The exponentially growing world population, which is estimated to reach 9.5 billion by 2050, will imply more food needs and more demand of agricultural productivity, particularly vegetable crops. Tomato (Solanum lycopersicum L.) is one of the most consumed and economically important vegetable crops in the world with a world production of over 186 million tons (FAOSTAT 2022). Plant nutrition is one of the major concerns for modern agriculture. Nitrogen (N), phosphorus (P), and potassium (K) are major growth-limiting macronutrients for plant growth. Sustainable agricultural practices should be encouraged, and development of new biological tools is needed. Application of beneficial soil microbia, either alone or combined with natural nutrient sources such as rock phosphate, represent a promising strategy. On the other hand, tomato is attacked by the gram-positive bacterium Clavibacter michiganensis subsp. michiganensis (Cmm), which causes bacterial canker on tomato stem, leaves and fruits. The disease is considered the major threat for tomato cultivation all over the world, as it can cause economic losses that can reach up to 100% of production (Chang et al., 1992; Wang et al., 2022). Nowadays, two main strategies are commonly adopted for enhancement of plant growth and disease management: (i) development of new cultivars with enhanced growth and nutritional qualities and resistance to phytopathogens (ii) use of chemical fertilizers and pesticides. However, for tomato bacterial canker, no resistant cultivars are available, and no chemical treatments are efficient to combat this disease.
Phosphorus is the second most important nutrient for the plant growth after nitrogen, which plays a crucial role in energy transfer, photosynthesis, signal transduction, and respiration (Ham et al., 2018; Bouizgarne, 2022). It accounts for between 0.2 and 0.8% of the dry weight of plants, and adequate availability of this nutrient is required in many physiological and biochemical plant activities. Phosphorus exists in two forms in the soil, organic and inorganic phosphates. Insoluble forms of iron, aluminum, and calcium as aluminum phosphate (Al3PO4) and ferrous phosphate (Fe3PO4) exist in acidic soils and as calcium phosphate (Ca3PO4)2 in calcareous soils. Natural rock phosphate (RP) is composed of calcium hydroxyapatite [Ca10 (PO4)6 OH], with only small fractions of phosphate anions, H2PO4−, HPO42−, and PO43−. In addition, soluble forms are rapidly converted into insoluble complex forms with iron, aluminum, or calcium. All these mechanisms render phosphorus into an insoluble form which is not directly available for plant uptake (Adnan et al., 2020). This conversion is also responsible for the loss of approximately 75% of chemical P fertilizer, added to soils each year, which rapidly becomes unavailable for plants. Consequently, although abundant in natural soils, P-availability in the soil solution is often insufficient as most soils are poor in phosphorus, which is considered a limiting factor for plant nutrition (Bouizgarne, 2022). Its deficiency severely restricts plant growth and yields. Under a limited P-availability, plant root system becomes concentrated in the topsoil, resulting in inhibited elongation of primary root and stimulation of lateral root initiation. In the other hand, direct application of rock phosphate as fertilizer is not effective in most soils as plants could not extract phosphorus from these forms, making increasing phosphorus use efficiency a major challenge in intensive agricultural production systems.
This issue could be addressed efficiently by using soil microorganisms, particularly those living in the vicinity of the roots, also called plant growth-promoting rhizobacteria (PGPR) in an eco-environmental context. PGPR have attracted more attention during last decades as they have the potential to contribute to acceptable crop yields while preserving soil health. They are crucial in maintaining agricultural soil function because they contribute to soil processes such as stabilizing soil aggregates, improving soil structure, breakdown of organic matter, and cycling of nutrients such as carbon, nitrogen, phosphorus, and sulfur. In addition, they act by providing growth compounds to plants, helping plants to uptake soil nutrients, or preventing them from being infected by phytopathogens (Bouizgarne, 2013; Riaz et al., 2021; Zhang et al., 2021).
Among PGPR, bacteria that are able to solubilize insoluble phosphate forms, named phosphate solubilizing bacteria (PSB), dwell in most soils and potentially represent 40% of the culturable population (Richardson, 2001). PSB have been considered important for making soluble phosphorus forms available to plants by solubilizing insoluble inorganic phosphates or mineralizing organic phosphate compounds into soluble forms (Gyaneshwar et al., 2002; Rodríguez et al., 2006). They also prevent the released P from being immobilized again (Richardson et al., 2011). The most known mechanism by which PSB act is by generating organic acids that are able to solubilize complex mineral phosphates, including calcium phosphates in alkaline soils and RP (Alori et al., 2017; Bargaz et al., 2021). The mechanisms of PGPR-mediated enhancement of plant growth and yield of many crops are not yet fully understood. However, some PSB might actively contribute to the plant health by other mechanisms such as producing phytohormones or active substances against some specific plant pathogens or reducing ethylene synthesis (Bouizgarne, 2013). Bacterial strains belonging to genera Bacillus, Pseudomonas, and Serratia were reported for their ability to solubilize insoluble mineral phosphate compounds, such as dicalcium and tricalcium phosphate and rock phosphate (Ahmad et al., 2022; Bouizgarne et al., 2023). In particular, bacteria belonging to Pseudomonas species have frequently been isolated from the plant rhizosphere, and several have been reported as plant growth-promoting rhizobacteria (PGPR), considering their ability to increase P-availability in plants, thus helping to sustain crop health and productivity (Bouizgarne, 2013). In addition, the opportunity of improving the P uptake of crops by artificial inoculation with P-solubilizing rhizobacteria has been shown as an attractive plan for research (Azaroual et al., 2020).
The demand for chemical-free food is rising among consumers and sparked the research for environmentally safe techniques in order to reduce the reliance on chemicals. It is therefore recommended to search for alternative methods such as using phosphate solubilizing PGPR adapted to the rhizosphere of plant roots combined with natural rock phosphate in order to enhance the effectiveness of rock phosphate as fertilizer. The application of phosphate solubilizing bacterial strains from Moroccan rock phosphate, for tomato growth promotion has been reported (Bouizgarne et al., 2023). This is the first report of the use of Pseudomonas or Bacillus, combined with rock phosphate, for tomato growth promotion and protection against the causal agent of bacterial canker, Clavibacter michiganensis subsp. michiganensis, by improving host resistance mechanisms.
Thus, the present study aims (i) to screen for efficient phosphate, potash, and zinc solubilizing bacteria from tomato rhizosphere soil in addition to indole acetic acid (IAA) production, (ii) investigate the beneficial effects of their combination with natural rock phosphate (RP) on tomato growth and nutrient uptake in greenhouse experiments, and (iii) biocontrol of tomato bacterial canker caused by Cmm through triggering induced systemic resistance in inoculated tomato seedlings.
2 Materials and methods 2.1 Isolation of bacteria and screening for P, K, and Zn solubilizing activity 2.1.1 Screening for phosphate solubilizing bacteriaBacterial strains were isolated from two soil areas in the Souss-Massa region, Morocco. Soil samples were collected from the root system of five healthy tomato plants. Soil dilutions were performed, and 0.1 mL was spread on the King B medium (per liter: proteose peptone 20 g; K2HPO4 1.5 g; MgSO4.7H2O 1.5 g; pH 7.2). After incubation at 28°C for 48 h, bacteria were stained with the Gram staining method and checked for cytochrome-C oxidase (oxidase test). Bacteria showing fluorescence under UV at 365 nm were considered as fluorescent pseudomonads. The capacity of isolates to solubilize inorganic phosphate was tested on National Botanical Research Institute’s phosphate (NBRIP) growth medium [per liter: 10 g glucose, 0.1 g (NH4)2SO4, 0.2 g KCl, 0.25 g MgSO4, 7H2O, 5 g MgCl2, 6H2O, and 5 g tri-calcium phosphate (TCP)] (Nautiyal, 1999). Appearance of halos around colonies reflected tri-calcium phosphate solubilization. The experiment was performed in four replicates. Halo diameter around colony and the colony diameter were measured, and the solubilization index (PSI) was calculated according to the following formula:
PSI=Diameterofsolubilizationhalo+Colonydiameter/ColonydiameterQuantitative estimation of phosphate solubilization was performed in liquid NBRIP medium; 20 mL of NBRIP containing TCP was inoculated with 100 μL of bacterial suspension from a 24-h preculture in King B medium (KB) at a final concentration of 106 cfu mL−1. The flasks were incubated at 28°C in the dark under shaking at 180 rpm in an incubator shaker (KS 3000 IC, IKA® Werke Staufen, Germany). After 48 h, phosphate solubilization was monitored by colorimetry in the culture supernatant at 600 nm, according to the Fiske and Subbarow (1925) method. A calibration curve was plotted using KH2PO4, and the pH variation was estimated by recording pH value at starting conditions and after 48 h of culture. The experiment was performed in six replicates, and the amount of P in the medium was expressed as mg L−1.
2.1.2 Identification of organic acid by HPLCHigh-performance liquid chromatography (HPLC) (Ultimate 3000 Dionex), coupled with mass spectrometry (Exactive Plus de Thermo Scientific) and equipped with a BDS Hypersil C18 (150 × 4.6 mm × 5 μm) HPLC Column, was used to identify and quantify organic acids. Flasks containing 20 mL of liquid NBRIP with TCP as P source were inoculated with 100 μL of standardized bacterial suspension at a final concentration of 106 cfu mL−1 and incubated at 28°C in the dark under shaking at 180 rpm. After 48 h, samples were collected, filtered through a 0.22 μm cellulose membrane, and injected into the chromatographic column. Detection of organic acids was performed using the following organic acids (Supelco/Sigma–Aldrich) as analytical standards with typical retention time means as follows: 2-ketogluconic acid (2.10 min), phytic acid (3.25 min), gluconic acid (3.75 min), tartaric acid (3.93 min), quinic acid (4.03 min), oxalic acid (4.25 min), succinic acid (4.85 min), DL-iso-citric acid (4.86 min), ascorbic acid (5.05 min), maleic acid (5.56 min), citric acid (7.51 min), fumaric acid (8.10 min), shikimic acid (8.71 min), propionic acid (9.00 min), malonic acid (9.27 min), and benzoic acid (12.5 min). The mobile phase was 0.1% H3PO4 (pH 1.81) with a flow rate of 0.5 mL min−1 and a 100 μL injection per sample, according to Marra et al. (2015). The acquisition time of the chromatograms was estimated to be 30 min with 30 min of intervals between runs. Detection was performed by UV at 210 nm with a diode array detector (DAD).
2.1.3 Screening for potassium solubilizationPotassium solubilization was carried out by bacterial isolates on Aleksandrow agar medium (Rajawat et al., 2014) containing 5.0 g/L glucose, 0.5 g/L MgSO4, 7H2O, 0.1 g/L CaCO3, 0.006 g/L FeCl3, 2.0 g/L Na2 HPO4, and feldspar (2.0 g/L) as sole K source. The plates were then incubated for 2 days at 28°C. Appearance of yellow halos around colonies reflected potassium solubilization. The experiment was performed in four replicates. Halo diameter around each colony and the colony diameter were measured. Potassium mineral solubilizing index (KSI) was calculated as follows:
KSI=Diameterofsolubilizationhalo+Colonydiameter/Colonydiameter 2.1.4 Screening for zinc solubilizationThe zinc solubilization was investigated in Bunt and Rovira medium (Bunt and Rovira, 1955) (glucose: 20.0 g, peptone: 1.0 g; yeast extract: 1.0 g, (NH4)2 SO4: 0.50 g; K2 HPO4: 0.40 g; MgCl2: 0.10 g, FeCl3: 0.01 g, distilled water 1.000 mL, and adjusted pH 6.7) containing 0.1% insoluble zinc oxide. Fresh cultures of bacteria were spot inoculated at the center of the plate in triplicates with toothpicks and incubated for 72 h in the dark at 26°C. The experiment was performed in four replicates. The appearance of halo zones around colonies reflected zinc solubilization. Halo diameter around each colony and the colony diameter were measured. The zinc solubilizing index (ZSI) was calculated as follows:
ZSI=Diameterofsolubilizationhalo+Colonydiameter/Colonydiameter 2.2 Screening for indole-3-acetic-acid productionProduction of IAA was demonstrated according to Bric et al. (1991). Isolates were cultivated in Luria–Bertani (LB) medium (per liter: tryptone 10 g; yeast extract 5 g; NaCl 5 g; pH 7.2) and incubated at 28°C for 2 days, and then, positive colonies were revealed by Salkowski reagent. For quantitative measurement, liquid LB medium containing 0.1 g/L of L-Trp was seeded with isolates and incubated for 3 days at 28°C. Amount of IAA was determined according to Gravel et al. (2007). A calibration curve was obtained by synthetic IAA (Sigma–Aldrich). The experiment was performed in six replicates, and amounts of IAA were expressed in μg mL−1.
2.3 Effect of bacterial isolates on tomato seed germinationBacterial isolates were cultivated for 48 h days in King B broth and incubated at 28°C on a rotating shaker at 180 rpm in an incubator shaker (KS 3000 IC, IKA® Werke Staufen/Germany). Tomato seeds (cultivar calvi) were surface sterilized using 2% sodium hypochlorite and subsequently washed with distilled water. Then, 50 tomato seeds were placed in sterile Petri dishes containing wet sterile paper filter. Seeds were coated with 1 mL of a mixture containing culture suspension from each of the six bacteria at a concentration of 106 cfu mL−1, 0.5% carboxymethyl cellulose (CMC), and 0.1% RP. The composition of RP was evaluated by using scanning electron microscopy (SEM–EDX, JSM IT-100, JEOL, USA) as follows: C: 7.1%, O: 48.6%, F: 11.0%, Na: 1.8%, Al: 1.7%; Si: 3.5%, P: 8.9%, and Ca: 17.1%. RP contains particles with an average size of 377 μm. pH of H2O (1.1) was 6.86 and electrical conductivity was 488 mmho cm−1. It contains approximately 5% total phosphorus and low amount of assimilable phosphorus (P-Olsen) (P2O5 < 18 mg/Kg). It contains low amount of organic carbon (0.23%) and organic matter (0.40%) and 99.5% of mineral matter with 5.72% total limestone. The C/N ratio was 3.55. RP contains low amount of nitrogen (0.012%), calcium (CaO) 1.93 g/kg, and potassium (0.01 g/kg). RP magnesium (MgO) content was 0.34 g/kg. The mixture was added to seeds under shaking condition until a fine coating appeared on seeds. The plates were placed in a germination chamber at 28 ± 2°C for 12 days. Germinated seeds of negative control were coated with bacteria-free CMC. The percent of germinated seeds was determined after 15 days (International Seed Testing Association, 2009). The experiment was performed with three replicates.
2.4 Greenhouse experiments 2.4.1 Rhizosphere colonization by selected bacteriaTo investigate the ability of the selected isolates to colonize the roots of tomato plants, six tomato seeds (cv. calvi) were first surface disinfected with 2.5% sodium hypochlorite solution for 3 min and then coated with 1 mL of a mixture containing culture suspension from each of six bacteria at a concentration of 106 cfu mL−1, 0.5% carboxymethyl cellulose (CMC), and 0.1% RP and germinated in Petri dishes. After germination, seedlings were transferred in a mixture of soil and peat (2,1 w,w) and cultured in axenic conditions. Soil characteristics were as follows: silt (6.70%), clay (4.15%), sand (87.32%), pH (H2O): 8.57, and electrical conductivity (EC) at 25°C (0.28 mS cm−1). Water retention capacity was 30.71%, and total organic carbon was 0.4%. Rhizosphere colonization was determined after 15 days by uprooting seedlings and removing root adhering soil by washing three times. The homogenate was then stirred in 9 mL sterile water for 5 min. Then, serial decimal dilutions were carried out in sterile physiological water. Overall, 0.1 mL of each dilution was spread on King B agar medium and incubated at 28°C for 24 h, and the growth of the bacterial isolates was estimated by counting colonies. Rhizosphere colonization was expressed as colony forming unit (cfu) g−1 soil. Each experiment was performed in triplicate.
2.4.2 Effects on plant growth parametersIn addition, after germination, seedlings were transferred to disinfected pots (one seedling per pot) containing 5 kg of a mixture of soil and peat (2,1 w,w) amended with RP at 1 g/kg mixture. Plants were placed in an experimental greenhouse where the average temperature was 35°C during the day and 10–18°C at night, a photoperiod of 16 h day/8 h night, and an average relative humidity of 67.5%. Seedlings were watered daily with sterilized water. To check for the ability of bacteria to promote the growth in tomato, the following experiments were carried out: (i) control seedlings: tomato seedlings were grown in the mixture of soil and peat and amended with RP (1 g/kg) as a source of phosphorus and (ii) bacterized seedlings: tomato seedlings were grown in the mixture of soil and peat and amended with RP (1 g/kg) and inoculated at plant rhizosphere with 20 mL of bacterial suspension from each selected isolate at 106 mL−1. Each month, the treatment with the bacteria at plant rhizosphere was renewed. Thirty seedlings were used for each experiment in three replicates. Tomato plants were harvested after 60 days, and then, the length of the seedling shoots and roots and the shoot and root fresh and dry weight were measured. In addition, the following physiological parameters were measured:
2.4.3 Effects on chlorophyll content and chlorophyll fluorescenceThe effect of the different treatments on total chlorophyll content was measured in youngest fully expanded leaves, which were detached from tomato plants immediately after harvesting. Measurements were performed according to Arnon (1949). Four repetitions were performed per treatment, and chlorophyll contents were expressed in mg g−1 DM. The chlorophyll fluorescence (Fv/Fm) (Maxwell and Johnson, 2000) was measured using a portable fluorometer (Handy PEA fluorimeter, Hansatech instruments, Norfolk, UK). This parameter was measured on well-developed leaves of the same rank (seven repetitions per treatment). Clamps were placed on the upper side of the leaves for 20 min, to keep the leaves in the dark before measurement.
2.4.4 Effects on proteins and total soluble sugarsThe extract was prepared by mixing 100 mg of the aerial part (stem+ leaves) of each plant sample with 4 mL of 0.1 M phosphate buffer pH 6 containing 5% insoluble polyvinylpyrrolidone and 0.1 mM ethylenediaminetetraacetic acid (EDTA). The protein content in the supernatant was measured according to Bradford (1976). Total soluble sugar was determined according to Dubois et al. (1956). The results were expressed in mg g−1 of fresh matter.
2.4.5 Effects on mineral elements contentsSodium (Na+), potassium (K+), calcium (Ca2+), and phosphorus (P) were measured after mineralization of the plant shoot and root material. Sodium (Na+), potassium (K+), and calcium (Ca2+) cations were measured by flame photometer PFP7 (Jenway, United Kingdom), according to Tuna et al. (2007). To measure phosphorus in leaves, the phospho-molybdate blue method was used (Olsen and Sommers, 1982). In brief, an aliquot of the solution (1 mL) was added to 5 mL of the following reagent mixture: ammonium molybdate (2.5%), hydrazine sulfate (0.15%), and 4 mL of distilled water. After heating in a water bath at 70°C for 10 min and cooling for 5 min, optical density of the samples was measured at λ = 820 nm with a UV/VIS spectrophotometer (UV-3100PC, VWR®, United States).
2.5 Biocontrol of tomato bacterial canker by induced systemic resistance 2.5.1 Ability of bacteria to grow inside root tissues and on root exudatesTomato seedlings were used to test the growth of six bacterial isolates, PsT-04c, PsT-94s, BaT-86s, PsT-116, PsT-124, and PsT-130, on root tissues and crude root exudates. Tomato seedlings (15 days old) were removed carefully from pots containing the mixture of soil and peat and washed three times with sterilized water. The roots were carefully cleaned with cotton soaked in alcohol, surface disinfected with 0.4% sodium hypochlorite, and rinsed extensively with sterile deionized water. 10 μL of each bacterial suspension at a concentration of 106 bacteria mL−1 was injected into the main roots of six tomato seedlings by using a Hamilton micro syringe. Seedlings were then transferred to aseptic flasks containing 450 mL of sterile distilled water, inoculated with 106 mL−1 of each of the six bacteria, and hydroponically grown in a growth chamber at 25 ± 2°C in natural light with 16-h photoperiod for 15 days. The control consisted of six seedlings injected only with sterile water and transferred to uninoculated flasks under the same conditions. To check for the ability of isolates to grow in root tissues, after 15 days, roots were ground in a mortar at 4°C; 1 g of the homogenate was stirred in 9 mL of sterile water for 5 min. Then, serial decimal dilutions were carried out in sterile physiological water. In total, 0.1 mL of each dilution was spread on nutrient agar plates and amended with cycloheximide (40 μg mL−1) to inhibit the fungal growth. Three replicates were carried out by dilution. The agar plates were then incubated at 28°C for 48 h. Colonies of bacteria grown from root extracts were identified by their phenotypic characteristics, and representative colonies were identified by 16S rRNA sequencing. Colonies were counted, and their growth was estimated as colony forming units (cfu) per gram roots.
For growth on root exudates, exudates from control seedlings were extracted and sterilized by filtration using a 0.22 μm Millipore filter. In total, 20 mL exudates were added to 80 mL sterilized distilled water and 1.5 g agar in order to prepare a solid medium (exudates agar). For each isolate, a serial dilution of non-sterilized exudate was performed, and 0.1 mL of each dilution was spread on the exudate agar, amended with cycloheximide, and incubated for 48 h at 28°C. Control consisted of root exudate-free medium. Three replicates were performed for each isolate. Colonies of bacteria grown on root exudates were counted, roots were weighed, and growth was estimated as colony forming units (cfu) per gram roots. No growth was recorded in the control without root exudates.
2.5.2 Ability of bacteria to protect tomato against Cmm and induce systemic resistance 2.5.2.1 Bacterial strains: Pseudomonas strains and the pathogen CmmA virulent strain of Clavibacter michiganensis subsp. michiganensis (Cmm) and the two selected Pseudomonas isolates, PsT-04c and PsT-130, were used for biocontrol experiments. Cmm colonies were cultivated on a slightly modified BCT medium (Ftayeh et al., 2011) containing per liter of deionized water: 2.5 g mannitol, 2.0 g yeast extract, 1.0 g K2HPO4, 0.1 g KH2PO4, 0.05 g NaCl, 0.1 g MgSO4. 7H2O, 0.015 g MnSO4. H2O, 0.015 g FeSO4. 7H2O, 0.6 g H3BO3 (pH 7.1), and 15 g agar. After autoclaving and cooling at 50°C under stirring, nalidixic acid (20 mg/L), trimethoprim (100 mg/L), and 50 μL of a solution of penconazole (80 g/L) were added to the medium. To confirm the pathogenicity of the Cmm isolate, tomato seedlings were infected with a suspension of Cmm at 106 bacteria mL−1 by depositing 50 μL droplets on the surface of the leaves and injecting 10 μL droplets into roots. After 15–20 days, Cmm was reisolated from infected seedlings, showing symptoms of bacterial canker.
2.5.2.2 Protection of tomato seedlings against bacterial cankerFor biocontrol experiments in greenhouse experiments, 1-month-old tomato seedlings obtained from seed germination were treated by either Cmm or Pseudomonas + Cmm and transplanted into the mixture containing soil and peat (2: 1). Treatments were carried out as follows:
• Negative control: seedlings for which roots were soaked only in sterilized water for 8 h and transplanted into pots.
• Positive control: seedlings infected by soaking in 10 mL of Cmm suspension at 106 bacteria mL−1 for 8 h and transplanted into pots.
• Bacterial treatment: seedlings for which roots have been soaked in a 10 mL suspension of each of the two Pseudomonas strains PsT-04c and PsT-130 and subsequently infected by Cmm. Treatments were performed as follows: The first treatment was carried out with Pseudomonas (1st immunization) by soaking seedlings in 10 mL of each Pseudomonas isolate at 106 bacteria mL−1 for 8 h before transplanted into pots. After 1 week, seedlings were infected by inoculating 10 mL of Cmm suspension at 106 bacteria mL−1 at root level. Finally, after 20 days, the second bacterial treatment, by inoculating 10 mL of each of the two Pseudomonas isolates at 106 bacteria mL−1 at root level, was performed (2nd immunization).
Each treatment consisted of 32 seedlings arranged in a complete random block. After 120 days of culture in greenhouse, symptoms were observed, and the mortality rate was calculated as follows:
Numberofdiedseedlingsineachtreatment/Numberofseedlingsincontrol%x100In addition, the following parameters were measured:
2.5.2.3 Total soluble phenolic compoundsAfter 1 month of the second bacterization, control tomato seedlings, Cmm-infected seedlings, and seedlings treated with the two isolates PsT-04c and PsT-130 were used for the evaluation of the root-soluble phenolic compound contents, which is carried out according to the protocol described by Macheix et al. (1990). Roots (200 mg) were ground in an ice bath with 2 mL of 80% methanol before stirring for 15 min and centrifugation at 7,000 g for 3 min. The extraction operation was repeated three times, and the recovered supernatants were stored in the freezer (−20°C). The supernatants were used for quantitative determination of the total soluble phenolic compounds by the Folin–Ciocalteu reagent. The reaction mixture consisted of 50 μL of phenolic extracts diluted in 2 mL of distilled water and 0.5 mL of the Folin–Ciocalteu reagent (10%, v/v). After shaking, 0.5 mL of a Na2CO3 solution (20%) was added, and the mixture was incubated at 40°C for 30 min. For the colorimetric measurement, blank was prepared with 0.5 mL of Folin–Ciocalteu reagent (10%, v/v) and 0.5 mL of 20% Na2CO3. The results were read on a UV/VIS spectrophotometer (UV-3100PC, VWR®, United States) at 765 nm. The experiment was performed with three replicates, and total soluble phenolic contents were expressed in mg g−1 of fresh material by using a standard curve of caffeic acid.
2.5.2.4 Phenylalanine ammonia-lyase activityAfter 1 month of the second bacterization, the phenylalanine ammonia-lyase activity was determined according to Mozzetti et al. (1995). Tomato roots were ground in 100 mmol/L potassium borate buffer, pH 8.8, with 14 mmol/L 2-mercaptoethanol. The homogenate was then centrifuged at 13,000 g for 30 min, and 100 μL of the supernatant (enzymatic extract) was added to 1 mL of 100 mmol/L potassium borate buffer, pH 8.8, with 200 μL of 100 mmol/L L-phenylalanine. After incubation at 40°C for 60 min, the reaction was stopped by the addition of 250 μL of 5 N HCl, and the absorbance was determined at 290 nm. Cinnamic acid was used to perform a standard curve, and the protein content was determined according to Bradford (1976). The experiment was performed with three replicates, and L-phenylalanine was expressed as μg cinnamic acid mL−1 enzymatic extract.
2.5.2.5 Hydrogen peroxide (H2O2) contentOne month after the second bacterization, the H2O2 assay was performed according to Velikova et al. (2000). The fresh plant material (500 mg) was homogenized in 2 mL of 1 g/L solution of trichloroacetic acid (TCA). After centrifugation at 10,000 g for 15 min, 0.5 mL of the supernatant was added to the reaction mixture containing 0.5 mL of 10 mM potassium phosphate buffer (pH 7) and 1 mL of a 1 M solution of KI. The absorbance was measured at 390 nm. The experiment was performed with three replicates, and the H2O2 content was expressed in μg g−1 of fresh weight using a standard curve of H2O2, which was established under the same conditions.
2.6 Identification of bacterial isolatesThe characterization of bacterial isolates was performed by observing phenotypic traits such as colony morphology, Gram staining, and fluorescence under UV light (254 nm). Furthermore, oxidase, tween, and Levan tests were assayed according to the methods described by Lelliott et al. (1966). Other traits were determined by using API 20NE strips (Biomérieux-France). All tests were performed in triplicate. Molecular identification of the six isolates was performed by using 16S rRNA gene sequences as follows: the selected bacteria were grown on King B broth on a rotary shaker at 180 rpm in an incubator shaker KS 3000 IC (IKA® Werke Staufen/Germany) at 28°C for 48 h. After centrifugation, total DNA was extracted with the “MagPurix Bacterial DNA extraction kit” using the MagPurix extractor robot. Purified bacterial DNA was estimated by Nanodrop 8,000 (Thermo Fisher Scientific, USA). The bacterial 16S rRNA gene was amplified by using the universal 16S rRNA primers FD1 (5′- AGAGTTTGATCCTGGCTCAG-3′) and RP2 (5’-TACGGCTACCTTGTTACGACTT- 3′) (Weisburg et al., 1991). The PCR was carried out by using an ABI “Veriti, Applied Biosystems™” thermal cycler in a mixture containing 10 μL of PCR Master Mix (2X) (HS MyTaq DNA polymerase kit from Bioline), 1.5 μL of each primer, 130 ng of DNA, and purified water in a final volume of 25 μL, and the PCR program is as follows: 95°C, 2 min; (95°C, 30 s; 52°C, 30 s; and 72°C, 30 s) 35x; 72°C, 3 min. In total, 8 μL of PCR products was deposited on 1% agarose gel in the presence of the ladder of 1 kb molecular weight, and the gel was visualized by the “G Box” documentation system. Cleaned PCR products were used as a template for the cycle sequencing. Sequencing was performed using the 3130XL Dye Terminator Cycle Sequencing (DTCS) Quick Start kit (Applied Biosystems). The thermocycling conditions were as follows: 25 cycles of 96°C for 1 min, 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min, followed by a 4°C infinite hold. The Sephadex G50 superfine (Sigma–Aldrich) was used to remove unincorporated dye terminators by washing the column with 300 μL of distilled water and centrifuging at 1,500 g for 3 min before applying the sample to the column. The sequencing reaction was performed using the forward and reverse primers FD1 and RP2. Forward and reverse sequencing were performed using Big Dye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Sequence trimming and assembly were performed using DNA baser. Then, the sequences were submitted to Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990) and ClustalW was used for multiple alignment with available 16S rRNA sequences in GenBank (National Center for Biotechnology Information NCBI, Bethesda, MD, USA). Phylogenic relationships were performed by Mega 11 software (Stecher et al., 2020; Tamura et al., 2021). The phylogenetic tree was built using the neighbor-joining method (Saitou and Nei, 1987) with statistic method of maximum likelihood, taking into account the correction of Jukes and Cantor (1969). Tree topology was evaluated by bootstrap analyses (1,000 replicates) (Felsenstein, 1985). The cutoff for species identification was 98.8%. The sequence data were deposited at the GenBank database under the following accession numbers PsT-04c (OP677775), PsT-94s (OP677776), PsT-116 (OP677777), PsT-124 (OP677861), PsT-130 (OP677782), and BaT-68s (OP677783).
2.7 Data analysisValues are means and standard deviation of three or more than three replicates. Statistical analyses were conducted using SPSS software package version 22 for Microsoft Windows. The data obtained were examined through analysis of variance (ANOVA), to test the statistical differences. Means were compared at the 5% significance level using Tukey’s post-hoc test, to detect statistically significant differences between treatments. Principal component analysis (PCA) was performed using GraphPad Prism 9 software.
3 Results 3.1 Isolation of bacteria and screening for P, K, and Zn solubilizing activity and IAA productionIn the present study, we screened isolated bacteria for effective phosphate solubilization, and we investigated in vitro PGP traits and their potential as tomato plant growth promoters. The primary isolation of bacteria from tomato rhizosphere soil was performed on King B medium. In total, 100 different bacterial strains were isolated. Among these bacteria, those which developed yellow-green fluorescent pigment under UV light (365 nm) are putatively considered, belonging to the fluorescent pseudomonads. The isolates were further screened for phosphate solubilizing ability. Out of 100 isolates tested on NBRIP agar medium, 16 isolates (PsT-04c, PsT-11b, PsT-35b, PsT-63k, BaT-68s, PsT-77, PsT-82k, PsT-88k, BaT-91k, PsT-92k, PsT-94s, PsT-96k, PsT-98k, PsT-116, PsT-124, and PsT-130) were able to solubilize phosphate on NBRIP (Table 1). These isolates exhibited clear halo zones on NBRIP after 24–72 h of incubation at 28°C (Figure 1). In NBRIP broth, the growth of these isolates was accompanied with increased amount of released P ranging from 73.22 to 195.42 mg mL−1. The maximum amount was showed by isolate PsT-94s, while the lowest amount was detected in isolate PsT-63k. All isolates lowered pH of the medium compared with control. All isolates brought the pH value of the medium below 6. The pH of the medium at initial conditions ranged between 6.7 and 6.9 and dropped to 3.22 as the lowest value for the isolate PsT-130 after 48 h, suggesting acidification as the main mechanism for solubilization. Organic acids were detected by HPLC/MS in NBRIP cultures of six bacterial isolates (Figure 2 and Supplementary Figure S1), showing highest phosphate solubilization (isolates PsT-04c, BaT-68s, PsT-94s, PsT-116, PsT-124, and PsT-130). Retention times for all isolates were compared with those of standards. The main organic acids detected were gluconic acid, maleic acid, and citric acid (Figure 2). The rest of peaks were not determined in this investigation.
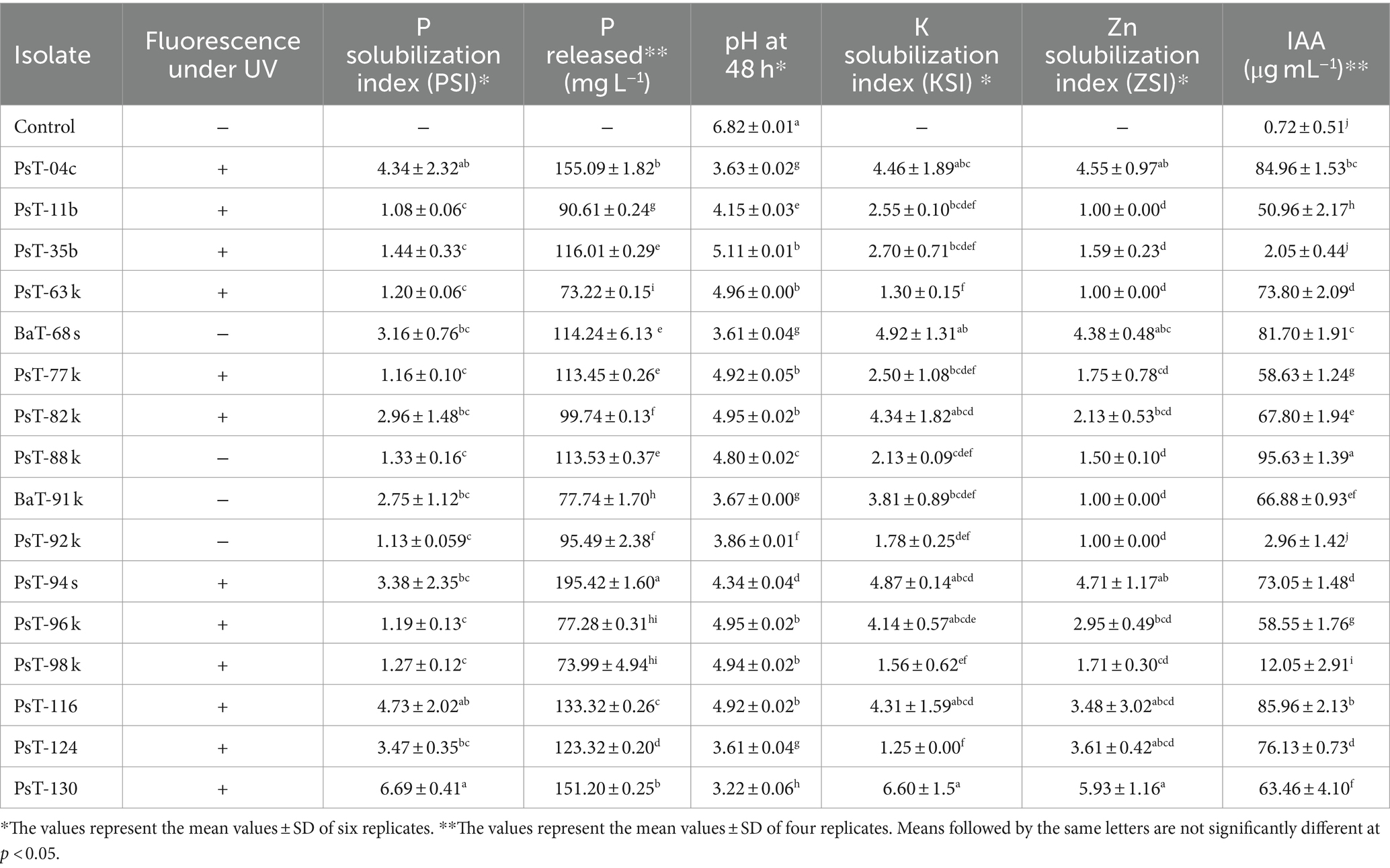
Table 1. Fluorescence under UV, phosphate, potash, and zinc solubilization and IAA production by 16 bacterial isolates.
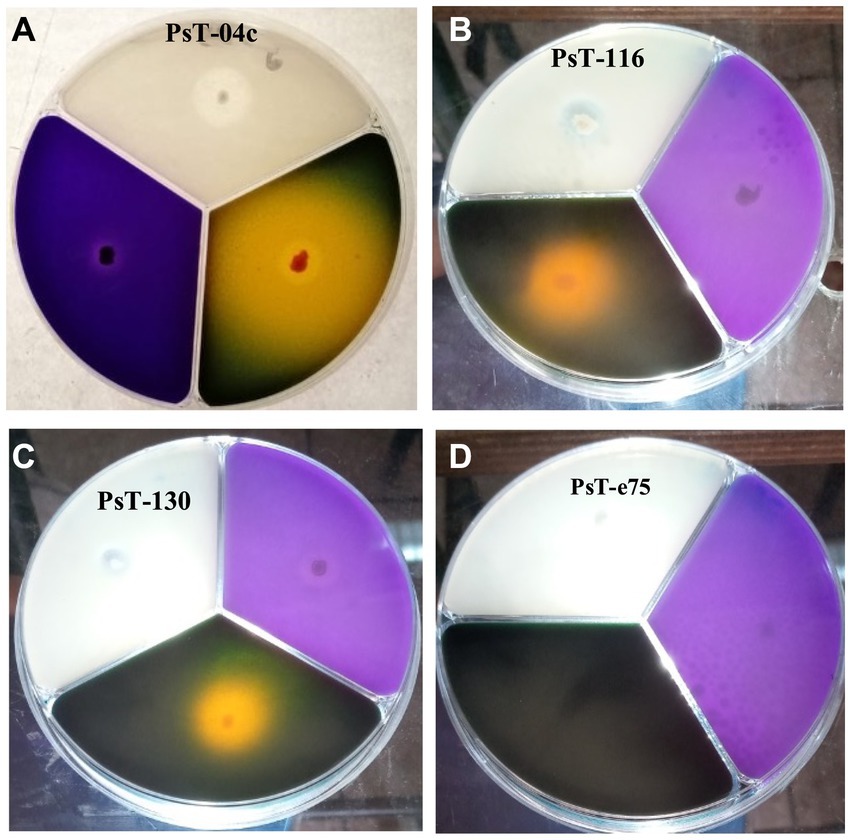
Figure 1. Solubilization of phosphorus, potassium, and zinc insoluble forms on NBRIP (purple medium), Alexandrov (green medium), and Bunt and Rovira media (translucid medium) respectively by three solubilizing strains PsT-04c (A), PsT-116 (B), and PsT-130 (C) compared with a non- solubilizing strain PsT-e75 (D).
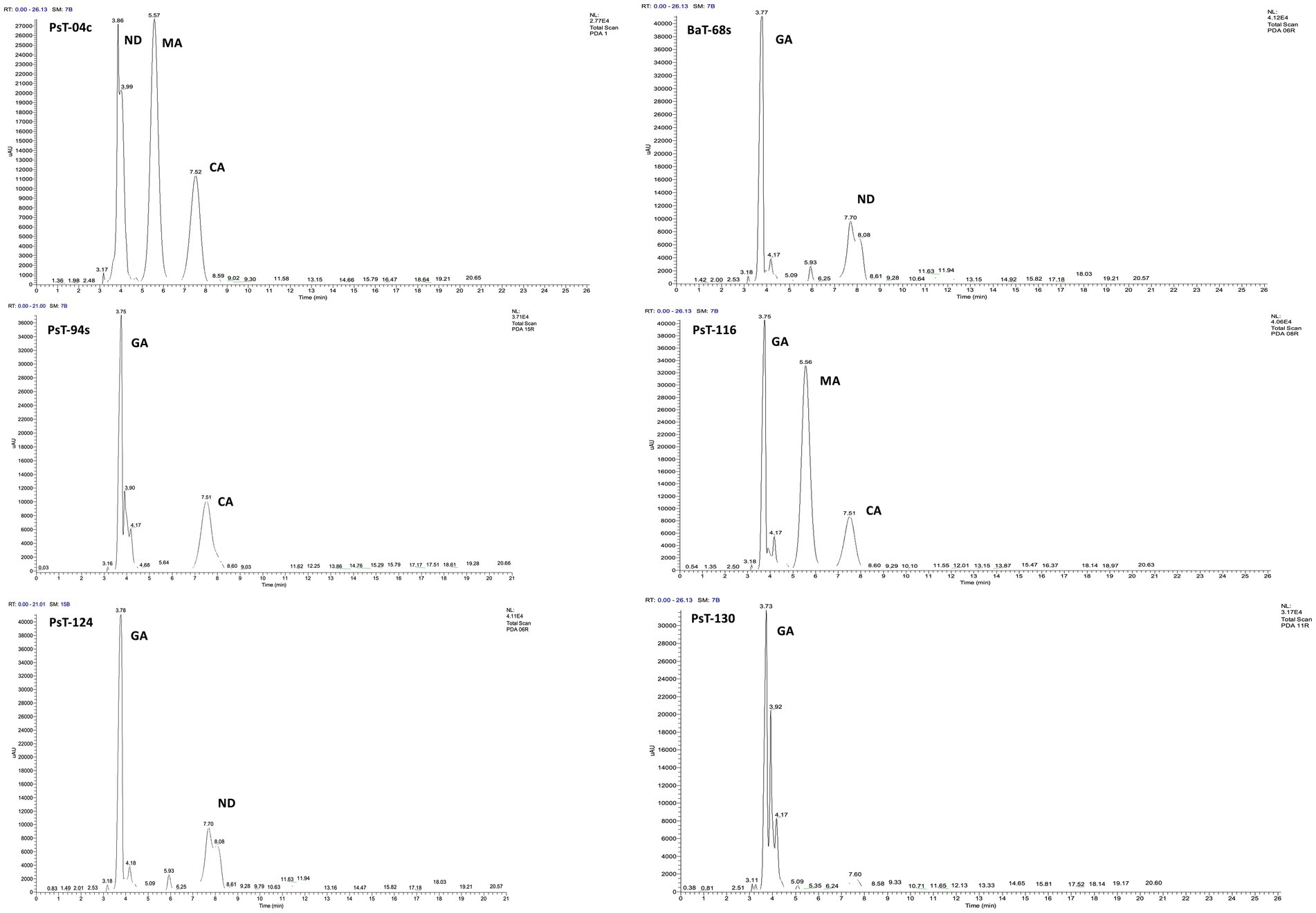
Figure 2. C18-HPLC profiles of the organic acids secreted by the six selected bacterial isolates PsT-04c, BaT-68s, PsT-94s, PsT-116, PsT-124, and PsT-130. GA, gluconic acid; CA, citric acid; MA, maleic acid; ND, not determined.
Most of the selected bacterial isolates for phosphate solubilizing ability also solubilized insoluble forms of potash and zinc and produced IAA (Figure 1 and Table 1). The isolates PsT-11b, PsT-63k, BaT-91k, and PsT-92k did not solubilize insoluble form of zinc. Isolates PsT-04c, PsT-94s, BaT-68s, and PsT-130 showed the highest potassium solubilization with index values of 4.46, 4.87, 4.92, and 6.60, respectively. The selected bacterial isolates showed significant levels of hormone IAA production (Table 1). The IAA amounts exceeded 80 μg mL−1 for several isolates. The six isolates PsT-88 k, PsT-116, PsT-04c, BaT-68s, PsT-124, PsT-63 k, and PsT-94s produced 95.63, 85.96, 84.96, 81.70, 76.13, 73.80, and 73.05 μg mL−1 of IAA, respectively, while only less amount was produced by isolates PsT-98 k (12.05 μg mL−1), PsT-92 k (2.96 μg mL−1), and PsT-35b (2.05 μg mL−1).
3.2 Plant growth-promoting effects on tomato 3.2.1 Effects on growth parametersThe six bacterial isolates, which showed the highest phosphate solubilization and IAA production abilities (isolates PsT-04c, BaT-68s, PsT-94s, PsT-116, PsT-124, and PsT-130), were selected for further experiments in order to test their abilities to show positive effects on seed germination and successfully colonize tomato rhizosphere (Table 2). The results showed that the germination rate of the seeds was improved in the presence of the isolates. Results of the effect of selected isolates combined with RP on seed germination showed that all treatments with bacterial isolates and RP had a significant positive effect on the germination rate at p < 0.05 compared with the controls consisting of seeds treated only with RP (RP amended, bacteria-free seeds). The maximum increase was obtained by the isolates PsT-04c and PsT-130 (Table 2). Greenhouse experiments were conducted by using these six isolates, and shoot and root length and fresh and dry weight were measured at the end of the experiment (Table 3). The maximum effect on shoot length and weight was showed by the isolates PsT-04c, BaT-68s, and PsT-94s, while the maximum positive effect on roots was showed by the isolates PsT-116 and BaT-68s. The results showed that the shoot length was increased by 45.51 and 43.25 % when seedlings were treated by the isolates PsT-04c and PsT-94s, respectively, while the root length reached up to 54.52% by the isolate PsT-116. Shoot and root fresh weight increased up to 63.68 and 72.96 %, respectively, while dry weight increased by 56.26 and 78.13%, respectively.
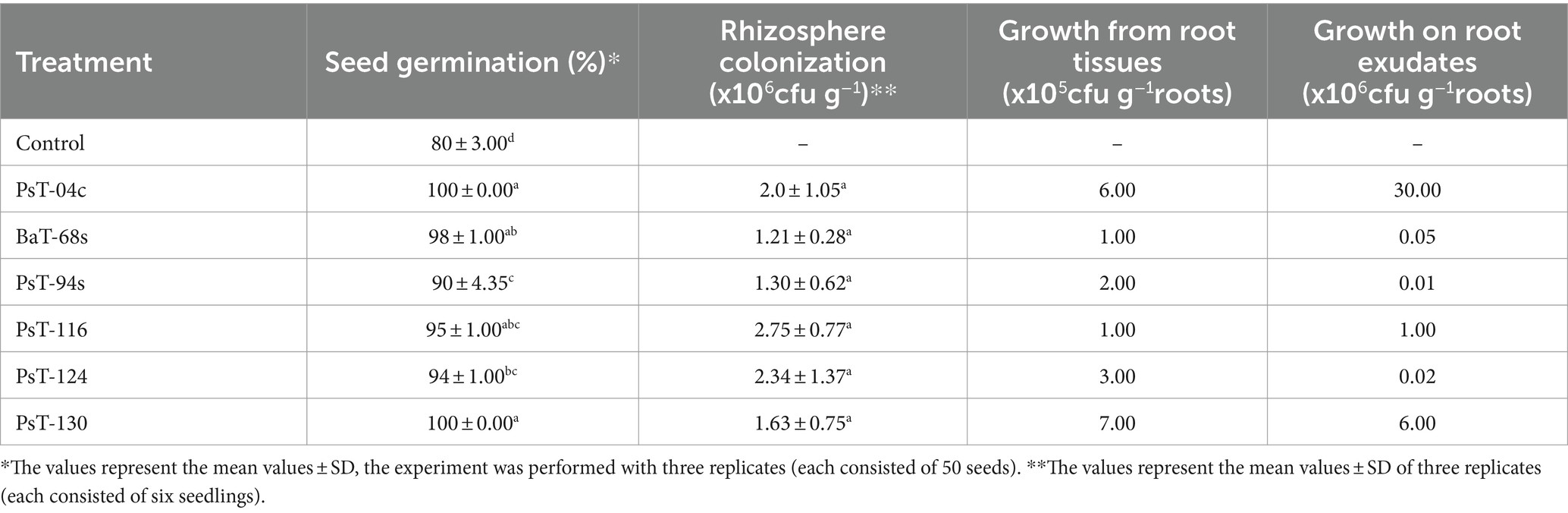
Table 2. Seed germination, rhizosphere colonization, growth from root tissues, and on root exudates displayed by the six bacterial isolates.
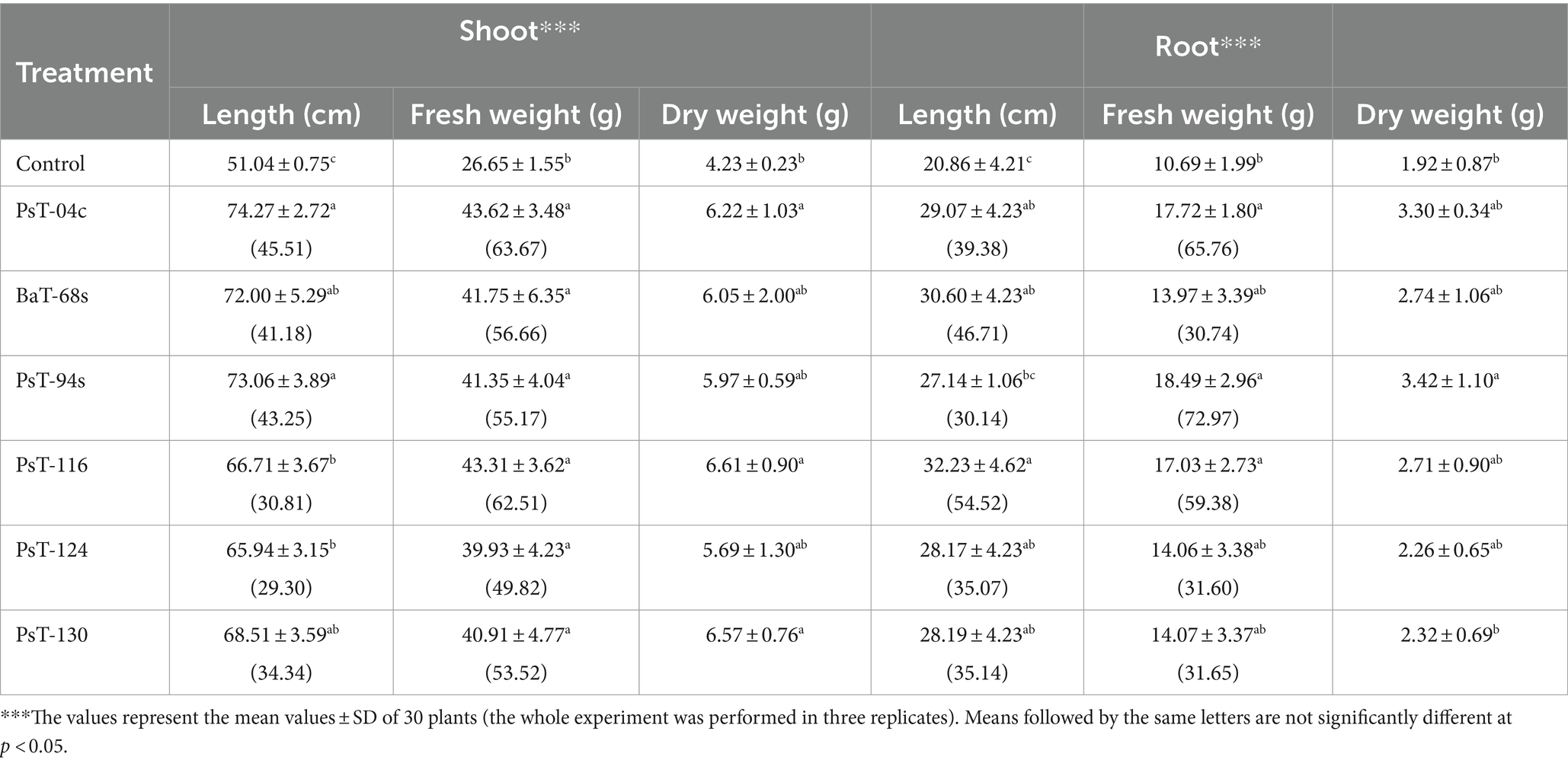
Table 3. Plant growth promotion displayed by selected bacterial isolates: on tomato (calvi cv.): effects on shoot and root length and fresh and dry weight, percentage between parenthesis correspond to increase (%) in shoot and root length and fresh weight.
3.2.2 Effects on mineral, sugars, proteins, and chlorophyll contents and chlorophyll fluorescenceThe bacterial isolates were further investigated for their abilities to enhance nutrient contents of protein, sugars, calcium (Ca), sodium (Na), potassium (K), phosphorus (P), and chlorophyll. Sugar contents enhanced by up to three-fold with the isolate PsT-116, while protein contents enhanced by 47.77, 46.90, and 45.55%, respectively, by isolates PsT-04c, PsT-94s, and PsT-130 (Figure 3). In comparison to the control, all bacterial isolates showed significant increases in K, Ca, and Na contents of the shoot and root, with the maximum increase by isolate BaT-68s for root K, and PsT-94s for root Na and Ca (Table 4). The maximum amounts of shoot Ca and K were detected following treatment with strain PsT-124 while the maximum amount of Na was obtained by isolates PsT-124 and PsT-130. The results also showed a significant increase in P content in leaves by up to three-fold in seedlings treated with PsT-94s, in comparison to non-inoculated control. Total chlorophyll content increased by up to 43–47% in seedlings treated with the isolates PsT-04c and PsT-94s, respectively, while Chl a and Chl b increased by 40 and 55%, following treatment by the isolate PsT-94s. The maximum increases in Chl a and Chl b were shown respectively by the isolate BaT-68s (47%) and PsT-04c (76%). No significant changes in chlorophyll fluorescence (photosynthetic quantum yield: Fv/Fm) were observed after 60 days of culture (Table 5).
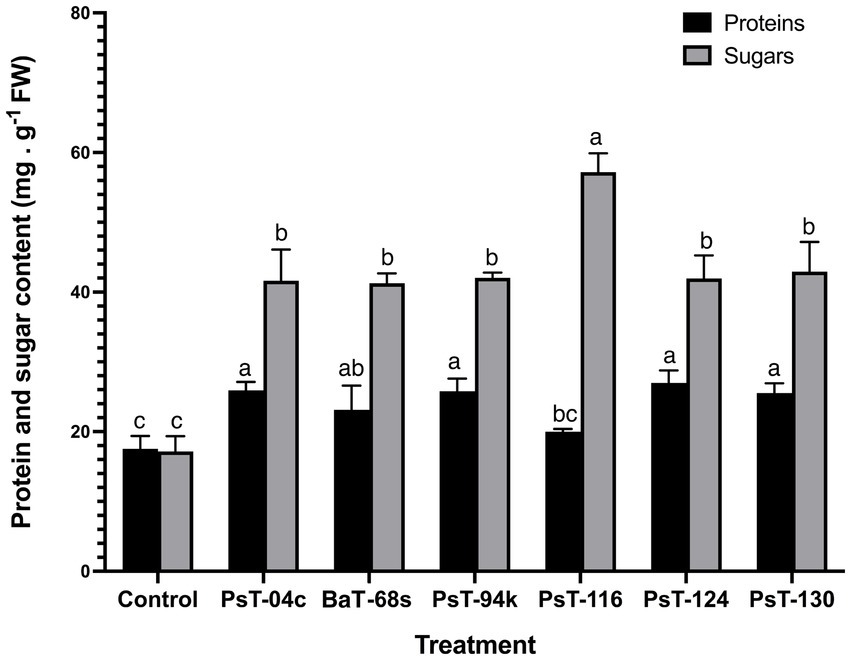
Figure 3. Effect of treatments by selected bacterial isolates on protein and sugar contents of tomato seedlings after 60 days. Different letters above the bars indicate the differences are significant at P < 0.05.
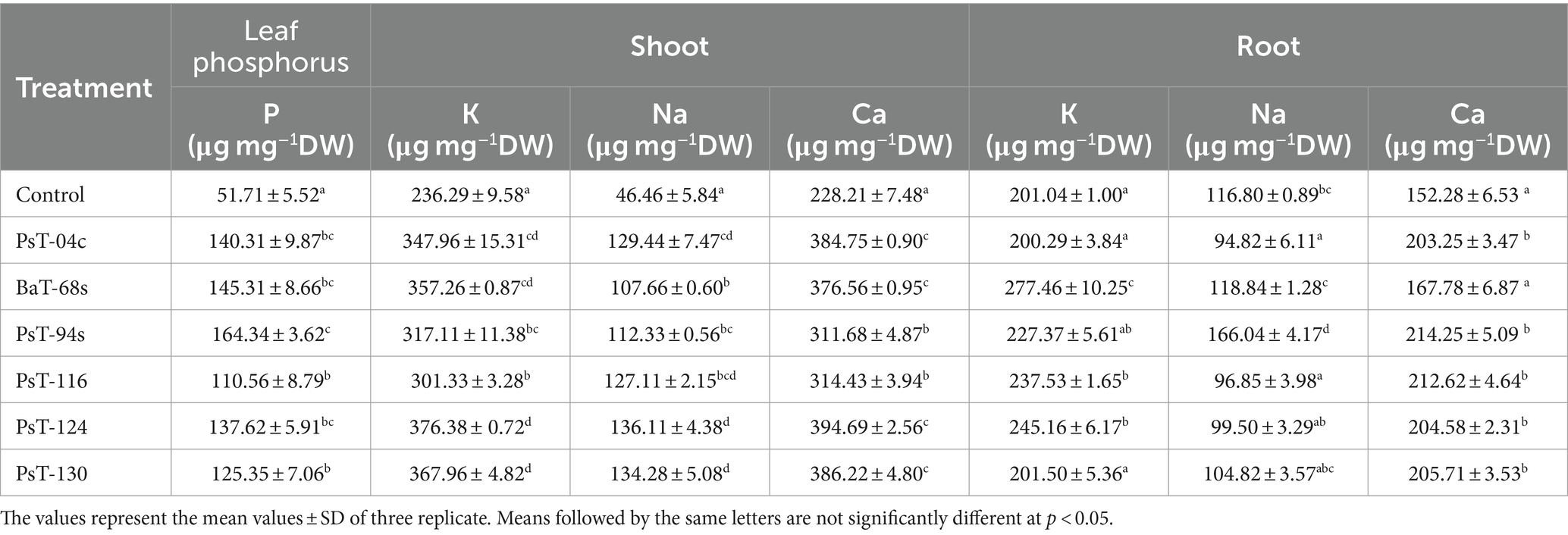
Table 4. Effect of inoculation by selected bacterial isolates on phosphorus content of tomato leaves and shoot and root cation element contents: potassium (K), calcium (Ca), and sodium (Na) after 60 days.
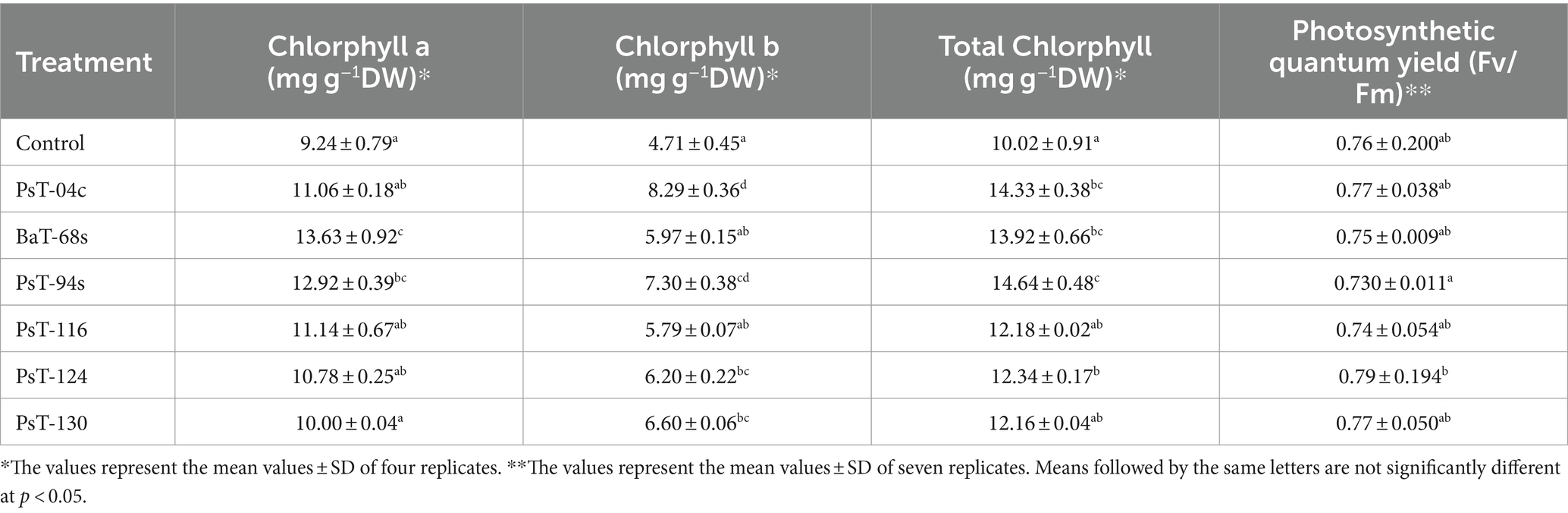
Table 5. Effect of inoculation by selected bacteria on the chlorophyll content of tomato leaves (expressed as mg/dry weight) and chlorophyll fluorescence (Fv/Fm).
3.2.3 Principal component analysisTo assess the contributions of each parameter to phosphate solubilizing bacteria-treated tomato plants compared with control plants, we performed a principal component analysis using plant growth and physiological parameters. The principal component analysis showed that bacterial treatments (red) and variables (blue) were associated with two top PCs, accounting for 78.50% of the total variation in traits under greenhouse conditions (Figure 4). PC1 explained 62.18% of the total variation and was strongly influenced by morphological and biochemical parameters, while PC2 accounted for 16.32% of the total variation and was strongly associated with physiological photosynthetic parameters. The PCA showed a positive correlation between applied phosphate solubilizing bacteria and growth parameters, photosynthetic pigments, and protein and sugar contents, which were positively correlated with each other and confirmed the positive impact of the used bacteria on growth, physiology, biochemistry, and nutrition parameters. In addition, PCA showed that all applied treatments were separated from their controls. Agro-morphological (shoot fresh weight and root fresh weight), physio-biochemical (total soluble sugar, protein, chlorophyll a, chlorophyll b and total chlorophyll contents), shoot and root minerals (Na, K, and Ca) and leaf phosphorus parameters grouped the applied treatments into three main groups; the best responses in terms of higher growth and physio-biochemical parameters were shown by treatments with strains PsT-04c, PsT-116, PsT-124, and PsT-130 (right side of the first axis PC1, upper panel). In addition, treatments with strains PsT-04c and BaT-68s (right side of the first axis, lower panel) resulted in better efficient photosynthetic system and phosphorus nutrition. In contrast, the control treatment without inoculation showed lower growth in both aboveground and root parts, in addition to lower mineral nutrition.
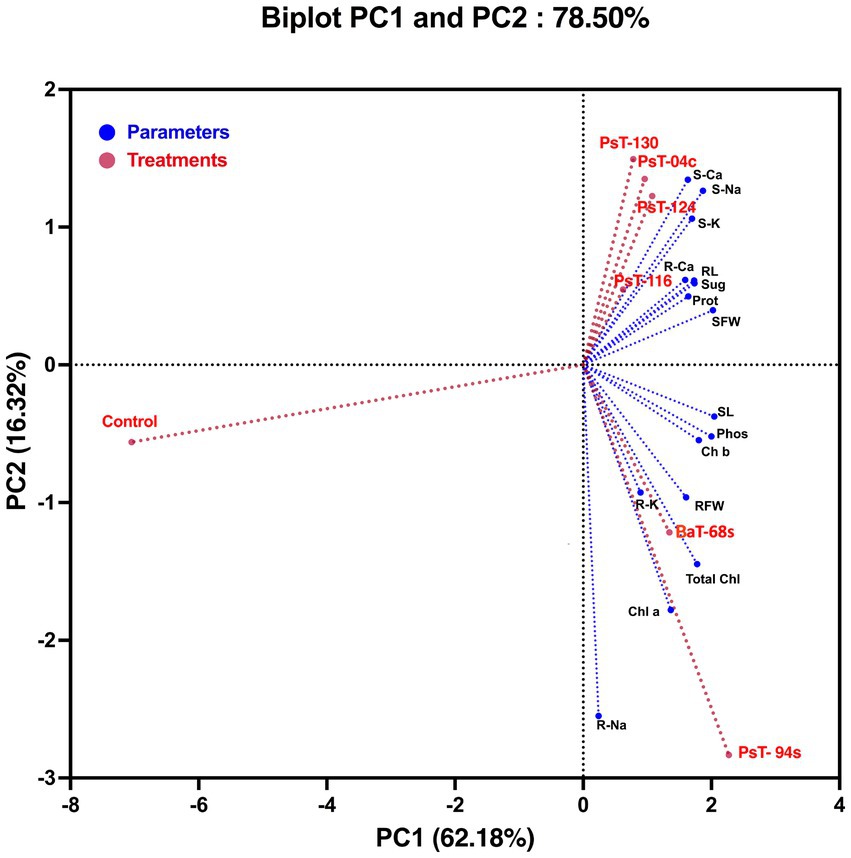
Figure 4. Principal component analysis (PCA) of tomato plants subjected to phosphate solubilizing bacteria. The variables (agro-morphological, physio-biochemical, and minerals including phosphorus) are presented in blue while bacterial treatments are presented in red. Control: absence of the phosphate solubilizing bacteria, PsT-04c, PsT-94s, PsT-116, PsT-124, and PsT-130: treatments with phosphate solubilizing Pseudomonas sp. isolates. BaT-68s: treatment with phosphate solubilizing Bacillus isolate. SL, shoot Lenght; RL, root length; SFW, shoot fresh weight; RFW, root fresh weight; S-Ca, shoot calcium content; S-Na, shoot sodium content; S-K, shoot potassium content; R-Ca, root calcium content; R-Na, root sodium content; R-K, root potassium content; Phos, plant leaf phosphorus; Sug, total soluble sugar content; Prot, protein content; Chl a, chlorophyll a; Chl b, chlorophyll b; Total Chl, total chlorophyll.
3.3 Protection of tomato against Clavibacter michiganensis subsp. michiganensis (Cmm) by induced systemic resistance 3.3.1 Ability of bacteria to grow inside root tissues and on root exudatesBacterial growth in the rhizosphere depends on their ability to positively interact with the root system, since rhizosphere represents the contact point of plant with major soil microorganisms. In this study, all the six isolates showed abilities to develop in root tissues and on root exudates. In root tissues, their numbers were: PsT-04c: 6.105, BaT-68s: 105, PsT-94s: 2. 105, PsT-116: 105, PsT-124: 3.105, and PsT-130: 7.105 cfu g−1 roots. Moreover, the two isolates Pst-04c and Pst-130 showed the highest ability to grow on tomato root exudates: 3.107 and 6.106 cfu g−1 roots, respectively (Table 2). Hence, the isolates PsT-04c and PsT-130 were selected and used in greenhouse experiments for application as biocontrol agents against the causal agent of bacterial canker, Clavibacter mich
留言 (0)