sensitivity and specificity in detection, resulting in false positive responses (104). Many approaches aimed at reducing background interference and nonspecific interactions have experienced significant progress (105). A sample dilution was used to reduce the background signals. Although effective in reducing the presence of non-target molecules, this method may not always be practical (106). Surface blocking has had significant success in targeting therapeutic sites in human physiological fluids by utilizing adsorbed and non-reactive substances like Tween 20, BSA (bovine serum albumin), and commercially available combinations (66). Polymeric separation membranes have made significant progress in minimizing nonspecific adsorption on gold surfaces, utilizing poly (ethylene glycol) (PEG) polymer (107), zwitterionic polymer (108), and peptide (109). Phosphocholine has emerged as a promising antifouling membrane material in recent years. Phosphocholine can imitate eukaryotic membrane properties (110), making it very resistant to protein or cell non-specific adsorption. This is achieved by creating a hydration layer through strong water binding (111). Phosphocholine-integrated layers are short, which increases the likelihood of fast electron transfer.
Serum-soluble folate binding protein (FBP) is a crucial tumor marker, and there is a strong demand for the creation of a straightforward biosensing technique. Bobo Fan et al. developed a photoelectrochemical (PEC) biosensor to detect FBP by creating an antifouling surface and utilizing unique ligand-protein recognition. The PEC sensing platform was created by covering TiO2 nanotube arrays (NTAs) with biomimetic polydopamine (PDA). The macroporous structures led to a substantial boost in PEC. Conjugating amino-group-terminated 8-arm polyethylene glycol (PEG) resulted in outstanding antifouling performance. Folic acid (FA) inclusion maintains the antifouling function and demonstrates recognition capabilities for FBP. The artificial photoelectrochemical biosensor demonstrates excellent analytical capabilities (Figure 4A) (112).
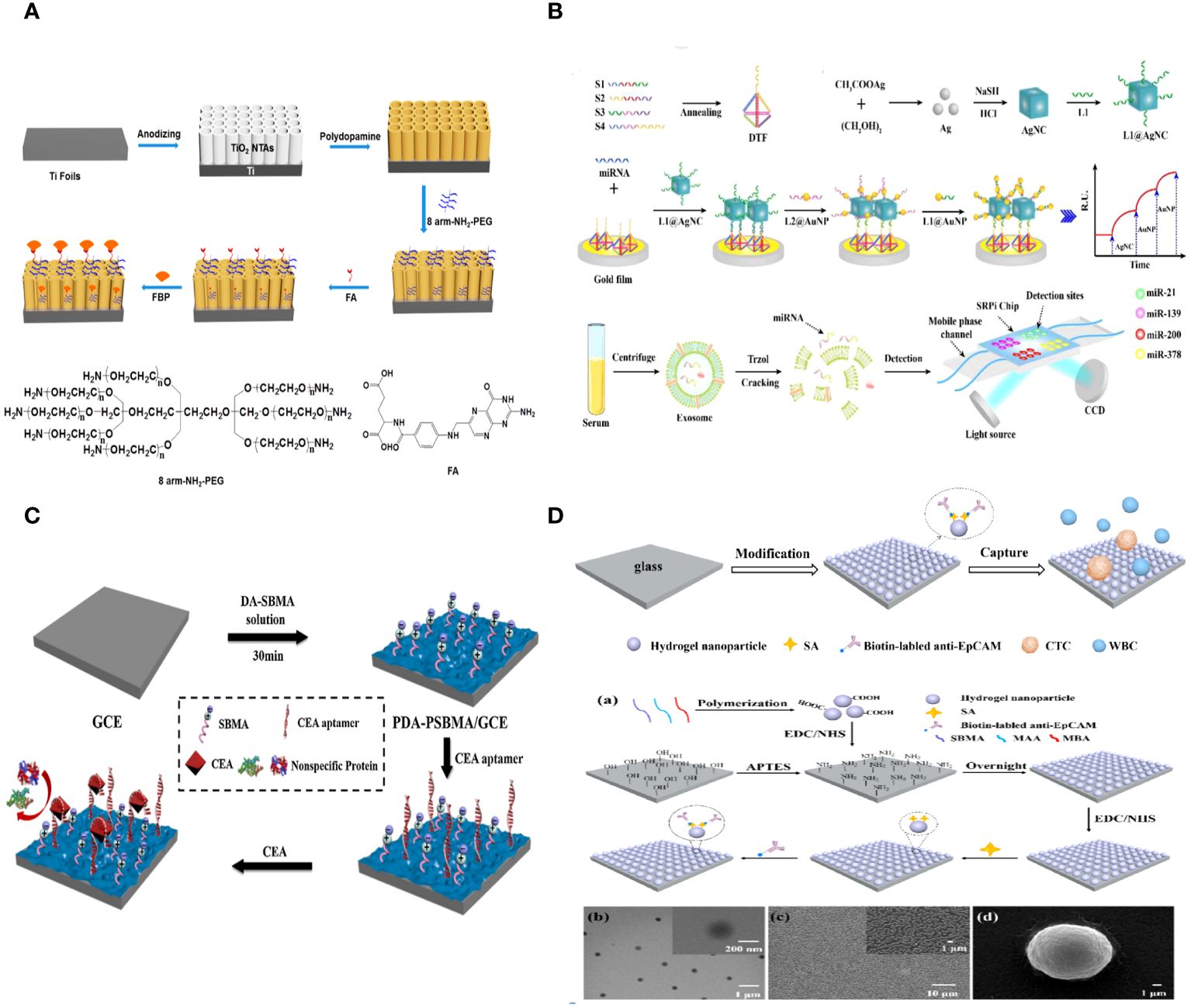
Figure 4 (A) Schematic Representation of the Fabrication of a PEC Biosensor for FBP Detection (112). (B) Schematic illustration of the multiplex exosomal miRNAs detection using SPRi biosensor (113). (C) Schematic illustration of the fabrication process of the PDA-PSBMA based sensing platform (114). (D) Schematic illustration for the capture of circulating tumor cells (CTCs) using the hydrogel nanoparticle substrate. Preparation and characterization of hydrogel nanoparticle substrate (115).
Exosomal miRNAs have the potential to serve as tumor biomarkers for the early detection of non-small cell lung cancer (NSCLC). A biosensor utilizing surface plasmon resonance imaging (SPRi) was created to identify several non-small cell lung cancers (NSCLC)-related exosomal miRNAs in a clinical sample. This biosensor combines an Au-on-Ag heterostructure with a DNA tetrahedral framework (DTF). Exosomal miRNAs are trapped by different DNA-templated fluorescent probes fixed on the gold array chip. The ssDNA-functionalized silver nanocube hybridizes with collected exosomal miRNAs. Subsequently, ssDNA-coated Au nanoparticles assemble on the AgNC surface, creating Au-on-Ag heterostructures that act as crucial markers for increased SPR response. The SPRi-based biosensor, utilizing DNA-programmed Au-on-Ag heterostructure and DTF, demonstrates a broad detection range of 2 fM to 20 nM, an extremely low limit of detection of 1.68 fM, increased capture efficiency, and enhanced antifouling capacity (Figure 4B) (113).
A new method was created to build highly sensitive and resistant biosensors that can detect the tumor marker CEA in complex biological samples. This method involves a one-step copolymerization of polydopamine (PDA) and poly (sulfobetaine methacrylate) (PSBMA). When copolymerized PDA and PSBMA are present, CEA aptamers with thiol groups can be connected to the PDA through the Michael addition reaction. The zwitterionic polymer PSBMA helps maintain the antifouling properties of the sensing interface. The electrochemical biosensor demonstrated effective efficacy in detecting CEA, with a linear range of 0.01–10 pg/mL and a low limit of detection (LOD) of 3.3 fg/mL. The presence of PSBMA in the sensing interface allowed the electrochemical biosensor to detect CEA in complex biological samples with a good antifouling effect, demonstrating the potential of biosensors using copolymerized PDA and PSBMA for analyzing human serum samples (Figure 4C) (114).
3.1.3 Application of antifouling materials in CTCsCirculating tumor cells (CTCs) are a small number of cancer cells that move from the primary or metastatic tumor site into the bloodstream and play a crucial role in cancer metastasis, which is the primary cause of cancer-related mortality (116). CTC detection, a non-invasive approach, could allow for early cancer diagnosis, prognosis, real-time treatment monitoring, and the identification of new treatment targets. Circulating tumor cells (CTCs) are cells that are released from solid tumor tissue into the bloodstream and are being studied as a diagnostic for early cancer diagnosis and prognosis. Various systems have been created to isolate circulating tumor cells due to their potential importance in clinical settings. Yet, effectively isolating CTCs poses considerable hurdles, particularly in attaining the required sensitivity and specificity because of their great scarcity and severe biofouling in blood, which includes numerous background cells and diverse proteins. Recent advancements in CTC detection technology have led to the development of extremely efficient and specific platforms for capturing CTCs, which have improved capture efficiency, purity, and sensitivity. To improve the purity and specificity of CTC capture, capture substrates like nanofiber mats need to be changed so they don’t allow nonspecific proteins to stick to them or blood cells to stick to them.
Zhili Wang et al. created an antifouling nanostructure substrate using hydrogel nanoparticles to efficiently capture circulating tumor cells (CTCs) from blood samples. The hydrogel nanoparticles were produced using a straightforward polymerization process using zwitterionic sulfobetaine methacrylate (SBMA), methacrylic acid (MAA), and N, N’-methylene bisacrylamide (MBA). SBMA can create an efficient antifouling layer on the substrate to prevent random cell adhesion. MAA can provide active carboxyl groups for attaching antibodies to enable specific capture of circulating tumor cells (CTCs). The nanostructured surface can improve the interaction between target cells and the substrate surface that has been modified by antibodies. This makes CTC capture more effective. Additionally, there was no need for any alterations to the antifouling molecules on the surface of the hydrogel nanoparticle substrate, which decreased the complexity and difficulty of preparing the substrate. The findings showed that the antibody-modified hydrogel nanoparticle substrate trapped about 87% of MCF-7 cells. Conversely, the substrate exhibited minimal adhesive capability for the nonspecific cells (K562 cells), capturing only 0.15% of the cells. 98% of the collected cells maintained high cell viability. 1–32 circulating tumor cells per milliliter were identified in the blood samples of five cancer patients, whereas no circulating tumor cells were observed in five healthy samples (Figure 4D) (115).
Tong Li et al. constructed a red blood cell membrane mimetic surface (CMMS) on various substrates to prevent blood cell attachment. Tumor cell-targeting ligands, folic acid (FA) and arginine-glycine-aspartic acid (RGD) peptides, are attached to the CMMS to enhance its ability to capture tumor cells, creating the decorated surface (CMMS-FA-RGD). The CMMS consists of a self-adhesive polydopamine layer inspired by mussels, together with a non-fouling or anti-cell adhesion layer made of a phosphorylcholine zwitterion polymer and poly (ethylene glycol) (PEG) that is covalently anchored. The extended sections of the PEG chains attached to the anchored CMMS are linked with FA and RGD ligands to provide targeted binding to tumor cells. Additionally, all elements of the systematically created surfaces can be precisely controlled to enhance non-specific cell repulsion and tumor cell adhesion. The intricately designed CTC capture surface boosts the HeLa cell enrichment factor to 18000-fold by preventing over 99.999% of blood cells from adhering, leading to a high capture efficiency (91%) and capture purity (89%) from spiked whole blood samples. This method of modifying surfaces to collect tumor cells independently of the substrate and reject blood cells could offer a simple, adaptable, and cost-effective technology solution for improved cancer diagnostics and targeted therapy (Figure 5A) (117).
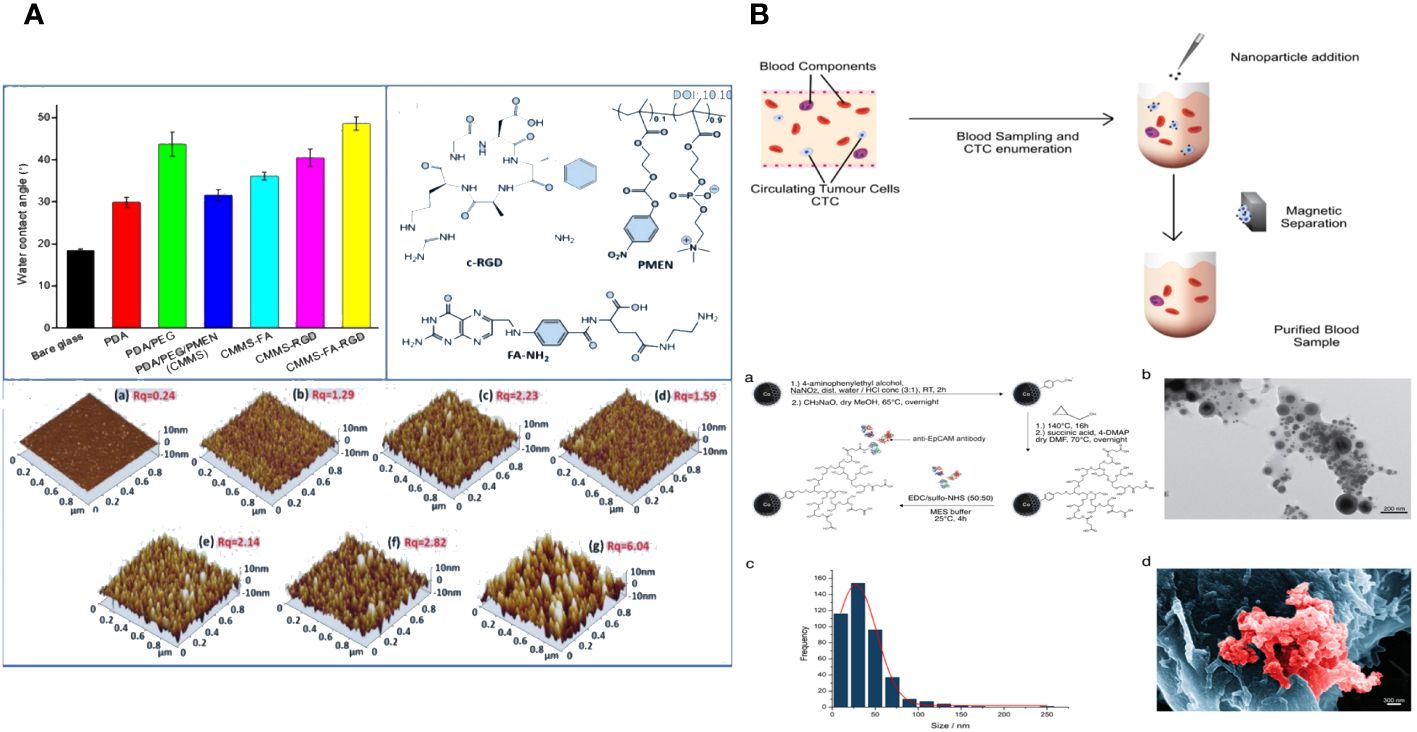
Figure 5 (A) The fabrication, blood cell repellence and tumor cell capture of ligands decorated cell membrane mimetic surface (CMMS-FA-RGD) (117). (B) Overview of working principle in blood from cancer patient; Nanoparticle synthesis and characterization (118).
Novel magnetic nanoparticles consisting of carbon-coated cobalt (C/Co) were created and linked with anti-epithelial cell adhesion molecule (EpCAM) antibodies to investigate their antifouling and separation characteristics. The recently created C/Co nanoparticles demonstrated outstanding separation and antifouling characteristics. The tumor cells added to healthy patients’ blood samples were effectively eliminated by an interaction with an anti-EpCAM antibody. The nanoparticles showed little interaction with other blood components, such as lymphocytes or the coagulation system. On average, at least 68% of circulating tumor cells (CTCs) were eliminated from the blood samples of carcinoma patients with metastatic illnesses. The nanoparticles have the potential to stimulate the creation of a blood purification technology, like a dialysis device, to remove circulating tumor cells from the blood of cancer patients after surgery and potentially enhance their outlook (Figure 5B) (118).
3.2 Enhancement of tumor treatmentNanoparticle-based cancer treatments have been extensively researched due to their ability to minimize side effects and enhance effectiveness (119). An ideal nanomaterial should possess prolonged blood circulation, increased accumulation in tumor tissue, and improved internalization by cancer cells (120). To accomplish this, “stealthy” coverings such as hydrophilic polymers, proteins, and cell membranes were utilized to shield nanomaterials, resulting in prolonged blood circulation and effective accumulation at tumor locations. This is achieved by a phenomenon called the increased permeability and retention (EPR) effect (121). Yet, these covert nanomaterials frequently experience diminished absorption by cancer cells, resulting in low levels within cancer cells and tissues.
One frequent technique to improve the absorption of nanomaterials by cancer cells is to incorporate targeted molecules. Adding targeting moieties like antibodies could unfortunately make nanomaterials less effective at passive targeting and extending their circulation time. It remains a significant problem to prolong the circulation of nanomaterials while simultaneously enhancing their uptake by cancer cells. A new targeting technique was created using nanoparticles designed with a responsive surface coating. This coating may be deactivated in tumor tissue under specific conditions, enabling cancer cell absorption (122). One effective approach involves attaching cancer-targeting components to nanoparticles and then applying antifouling coatings to inhibit non-specific interactions while circulating (123). Removing the shielding layer would reveal the targeted moieties and trigger cell uptake once the nanomaterials reach the tumor microenvironment.
3.2.1 Enhancing tumor targetingThe most crucial variables in cancer diagnostics and therapy are tumor targetability and site-specific release. Biofouling is the unintentional accumulation of organisms, proteins, or biomolecules on the surface of metal nanocomplexes. The phenomenon is a significant concern in bioinorganic chemistry as it causes the formation of a protein corona, leading to the destabilization of a colloidal solution and triggering unwanted macrophage-driven clearance, ultimately resulting in the unsuccessful delivery of a specific therapeutic payload.
For successful tumor treatment, the nanoplatforms gathered in the tumor area must exhibit improved cellular absorption and penetration into the tumor. Antifouling nanoplatforms like zwitterionic near the tumor site may not be optimal for increasing tumor cell uptake and penetration. Furthermore, when the nanoplatforms are taken up by the tumor cells, the medication molecules they contain should be quickly released to prod
留言 (0)