It was a set of staggerer mice that drew the attention of Sidman et al. in 1962 to their underdeveloped cerebellar cortex (1). The staggerer mice were a set of mouse mutants, maintained at the Jackson Laboratory, after an F1 female (BALB/cHm crossed with C3H/HeJ) had bred with the male of an obese (Lepob) stock mouse (https://www.jax.org/strain/000237). Sixteen years later, in 1978, Landis and Sidman studied the developmental stages of the staggerer mice mutants and noted pathological changes in the cerebellar cortex of these mice (2). Earlier in 1978, Roffler-Tarlov and Sidman noted reduced concentrations of glutamic acid in the staggerer mice (3). The glutamine→glutamate→α-ketoglutarate cycle stands at the crossroads of carbohydrate, protein, and lipid metabolism, serving as an essential precursor to the synthesis of purines and pyrimidines, which is the mainstay in the uninterrupted synthesis of genes on chromosomes (Figure 1). It was no wonder then that Roffler-Tarlov and Sidman noted reduced glutamic acid, encoded by the purine codons GAA and GAG, which might have affected the transcription and translation of the ligand binding domain (LBD) of RORα (3, 4). However, it was not until 1996 when Hamilton et al. deciphered the genetic reasoning behind the characteristic clinical observations in the staggerer mice—the deletion of 160 kilobases on chromosome 9—that resulted in a truncated version of the RORα protein, devoid of its LBD (4). The deletional mutation affecting the LBD of the RORα gene led to the birth of the staggerer (sg−/sg−) mice exhibiting symptoms associated with a loss of function to the downstream cascade of signaling events promoted by ligand-induced RORα. Essentially a deletion of RORα’s LBD region manifested itself as pronounced developmental and metabolic alterations in the cerebellum, adipose tissue, bones, cardiac and skeletal muscles, liver, thymus, and spleen of the staggerer mice (1, 5–9).
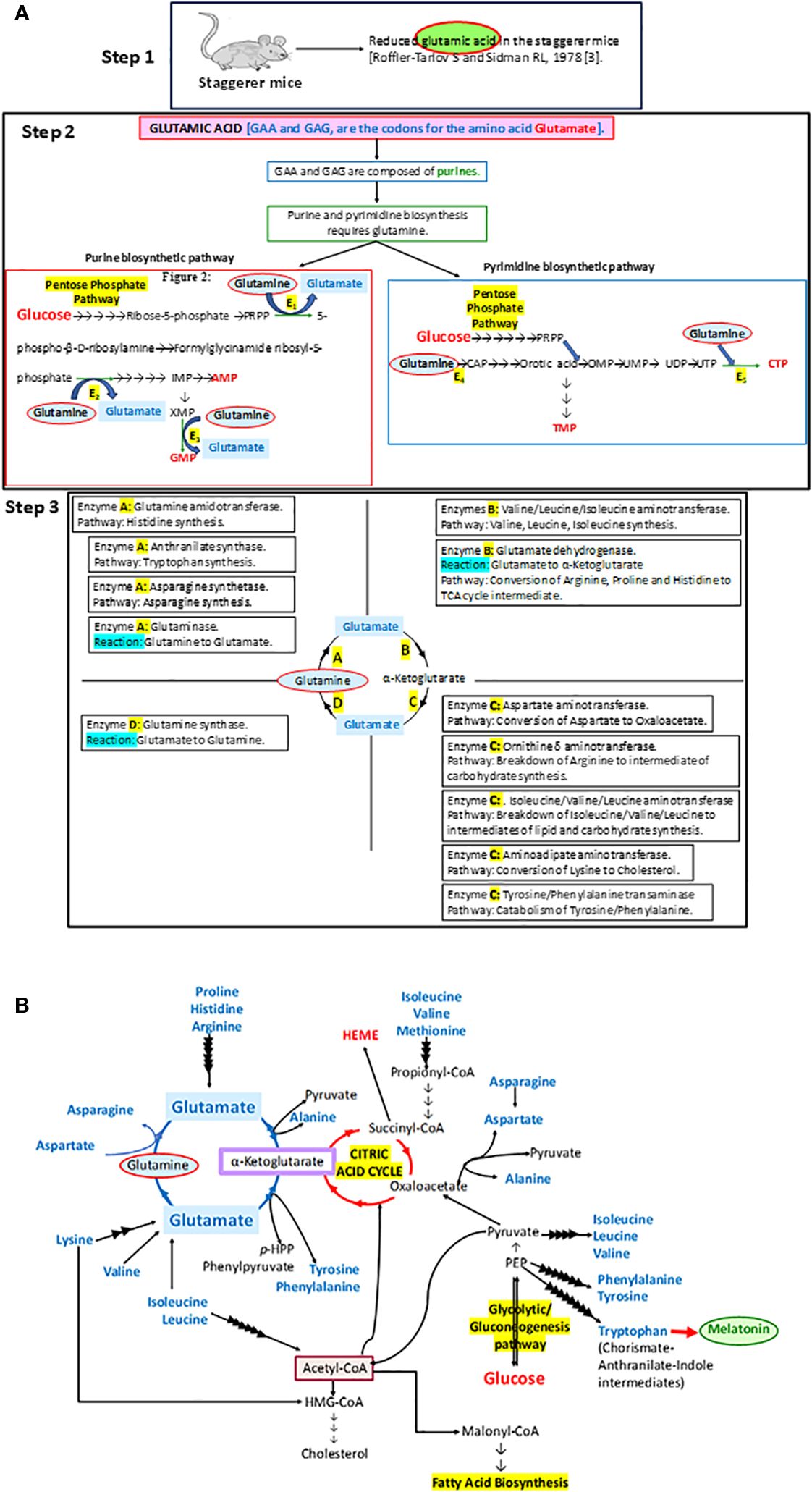
Figure 1 (A) A step-by-step analysis of the observations by Roffler-Tarlov and Sidman (1978) (3). The enzymes E1, E2, E3, E4, and E5 in step 2 that require glutamine as a substrate in the synthesis of purines and pyrimidines stand for phosphoribosyl pyrophosphate (PRPP) glutamyl amidotransferase (E1), formylglycinamide ribosyl-5-phosphate synthetase (E2), xanthosine monophosphate (XMP) transamidinase (E3), cabamoyl phosphate (CAP) synthase II (E4), and cytosine triphosphate (CTP) synthase (E5), respectively. PRPP is phosphoribosyl pyrophosphate, IMP is inosine monophosphate, GMP is guanosine monophosphate, AMP is adenosine monophosphate, CAP is carbamoyl phosphate, OMP is orotidine monophosphate, UMP is uracil monophosphate, UDP is uracil diphosphate, UTP is uracil triphosphate, CTP is cytosine triphosphate, and TMP is thymine monophosphate. Some of the other enzymes involved in metabolic pathways, as part of the glutamine→glutamate→α ketoglutarate cycle: A, B, C and D, are denoted in the four quadrants at step 3. (B) The importance of the glutamine→glutamate→α ketoglutarate cycle and its association with carbohydrate, lipid, and protein metabolism has been illustrated in this diagram.
The staggerer mice, with a defunct RORα LBD that led to the disruption to RORα-mediated crucial genetic networks, were endowed with specific redundant genetic mechanisms that permitted viability of these mice. The redundancy of RORα to the transcription factors DHR3, RARα1, RXRα, Rev-ERBα, T3Rβ, PPARα, VDR, or GR, at specific anatomical and pathological sites, upon activation with a specific ligand, was essential to the survival and longevity of the staggerer mice (10–12). The activation of RORα and their redundant transcription factors within specific cells and tissues, with the specific ligand, leads to the activation of a cascade of signaling molecules and the organization of cells within tissues during the stages of embryogenesis, an absence or the inability of which leads to the staggerer mice (4, 10–14). The known ligands of RORα are melatonin, tri-iodothyronine (T3), the steroid hormones (estrogen, testosterone and the adrenal gland hormones, cortisol, and adrenocorticotrophic hormones), cholesterol and cholesterol derivatives (15–20). These ligands, sometimes known as hormones, are mainly under the control of the hypothalamic–pituitary axis (HPA) and function as an integral part of the circadian rhythm. In response to the cyclical release of hormones by the HPA, a “molecular circadian clock” operates within cells, composed of the clock genes and RORα (21–26). Sato et al. (2004) along with Akashi et al. (2005) clearly substantiated RORα as a circadian clock component (21, 23).
In essence, the loss of RORα, a crucial molecular circadian clock gene, led to a tsunamic effect on RORα-dependent molecular events and functions. Sidman et al. (1962) more precisely described the staggerer mice as recognized with a “staggering gait, mild tremor, hypotonia, and small size”, and that their “cerebellar cortex is grossly underdeveloped, with too few granule cells and unaligned Purkinje cells” (1). Moreover, glutamic acid—an amino acid intermediate of the essential and non-essential amino acids (aspartate, alanine, isoleucine, leucine, valine, methionine, asparagine, phenylalanine, tyrosine, arginine, lysine, histidine, and proline), carbohydrate metabolism at the α-ketoglutarate and oxaloacetate intermediate stages (Krebs tricarboxylic citric acid cycle pathway), and lipid metabolism (free fatty acids metabolize into acetyl-CoA→citrate→α-ketoglutarate→glutamate)—was noted to be reduced in the staggerer mice (Figure 1) (3). Thus, nutrition and the ability of an enzymatic machinery in the pre- and post-natal cells to assimilate and metabolize carbohydrates, lipids, proteins, and nucleotides play an important role in the development of wild-type mice without any mutations. An unhealthy diet, bereft of the essential amino acids, is the main cause of a disrupted molecular–circadian rhythm, dysregulated endocrine HPA, anomalous metabolism, mutations, and an immune system susceptible to infections.
This review had been conceptualized due to RORα being identified as a signal transducer and activator of transcription 5 (STAT5) target gene during my doctoral studies at King’s College London, although the review in its present form has been compiled after literature surveys led me to the staggerer mice and melatonin as the keywords, during searches regarding the genetics of RORα.
RORα: gene and protein structureRORα belongs to a group of nuclear receptor (NR) superfamily of transcription factors that elicit their response upon binding to a hormone response element (HRE), more commonly known as a RORE site. The basic structure of RORα, like all other members of the nuclear family of receptors, contains the following domains: an N-terminal transactivation domain (NTTD), a DNA binding domain (DBD) that determines its specificity for specific DNA sequences within genes, a hinge domain, and a carboxy terminal LBD that dictates crucial conformational changes in the structure (27). A grouping of the NR family of proteins, based on the HUGO nomenclature, is listed in Supplementary Table 1 (HUGO Gene Nomenclature Committee). RORα is located on the long arm, at the q22.2 loci on human chromosome 15, as per the human assembly, March 2006 build NCBI36/hg18 (www.ncbi.nlm.nih.gov28). The carboxy terminus on the RORα protein and gene, in mice and humans, is close to two other genes of significance: NMDA receptor-regulated 2 (NARG2) and Annexin A2 (ANXA2) (4, 28). A schematic representation of the chromosomal mapping of RORα in humans and mice is depicted in Figure 2. Notably, while RORα is transcribed in a 5′ to 3′ direction away from the centromere in the “nocturnal mouse”; in the “diurnal humans”, RORα is transcribed in a direction towards the centromere (28). There are at least four known isoforms of RORα—RORα1, RORα2, RORα3, and RORα4—that belong to the group 2 NRs and most studies have noted a predominant role for RORα1 and RORα4 (27–31). The four isoforms of RORα as per the UCSC genome browser databases are listed in Table 1 (28). Each of these four isoforms display differential activities depending on their differential expression in specific cells within specific tissues (14, 15, 21–24).
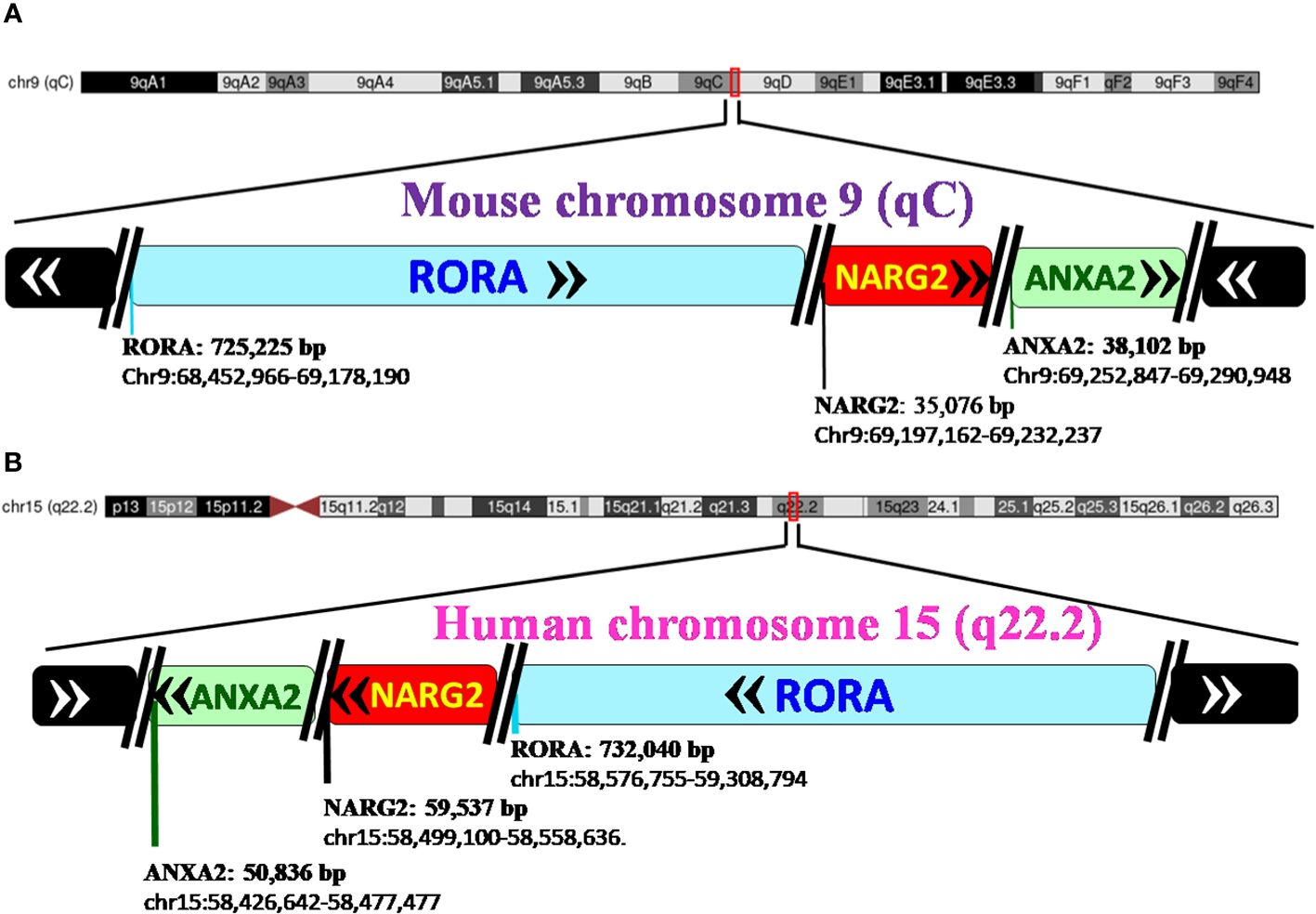
Figure 2 Pictorial representation of the chromosomal mapping of RORα gene in mice and humans. (A) RORα in the mouse maps to qC on chromosome 9, and its length and precise location are denoted below the name of the gene, as per the UCSC Genome Browser on Mouse Feb 2006 (NCBI36/mm8). The gene transcribes away from the centromere. RORα is followed by NARG2 and ANXA2, all the three genes being transcribed in a direction away from the centromere. (B) In humans, RORα maps to q22.2 on chromosome 15, as per the UCSC Genome Browser on Human Mar. 2006 (NCBI36/hg18) build, and transcribes towards the centromere (28). [The length of the gene is mentioned alongside the name of the genes as in panel (B) RORA: 732,040 base pairs and the region that the gene spans on the chromosome, as chr15:58,576,755-59,308,794. In humans, the three genes, RORα, NARG2, and ANXA2, transcribe themselves in a 5′–3′ direction towards the centromere. RORA, NARG2, and ANXA2 refer to the isoform 1 of each of these genes.].
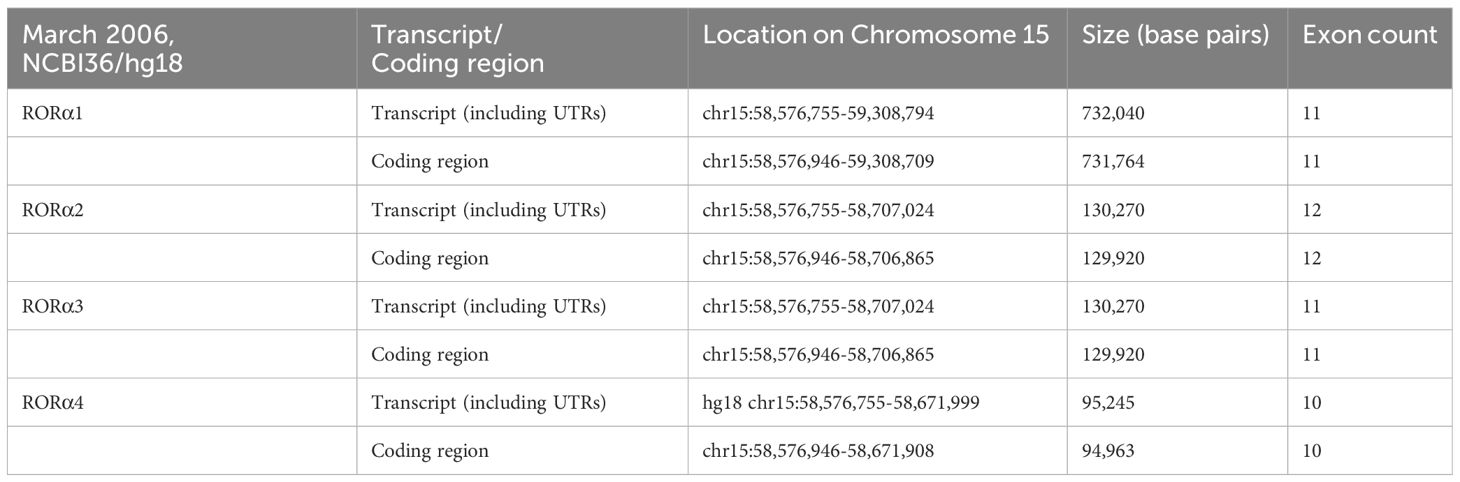
Table 1 The four isoforms of RORα, and their respective loci on human chromosome 15 (28).
RORα was first cloned and functionally characterized by Giguere et al. in 1994 and they identified a notable difference, in that the N-terminal domain dictates the specificity for DNA binding by each of the variants (11). X-ray crystallography at a resolution of 1.63 Å and 2.2 Å identified cholesterol and its derivative as RORα’s natural ligands at the LBD of RORα (19, 20). A study by McBroom et al. (1994) revealed that the hinge region, located between the DBD and the LBD, determines the angle of bend that occurs to stabilize RORα–DNA interactions (29). Of essence is also the study where sequence alignment studies detected homology in the DBD, between RORα and several other NRs: Retinoid X receptor (RXRα), V-ErbA-Related Protein 1 (Rev-Erbα), thyroid hormone receptor (T3Rβ), peroxisome proliferator-activated receptor (PPARα), vitamin D receptor (VDR), and the glucocorticoid receptor (GR) (11). One of the reasons behind the ability of staggerer mice to survive embryogenesis past adulthood is a result of the compensatory mechanisms exercised through the redundant role sharing between RORα and few other NR family members. It was the DNA binding sequence of Rev-Erbα that is very similar to RORα1 and RORα2 (11, 12). Sequence alignment of RORα1 with Rev-Erbα showed 60% similarity of its DBD to RORα1 and only 30% of its LBD to RORα1 (11, 12). Again, amino acid sequence alignment revealed similarities between the mouse Rev-Erbβ, rat Rev-Erbα, human RORα, and rat RZRβ (10). Rev-Erbβ, Rev-Erbα, and RORα1 bind to the same extended binding site (10). Additionally, Giguere et al. go on to state that the RORα and Rev-ErbAα-binding sites are practically indistinguishable, and the two receptor systems should be expected to control overlapping gene networks (11). In support of the above, a comprehensive study by Mukherjee et al. (2021) has clearly demonstrated that RORα along with the other NRs, Rev-Erbα, PPARβ, Erβ, and PPARα are expressed uniquely in the intestinal compartments, ileum, and colon, although there is a certain degree of redundancy between these NRs (12).
Basically, RORα mediates its actions through the activation of the ligand–RORα complex, which then binds to consensus sequences within target genes, namely, the ROR response elements (ROREs) (11). The ROREs are composed of a 6-bp-long A/T-rich sequence followed by (A/T)GGTCA (11). It is important to note that Odawara et al. identified a novel RORα binding site within the aromatase gene, more specifically at exon 1.4 in MCF7 cells, although occupancy by RORα within the exon would possibly be expected to block the transcriptional activity of the aromatase gene (30). Upon ligand-induced RORα binding to the RORE sites within genes, transcriptional activity ensues, which is attained through post-translational and structural modifications, aided through the binding of an array of proteins to the regulatory sites of genes.
RORα: post-translational modificationsUpon binding of a ligand to the LBD of the RORα gene, a conformational change ensues, thereby promoting its binding to regulatory regions within their target genes and subsequent effects on the regulation of embryogenesis, with modulation of a molecular–circadian rhythm being one of the first and foremost in the cascade of regulatory processes affected (21, 23). RORα, akin to other transcriptional factors, promotes its action through post-translational modifications: phosphorylation, acetylation, ubiquitination, and SUMOylation (27). The post-translationally modified sites of RORα are depicted in Figure 3. According to Hornbeck et al. (2015), RORα is phosphorylated at S35, S100, S155, T183, S201, and N202; ubiquitinated at K162 and K469; acetylated at K79; and SUMOylated at K240 (27).
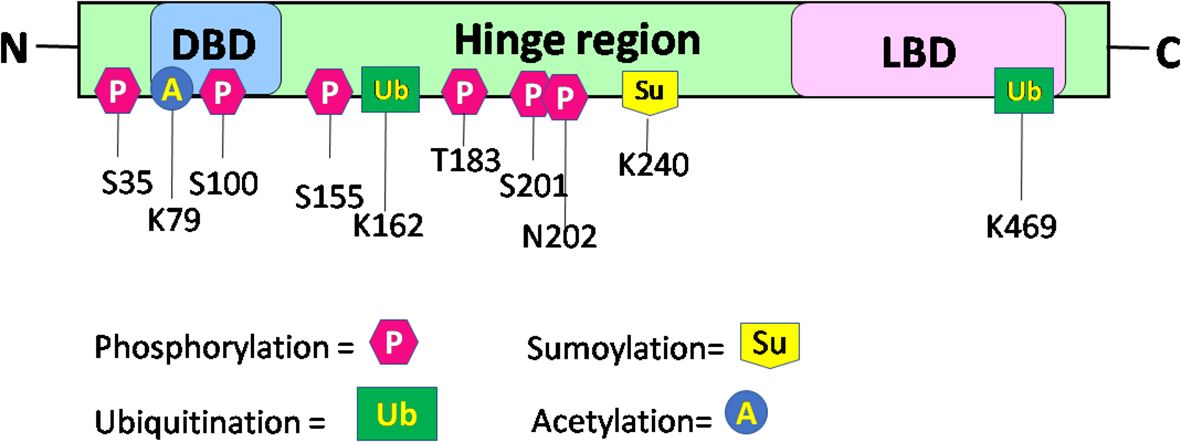
Figure 3 Schematic structure and post-translational modification sites of RORα in humans. This figure for RORα has been modified and reproduced from www.phosphosite.org.
Post-translational modification of RORα promotes the activation or repression of its intrinsic activity and the target genes, thereby promoting successful embryogenesis, growth, and development (1, 2, 4, 31–35). Phosphorylation of RORα at serine 35 is promoted by the activation of protein kinase C (PKC) (31). Ermisch et al. identified protein kinase A (PKA) as one of the kinases phosphorylating RORα and a mutation at the phosphorylating site led to an abolishment of the phosphorylating capacity by PKA at that site (32). Ermisch et al. subsequently concluded that RORα4 is regulated by PKA and calcium/calmodulin-dependent protein kinase IV (CaMK-IV) (32). Extracellular signal-regulated kinase 2 (ERK-2) is another RORα4 phosphorylating protein, where the hinge region of RORα4 contains an ERK-2 binding motif at the threonine residue 128, and a mutation of T128A leads to enhanced transcriptional activity (33). Using the HEK293 cells, RORα was identified to be phosphorylated by protein kinase C alpha (PKCα), within the N terminal domain (31). RORα is also phosphorylated at S100 (34). More specifically, chromatin immunoprecipitation (ChIP) studies, Western blotting, and luciferase assays performed on the S100 site of RORα, using transfection of the hRORαWT100, hRORαS100A (alanine = A), or hRORαS100D (aspartate = D) sequences into pGL3 vectors containing the SULT1e1 promoter, validated the phosphorylation of RORα at the S100 locus (34). While RORα is phosphorylated by ERK-2, PKA, and PKCα, it is also SUMOylated by small ubiquitin-like modifier 1 (SUMO1) and SUMO2 at the Lysine 240 residue, by the protein inhibitor of activated STAT (PIAS) proteins (Figure 3) (27, 31–35). Furthermore, RORα is acetylated at K79 and ubiquitinated at K162 and K469, respectively (27). In addition to these modifications within the RORα protein, which generates a structural configuration aimed at accomplishing distinct functions within distinct cells in a tissue, RORα also promotes the recruitment of transcriptional coregulators.
RORα: transcriptional coregulators and regulationRORα mediates its actions through its association with co-activators and co-repressors that act as a conglomeration of proteins to promote transcriptional functions, with one of its first roles materializing during embryogenesis. The activation/repression of upstream/downstream genes is determined through a fine cross-talk between transcriptional coregulators that regulate RORα. RORα is regulated by glucocorticoid receptor-interacting protein-1 (GRIP-1), with GRIP1 itself being phosphorylated by glucocorticoid-mediated glucocorticoid receptor (GR) interactions (36, 37). Atkins et al. (1999), using a two-hybrid screen, had identified RORα coactivators that bind as a complex of proteins: GRIP-1 and peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP/TRAP220/DRIP205) (36). The same study also identified TRIP-1, TIF-A, TRIP230/TRIP-11, and PBP as few other RORα modulators (36). Subsequently, Ji Min Lee et al. in 2001 identified β-catenin and GRIP1, along with several other protein binding partners of RORα, with molecular weights of 270, 220, 160, 140, 65, and 45 kDa, respectively (31). A GRIP1 knockout study defined the importance of GRIP1 during embryogenesis (38). Another co-activator of RORα is PPARG coactivator 1 alpha (PGC1α), a regulator of energy metabolism (39). The PGC1α-RORα pathway regulates the circadian genes BMAL1, clock, and Rev-ERBα, possibly through a self-regulatory “molecular circadian rhythm” (39, 40). While the coregulator PGC1α activates the transcription of RORα, Hairless (Hr) acts as a repressor of RORα’s transcriptional activity (40, 41). In a separate study, p300 and PGC1α were identified as the upstream coactivators of RORα; these coactivators along with RORα regulate caveolin-3 (CAV3) and the enzyme carnitine palmitoyltransferase-1 (CPT1) in the muscle (42). While some transcription factors act as coregulators of RORα, RORα itself serves as a coregulator.
In the liver, RORα acts as a coactivator with SRC2 to regulate the functions of the enzyme Glucose-6-phosphatase (43). RORα also binds to the promoters of Slc1a6, Itpr1, Pcp4, and Pcp2 to regulate their expression (44). The string database has assembled and organized a few of the proteins that interact with RORα, some of them as of July 2023 are as follows: NPAS2, CLOCK, NRIP1, ARNTL, HIF1A, KAT5, WNT5A, STAT3, BCL6, and BATF (www.string-db.org). The protein N-MYC expressed highly in the brain is regulated by RORα (45, 46). The staggerer mice, an example of a molecular–endocrine–circadian rhythm dysregulated model of mice, expressed reduced levels of the sonic hedgehog (Shh) transcript and RORα was ascertained to bind to a RORE sequence in the promoter of Shh, along with the coactivators β catenin and p300 (44). Studies in mice have shown that RORα, ataxin 1 (ATXN1), and the RORα coactivator lysine acetyltransferase (Tip60) exist as a complex of proteins, whereby cerebellar development is affected (47–49). Specific isoforms of RORα, in the presence/absence of specific activators/repressors, undergo specific modifications and perform specific functions, in the specific tissue. The RORα1 isoform interacts with co-repressors nuclear receptor corepressor 1 (NCOR1) and nuclear receptor corepressor 2 (SMRT/NCOR2) (48). It is again the RORα1 isoform that forms a complex with myogenin (myoD) and p300 (50). While the network of proteins regulating RORα and its isoforms are dependent on the pathological site of action, the sequence of genes and transcription factors regulated by RORα and its isoforms are also dependent on the site of action.
RORα: gene expression profiling studiesRORα is expressed differentially depending on the activating ligand, the anatomical site of action, the time of the day, and the stage of embryonic development. The staggerer mice with a deleted LBD within the RORα had affected the cerebellar cortex of the mice (1, 4, 49). In agreement with the findings of Sidman et al. (1962) and Hamilton et al. (1996), along with recent genomic studies on the human brain, RORα is expressed copiously in the cerebellum of humans (1, 4, 46). The normalized transcript expression (nTPM) data for RORα in the brain, available from the Human Protein Atlas, revealed the highest expression in the cerebellum (nTPM = 51.1), followed by the thalamus (49.6), midbrain (36), amygdala (19.9), cerebral cortex (13.1), hypothalamus (12.6), and basal ganglia (11.7), respectively. Few other organs presenting with high expression of RORα were the adrenal gland (nTPM = 38), liver (28.7), bone marrow (17.5), skeletal muscle (14.4), and the thyroid gland (12.8) (Human Protein Atlas dataset) (46). Northern blot analysis done on several human tissues indicated that Rev-Erbβ, Rev-Erbα, and RORα1 are expressed in the brain and skeletal muscle, while Rev-Erbβ and Rev-Erbα are expressed in the lung, liver, and kidneys too (10). Gene expression analysis on skeletal muscle, liver, and epididymal fat of staggerer mice reported diminished expression of RORα1 and RORα4, when compared to the WT mice (7). Understandably, the four isoforms of RORα perform independent functions and, under altered circumstances, play redundant roles. Thus, although RORα is expressed in several other organs, the mountain of evidence for the role of RORα, an endocrine-like-mediator of a "molecular circadian rhythm", in organogenesis is irrefutable (1, 49).
Activation of RORα upon the formation of a ligand–RORα complex leads to the binding of RORα to RORE sequences, thereby mediating the activation/repression of several genes. Some of the genes known to be activated/repressed are: NMYC, CAV-3, CABP3, FAS, ADRP, ACS4, SCD1, SCD2, VEGF, HIF1α, ALOX5, apoa5, srebp1, ampk, apocIII, apocI, shh, slc1a6, Itpr1, pcp4, pcp2, CIDEC, CIDEA, GPAM/GPAT1, AGPAT9, MOGAT1, ACOT3, acyl-coenzyme A thioesterase 4 (ACOT4), FABP5, ADFP/PERILIPIN 2, LPIN2, ANGPT14, FGF21, CYP19A1, MIF, ABCA1, SEMA3E, NEPH, ADCY8, NR2F1, Netrin G1, CD47, CSPG5, LXRα, CYP7B1, IkBα, c-jun, E4BP4, IL-6, REG3Y, STAT3, and TNFα (42, 44, 45, 51–61). A list of the genes regulated by RORα is listed in Supplementary Table 2.
RORα: circadian rhythm–HPAA molecular circadian rhythm that regulates the expression of RORα through the developmental stages of life is a determinant of uninterrupted embryogenesis, the birth of a fetus, without cerebellar defects, and the obvious stagger (1, 2). Years later, the research by Akashi and Takumi (2005) and Sato et al. (2004) then laid the foundation for a role of RORα as a core component of the mammalian circadian clock (21, 23). In 2006, Yang et al. cemented the findings of Akashi and Takumi (2005) and Sato (2004) when they studied the rhythmic expression of RORα, RORγ, Clock, Bmal1, Per2, Cry1, Rev-ERBα, Rev-ERBβ, PPARγ, PPARα, PPARδ, PGC1α, and TRα in the white adipose tissue (WAT), brown adipose tissue (BAT), liver, and skeletal muscle of mice during a 28-h day–night period (21, 23, 40). While the expression of RORα was the highest in the WAT, at the 12-h Zeitbeger time (ZT), the well-known circadian genes, Clock and Bmal1, dipped to their lowest (40). Earlier, Clock, Bmal1, Per1, and Per2 had been identified as circadian rhythm clock genes (62).
It is the rhythmic circadian cycle that plays a crucial role in maintaining balance and normal gait, which was absent from the staggerer mice. In 2017, three researchers, Jeffrey Hall, Michael Rosbash, and Michael Young, were awarded the Nobel Prize for their studies on the circadian gene “period” (63, 64). The original concept of circadian rhythm was said to have been derived from the rotation of the earth around its axis in 24 h. The circadian rhythm is responsible for several rhythmic variations observed in humans, with the hormonal and temperature fluctuations, the metabolic changes, and the sleep–wake hours being some of the obvious rhythmic changes observed between various times of the day (21, 22, 62, 65, 66). Some of the external factors that maintain the biological rhythm are light, diet, sleep and wake hours (time of the day), and sedentary/active lifestyle, among many other known and unknown factors (65–68). A synchronized HPA, with positive and negative feedback mechanisms, allows for specific hormonal release at specific times of the day, thereby regulating a spontaneous and balanced circadian rhythm, and vice versa (66, 67). The suprachiasmatic nucleus (SCN) of the hypothalamus serves as a pacemaker of circadian oscillation (66, 67). RORα is expressed in the SCN of the hypothalamus and the transcript levels of RORα follow a cyclical day–night harmonic trend (40, 66). After numerous studies on RORα and the staggerer mice, Vitalis and Mariani in 2018 had concluded that “RORα acts initially in various aspects of brain construction and later on in neuroprotection” (69). My hypothesis, based on already published data, that led to the birth of the staggerer mice is depicted pictorially in Figures 4A, B. Some of the hormones known to affect RORα regulation rhythmically/arrhythmically are discussed below.
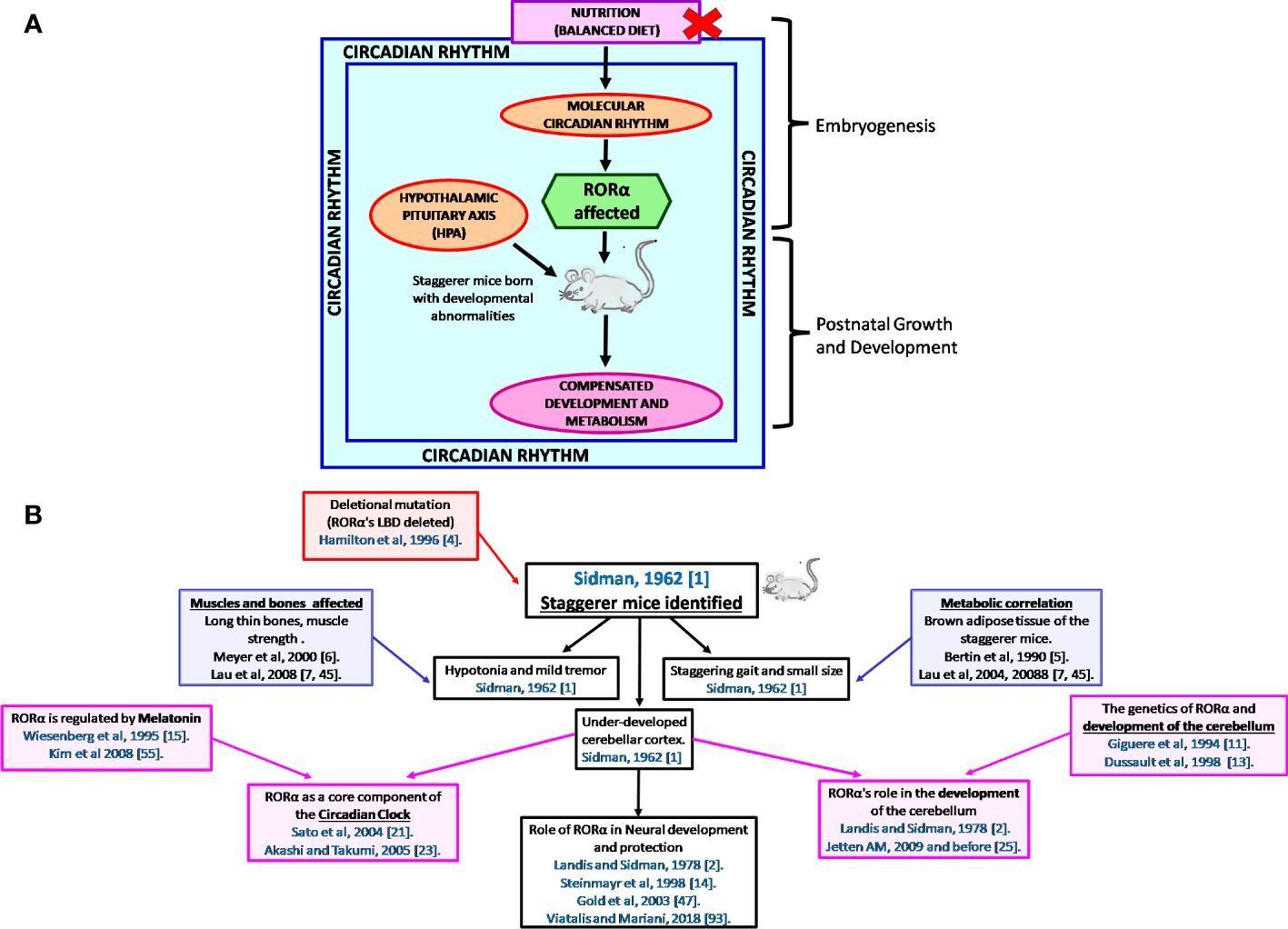
Figure 4 (A) A schematic representation of the thought process behind the birth of the staggerer mice and its compensatory mechanism. A nutritionally challenged/imbalanced diet led to error-prone DNA replication, unable to direct the systematic regulation of the molecular circadian events, with the RORα gene being affected. A mutated RORα ligand binding domain led to the birth of the staggerer mice. The staggerer mice were endowed with a set of compensatory mechanisms to withstand the mutation, through adiposity resistant metabolic pathways, along with following alternate transcriptional (Rev-ERBα/Rev-ERBβ) pathways. (B) A flowchart of the milestones after the discovery of the staggerer mice in 1962 by Sidman, Lane, and Dickie in 1962 (1). The foundations of this review lie on the substantial research findings by all the authors mentioned in this flowchart (1, 2, 4–7, 11, 13–15, 21, 23, 25, 45, 47, 55, 70).
RORα: melatoninThe circadian rhythm is mainly maintained through an HPA-regulated homeostatic mechanism, composed of hormones, one of which is melatonin, released by the pineal gland. Although melatonin is predominantly secreted by the pineal gland, melatonin is also produced by the lymphocytes and the bone marrow (71, 72). Melatonin is a metabolite of the nutritionally essential amino acid tryptophan. The essential amino acid tryptophan is hydroxylated to 5-hydroxytryptophan, which is further metabolized to serotonin and melatonin. Melatonin was identified as a ligand for RORα1, when studying the effects of the synthetic compound CGP52608 (15). The circadian hormone melatonin, also known as the “hormone of darkness”, was identified as a transcriptional regulator of RORα and RORα1 (15, 16, 54). Eun-Jin Kim et al. (2008) successfully demonstrated that melatonin enhances the transcriptional activity of RORα1, RORα4, HIF1α, and VEGF (54). Melatonin is a circadian rhythm hormone, fluctuating rhythmically, with a peak occurring during the early hours after midnight, and serves as a neuroprotective hormone through the brain glutathione peroxidase (73–75). The antioxidant enzymes glutathione peroxidase 1 (gpx1) and peroxiredoxin 6 (prx6) promote neuroprotection through the human RORα1 (76). In MCF7 cells, melatonin acts on MT1 and RORα to inhibit cell proliferation (77, 78).
RORα: steroidogenic hormonesAn interesting study by Komatsubara et al. (2017) deciphered several interactions between adrenocortical steroid hormones, melatonin, BMP-4, and catecholamine synthesis in rat pheochromocytoma (PC12) cells (79). Cholesterol is a precursor to the steroidogenic hormones and Kallen et al. (2002) discovered cholesterol as a natural ligand of RORα (19, 80). RORα is postulated to be constitutively activated in a specific conformation, and evidence from HepG2 cells treated with cholesterol sulfate, 22®-hydroxycholesterol, and 7-dehydrocholesterol demonstrated enhanced expression of RORα4, HIF-1α, and VEGF (19, 54). Assays to study the effects on RORα’s LBD identified maximum activation of RORα by cholesterol sulfate, followed by 7-dehydrocholesterol, cholesterol, epicholesterol, and cholestenol, respectively (19). An interesting study by Kallen et al. (2004) identified cholesterol 3-o-sulfate as a stronger ligand of RORα, when compared to cholesterol, the reason being additional hydrogen bonds at the RORα LBD site (20). The angle of bend between the DBD and the LBD that is plausible due to the hinge region and leads to stabilization of RORα complexes is determined by specific ligands and hormones that act either transiently or constitutively (29). Since cholesterol is a precursor of the steroid hormones that serve as critical mediators in the HPA axis and is a natural ligand of RORα, any synthetic modifications to cholesterol might have deleterious effects on the pathophysiology of the RORα regulatory pathway (80). However, estradiol (E2), a natural cholesterol metabolite, was established to activate RORα and plays a crucial role in bone mineralization (18). Osteopenic bones were a hallmark of the staggerer mice (6). E2, the female reproductive hormone, activates RORα, and the estrogen receptor β (ESR2) along with estrogen receptor-related protein 3 (ESRRG) were identified as potential RORα target genes by Sarachana and Wu (2013) (18, 81). The same study also identified progesterone receptor membrane component 2 (PGRMC2), the hydroxysteroid 17-beta dehydrogenase 3 (HSD17B3), and hydroxysteroid 17-beta dehydrogenase 10 (HSD17B10) as RORα targets in their genome-wide study on a neuronal cell line (81). Moreover, upon stimulation of MC3T3-E1 osteoblastic cells with E2, there was a RORα-mediated upregulation of bone morphogenetic protein 2 (BMP2) and RUNX family transcription factor 1 (RUNX1) (18). Testosterone, another cholesterol metabolite, serves as a ligand for RORα in the skeletal muscles (82).
The staggerer mice showed enhanced responsiveness to novelty stress when compared to the wild-type mice, with enhanced levels of adrenocorticotropic hormone (ACTH) (83). Basically, the hormones produced by the adrenal cortex are controlled by the corticotropin-releasing hormone (CRH) and the anterior pituitary, under positive and negative feedback mechanisms. At an nTPM of 38, the adrenal gland expresses RORα only next, after the cerebellum (51.1) and the thalamus (49.6) (46).
Apart from the HPA-regulated positive and negative feedback mechanisms, cyclical variations influenced by seasonal changes, varied stages of development/aging, sleep, and psychological changes are among few of the known factors that affect RORα expression and regulatory pathways. Under stressed conditions, the staggerer mice exhibited enhanced endocrine activity, in a study of the ACTH levels in these mice (83). Additionally, the expression of cytochrome P450 family 7 b1 (CYP7B1), which is a RORα target gene, was reduced in the staggerer mice (59, 81). The cytochrome p450 family of enzymes are present in the adrenals as well as the liver and a number of genes in this family of proteins were identified as putative RORα target genes (81). The cytochrome family of genes identified as part of the Sarachana and Wu study were as follows: CYP2R1, CYP7B1, CYP2D7P1, CYP26B1, CYP26A1, CYP27C1, CYP19A1, CYP11A1, CYP2B6, CYP2B6, CYP2A13, CYP2U1, CYP2A7, CYP4A22, and CYP4F3 respectively (81).
RORα: eicosanoid hormonesSince the RORα–HPA molecular circuitry is a complex association, some of the other pathways regulated are the NF-κB-IkBα and TNFα-COX2 (60, 84). RORα1 activates the NF-kB-IkBα pathway, upon binding to a IkBα RORE, although melatonin-induced RORα inhibits NF-kB activity (60, 84). RORα1, through a TNFα-mediated pathway, leads to suppression of cyclooxygenase-2 (COX-2) (60). The cyclooxygenases are involved in prostaglandin synthesis. Additionally, 5-lipoxygenase is a RORα1 target gene, presenting with a RORE site (55, 56). The lipoxygenase is an enzyme crucial to the formation of leukotrienes. Again, the prostaglandin F2 receptor negative regulator (PTGFRN) and prostaglandin E synthase (PTGES) were both identified as RORα target genes during a genome-wide study on the human neuronal cell line SH-SY5Y (81). Essentially, the cyclooxygenases and lipoxygenases are enzymes that synthesize prostaglandins and leukotrienes from nutritionally essential fatty acids, namely, linoleic and α-linolenic acid, which go on to synthesize arachidonate, the precursor to prostaglandins, thromboxanes, and leukotrienes.
RORα: thyroid hormonesAnother HPA-controlled axis of hormones is the thyroxine-releasing hormone (TRH)–thyroid-stimulating hormone (TSH)–T3/T4 pathway. The transcript expression of RORα in the thyroid gland is at 12.8 nTPM as per the Human Protein Atlas (46). Boukhtouche et al. (2010) studied the importance of tri-iodothyronine (T3) as a mediator of dendritic branching in the cerebellum, through their studies on the RORα-deficient staggerer mice (17). Only the RORα1 isoform is regulated by tri-iodothyronine (T3), in the cerebellum of Swiss mice when compared to the staggerer mice, which failed to express RORα (17).
The TRH-TSH-T3/T4 pathway of hormones is one of the most important hormonal pathway in the HPA, controlling metabolism of carbohydrates, proteins, and lipids, and T3 has long been documented to play a pivotal role in metabolism at the cellular level (85, 86). Regulation of the thyroid gland is under the influence of the pituitary that secretes the TSH and the hypothalamus, which releases the TRH. The thyroid hormone receptor (THRB) gene is a potential RORα target, as identified during a genome-wide study on a neuronal cell line (81).
RORα: metabolismWith developmental defects being the hallmark of the staggerer mice, the most significant study was that of Roffler-Tarlov and Sidman (1978), wherein they noted reduced glutamic acid, a prerequisite in the uninterrupted synthesis of the building blocks of the RORα gene: the purines adenine and guanine, and pyrimidines thymine and cytosine (Figure 1). Glutamic acid is a metabolic by-product of carbohydrate, lipid, and protein metabolism, the glutamine→glutamate→α-ketoglutarate cycle serving as the constant source of metabolic fuel that governs normal embryogenesis, growth, and development. As expected, the staggerer mice revealed aberrant metabolic signaling in addition to the observations of cerebellar defects and a staggering gait by Sidman et al. (1, 51–53, 87–93). Lau et al. (2008) observed that the staggerer mice were leaner, with reduced fat pads; Trenkner and Hoffman (1986) noted that the staggerer mice presented with smaller thymus and spleen when compared to their wild-type counterparts, and Lau et al. (2004) directly implicated RORα to lipid metabolism (7, 9, 42). These macroscopic, microscopic, and metabolic observations in the staggerer mice had set the stage for researchers looking for answers to the metabolic pathways affected (3, 5–7, 18, 25, 51–53, 56–59, 70, 79, 84, 87, 94–98). The staggerer mice exhibit altered protein, lipid, and glucose metabolism (3, 5–7, 25, 42, 94). In support of the above statement, Kang et al. (2011) noted that the levels of serum cholesterol, HDL, and glucose were lower in the staggerer mice, with implications on a homeostatic mechanism to control adiposity/weight (5, 7, 9, 42, 56). Liver being one of the most metabolically active organs serves as a hub for control of lipid, protein, and glucose metabolism (95, 98). One of the liver enzymes PNPLA3 (1-Acylglycerol-3-Phosphate O-Acyltransferase), a RORα target gene, is regulated through a c-JUN-RORα-PNPLA3 pathway and is inactivated in the presence of a high-fat diet (97). Several other phospholipase proteins, PNPLA2, PNPLA7, and PNPLA8, were identified as RORα target genes (81). Mice on a high-fat diet undergo hepatic stress, which leads to an activation of c-Jun and the subsequent inactivation or deregulation of RORα-PNPLA3 (97). Essentially, the cJun : RORα:PNPLA3 pathway is activated under hepatic duress or an overload of a diet rich in fats, and a study on the HepG2 cells identified significance for the RORα:HIF1α and RORα:VEGF pathways (54, 97). RORα4 is the predominant isoform in the liver of mice and has also been validated on the HepG2, human liver cells (99). Cyp7b1 was identified as a RORα target in the liver and the cytochromes, a group of heme-like enzymes that are present abundantly in the liver (59). The liver, being a metabolically active organ, is a hub for the storage, distribution, and utilization of carbohydrates, lipids, and proteins, including disposal of metabolic waste. The cytochromes, which are also integral to the respiratory chain, are essential to carbohydrate, lipid, and protein metabolism. Carbohydrate metabolism-associated genes/proteins, PEPCKc, and Glucose-6-Phosphatase (G6PC) are dysregulated in the staggerer mice (95). Most of the dietary non-essential proteins give rise to intermediates of the Krebs tricarboxylic citric acid cycle, and the dietary intake of a “nutritionally balanced diet” is assimilated into the various organs and tissues through the metabolic enzymes involved in carbohydrate, protein, and lipid metabolism.
Staggerer mice: lipid metabolismNot long after the discovery of a mutant RORα being identified as the cause of developmental defects in the staggerer mice, Patrick Lau et al. (2004, 2008), through a series of extensive studies, published their research entitled “RORalpha regulates the expression of genes involved in lipid homeostasis in skeletal muscle cells (2004)”, after studying several genes involved in lipid metabolism within the mice myogenic cell line C2C12 (7, 42). The prelude to the studies by Patrick Lau et al. were the discoveries by Bertin et al. (1990), Vu-Dac et al. (1997), and Raspe et al. (2001) (5, 7, 42, 51, 53). Bertin et al. (1990), during studies on the staggerer mice, noted differential expression/levels of the BAT and their energetic metabolism at 28°C, when compared to nonmutant controls, the adipose tissue being the main storage tissue for lipids (5). Vu-Dac et al. (1997) and Raspé et al. (2001), while studying metabolism, identified RORα as a transcriptional regulator of apolipoprotein A-I (APOA1) and apolipoprotein C-III (APOC3), the transporters of lipids in blood (51, 53). Patrick Lau et al. (2008) subsequently published their work on the role of lipid metabolism in the staggerer mice and summarized their findings with the following sentence: “In summary, our study suggests RORα is an important factor in the regulation of genes associated with lipid homeostasis and adiposity” (7). The staggerer mice were leaner and were obesity resistant, the pads of WAT and BAT offering the staggerer mice a better chance of survival (5, 7). Moreover, the expression of ADRB2, an adrenergic receptor, was increased in the WAT, BAT, and skeletal muscle of staggerer mice (7). The levels of plasma triglycerides, non-esterified fatty acids (NEFA), total cholesterol, and high-density-lipoprotein (HDL) were reduced in the staggerer mice (7). Additionally, expression of RORα1 and RORα4 was also reduced in the liver, WAT (epididymal fat), and skeletal muscles of the staggerer mice (7). Expression profiling on genes involved in lipid metabolism and transport indicated differential transcript levels in the staggerer mice, with SREBP1c and FAS reduced in the liver, while there was an increase in PGC1α and lipin 1 (7). The relative expressions in the WAT of the staggerer mice for PGC1α were higher, while the BAT expressed higher levels of PGC1β, although PGC1α, PGC1β, and lipin1 were increased in the BAT and WAT (7). The skeletal muscles had reduced levels of SREBP1c, GcK, and FAS in the staggerer mice when compared with WT mice (7). The adipose tissue and skeletal muscles are also lipid storage tissues, during increased dietary intake of carbohydrates, proteins, and lipids. The staggerer mice also displayed lower levels of plasma triglycerides and apo C-III levels when compared with the WT (51). The decrease in plasma lipoproteins and apo C-III corresponds to lower mRNA levels of apo C-III in the liver and proximal and distal intestines of the staggerer mice (51). The lipids, cholesterol, and cholesteryl esters are transported in plasma as water-miscible lipoproteins, which are composed of the protein moiety termed apolipoprotein, and these apolipoproteins can function as ligands, enzyme cofactors, and enzyme inhibitors. The staggerer mice, when maintained on a high-fat atherogenic diet, elucidated reduced plasma levels of apolipoproteins, Apo-AI and Apo-AII (87). Further inspection identified reduced expression of the Apo-A1 mRNA in the intestinal cells of the staggerer mice (53, 87). Indeed, Apo-A1 is a RORα1 target gene and contains a RORE site within the promoter region of the intestinal caco-2 cells studied (53). The RORα1 and RORα4 isoforms of RORα regulate the transcriptional activity of apolipoprotein A5 (ApoA5) in HepG2 and HUH7 cells; however, RORα has no effect on ApoA5 in the staggerer mice (52). A compensatory mechanism exists within the staggerer mice, whereby metabolic genes are dysregulated to overcome the loss of RORα activity. Additionally, it was revealed that there was an association between hypo-alphalipoproteinemia and decreased high-density lipoprotein (HDL) levels due to a downregulation of the apoA-I gene in the intestine only, with the apoA-II being unaffected at the genetic level in the intestine (87).
Delving further into the role of RORα in lipid metabolism, crucial enzymes involved in the cholesterol and fatty acid synthesis pathway, namely, acetyl-coenzyme A acetyltransferase 1 precursor (ACAT1), acetyl coenzyme A acetyltransferase 2 (ACAT2), and short-chain acyl-CoA dehydrogenase precursor (ACADS), were identified as potential RORα target genes in the Sarachana and Wu genome-wide study (81). Kang et al. (2011) also defined a prominent role for RORα in lipid metabolism (56). Studies on the RORα staggerer (RORA sg/sg) mice demonstrated that genes involved in lipid metabolism, like GPAM/GPAT1, AGPAT9, CIDEC, CIDEA, and MOGAT1, were significantly downregulated (7, 42). The genes are direct targets of RORα (64). In the liver of RORα staggerer mice, several other genes involved in fatty acid metabolism were suppressed, including ACOT3 and ACOT4 (acyl-coenzyme A thioesterases), FABP5, ADFP/PERILIPIN 2, LPIN2, ANGPT14, and FGF21 (7, 42). After extensive studies on the staggerer mice, the levels of biochemical lipids in the serum of the staggerer mice, and specific sets of metabolic enzymes, Patrick Lau et al. (2008) wrote: “The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism”. Evidence for the above statement lies in the fact that α-ketoglutarate in the glutamine→glutamate→α-ketoglutarate cycle is central to the Krebs citric acid cycle, and acetyl-CoA along with oxaloacetate biosynthesize citrate, catalyzed by citrate synthase (Figure 1). Citrate synthase had been identified as a RORα target gene and the enzymatic activity was diminished in the staggerer mice (100). More importantly, the lipid metabolites that are crucial to the glutamine→glutamate→α-ketoglutarate cycle are acetyl-CoA, malonyl-CoA, propionyl-CoA, and HMG-CoA, with Kallen et al. (2002, 2004) having identified cholesterol, the Acetyl-CoA→HMG-CoA→squalene pathway metabolite, as a natural ligand of RORα (19, 20).
Staggerer mice: carbohydrate metabolismAfter delineating the role of RORα in lipid metabolism, Patrick Lau et al. (2011) delved into studying the role of RORα in carbohydrate metabolism, through an understanding of the role of insulin in glucose uptake by the tissues in the staggerer mice when compared to wild-type littermates (94). The staggerer mice exhibited increased insulin sensitivity upon being challenged with intraperitoneal insulin on time-course assays (94). Glucose tolerance tests on the staggerer versus wild-type control mice detected an enhanced ability of glucose clearance from the blood within 90 min in the staggerer mice (94). The skeletal muscles being a suitable tissue for studies on glucose metabolism, utilization, gluconeogenesis, glycogenesis, and glycogenolysis have been used as an ideal tissue to study the effect of mutations and hormones (94, 95). The glucose uptake transporter Glut4 was significantly increased in the skeletal muscles of staggerer mice studied, and the increase corresponded to an increase in Akt and that of phosphorylated Akt upon stimulation with insulin (94). A noteworthy observation was that the glucocorticoid-inducible kinase 1 (Sgk1) transcript was upregulated in the skeletal muscles of the staggerer mice, with Sgk1 being considered as a regulator of transport channels (95). A regulator of energy metabolism, PGC1β was differentially regulated in the skeletal muscles and liver of staggerer mice when compared to the wild-type littermates, with reduced expression being observed in the skeletal muscles (7). Basically, the skeletal muscles serve to store excess blood glucose as glycogen. While insulin enhances glucose uptake and glycogen synthesis by the skeletal muscles, glucagon and epinephrine (a metabolite of the essential amino acid phenylalanine) promote the breakdown of glycogen into readily available forms of glucose (101). Glucose-6-phosphatase (G6PC), an enzyme essential in the breakdown of glycogen→glucose-6-phosphate→glucose, is regulated by RORα (95). The staggerer mice had reduced expression of G6PC and PCK1, both of these being involved in gluconeogenesis/glycogenolysis (95). Apart from the staggerer mice being sensitive to insulin, the adrenergic receptor β2 (ADRB2) was also observed to be upregulated in the skeletal muscles of staggerer mice (7). Nevertheless, the importance of carbohydrate metabolism in the staggerer mice and embryogenesis lay in the biochemical evidence that pyruvate and α-ketoglutarate are the center points in the synthesis of glutamine: the crux in purine and pyrimidine synthesis (Figure 1).
RORα: the mitochondriaThe staggerer mice expressed differential amounts of BAT and WAT, which is an indicator of the number of mitochondria within these specific cell types (5). Yang et al. (2006) noted differential expression of several oscillatory genes within the BAT and WAT of mice (40). RORα was the predominant oscillatory gene in the WAT of control mice, with the relative mRNA levels fluctuating between the Zeitgeber hours of 0 to 24 (40). Upon mining for information using the UCSC genome browser, a study for the protein RORα yielded information regarding an interaction with nucleoside diphosphate kinase, nm23-2 (28). Nucleoside di-phosphate kinase enzyme (NDPK) isoforms are essential to cellular differentiation and proliferation, and thereby in organogenesis; while nm23-M1, nm23-M2, and nm23-M3 were expressed in the cerebellum and cerebral cortex, nm23-M4 is present in the proliferating layer during organogenesis (70). Basically, the NDPK are a group of enzymes that catalyze conversion of diphosphate energy molecules into their triphosphate form, e.g., UDP is catalyzed to UTP and ADP is catalyzed to ATP. Interestingly, genes involved with components of the ATP synthase and ATPase genes—ATP synthase H+ transporting, mitochondrial F0 complex subunit F (ATP5J), ATPase H+/K+ transporting subunit alpha (ATP4A), ATPase plasma membrane Ca2+ transporting 2 (ATP2B2), ATPase phospholipid transporting 11A (ATP11A), and probable cation-transporting ATPase 13A3 (ATP13A3)—were identified as putative RORα targets (81).
The mitochondrion, the powerhouse of a cell, serves as the source of adenosine triphosphate (ATP) synthesis, and the ATP synthase complex along with ATPase act as its mediators. More specifically, ATP, the energizer of biochemical reactions within cells, is synthesized by the ATP synthase complex composed of several subunits spanning the mitochondrial membrane (102). The genome-wide study on a neuronal cell line identified adenylate cyclase (ADCY5) and ADP-dependent glucokinase (ADPGK) as putative target genes of RORα (81). The oxidative phosphorylation in the respiratory chain, of which ATP is a component, requires oxygen, and hypoxic conditions lead to defective synthesis of ATP; the requirement of ATP is essential in the synthesis of lipids, carbohydrates, and proteins (103). Hypoxia induces several signaling pathways, some of which are the HIF, PI3K/AKT/mTOR, MAPK, and NFκB pathways (104, 105). It is the RORα4 isoform that is activated under hypoxic conditions, and the effect is through molecular level interactions regulating the hypoxia-responsive element/site (106). Miki et al. (2004) had concluded that the RORα4 promoter is a site that is activated under hypoxic stress and its activation is through the HIF-1 and Sp1/Sp3 pathway (107). While Chauvet et al. showed that RORα is a HIF-1 target gene, Kim et al. demonstrated that melatonin-induced RORα activates HIF-1α in a dose-dependent manner (54, 108). The transcript levels of RORα1 and RORα4 increased progressively under hypoxic conditions (107, 108). A study on keratinocytes showed that under hypoxic conditions, gene silencing of RORα led to a reduction in the expression of HIF1α, the hypoxia-associated gene (108). In essence, RORα is a crucial factor in cellular stress response/hypoxia, and the expression of RORα1 and RORα4 increased proportionately to HIF1α and VEGF, upon activation with melatonin on a time-course assay (54, 106–108). Thus, it appears that there is a fine cross-talk of positive and negative feedback circuits that dictate the regulation of both RORα and hypoxia-associated genes.
Staggerer mice: protein metabolismProtein metabolism is at the core of all metabolic pathways, since their by-product acts as enzymes, and of the genes/proteins regulated. Glutamate, a metabolite of glutamic acid, which is also derived from essential amino acids histidine and arginine, was reduced in the cerebellar cortex and cerebellar deep nuclei of the staggerer mice (Figure 1) (3). Glutamate is encode
留言 (0)