Autoimmune diseases represent a group of chronic and systemic disorders characterized by an excessive immune response, abundant inflammation, and the extensive depositions of immune complexes to tissues and organs. Epidemiological investigations have revealed a global incidence of 5~8% for autoimmune diseases (1, 2). The pathogenesis of autoimmune disease is multifaceted involving genetic predisposition, immune dysregulation, and environmental factors like lifestyle, dietary patterns, and medications (3). Despite advancements in diagnosis and treatment, early diagnosis and precise therapeutic strategies of autoimmune diseases remain significant challenges (4). Increasing evidence has highlighted the pivotal role of the gut microbiota in maintaining immune balance and homeostasis in autoimmune diseases, particularly including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic scleorosis, and type I diabetes mellitus (T1DM). Disturbances in the composition and diversity of the gut microbiota are strongly associated with autoimmune disorders (5–7). This review aims to elucidate the regulatory mechanisms by which the gut microbiota influence autoimmunity and inflammation, consolidating available evidence on their association with autoimmune diseases. Additionally, it seeks to offer novel insights into early diagnosis and precise treatment strategies for autoimmune diseases.
2 Compositions and functions of the gut microbiotaThe human gut harbors a diverse array of microbiota, encompassing bacteria, fungi, viruses and assorted microorganisms (8).
With advances in sequencing techniques, such as 16S rRNA and metagenomics, certain intestinal microbiota closely associated with human health have been identified, such as Phyla Firmicutes, Bacteroidetes, Pseudomonadota, and Actinomycetota, while others like Fusobacteria are relatively less studied (9, 10).
Microbiota populations colonize not only the gut but also the skin, respiratory tract, and reproductive system, influencing various physiological processes, including nutrition, tumorigenesis and immune homeostasis (11). It has been well established that the composition and abundance of the gut microbiota can be influenced by various factors, including environmental factors, diet and the host’s immune system (12, 13). The gut microbiota is not static but dynamic throughout human life. The study by Xie et al. has shown that the gut microbiota diversity changes over time, notably in genetically identical twins living apart (14). Dietary habits also significantly impact microbiota; for instance, high-fiber diets positively correlate with increased abundance of Lachnospiraceae (15), while Western diets rich in red meat and low in fiber are associated with Bacteroides spp and Ruminococcus torques dominance (16). Consequently, microbial compositions vary with dietary habits, heredity and other factors.
The gut microbiota, evolved alongside its host, profoundly influences various physiological and pathological processes, including nutrient production, drug effects, resistance to pathogens, and immune regulation (17–19). Gut bacteria ferment indigestible carbohydrates, generating short-chain fatty acids (SCFAs) like acetate, propionate and butyrate. These SCFAs act as biologically active compounds, providing energy for colonic epithelial cells (20, 21). In particular, butyrate acts as a primary energy source for the colonic epithelial cells (22). Furthermore, the gut microbiota significantly impacts medication metabolism. For instance, certain bacteria convert gemcitabine metabolites and irinotecan HCl, affecting therapeutic efficacy and potential side effects (23). It has been demonstrated that Escherichia coli (E. coli), Staphylococcus and Clostridium sporogenes produce an enzyme called beta-glucuronidase, converting the harmless form of the chemotherapeutic drug irinotecan HCl (CPT-11), known as SN-38 glucuronide, to its active form, SN-38 (24). Moreover, the gut microbiota exerts crucial effects on the immune system, including impacts on the proliferation and activation of immune cells, autoantibodies generation, and the onset of autoimmune diseases. Studies comparing germ-free (GF) and specific pathogen-free (SPF) mice highlight the microbiota’s impact on innate immune cell modulation and host defense against bacterial infections (25). Additionally, the microbiota contributes to the production of neurochemicals like gamma-aminobutyric acid (GABA), impacting the central nervous system and the gut through the brain-gut axis, ultimately influencing the immune microenvironment (26). Exploring the makeup, diversity, and structural alterations in the body’s microorganisms, along with interactions with the immune system, holds promise for understanding the fundamental processes of autoimmune diseases. All these findings offer the possibilities of identifying new biomarkers and developing effective therapeutic strategies for various diseases.
3 Role of microbiota in establishing and maintaining a stable immune system3.1 Microbiota and innate immunityThe correlation between the gut microbiota and innate immunity has attracted significant attention in academic research. The gut-associated lymphoid tissues (GALT) play a crucial role in protecting the intestinal mucosa, working in coordination with the mucosa-associated lymphoid tissues (MALT). Innate immune cells within these tissues employ non-specific pathogen recognition, innate immune reaction initiation and antigens presentation to activate the adaptive immune system (27, 28). It has been shown that the gut microbiota is important in regulating the physiological functions of GALTs in germ-free (GF) models, aiding in their development and maturation (27). Additionally, metabolic by-products produced by commensal microbiota, such as SCFAs, influence the immune response of GALTs through epigenetic mechanisms, supporting the defensive functions and immune tolerance (27).
Innate lymphoid cells (ILCs) are integral to GALTs. The development of ILCs occurs independently of the gut microbiota, while their specific functions rely on commensal microbiota (29, 30). For instance, ILC3, a prominent ILC class, supports epithelial cells survival, antimicrobial peptides production, and the generation of IL-22 (31). IL-22 is a key cytokine essential for the host’s immune response to Citrobacter (32, 33). It has been well documented that SCFAs promote IL-22 production of ILC3 by activating aromatic hydrocarbon receptor (AHR) through the AKT-STAT3 and ERK-STAT3 signaling pathways (34, 35) (Table 1). Moreover, SCFAs stimulate the proliferation of intestinal ILCs by affecting G protein-coupled receptor (GPCR) activity (45) (Table 1). The gut microbiota also facilitates interactions between ILC3 and other cell types (31), promoting the expression of protective proteins like fucosyltransferase 2 (FUT2) that strengthen the intestinal mucosal barrier (60). As the gut microbiota matures, ILC1 levels increase, indicating their reliance on commensal microbiota for development (30).
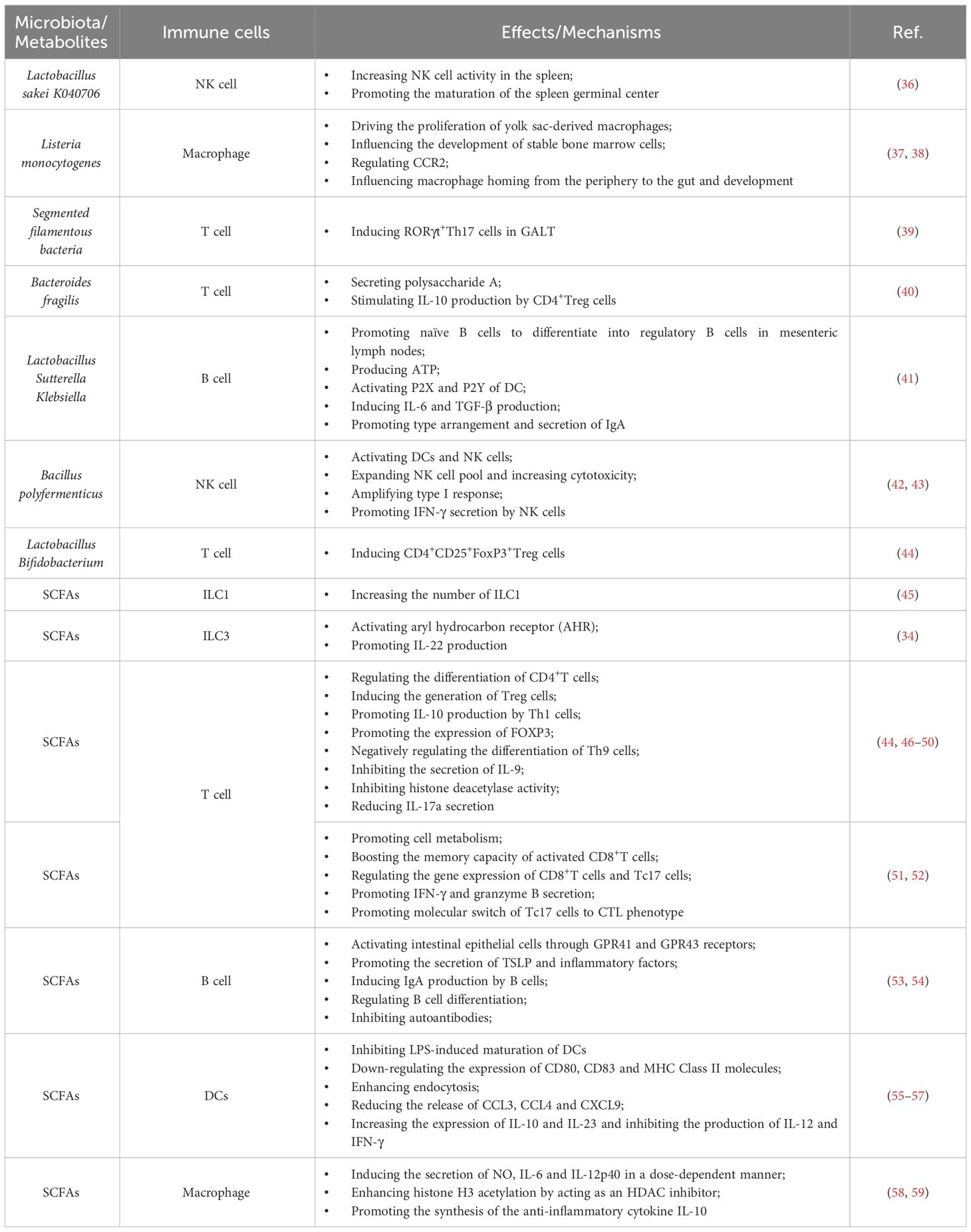
Table 1 Effects of the gut microbiota and microbiota-derived metabolites on the immune system.
Conventional natural killer (NK) cells, the sole cytotoxic cell population within innate immunity, detect pathogens and carry out cytotoxic functions by releasing proteins like granzymes (61). The precise mechanisms regulating NK cell equilibrium between alloreactivity and autotolerance remain unclear. The gut microbiota, containing ligands for NK cell receptors, influences NK cell function and cytotoxicity, as observed in GF mice with the absence of interaction with commensal bacteria.
Macrophages in the gut are crucial for defending against infections and play a pivotal role in maintaining the integrity of the intestinal mucosa. The gut microbiota influences the development of myeloid-derived macrophages and the intestinal inflammation by modulating myeloid cell hematopoiesis, migration, and population sustenance within the gut (37, 38, 62–64) (Table 1).The relationship between the gut microbiota and innate immune cells underscores the profound influence of microbial communities on the development, differentiation, and functionality of these vital immune components, thus significantly contributing to the gut’ s microecology balance.
The intricate interplay between the gut microbiota and the local innate immune system contributes to the shaping of the gut microenvironment. Increasing evidence has demonstrated that the dysregulation of gut microbiota can result in disruptions and imbalance of the innate immune system by regulating the TLR signaling activation, inflammasome response, and ILC alterations, ultimately contributing to the onset of autoimmune diseases, such as RA and SLE (27, 65). Furthermore, fecal transplantation from healthy mice to certain disease-model mice has been shown to alleviate symptoms and ameliorate metabolic irregularities due to impaired activation of innate immune receptors, such as type 1 diabetes mellitus (T1DM) and inflammatory bowel disease (IBD) (31, 66). These findings have suggested the crucial role of gut microbiota in regulating autoimmune disorders through influencing innate immunity.
3.2 Microbiota and adaptive immunityThe gut microbiota plays a pivotal role in establishing and sustaining adaptive immunity in the gut, orchestrating interactions between varieties of immune cell types like T cells and B cells to ensure immunological equilibrium. T cells within the gut, specifically the CD4+T cells known as Th cells, display remarkable diversity and functions shaped by the unique metabolic characteristics of the gut microbiota (67). Notably, the gut microbiota influences the differentiation of naïve CD4+T cells into subsets like Th17 cells and Treg cells. Th17 cells are vital in defending against bacterial and fungal infections in the lamina propria (LP) of the small intestine by producing IL-17A, IL-17F, and IL-22. They also promote intestinal epithelial cells for more production of antimicrobial peptides (AMP), activate endothelial cells (ECs), and aid in neutrophils recruitment (68, 69). Segmented filamentous bacteria (SFB), a relatively low-abundance microbial population in the ileum, can induce the generation of RORγt+Th17 cells in the tissue-associated lymph nodes in the gut, although the excessive activation of RORγt+Th17 cells might lead to autoimmune diseases (70).
In contrast, regulatory T (Treg) cells contribute to immunological tolerance by promoting self-tolerance and suppressing excessive immune activation (71). The forkhead box P3 (Foxp3) is the key transcription factor for CD4+CD25+Treg cells, inhibiting excessive immune reactions. Certain probiotics, like Lactobacillus and Bifidobacterium infantis induce the production of anti-inflammatory CD4+CD25+Foxp3+ Treg cells, while Bacteroides fragilis and its polysaccharide A (PSA) stimulate IL-10 production by CD4+Treg cells depending on IL-2 pathway, suppressing inflammation (72). In addition, some bacterial metabolites, such as adenosine and inosine, can interact with T cells’ adenosine A2A receptor (A2AR), enhancing Treg cell activity while inhibiting Th1 and Th17 inflammatory responses (73).
Several pathways, including B cell receptor (BCR), CD40, Toll-like receptors (TLRs), B cell activating factor receptor (BAFF), and proliferation-inducing ligand (APRIL) receptors, primarily control the activation and differentiation of B cells (74). Activation of TLRs and BAFF/APRIL receptors significantly impacts T cell-dependent B cell antibody production (74). It is firmly established that the gut microbiota modulates B cell function by interacting with BCR through antigenic determinants and activating TLRs and NOD-like receptors (NLRs) via specific metabolites (74). Moreover, the gut microbiota stimulates dendritic cells (DCs) and the intestinal tissue cells to release cytokines like IL-1β and IL-6, which enhances the differentiation of naïve B cells into regulatory B cells (Bregs) within mesenteric lymph nodes (MLN) (41) (Table 1). Additionally, microbiota-derived ATP in the gut is converted to adenosine, activating adenosine receptors on B cells and promoting the production of IgG and IgA antibodies (74). Beyond these direct effects, the gut microbiota indirectly influences B cell differentiation and activation. As gut microbiota-derived metabolites, SCFAs can activate the intestinal epithelial cells via the cell receptors, such as GPR41, GPR43, and FFAR2, which prompts the release of thymic stromal lymphopoietin (TSLP) by epithelial cells and the release of TNF-α and iNOS of dendritic cells. This cascade triggers the expression of APRIL and IL-10, further enhancing IgA antibody production by B cells (75). Additionally, SCFAs can also activate receptors on the intestinal epithelial cells, like FFAR2, FFAR3 or GPR109a, and modulate the nuclear factor-κ-light chain enhancer (NF-κB) pathway involved in B cell activation, thereby suppressing inflammatory responses (Figure 1). Consequently, the gut microbiota and its metabolites are closely intertwined with the development, differentiation, and activation of B cells, exerting a substantial influence on immune responses and inflammation.
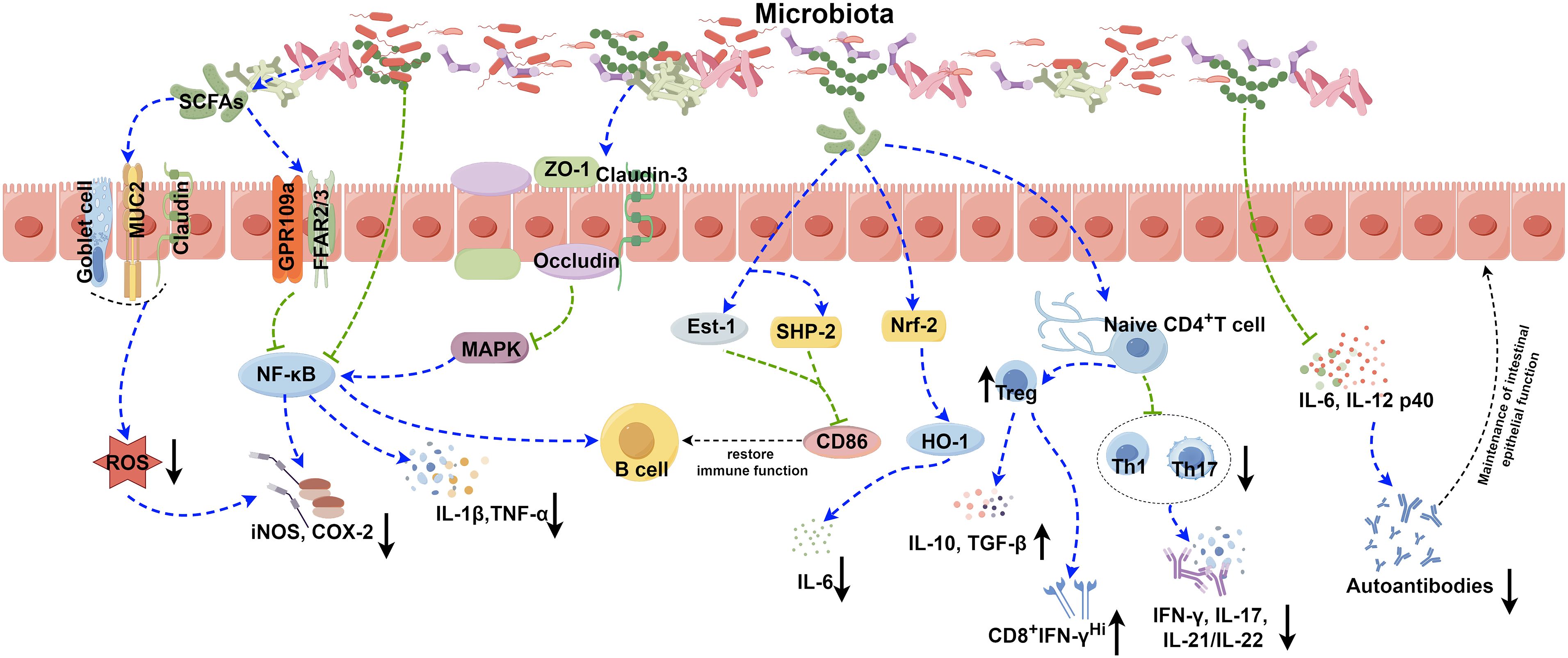
Figure 1 Role of the gut microbiota in autoimmune disease. The gut microbiota contributes to the pathogenesis of autoimmune diseases through various complicated mechanisms, such as the secretion of SCFAs, modulation of NF-κB and Nrf2 signaling pathways, disruption of the balance between Treg, Th1, and Th17 cells, and modulation the release of inflammatory factors. The image was created utilizing the FigDraw online platform (https://www.figdraw.com/#/) and was identified by the in-house image ID: UPORSc4cf4. SCFAs: Short chain fatty acids; MUC2: Recombinant Mucin 2; ROS: Reactive oxygen species; GPR109a: G protein-coupled receptor 109a; FFAR2/3: Recombinant Free Fatty Acid Receptor 2/3; NF-κB: Nuclear factor kappa-B; COX-2: Cyclooxygenase 2; iNOS: Inducible nitric oxide synthase; ZO-1: Zona Occludens 1; MAPK: Mitogen-activated protein kinase; IL-1β: Interleukin-1 beta; TNF-α: Tumor necrosis factor alpha; Est-1: Estrogen sulfotransferase-1; SHP-2: SH2 domain-containing protein-tyrosine phosphatase-2; Nrf-2: NF-E2-related factor 2; HO-1: Recombinant Heme Oxygenase 1; IL-6: Interleukin-6; IL-10: Interleukin-10; TGF-β: Transforming growth factor beta; IFN-γ: Interferon gamma; IL-17: Interleukin-17; IL-21/IL-22: Interleukin-21/Interleukin-22; IL-12 p40: Interleukin-12 p40.
It has been well documented certain gut microorganisms harboring epitopes resembling host proteins, such as the RNA-binding protein Ro60, are capable of activating T and B cells in SLE, thereby triggering abnormal adaptive immune response and inducing production of pathogenic autoantibodies (76). Besides, the roles of Th9 cells and IL-9 have been demonstrated in UC, functioning to impair intestinal barrier function, prevent mucosal wound healing in vivo and compromise tolerance to commensal bacteria by inducing inflammation and adaptive immune disorders (77–79). Therefore, the gut microbiota may contribute to autoimmune diseases by regulating T- or B-cell mediated adaptive immunity. The complex interplay between the gut microbiota and immune cells influences immune responses and homeostasis. Metabolites like SCFAs significantly impact cytokine and immunoglobulin production, affecting the progression of autoimmune diseases. This close interaction between the microbiota and the immune system forms a protective barrier against various threats. Alterations in the microbiota can lead to immune dysfunction and the onset of autoimmune diseases.
4 Role of microbiota and autoimmune diseasesThe integrity of human gut microbiota has been shown to correlate with susceptibility and outcomes of various diseases, such as metabolic, infectious and autoimmune diseases (80). As suggested in a groundbreaking article in 2002, the incidences of some classic infectious diseases such as tuberculosis and measles as well as intestinal infections have declined significantly but with a higher incidence of autoimmune diseases like T1DM and asthma in western countries in the last 50 years of the 20th century due to the improved sanitation, antibiotics usage and vaccination (81). This trend of change could still be observed in the last few years even until now that the incidence of autoimmune diseases is still steadily increasing, accompanied by a steady decline in the incidence of primary infectious diseases (82). According to “old friend hypothesis”, limited exposure to specific microbes (“old friends”) prevent immune system from forming a tolerogenic microenvironment, especially in early childhood phase (83–86). For children, the delivery mode, diet, and exposure to antibiotics and antacids are common factors for the contact with microbiota. Researches have shown that infants undergoing vaginal delivery and exclusive breastmilk feeding usually have a lower cumulative allergic burden, while those who exposed to antibiotic and antacid have an increased cumulative allergic burden conversely, thereby demonstrating the importance of microbiota in resisting excessive autoimmune response (87). Therefore, investigating the development, management, and prognosis of autoimmune disorders holds paramount importance due to the potential impact of microbiota dysfunction on both immunodeficiency syndromes and autoimmune diseases.
Dysbacteriosis, a commonly recognized term, refers to disruptions in the composition or activity of the microbiota within specific anatomical areas. This disruption encompasses alterations in both α-diversity and β-diversity. α-diversity reflects the variability in types and quantities of microorganisms of the host, while β-diversity delineates differences in microbial community makeup between individuals (88). Recent research has increasingly linked microbiota dysregulation to compromised intestinal barrier integrity, diminished functionality, and enhanced inflammation in autoimmune diseases (89, 90). Therefore, a growing body of evidence has underscored the intimate relationship between microbiota compositions and these conditions.
4.1 Microbiota and systemic lupus erythematosusSLE is one of the most prevalent autoimmune diseases with multisystemic clinical manifestations caused by abundant immune complex depositions to tissues and target organs, leading to long sustained inflammation, immune disorders and ultimately multi-organ damages (91). A previous study has suggested that transferring microbiota from the cecum of lupus-prone mice into healthy mice can induce lupus (92). Similarly, GF mice receiving fecal matter from lupus mice exhibit increased levels of anti-DsDNA antibodies in serum, implicating the crucial role of the gut microbiota in autoantibody formation and immune reactions (93). Consequently, the gut microbiota is recognized as a pivotal factor contributing to SLE (27, 94, 95). The migration of microbial products from the colon and elevated permeability of the intestinal mucosal barrier are well recognized mechanisms implicated in the initiation and progression of SLE. Research in lupus-susceptible (NZB×BXSB) F1 mice has shown the migration of Enterococcus gallinarum from the intestine to mesenteric veins, intestinal draining lymph nodes, liver, and spleen (96). Enterococcus gallinarum has been also detected in liver biopsy specimens from SLE patients (96). Calprotectin, a calcium-containing protein in neutrophils and macrophages, acts as a crucial biomarker for impaired intestinal barrier (97). A close association between damaged intestinal barrier function in SLE patients and elevated levels of fecal calprotectin has been well documented (96, 98). Furthermore, certain gut microorganisms harboring epitopes resembling host proteins are capable of activating T and B cells, thereby triggering abnormal immune responses and abundant production of pathogenic autoantibodies. For instance, the RNA-binding protein Ro60, found in various gut microorganisms, shares homologous sequences with human Ro60 epitopes (76, 99). Anti-Ro60 antibodies can induce the generation of autoantibodies against Ro52, Smith, or U1RNP by spreading their epitopes (76, 99). Therefore, the intestinal microbiota containing sequences akin to human Ro60 epitopes may contribute to SLE by exacerbating pathological damages and dysregulated autoimmune responses in lupus-susceptible individuals.
It has been reported that certain gut microbiota can exert inhibitory effects on SLE through various mechanisms (Figure 1). Lactobacillus has been demonstrated to possess the capability to diminish the number of ILC3 cells and Th17 cells, suppress the pro-inflammatory factor IL-17 production, shift the Treg/Th17 balance in favor of the Treg phenotype, stimulate IL-10 production, and limit the accumulation of IgG-2a in the kidney (94, 100). This cascade ultimately reduces the kidney injures induced by dysregulated autoimmune responses. Moreover, it has been reported that Lactobacillus can significantly enhance the expression of key molecules associated with the intestinal mucosal barrier, such as ZO1, occludin and Cldn1 (100). Lactobacillus helps to enhance the intestinal barrier’s functionality without affecting the expression of Cldn2, responsible for creating lining pores (100) (Table 2). MicroRNAs (miRNAs) play pivotal roles in diverse biological processes, including immune cell maturation, the establishment of central and peripheral tolerance, and the differentiation of T helper (Th) cells. Alterations in miRNA expression can lead to immune system dysfunctions (123). Earlier studies have indicated a positive correlation between elevated expression levels of miR-155 and miR-181a and increased disease severity of SLE patients. Conversely, miR-155 deficiency reduces anti-dsDNA IgG titers and alleviates disease symptoms (124, 125). Interestingly, it has been discovered that Lactobacillus rhamnosus and Lactobacillus delbrueckii can attenuate the activity of miR-155 and miR-181a in peripheral blood mononuclear cells (PBMCs) of SLE patients (126). However, it’s noteworthy that Lactobacillus reuteri can exacerbate SLE by increasing the expression of type I interferon gene in the spleen and ileum of C57/B6 mice, resulting in anemia, increased intestinal permeability, and immune dysfunctions (127). These findings have suggested that different Lactobacillus strains may induce distinct immune responses in varying conditions in mice, leading to diverse outcomes (128). Additionally, the Firmicutes/Bacteroidetes (F/B) ratio is found to be significantly lower in SLE patients compared to non-SLE individuals (129–131), with Firmicutes showing a negative correlation with SLE Disease Activity Index (SLEDAI) scores (132). This suggests that Firmicutes may potentially delay the progression of SLE. Butyric acid and propionic acid, produced by Firmicutes, directly impact B cells by promoting the differentiation and proliferation of extrathymic Treg cells. Additionally, they suppress the expression of LPS-induced inflammatory cytokines such as IL-6, IL-12, and p40, thereby reducing the production of autoantibodies. Furthermore, these acids enhance and sustain the integrity of the intestinal epithelial barrier function in lupus-prone animals (57, 133, 134).
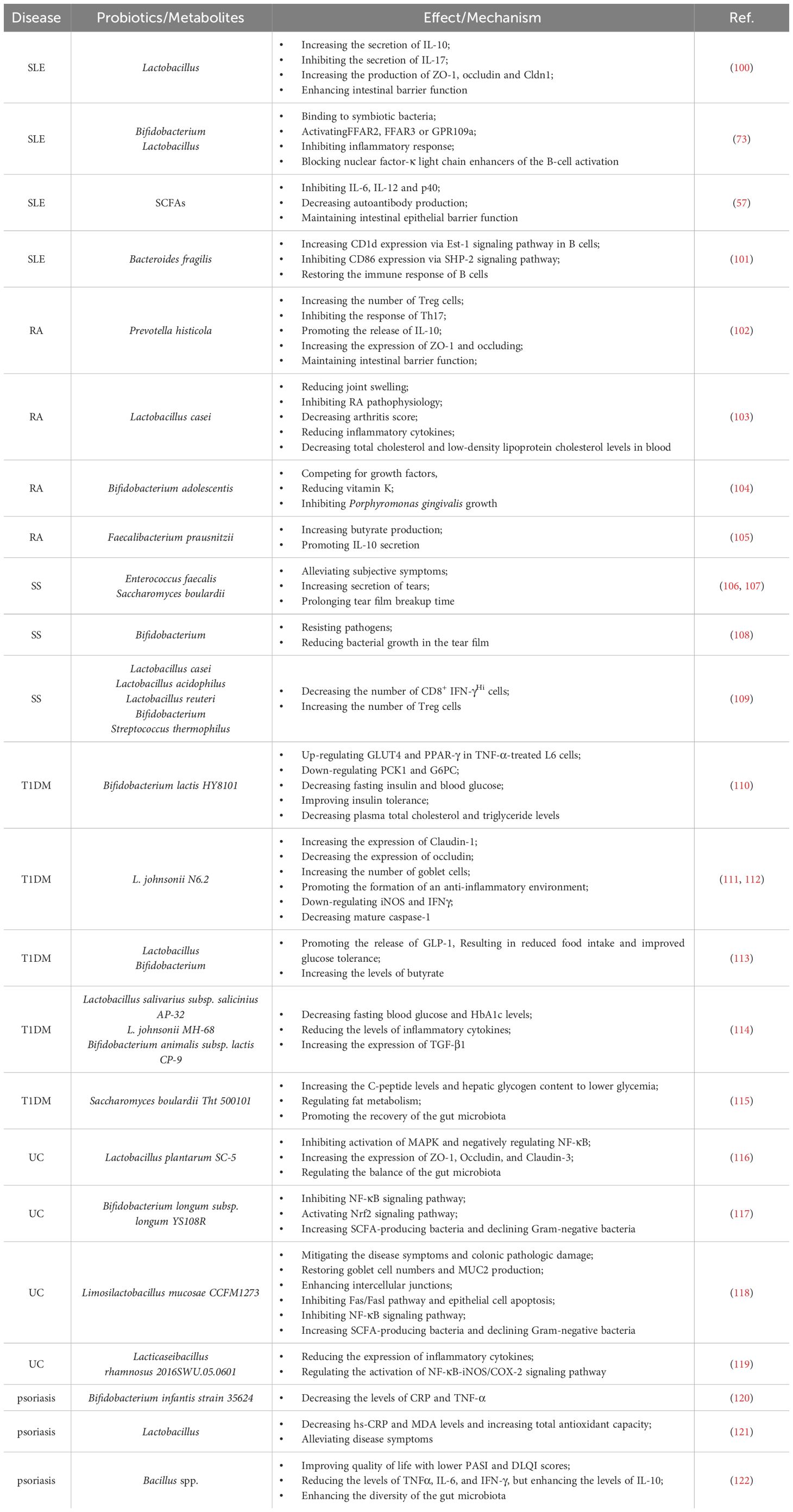
Table 2 Roles and mechanisms of probiotics in regulating autoimmune diseases.
4.2 Microbiota and rheumatoid arthritisThe gut microbiota composition in early RA patients differs significantly from that of healthy individuals, characterized by a significant reduction in Bifidobacterium and Bacteroides families and a notable increase in Prevotella species (135–137). These distinctions suggest a potential contribution of the gut microbiota-host interaction to the onset and progression of RA. However, the precise mechanism remains unclear. Recent studies propose that the influence of the gut microbiota on RA might involve various mechanisms, including the activation of antigen-presenting cells, production of citrullinated peptides through interactions with Toll-like receptors (TLRs) or NOD-like receptors (NLRs), induction of antigen-mimicking cross-reactivity, alterations in the intestinal mucosal permeability, and the promotion of Th17 cell-mediated inflammation in the mucosa (138, 139) (Figure 1).
Citrullinated peptides, formed when arginine residues are converted into citrullinated residues by protein arginine deiminase (PAD), can disrupt immune tolerance and trigger autoimmune reactions in individuals genetically predisposed to RA under specific conditions (140–142). The gut microbiota dysregulation may compromise PAD function, impacting immunological tolerance and contributing to autoimmune diseases (138, 143–145). Additionally, the gut microbiota itself can encode bacterial PAD enzymes, facilitating citrullination (146, 147). Different species of Prevotella play varying roles in RA. Overcolonization of Prevotella copri (P. copri) might intensify mucosal inflammation and induce immune responses, potentially leading to arthritis (138). P. copri can also compromise the intestinal barrier integrity by disrupting the tight junctions (TJs) between intestinal epithelial cells, facilitating disease occurrence (139, 148). Conversely, Marietta et al. have demonstrated that Prevotella histicola (P. histicola) can prevent and treat collagen-induced arthritis (CIA) in HLA-DQ8 transgenic mice by boosting Treg cells, suppressing Th17 responses, enhancing IL-10 release, and stabilizing the intestinal barrier (102) (Table 2, Figure 1).
Various strains of Lactobacillus casei (L. casei) in preclinical studies have shown efficacy in treating RA, including reducing joint swelling, arthritis scores, and serum inflammatory cytokine levels, highlighting probiotics’ potential in RA remission (103, 149, 150). Additionally, the interplay between periodontal disease and RA involves shared pathogenic mechanisms and immunological pathways. RA patients often display elevated antibody levels against Porphyromonas gingivalis (P. gingivalis) and Prevotella, with P. gingivalis antibodies correlating with the levels of RA-specific anti-CCP antibodies (151, 152).
The enzyme PAD from P. gingivalis in periodontal disease can citrullinate human fibrinogen and α-enolase. Antibodies generated against these citrullinated antigens may cross-react with joint antigens, exacerbating RA-related inflammation (153). Researchers are actively exploring strategies to mitigate microbial self-antigen cross-reactivity and curb excessive citrullination, aiming to develop microbiota-based treatments for RA.
4.3 Microbiota and Sjögren syndromeSS is one of the most prevalent autoimmune diseases, primarily affecting the lacrimal and salivary glands. The precise pathogenesis of SS remains largely unknown. Dysfunction of T cells and B cells play a critical role in the onset and progression of SS (154, 155). An imbalance, marked by an increase in Th17 cells and a decrease in Treg cells, can prompt lymphocyte infiltration, epithelial cell activation, enhanced proinflammatory cytokines production (e.g., IFN-γ and IL-17), exposure to autoantibodies, and damages to the corneal barrier, contributing to the development of SS (156–158). Furthermore, Th1 cells, known contributors to the pathogenesis of SS, partake in ocular inflammation by secreting pro-inflammatory cytokines like IFN-γ, IL-1β, IL-6 and TNF-α (159). Elevated expression of B cell activating factor (BAFF) is mainly induced by type I and type II interferons (IFN) (160). Significantly elevated levels of BAFF in the peripheral circulation and the salivary gland tissues have been observed in 55% of SS patients, highlighting increased B cell activation in SS (154, 161). A significant reduction in the diversity of the gut microbiota has been reported in SS patients, characterized by diminished symbiotic bacteria and increased potentially pathogenic strains, positively correlated with disease severity (158). Fecal transplantation (FMT) effectively ameliorates ocular symptoms in germ-free (GF) mice with SS, highlighting the strong link between gut microbiota and SS pathogenesis (162–164). The protective role of the gut microbiota in SS operates through two main mechanisms (Figure 1). Firstly, microbiota-produced metabolites like SCFAs exert anti-inflammatory properties, alleviating ocular inflammation (165–167). Secondly, the gut microbiota plays a critical role in regulating the development, differentiation and activation of ocular immune cells, effectively modulating the balance between pro-inflammatory Th17 cells and anti-inflammatory Treg cells (109, 165, 168). As a result, strategies aiming at maintaining microbial balance may hold promise for effective biological prevention approaches for SS.
4.4 Microbiota and T1DMT1DM emerges from a multifaceted autoimmune process, characterized by the T-cell-mediated destruction of insulin-producing β-cells. It is the most commonly diagnosed diabetes in young population, while the incidence of T1DM in adult has also increased rapidly in the past 15 years (169, 170). More and more evidence provide supports that the composition of the gut microbiota, as an environment factor, is different between diabetes patients and healthy individuals. Bacteroidetes has been recognized as the most common microbial phylum in T1DM patients, while the number of butyrate-producing species from Clostridium clusters IV and XIVa, as well as mucin-degrading bacteria such as Prevotella and Akkermansia in T1DM patients has significantly reduced (171). The importance of butyrate in preserving the integrity of the intestinal mucosal barrier has been highlighted (172) (Figure 1). The reduction in butyrate-producing bacteria leads to increased gut permeability. And it will allow the passage of microbial antigens, products, and even the microorganisms themselves, which may promote the inflammation and the progression of T1DM (173, 174). In addition to this, there are many kinds of microbiota that can have an impact on the development of disease. An increase in the gut microbiota and abundance of Firmicutes was observed in non-obese diabetic (NOD) mice fed with human lacto-oligosaccharide, helping to inhibit islet inflammation and diabetes (175). Although Prevotella has been associated with the development of chronic inflammatory diseases by augmenting mucosal Th17-mediated immune responses, it has also been established that Prevotella play a role in protecting against Bacteroides-induced glucose intolerance, promoting glycogen storage, and enhancing glucose metabolism (176, 177). When transplanting human amniotic mesenchymal stem cells (hAMSC) into T1DM mice, scientists noticed that the therapeutic effect of MSC strongly depends on the modification of the beneficial gut microbiota, including Bifidobacterium, Providencia, Veillonella, and Prevotella, indicating the significant role of the gut microbiota in alleviating symptom and controlling the development of T1DM (178). Except for forming the intestinal mucosal barrier, the metabolic products of the gut microbiota, such as tryptophan derivatives and SCFAs, also regulates intestinal immunity. SCFAs protect NOD mice from insulitis and slow down the development of T1DM by inhibiting inflammatory responses and the accumulation of IFN-γ+T cell in the pancreas (179, 180). As the precursor of SCFAs and an important by-product, intracellular succinic acid could activate intestinal gluconeogenesis positively to regulate gluconeogenesis and blood glucose levels (181, 182). Hence, clinical treatments based on the gut microbiota show promise in maintaining intestinal homeostasis, slow down disease progression, and even reverse T1DM.
4.5 Microbiota and ulcerative colitisUC is a kind of IBD characterized by unpredictable and chronic clinical symptoms, with alternating periods of exacerbation and remission (183). Based on next-generation sequencing technique, scientists have found reduced bacterial diversity and imbalance between beneficial and aggressive bacteria in UC cases (184). Species from the Proteobacteria including E. coli, Enterobacteriaceae, Klebsiella, and Proteus spp., as well as members from Fusobacteria, could enhance inflammatory response and aggravate symptoms, and have been demonstrated to be positively associated with UC (185–188). The microbial metabolites also vary between UC patients and the healthy. SCFAs plays an important role in suppressing intestinal inflammation (34, 189). SCFAs could also induce the production of T-cell-dependent IgA and maintain mucosal homeostasis by regulating the localization of commensal bacteria (190). It has been determined that acetic acid and butyric acid in feces of UC patients were lower than those in the healthy individuals (191). Taken together, all of the changes reveal the close relationships between the gut microbiota and the development of UC (Figure 1). However, given the individual phenotypic differences in the gut microbiota, it is still necessary to further study the exact effect mechanisms between individual microbiota and the host (192).
4.6 Microbiota and psoriasisThe pathogenesis of psoriasis is complex and not very clear. For now, Th17/IL-23 axis has been established as a crucial immunological mechanism in the development of psoriasis, which is also the basis of biologics treatment (193–195). The gut microbiota and their metabolites could adjust the balance between immune tolerance and inflammation, such as acting on differentiation of naïve T cells into either regulatory or Th17 lineages, so that affecting the progression of psoriasis (168) (Figure 1). It has been found that the gut microbiota has impact on the manifestation of the psoriatic phenotype through a Th17-mediated T-cell response on imiquimod-induced mouse models; meanwhile, germ-free mice or conventionally housed mice treated with antibiotics showed protective effect on skin (196) (Figure 1). Scientists also noticed an interesting phenomenon that the gut microbiota dysbiosis in psoriasis patients is similar to those of IBD, both with reduced Eubacterium rectale (E. rectale), Alistipes finegoldii (A. finegoldii) and Alistipes shahii (A. shahii) species (197–200). Based on this consistency, using probiotics to restore the gut microbiota homeostasis and reduce inflammation to achieve therapeutic purpose for psoriasis is possible. Probiotic could suppress the expression of TNF−α, IL−6 and proinflammatory cytokines in the IL−23/IL−17 cytokine axis and enhance gut barrier function to prevent further infection (201). In another case, patients with severe pustular psoriasis showed obvious clinical improvement within 2 weeks after applying Lactobacillus sporogenes supplementation 3 times per day, and almost get remission after 4 weeks (202).
In this manuscript, our focus was directed towards examining the involvement of certain prevalent autoimmune diseases of the gut microbiota, including SLE, RA, SS, T1DM, UC, and psoriasis. However, diseases such as multiple sclerosis (MS), autoimmune thyroid disease (AITD), celiac disease (CeD), among others, were not addressed. Further research is warranted to explore the underlying mechanisms governing immune disorders.
5 Conclusions and prospectsThe interaction between human microbiota and the host plays a crucial role in maintaining health and influencing disease onset. The complex relationship encompasses multiple facets, with microbiota and their metabolites wielding significant influence over host inflammation and immune responses. The gut microbiota participates in regulating immune cell proliferation, differentiation, activation, intestinal permeability, and the integrity of mucosal barriers. Probiotics have emerged as a promising strategy for managing autoimmune diseases, such as SLE and RA. They operate by promoting a healthy gut microbiota and fostering a balanced interaction with the host’s immune system. However, further investigations are warranted to identify specific biomarkers that can accurately distinguish between healthy and compromised microbiota states. Additionally, understanding how microbiota and their metabolites impact normal balanced states versus inflammatory conditions, and discerning potential differences between effects on mucosal surfaces and systemic tissues, remains crucial. In-depth studies investigating the role of microbiota in autoimmune diseases provide insights into the underlying mechanisms of diseases. These insights may reveal prominent diagnostic markers and therapeutic targets, ultimately help us to understand the pathogenesis of autoimmune diseases and explore novel diagnostic and therapeutic strategies.
Author contributionsXW: Writing – original draft. WY: Writing – review & editing. CY: Writing – review & editing. ZW: Writing – review & editing. JZ: Writing – review & editing. DX: Writing – review & editing. XS: Writing – review & editing. WS: Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation, China [82201925 and 82171790] and Natural Science Foundation, Shandong Province, China [ZR2022QH203 and ZR2020KC001].
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. (2020) 181:63–80. doi: 10.1016/j.cell.2020.03.007
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Miyauchi EA-OX, Shimokawa C, Steimle A, Desai MA-O, Ohno HA-O. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol. (2023) 23:9–23. doi: 10.1038/s41577-022-00727-y
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Seekatz AM, Schnizlein MK, Koenigsknecht MJ, Baker JR, Hasler WL, Bleske BE, et al. Spatial and temporal analysis of the stomach and small-intestinal microbiota in fasted healthy humans. mSphere. (2019) 4:e00126-19. doi: 10.1128/mSphere.00126-19
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Leite GGS, Weitsman S, Parodi G, Celly S, Sedighi R, Sanchez M, et al. Mapping the segmental microbiomes in the human small bowel in comparison with stool: A reimagine study. Digestive Dis Sci. (2020) 65:2595–604. doi: 10.1007/s10620-020-06173-x
CrossRef Full Text | Google Scholar
12. Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, et al. Diet and the microbiota-gut-brain axis: sowing the seeds of good mental health. Adv Nutr (Bethesda Md). (2021) 12:1239–85. doi: 10.1093/advances/nmaa181
CrossRef Full Text | Google Scholar
13. Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. (2022) 15:47. doi: 10.1186/s13045-022-01273-9
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Xie H, Guo R, Zhong H, Feng Q, Lan Z, Qin B, et al. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. (2016) 3:572–84 e3. doi: 10.1016/j.cels.2016.10.004
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. (2021) 184:4137–53 e14. doi: 10.1016/j.cell.2021.06.019
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al. Western diet induces dysbiosis with increased E coli in ceabac10 mice, alters host barrier function favouring aiec colonisation. Gut. (2014) 63:116–24. doi: 10.1136/gutjnl-2012-304119
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life sciences: CMLS. (2019) 76:473–93. doi: 10.1007/s00018-018-2943-4
CrossRef Full Text | Google Scholar
19. Lee JS, Wang RX, Alexeev EE, Colgan SP. Intestinal inflammation as a dysbiosis of energy procurement: new insights into an old topic. Gut Microbes. (2021) 13:1–20. doi: 10.1080/19490976.2021.1880241
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. (2016) 7:189–200. doi: 10.1080/19490976.2015.1134082
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. (2017) 357:1156–60. doi: 10.1126/science.aah5043
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Yue B, Gao R, Wang Z, Dou W. Microbiota-host-irinotecan axis: A new insight toward irinotecan chemotherapy. Front Cell infection Microbiol. (2021) 11:710945. doi: 10.3389/fcimb.2021.710945
CrossRef Full Text | Google Scholar
25. Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science. (2012) 336:86–90. doi: 10.1126/science.1219179
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Chen M, Ruan G, Chen L, Ying S, Li G, Xu F, et al. Neurotransmitter and intestinal interactions: focus on the microbiota-gut-brain axis in irritable bowel syndrome. Front Endocrinol. (2022) 13:817100. doi: 10.3389/fendo.2022.817100
CrossRef Full Text | Google Scholar
27. Jiao Y, Wu L, Huntington ND, Zhang X. Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases. Front Immunol. (2020) 11:282. doi: 10.3389/fimmu.2020.00282
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Luciani C, Hager FT, Cerovic V, Lelouard H. Dendritic cell functions in the inductive and effector sites of intestinal immunity. Mucosal Immunol. (2022) 15:40–50. doi: 10.1038/s41385-021-00448-w
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Shi Z, Ohno H, Satoh-Takayama N. Dietary derived micronutrients modulate immune responses through innate lymphoid cells. Front Immunol. (2021) 12:670632. doi: 10.3389/fimmu.2021.670632
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Cox CB, Storm EE, Kapoor VN, Chavarria-Smith J, Lin DL, Wang L, et al. Il-1r1-dependent signaling coordinates epithelial regeneration in response to intestinal damage. Sci Immunol. (2021) 6:eabe8856. doi: 10.1126/sciimmunol.abe8856
留言 (0)