Cervical artery dissection (CeAD) is defined by the evidence of a mural hematoma within the arterial wall of either the carotid or vertebral arteries and can occur spontaneously (sCeAD) or in timely association with trauma. In the general stroke population, a sCeAD is rare and attributes for 1–2% of all strokes. In young individuals (i.e., under the age of 50 years of age) however, sCeAD accounts for 10–25% of ischemic strokes, making it one of the primary causes in this age group (1–4). The clinical presentation of sCeAD varies considerably with local signs and symptoms (such as head/neck pain, Horner’s syndrome, cranial nerve palsies, and pulsatile tinnitus), typically preceding ischemic stroke. As early detection of local signs and symptoms due to sCeAD followed by early treatment offers the opportunity for primary stroke prevention, understanding the clinical spectrum of sCeAD is of utmost importance. Over the years, studies and narrative reviews have focused on the frequent local signs and symptoms such as head/neck pain and Horner’s syndrome, neglecting cranial nerve palsies attributable to sCeAD (5–7). Therefore, we aimed to put the current evidence into perspective and to give an overview of the pathomechanistic as well as clinical aspects of sCeAD-related CN palsies.
MethodsA search term-based literature review of PubMed was conducted to identify articles investigating cranial nerve palsies due to sCeAD. Search terms were “cervical artery dissection” AND “cranial nerve palsy.” Additionally, a search with the terms “cervical artery dissection” AND “insert cranial nerve” (e.g., “facial nerve”) was performed for all 12 cranial nerves. Titles and abstracts were screened, and the full texts of potentially relevant articles were obtained for review. The inclusion criteria comprised cranial nerve palsies due to spontaneous cervical artery dissection (sCeAD). Articles concerning cranial nerve palsies due to other causes such as traumatic cervical artery dissection, stroke, cancer, surgery, or local inflammation were excluded. A review of the literature was performed by the two main authors (BD and LMS). In total, 75 search results matched the inclusion criteria for this review; 22 were duplicates of different search terms. Finally, 53 publications were considered (Supplementary Table S1).
Epidemiology/pathophysiologyIn line with the increasing availability of magnetic resonance imaging (MRI), absolute sCeAD diagnoses, especially in the vertebral arteries, have become more frequent (5, 8). The cause for sCeAD is essentially unknown. As environmental factors such as mild, non-penetrating head/neck trauma, or systemic infection are reported to be potential triggers for sCeAD, a multifactorial pathogenesis is likely (9–18). In addition, a subclinical connective tissue disorder has been proposed as a potential disease-promoting factor (19–28). Depending on the location of the pathognomonic vessel wall hematoma, two types of dissections can be differentiated: subintimal or subadventitial (29). It is assumed that subintimal dissections, which are present in over 80% of the cases, are associated with an intimal tear and subsequent anterograde blood flow from the vessel to the false lumen (inside-out theory) resulting in steno-occlusive vessel pathologies characterized by no significant diameter expansion, hence unlikely to affect nearby anatomical structures. A subadventitial wall hematoma originates from a rupture of the vasa vasorum and typically results in expansive vessel pathologies (outside-in theory) (schematic Figure 1) (4, 30–36).
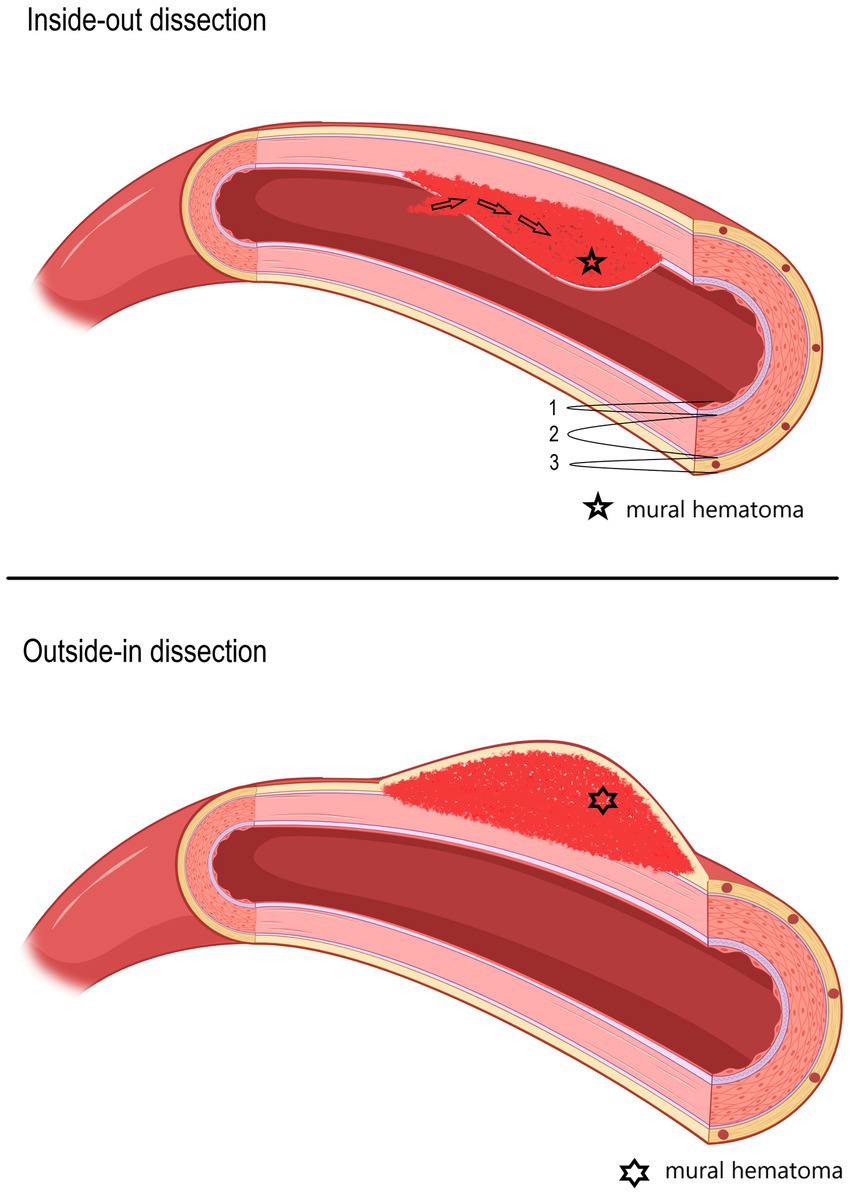
Figure 1. Schematic picture of two different hypothesized types of dissections (upper: inside-out; lower: outside-in), depending on the location of the mural hematoma. In the upper picture, an inside-out dissection is shown, and in the lower picture, an outside-in dissection is shown. 1—Tunica intima, 2—Tunica media, 3—Tunica externa (tunica adventitia) with vasa vasorum.
Clinical presentation—overallThe clinical presentation of patients with sCeAD ranges from asymptomatic to severe cerebral ischemic events. Hospital-based cohorts indicate a likelihood of sCeAD-related cerebral ischemia (TIA or stroke) ranging from 65 to 80%. These events usually exhibit an embolic pattern and, less frequently, occur in cases with high-grade stenosis or occlusion, including hemodynamic watershed infarcts (29, 30, 37–41). More than 80% of cases report at least one local symptom, with head/neck pain being by far the most frequent (5). The pain typically is characterized as pulling and/or dull, with mild-to-moderate progressive intensity. It often responds poorly to oral at-home analgesia and is specific to the ipsilateral side of the dissection (6). Another common local sign is the ipsilateral Horner’s syndrome, which is present in 28 to 58% of patients with internal carotid artery (ICA) dissection and can be the sole symptom in 10–12% of cases (1, 42–50). Tinnitus, often of pulsatile nature, is another possible local sign, occurring in 7–27% of cases and stems most likely from non-laminar flow in steno-occlusive sCeAD pathologies near the tympanic membrane (5, 43, 49, 51, 52).
sCeAD-related cranial nerve palsyCranial nerve palsies occur in 3–12% of all patients with sCeAD and can be the sole clinical sign in 0.5% (1–4, 53, 54). Table 1 presents the clinical characteristics of published case reports with isolated CN palsies due to sCeAD.
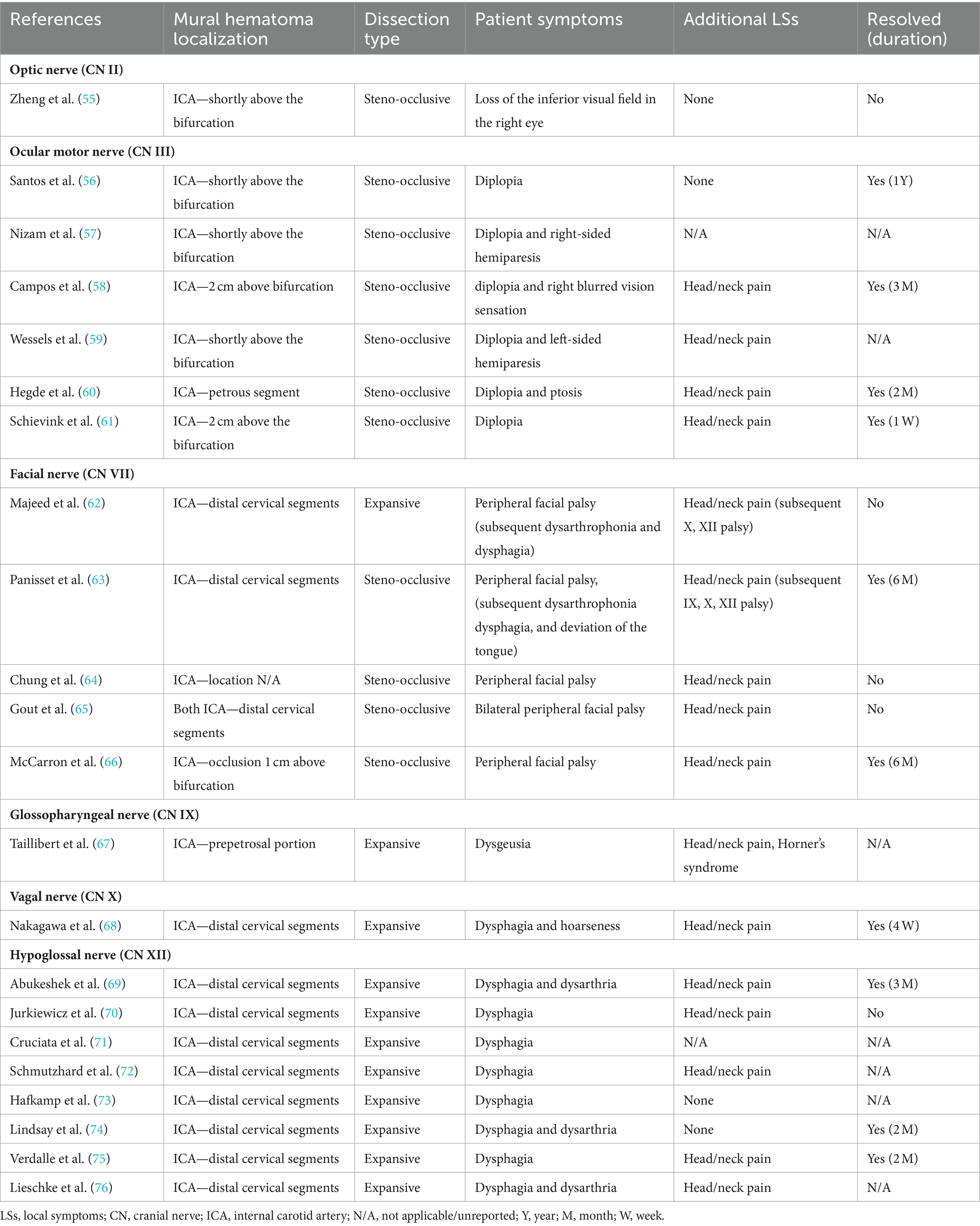
Table 1. Available data of cases reporting isolated cranial nerve palsies due to sCeAD.
In summary, isolated CN II, III, VII, IX, X, and XII palsies due to sCeAD have been reported in the literature. Patients with CN II or III palsy exclusively had steno-occlusive sCeAD-related vessel pathologies, while those with isolated CN IX, X, or XII palsy primarily had expansive mural hematoma. Furthermore, the mural hematoma localization typically involved more proximal segments of the ICA in those with CN II or III palsy compared to others. Solely those with sCeAD-related VII palsies had different mural hematoma localization and dissection types. In total, 80% of cases where clinical data were available reported head/neck pain as an additional sCeAD-related local symptom.
CN palsies due to sCeAD can also be present as clinical syndromes, namely, Collet–Sicard, Villaret, or Tapia syndrome (1–5, 36, 43, 54, 63, 77). Table 2 holds clinical information on such syndromes previously described as attributable to sCeAD.
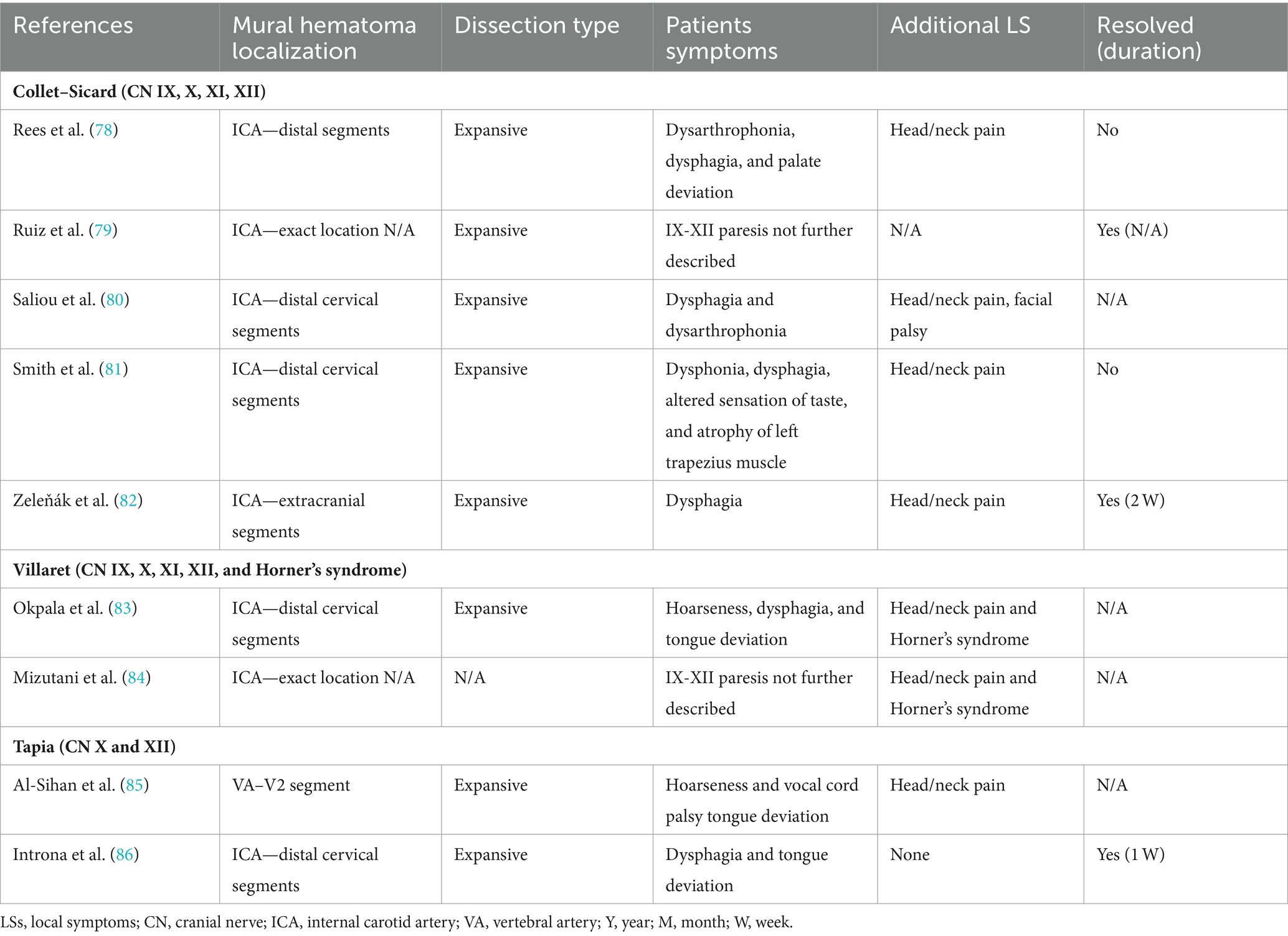
Table 2. Available data of articles reporting clinical syndromes of cranial nerve palsies due to sCeAD.
Clinical outcome was better in those with isolated CN palsies than those with clinical syndromes as 75% of patients with isolated palsies had complete resolution of symptoms compared to 60% of those with either Collet–Sicard, Villaret, or Tapia syndrome. However, the considerable amount of missing data on outcomes has to be mentioned.
DiscussionHospital-based cohorts report that approximately three in four sCeAD cases suffer cerebral ischemia (1). However, local symptoms, such as head/neck pain, Horner’s syndrome, pulsatile tinnitus, and CN palsies, are the most frequent sCeAD-related symptoms and typically precede stroke (5, 47). Therefore, swift identification and management would enable primary stroke prevention. As previous studies and reviews have extensively covered more frequent local signs and symptoms in sCeAD, our review emphasizes that CN palsies are presentations that clinicians should not miss (5–7). In the literature, isolated palsies of CN II, III, VII, IX, X, and XII have been reported, while CN XI palsy only occurs in combination with other caudal CN palsies (Tables 1, 2). In view of the available literature, these palsies originate either from an expansive vessel wall hematoma causing a local mass effect on adjacent structures or as a consequence of peripheral nerve ischemia (i.e., microembolism or hypoperfusion of vasa nervorum) (36, 54, 62, 87). In cases where isolated CN palsies occur due to sCeAD, the available data depicted in Table 1 support such hypothetical pathomechanisms. CN IX, X, XI, and XII have a close anatomic vicinity to the ICA at the base of the skull and are therefore susceptible to mechanical stress (schematic Figure 2). As suggested by the published case reports in Table 1, patients who have isolated palsy of these CN also have a primarily expansive sCeAD-related vessel pathology, such as aneurysm formations (schematic Figure 1). On the other hand, those with isolated CN II or III palsy show steno-occlusive ICA pathologies due to sCeAD throughout. Therefore, the available literature supports the pathomechanism of microembolism or hypoperfusion of vasa nervorum in these cases. In addition to the solely mechanistic hypothesis of either local mass effect or hypoperfusion of vasa nervorum being causal to CN palsy, the localization of the sCeAD-related mural hematoma further supports this theory. Table 1 emphasizes that the mural hematoma in patients with CN II or III palsy involves more proximal parts of ICA, while in patients with CN IX, X, XI, or XII, the mural hematoma is primarily located at the base of the skull (schematic Figure 2). The only singular sCeAD-related CN palsy where different dissection types or mural hematoma localizations are reported is in CN VII palsy. In these cases, clinical presentation and patient history are crucial for accurate diagnosis and management.
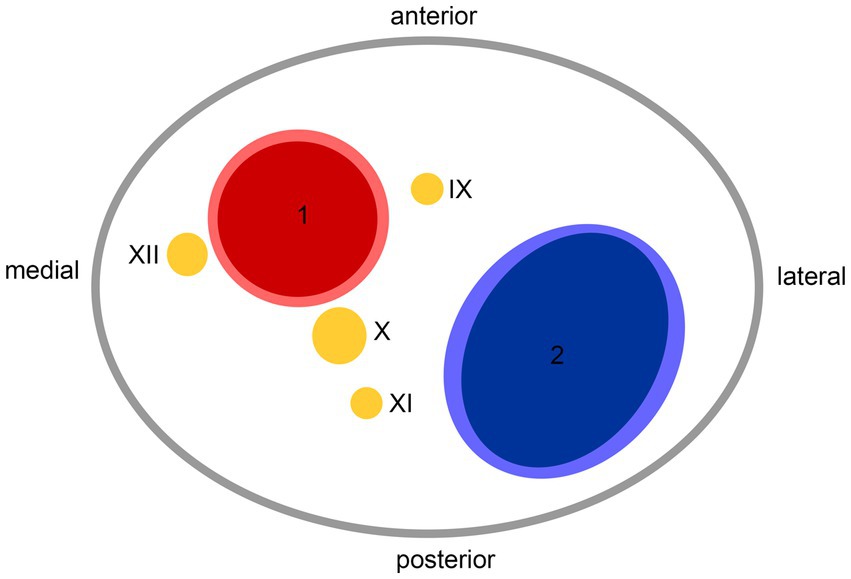
Figure 2. Anatomic scheme of axial view on left carotid sheath from caudal at the level of atlas; 1—internal carotid artery, 2—internal jugular vein, IX—glossopharyngeal nerve, X—vagus nerve, XI—accessory nerve, XII—hypoglossal nerve.
Careful clinical examination in individuals with CN palsy can reveal additional symptoms suggestive of sCeAD. In the reported cases, 80% had additional local symptoms (i.e., head/neck pain, pulsatile tinnitus, and Horner’s syndrome), with head/neck pain being the most frequent (16 of 20). Recently, a specific type of head/neck pain associated with sCeAD (acute onset, pulling pain with mild-to-moderate intensity, which continuously increases and does not respond to oral analgesia) has been reported (6). If available, imaging should be done using MRI as sCeAD with expansive vessel pathologies may be missed by ultrasound, especially if located at more distal ICA segments at the base of the skull (i.e., where CN are adjacent—schematic Figure 2) (88). Additionally, the most important differential diagnosis—brainstem ischemia-related CN palsies—could be revealed by MRI, as this distinction is sometimes difficult to identify clinically. A key difference is that peripheral CN palsies in sCeAD mostly occur in ICA dissections, while those caused by brainstem infarction relate to vertebral artery sCeAD (5). This emphasizes the necessity of a clear diagnosis, which, given the typical localizations of sCeAD primarily in the distal segments of ICA at the base of the skull and the vertebral artery (V3), should involve T1-weighted fat-saturated axial MRI imaging, if available. Here, surrounding the vessel lumen, either an isointense (first 5 days) or hyperintense crescent-shaped rim (>5 days after onset) can be found (89). However, a reported potential false-negative rate of MRI-based infra-tentorial ischemia detection of ~10% within the first 24 h after symptom onset has to be kept in mind (90). If MRI is not available, a combination of computed tomography angiography (CTA) and ultrasound can detect other, less specific signs of sCeAD, such as long tapered stenosis, false lumen and/or intima flap, and dissecting aneurysm (1, 91, 92). This is of clinical importance, especially in counseling patients, as a small hospital-based cohort analysis has shown that brainstem stroke-related CN palsies do not resolve over time, while peripheral CN do within a follow-up of 5 months (5). This was also true for the cases discussed within this review, as 72% of patients with available clinical follow-up information showed complete resolution of symptoms over time (Table 1). Even though observational studies have shown that planned stenting of sCeAD in the subacute setting is safe, we recommend a conservative approach in accordance to current treatment recommendations due to the benign prognosis of sCeAD-related peripheral CN palsies, which is in line with current guidelines (93).
Overall, CN palsy in sCeAD is evident in approximately 10% of cases. Although their prognosis is benign, it is important to consider sCeAD and the appropriate diagnostic pathways. This awareness can guide clinicians to make an early sCeAD diagnosis, offering the chance of primary stroke prevention.
Author contributionsBD: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. MK: Conceptualization, Supervision, Writing – review & editing. SK: Supervision, Writing – review & editing. LM-S: Supervision, Writing – review & editing, Conceptualization, Methodology.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by VASCage—Research Centre on Clinical Stroke Research. VASCage is a COMET Centre within the Competence Centers for Excellent Technologies (COMET) program and funded by the Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation, and Technology, the Federal Ministry of Labour and Economy, and the federal states of Tyrol, Salzburg, and Vienna. COMET is managed by the Austrian Research Promotion Agency (Österreichische Forschungsförderungsgesellschaft). FFG Project number: 898252.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1364218/full#supplementary-material
References1. Debette, S, and Leys, D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. (2009) 8:668–78. doi: 10.1016/S1474-4422(09)70084-5
Crossref Full Text | Google Scholar
2. Robertson, JJ, and Koyfman, A. Cervical artery dissections: A review. J Emerg Med. (2016) 51:508–18. doi: 10.1016/j.jemermed.2015.10.044
Crossref Full Text | Google Scholar
3. Fusco, MR, and Harrigan, MR. Cerebrovascular dissections—A review part I: spontaneous dissections. Neurosurgery. (2011) 68:242–57. doi: 10.1227/NEU.0b013e3182012323
Crossref Full Text | Google Scholar
4. Tentschert, S, Kreuzer, S, Lalouschek, W, Lang, W, Nasel, CH, et al. Dissektion der Arteria carotis als Ursache ischämischer zerebrovaskulärer Ereignisse - Fallberichte und Diskussion. J Neurol Neurochir Psychiatr. (2003) 4:13–20.
5. Mayer, L, Boehme, C, Toell, T, Dejakum, B, Willeit, J, Schmidauer, C, et al. Local signs and symptoms in spontaneous cervical artery dissection: A single Centre cohort study. J Stroke. (2019) 21:112–5. doi: 10.5853/jos.2018.03055
PubMed Abstract | Crossref Full Text | Google Scholar
6. Mayer-Suess, L, Frank, F, Töll, T, Boehme, C, Gizewski, ER, Ratzinger, G, et al. Head/neck pain characteristics after spontaneous cervical artery dissection in the acute phase and on a long-run. Cephalalgia. (2022) 42:872–8. doi: 10.1177/03331024221079298
PubMed Abstract | Crossref Full Text | Google Scholar
7. Arnold, M, Baumgartner, RW, Stapf, C, Nedeltchev, K, Buffon, F, Benninger, D, et al. Ultrasound diagnosis of spontaneous carotid dissection with isolated Horner syndrome. Stroke. (2008) 39:82–6. doi: 10.1161/STROKEAHA.107.492652
Crossref Full Text | Google Scholar
8. Mayer-Suess, L, Geiger, M, Dejakum, B, Boehme, C, Domig, LM, Komarek, S, et al. Sex-differences in psychosocial sequelae after spontaneous cervical artery dissection. Sci Rep. (2022) 12:611. doi: 10.1038/s41598-021-04686-7
PubMed Abstract | Crossref Full Text | Google Scholar
9. Dittrich, R, Rohsbach, D, Heidbreder, A, Heuschmann, P, Nassenstein, I, Bachmann, R, et al. Mild mechanical traumas are possible risk factors for cervical artery dissection. Cerebrovasc Dis. (2007) 23:275–81. doi: 10.1159/000098327
PubMed Abstract | Crossref Full Text | Google Scholar
11. Haldeman, S, Kohlbeck, FJ, and McGregor, M. Risk factors and precipitating neck movements causing vertebrobasilar artery dissection after cervical trauma and spinal manipulation. Spine. (1999) 24:785–94. doi: 10.1097/00007632-199904150-00010
PubMed Abstract | Crossref Full Text | Google Scholar
12. Genius, J, Dong-Si, T, Grau, AP, and Lichy, C. Postacute C-reactive protein levels are elevated in cervical artery dissection. Stroke. (2005) 36:e42–4. doi: 10.1161/01.STR.0000158911.74006.d6
Crossref Full Text | Google Scholar
13. Grau, AJ, Buggle, F, Ziegler, C, Schwarz, W, Meuser, J, Tasman, AJ, et al. Association between acute cerebrovascular ischemia and chronic and recurrent infection. Stroke. (1997) 28:1724–9. doi: 10.1161/01.STR.28.9.1724
PubMed Abstract | Crossref Full Text | Google Scholar
14. Guillon, B, Berthet, K, Benslamia, L, Bertrand, M, Bousser, MG, and Tzourio, C. Infection and the risk of spontaneous cervical artery dissection: a case-control study. Stroke. (2003) 34:e79–81. doi: 10.1161/01.STR.0000078309.56307.5C
PubMed Abstract | Crossref Full Text | Google Scholar
15. Rubinstein, SM, Peerdeman, SM, van Tulder, MW, Riphagen, I, and Haldeman, S. A systematic review of the risk factors for cervical artery dissection. Stroke. (2005) 36:1575–80. doi: 10.1161/01.STR.0000169919.73219.30
PubMed Abstract | Crossref Full Text | Google Scholar
17. Gutowski, NJ, Murphy, RP, and Beale, DJ. Unilateral upper cervical posterior spinal artery syndrome following sneezing. J Neurol Neurosurg Psychiatry. (1992) 55:841–3. doi: 10.1136/jnnp.55.9.841
PubMed Abstract | Crossref Full Text | Google Scholar
18. Mokri, B, Sundt, TM, Wayne, OH, and Piepgras, DG. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol. (1986) 19:126–38. doi: 10.1002/ana.410190204
Crossref Full Text | Google Scholar
19. Brandt, T, Orberk, E, Weber, R, Werner, I, Busse, O, Müller, BT, et al. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology. (2001) 57:24–30. doi: 10.1212/WNL.57.1.24
Crossref Full Text | Google Scholar
20. de Bray, JM, Marc, G, Pautot, V, Vielle, B, Pasco, A, Lhoste, P, et al. Fibromuscular dysplasia may herald symptomatic recurrence of cervical artery dissection. Cerebrovasc Dis. (2007) 23:448–52. doi: 10.1159/000101470
PubMed Abstract | Crossref Full Text | Google Scholar
21. Tzourio, C, Cohen, A, Lamisse, N, Biousse, V, and Bousser, MG. Aortic root dilatation in patients with spontaneous cervical artery dissection. Circulation. (1997) 95:2351–3. doi: 10.1161/01.CIR.95.10.2351
PubMed Abstract | Crossref Full Text | Google Scholar
22. Calvet, D, Boutouyrie, P, Touzé, E, Laloux, B, Mas, JL, and Laurent, S. Increased stiff ness of the carotid wall material in patients with spontaneous cervical artery dissection. Stroke. (2004) 35:2078–82. doi: 10.1161/01.STR.0000136721.95301.8d
PubMed Abstract | Crossref Full Text | Google Scholar
23. Lucas, C, Lecroart, JL, Gautier, C, Leclerc, X, Dauzat, M, Leys, D, et al. Impairment of endothelial function in patients with spontaneous cervical artery dissection: evidence for a general arterial wall disease. Cerebrovasc Dis. (2004) 17:170–4. doi: 10.1159/000075787
PubMed Abstract | Crossref Full Text | Google Scholar
24. Brandt, T, Hausser, I, Orberk, E, Grau, A, Hartschuh, W, Anton-Lamprecht, I, et al. Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol. (1998) 44:281–5. doi: 10.1002/ana.410440224
PubMed Abstract | Crossref Full Text | Google Scholar
25. Ulbricht, D, Diederich, NJ, Hermanns-Le, T, Metz, RJ, Macian, F, and Pierard, GE. Cervical artery dissection: an atypical presentation with Ehlers-Danlos-like collagen pathology? Neurology. (2004) 63:1708–10. doi: 10.1212/01.WNL.0000142970.09454.30
Crossref Full Text | Google Scholar
26. Uhlig, P, Bruckner, P, Dittrich, R, Ringelstein, EB, Kuhlenbaumer, G, and Hansen, U. Aberrations of dermal connective tissue in patients with cervical artery dissection (sCAD). J Neurol. (2008) 255:340–6. doi: 10.1007/s00415-008-0585-4
Crossref Full Text | Google Scholar
27. Mayer, L, Pechlaner, R, Barallobre-Barreiro, J, Boehme, C, Toell, T, Lynch, M, et al. Extracellular matrix protein signature of recurrent spontaneous cervical artery dissection. Neurology. (2020) 95:e2047–55. doi: 10.1212/WNL.0000000000010710
Crossref Full Text | Google Scholar
28. Popov, P, Chapot, R, Tanasković, S, Vekić, B, Sotirovic, V, Ilijevski, N, et al. Vocal cord paralysis as the first sign of spontaneous carotid dissection in a patient with extracranial internal carotid artery aneurysm. Vasc Endovasc Surg. (2016) 50:52–6. doi: 10.1177/1538574415627698
PubMed Abstract | Crossref Full Text | Google Scholar
29. Mayer-Suess, L, Dejakum, B, Ratzinger, G, Gizewski, ER, Kiechl, S, and Knoflach, M. Clinical characteristics and outcome in expansive compared with steno-occlusive mural hematoma in spontaneous cervical artery dissection. Int J Stroke. (2023) 18:1186–92. doi: 10.1177/17474930231185032
PubMed Abstract | Crossref Full Text | Google Scholar
30. Lucas, C, Moulin, T, Deplanque, D, Tatu, L, and Chavot, D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. (1998) 29:2646–8. doi: 10.1161/01.STR.29.12.2646
PubMed Abstract | Crossref Full Text | Google Scholar
31. Völker, W, Dittrich, R, Grewe, S, Nassenstein, I, Csiba, L, Herczeg, L, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. (2011) 76:1463–71. doi: 10.1212/WNL.0b013e318217e71c
PubMed Abstract | Crossref Full Text | Google Scholar
32. Morel, A, Naggara, O, Touzé, E, Raymond, J, Mas, JL, Meder, JF, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. (2012) 43:1354–61. doi: 10.1161/STROKEAHA.111.643338
PubMed Abstract | Crossref Full Text | Google Scholar
33. Perry, BC, and Al-Ali, F. Spontaneous cervical artery dissection: the borgess classification. Front Neurol. (2013) 4:133. doi: 10.3389/fneur.2013.00133
Crossref Full Text | Google Scholar
34. Schievink, WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. (2001) 344:898–906. doi: 10.1056/NEJM200103223441206
Crossref Full Text | Google Scholar
35. Völker, W, Besselmann, M, Dittrich, R, Nabavi, D, Konrad, C, Dziewas, R, et al. Generalized arteriopathy in patients with cervical artery dissection. Neurology. (2005) 64:1508–13. doi: 10.1212/01.WNL.0000159739.24607.98
Crossref Full Text | Google Scholar
37. Benninger, DH, Georgiadis, D, Kremer, C, Studer, A, Nedeltchev, K, and Baumgartner, RW. Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke. (2004) 35:482–5. doi: 10.1161/01.STR.0000109766.27393.52
PubMed Abstract | Crossref Full Text | Google Scholar
38. Binaghi, S, Saint-Maurice, JP, Laurian, C, and Houdart, E. Embolic stroke complicating cervical aneurysm. Cerebrovasc Dis. (2006) 22:196–8. doi: 10.1159/000093806
Crossref Full Text | Google Scholar
40. Peeters, A, Goffette, P, Dorban, S, Sindic, CJ, and Cosnard, G. An old dissecting aneurysm of the internal carotid artery presenting as acute stroke. Acta Neurol Belg. (2003) 103:179–82.
PubMed Abstract | Google Scholar
41. Lee, VH, Brown, RD, Mandrekar, JN, and Mokri, B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. (2006) 67:1809–12. doi: 10.1212/01.wnl.0000244486.30455.71
Crossref Full Text | Google Scholar
42. Dziewas, R, Konrad, C, Dräger, B, Evers, S, Besselmann, M, Lüdemann, P, et al. Cervical artery dissection: clinical features, riskfactors, therapy and outcome in 126 patients. J Neurol. (2003) 250:1179–84. doi: 10.1007/s00415-003-0174-5
PubMed Abstract | Crossref Full Text | Google Scholar
43. Baumgartner, RW, Arnold, M, Baumgartner, I, Mosso, M, Gönner, F, Studer, A, et al. Carotid dissection with and without ischemic events: local symptoms and cerebral artery findings. Neurology. (2001) 57:827–32. doi: 10.1212/WNL.57.5.827
Crossref Full Text | Google Scholar
44. Béjot, Y, Aboa-Eboulé, C, Debette, S, Pezzini, A, Tatlisumak, T, Engelter, S, et al. Characteristics and outcomes of patients with multiple cervical artery dissection. Stroke. (2014) 45:37–41. doi: 10.1161/STROKEAHA.113.001654
PubMed Abstract | Crossref Full Text | Google Scholar
45. Arnold, M, Cumurciuc, R, Stapf, C, Favrole, P, Berthet, K, and Bousser, MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. (2006) 77:1021–4. doi: 10.1136/jnnp.2006.094359
Crossref Full Text | Google Scholar
46. Biedermann, B, Sojer, M, Stockner, H, Spiegel, M, and Schmidauer, C. Dissektionen der Arteria carotis interna und vertebralis: Ursachen, Symptome, Diagnostik und Therapie. J Neurol Neurochir Psychiatr. (2007) 8:7–18.
47. Biousse, V, D’Anglejan-Chatillon, J, Touboul, PJ, Amarenco, P, and Bousser, MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. (1995) 26:235–9. doi: 10.1161/01.STR.26.2.235
PubMed Abstract | Crossref Full Text | Google Scholar
48. Bogousslavsky, J, Despland, PA, and Regli, F. Spontaneous carotid dissection with acute stroke. Arch Neurol. (1987) 44:137–40. doi: 10.1001/archneur.1987.00520140009010
Crossref Full Text | Google Scholar
49. Silbert, PL, Mokri, B, and Schievink, WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. (1995) 45:1517–22. doi: 10.1212/WNL.45.8.1517
Crossref Full Text | Google Scholar
52. Hart, RG, and Easton, JD. Dissections of cervical and cerebral arteries. Neurol Clin. (1983) 1:155–82. doi: 10.1016/S0733-8619(18)31177-0
Crossref Full Text | Google Scholar
53. Leys, D, Lucas, C, Gobert, M, Deklunder, G, and Pruvo, JP. Cervical artery dissections. Eur Neurol. (1997) 37:3–12. doi: 10.1159/000117396
Crossref Full Text | Google Scholar
54. Mokri, B, Silbert, PL, Schievink, WI, and Piepgras, DG. Cranial nerve palsy in spontaneous dissection of the extracranial internal carotid artery. Neurology. (1996) 46:356–9. doi: 10.1212/WNL.46.2.356
PubMed Abstract | Crossref Full Text | Google Scholar
55. Zheng, X, Wang, Y, Chen, G, Ma, C, Yan, W, and Chen, M. Diagnosis of ischemic optic neuropathy caused by dissection of the internal carotid artery: A case report. Medicine (Baltimore). (2020) 99:e20034. doi: 10.1097/MD.0000000000020034
PubMed Abstract | Crossref Full Text | Google Scholar
留言 (0)