Nowadays, the main ways of tumor treatment include radiotherapy, chemotherapy, surgery, and targeted therapy (Chen et al., 2023). Of these methods, radiotherapy and chemotherapy are the most common non-surgical treatments, and they are often used in combination with surgery. Radiotherapy uses high-energy radiation to kill cancer cells, which can accurately target the tumor area and cause minimal damage to surrounding normal tissues, making it suitable for the treatment of many tumors. Chemotherapy uses drugs to interfere with the growth of tumor cells, and chemotherapy agents are usually administered orally or intravenously. Chemotherapy is also suitable for the treatment of a variety of malignant tumors and some benign tumors. Surgery is one of the important ways to treat malignant tumors. Open surgery or minimally invasive surgery can be selected according to the specific situation of the patient, and resection of the tumor can achieve the treatment goal. However, the toxicity of traditional therapy seriously affects the patient’s tolerance and compliance (Mun et al., 2018). Drug resistance of tumor cells is one of the main reasons for the failure of traditional therapies and targeted therapies can help break through this situation (Holohan et al., 2013).
Targeted therapy is a kind of treatment that has emerged in recent years. By targeting specific tumor targets, targeted therapy can effectively reduce the side effects of treatment and improve the therapeutic effect (Singh, 2023). With the rapid development of precision medicine, molecular targeted therapy has been widely used in clinical tumor treatment because of its few side effects and high accuracy compared with traditional strategies (Zhang XN. et al., 2023).
The development of the tumor is highly correlated with the physiological state of the tumor microenvironment (TME). Although tumors from the same or different anatomical locations exhibit heterogeneity, common features can be found in mature TME of epithelial metaplastic tumors. TME predominates in terms of prognosis and influences the efficacy of anti-tumor therapies. Targeting TME more efficiently is of great significance for treating tumors (Roma-Rodrigues et al., 2019). This kind of targeted therapy has already achieved a lot in tumors. For example, targeted therapy has become an indispensable part of breast cancer treatment, and HER2 has been widely applied in the clinical treatment of breast cancer and achieved remarkable results (Zhang XN. et al., 2023). Similarly, in the treatment of non-small cell lung cancer, combination targeted therapy has more advantages than traditional chemotherapy and anti-PD-1 immunotherapy alone (Lv et al., 2019). For infants and young children, traditional chemotherapy and radiation therapy may have toxic side effects on brain development, while targeted therapy with biological agents has been proven to reduce these toxic side effects and have the same or higher efficacy (Nageswara Rao et al., 2012). Recently, it has been found that transforming growth factor-β (TGF-β) affects the effects of tumor immunotherapy, and targeted treatment with TGF-β can effectively improve the survival time of patients and reduce side effects (Batlle and Massagué, 2019). The achievements of targeted therapies are numerous.
The momentous discovery in 2010 of the function of mechanosensitive ion channels protein 1 (Piezo1) and protein 2 (Piezo2) channels not only won the 2021 Nobel Prize in Physiology or Medicine but also brought the understanding of mechanical transduction a step closer. Mechanical stimulation drives many physiological processes, including the perception of pain, temperature, pressure and the regulation of vasoconstriction and relaxation. Studies have confirmed that Piezo1 is necessary for mechanically activating cation channels (Coste et al., 2010). Piezo1 channels are proteins associated with mechanical stress-sensitive ion channels. It is widely present in many cell types and plays a key role in the regulation of the intracellular and extracellular environment. Piezo1 has special ion channel characteristics, that is, once the cell senses mechanical stimulation, it immediately opens the ion channel and non-selectively allows cations, especially Ca2+, to pass through (Qin et al., 2021). This influx and outflow of cations in turn affects downstream signal transduction, thereby promoting internal and external cell signal transduction (Yarishkin et al., 2021). It is precisely because of this characteristic that Piezo1 can serve as a bridge connecting mechanical forces and biological signals, and act as a bio-mechanical converter to mediate these mechanical reactions, affecting various cellular physiological activities (Köster et al., 2022), which are closely related to various pathological conditions such as idiopathic polycythemia (Filser et al., 2021) and renal fibrosis (Zhao X. et al., 2022). In addition, the activity of Piezo1 channels is regulated by mechanical stress rather than chemical signals, and this unique characteristic has attracted widespread attention in the fields of mechanical sensing, cell migration, hemorheology, and pain perception (Kim et al., 2012; Beech and Kalli, 2019a; Syeda, 2021; Mukhopadhyay et al., 2024). In recent years, several studies have linked mutations in Piezo1 to the onset and development of a variety of diseases (Dienes et al., 2023). For example, the Piezo1 gene plays an important regulatory role in dental pulp stem cells, suggesting that it may be involved in the development of pulpitis and other oral diseases (Xing et al., 2022). In addition, studies have found that the Piezo1 gene plays an important role in T cell receptor signal transduction, revealing its possible association with autoimmune diseases (Lukacs et al., 2015). Similarly, the activation of Piezo1 can cause relaxation of the pubic arteries and corpus cavernosum (Dela Justina et al., 2023), as well as regulate the migration of microglia and immune responses (Zhu et al., 2023). It follows that Piezo1 is closely associated with cellular life and that the proper opening of the Piezo1 is important for the human body.
In recent years, an increasing number of studies have linked the Piezo1 channel with tumor development. Tumors are a serious threat to human health, and their development process involves the abnormal regulation of multiple cell signaling pathways. The research suggests that the Piezo1 channel plays an important regulatory role in tumor development. First, Piezo1 is linked to the ability of tumor cells to migrate and invade. Multiple studies have found that the Piezo1 channel can increase the migration and invasion ability of tumor cells, and correspondingly, inhibiting its activity can weaken this migration and invasion ability. For example, in gastric cancer, Piezo1 is a novel trilobal-family-1-binding protein that promotes cancer cell migration in vitro (Yang et al., 2014). In osteosarcoma, Piezo1-shRNA was found to inhibit invasion of osteosarcoma cells, and the Piezo1 protein may be a new, potential therapeutic target (Jiang et al., 2017). Second, overexpression of the Piezo1 channel was associated with the proliferation and growth of tumor cells. Some studies have found that suppressing the Piezo1 channel inhibits the proliferation and growth of tumor cells. In synovial sarcoma, Piezo1 is highly expressed in SW982 cells, and knocking it down affects cell viability, making it a potential target against synovial sarcoma (Suzuki et al., 2018). In gliomas, high Piezo1 expression is associated with reduced survival time and serves as a strong biomarker for poor prognosis in gliomas (Zhou et al., 2020). Finally, the Piezo1 channel has also been linked to the transformation of tumor cells and drug resistance. Knowing the important role of the Piezo1 channel in tumor development, the researchers began exploring using the Piezo1 channel as a new target for tumor treatment.
Some studies have found that inhibiting the activity or expression of the Piezo1 channel can inhibit the growth and metastasis of tumor cells and enhance their sensitivity to chemotherapy drugs or ultrasonic therapy. As a result, the Piezo1 channel could be a potential target for tumor therapy. A large number of other tumor developments are underway or have been found to be connected to Piezo1, and Piezo1 has great potential as an anti-tumor target.
2 Basic information on Piezo1The family of Piezo channels, divided into Piezo1 and Piezo2, plays an irreplaceable part in the mechanical stimulation transduction in mammals. Research has found that these channels were evolutionarily conserved and played key roles in various mammalian physiology, including pain and temperature allometry, proprioception, regulation of blood pressure, and vascular development (Gottlieb et al., 2012; Xu, 2016). Although both Piezo1 and Piezo2 can convert physical mechanical stimuli into biochemical information between cells, Piezo2 is mainly expressed in cells of neuronal origin (Stewart and Davis, 2019). This paper focuses on the structure, characteristics, function, and distribution of Piezo1.
Piezo1, a mechanosensitive ion channel protein, is a large homotrimeric membrane protein capable of converting force into chemoelectric signals (Mulhall et al., 2023). Piezo1 was also found to undergo potential reversal at around 0 mV, and more specifically, Piezo1 undergoes voltage-dependent inactivation. Heterologous expression of this protein can also produce mechanical sensitivity in otherwise insensitive cells (Fang et al., 2021). Studies have shown that Piezo1 channels are able to open in response to mechanical force stimulation, resulting in an increase in ion flux. This mechanical sensitivity allows Piezo1 channels to sense and respond to extracellular physical forces, such as mechanical stretch and shear (Saotome et al., 2018). Piezo1 is distributed in plenty of tissues and cells, especially the muscle, heart, nervous system, and vascular system; Related animal experiments also detected increased Piezo1 expression in the epithelial tissues of the bladder, colon, kidneys, lungs and skin of mice. This side verifies the universality of Piezo1 distribution (Coste et al., 2010).
As for the structure of the Piezo1 protein, the Piezo1 that has now been discovered is quite complex and ingenious, with multiple domains. These different domains have a variety of functions, working together to maintain the body’s physiological processes, such as sensing mechanical forces and maintaining cell proliferation. Piezo1 consists of an N-terminal region comprising approximately 3,000 amino acids, a transmembrane region, and a short C-terminal region, where the transmembrane region contains multiple transmembrane domains. Each protomer contacts the central pore and cap domain near the C terminus, and projects a leaf of 36 transmembrane domains that extend outward and upward toward the N terminus (Mulhall et al., 2023). Among them, the central pore module includes the C-terminal extracellular domain, transmembrane inner helices and outer helices, and the intracellular C-terminal domain. The combination of the three C-terminal extracellular domains forms the extracellular cap domain (Fang et al., 2021). The transmembrane region contains multiple transmembrane domains that collectively form a tube through which ions can pass, generating an electric current. This transmembrane region can be further subdivided into two parts: the jumping helical structure and the membrane insertion region. The jumping helices represent a unique topology with 38 transmembrane helices, forming an autotrimer propeller-shaped structure. This structure consists of a central ion-conducting hole and three peripheral mechanical sensing blades. Notably, the abnormally curved transmembrane regions of these three peripheral mechanical sensing blades create a nanobowl configuration (Jiang Y. et al., 2021). Recent research has revealed that both lipids and modified lipid bilayer compounds play a regulatory role in Piezo1, while the sufficient rigidity of the propeller like structure can bend the surrounding membrane to form a dome (Jiang Y. et al., 2021), which subsequently affects the components of the extracellular matrix (ECM) and the cytoskeleton (Vasileva and Chubinskiy-Nadezhdin, 2023).
In addition, Piezo1 protein encompasses several extracellular domains integral to its function. The switching of Piezo1 ion channels has been found to be influenced by external mechanical stimuli. For instance, the mechanosensitive protein Piezo1 plays a crucial role in regulating bone homeostasis through osteoblast-osteoclast crosstalk (Cai et al., 2023). When Piezo1 is activated, it is these extracellular domains that are initially affected by mechanical force. This force is then transmitted to the transmembrane domain, leading to the opening of channels through which ions can pass (Guo and MacKinnon, 2017). This mechanism elucidates the relationship between the opening of Piezo1 channels (Guo and MacKinnon, 2017; Saotome et al., 2018). In summary, the Piezo1 channel employs a mechanical transduction and ion conduction module to carry out its functions of sensing mechanical forces, gating, and facilitating ion conduction (Zhao F. et al., 2022).
The novel and intricate structure of the Piezo1 protein holds the potential to inspire new avenues for drug development and the treatment of diseases. Due to its unique configuration, Piezo1 possesses the capability to detect and respond to various mechanical stimuli, including extracellular pressure, shear force and hydrostatic pressure. This mechanical sensing ability regulates the opening of ion channels, facilitating the flow of ions into and out of cells. Consequently, it initiates physiological cellular responses, such as muscle contraction, vasodilation, and nerve signaling. The alterations in physiological functions resulting from Piezo1 activation can be illustrated as follows (Figure 1).
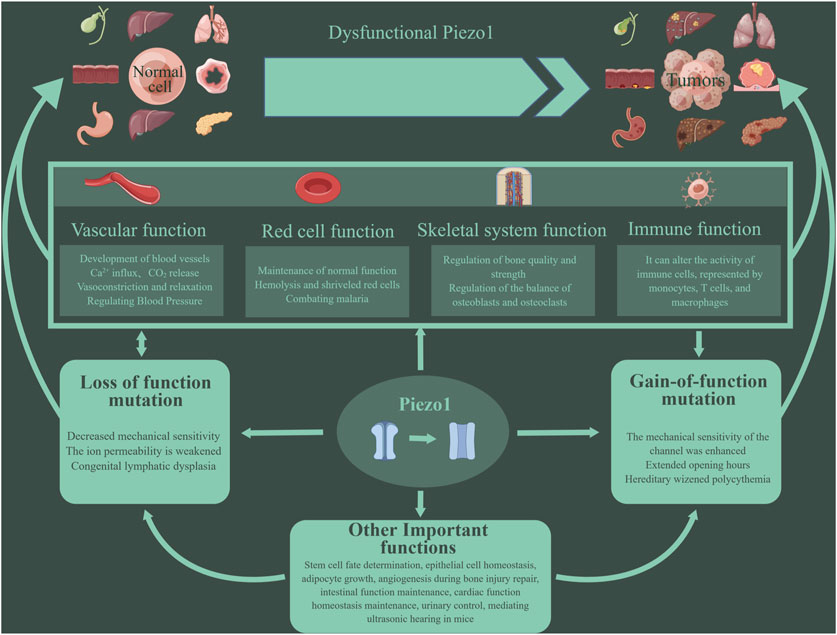
Figure 1. Piezo1 has multiple functions. Piezo1 can affect the cardiovascular system, red blood cell function, bone repair and reconstruction, immune function, etc. More and more studies have shown that the abnormal function of Piezo1 is related to the occurrence and development of tumors. Mutations in the Piezo1 gene can lead to inhibition or enhancement of Piezo1 function, eventually leading to various pathological changes. Such mutations are divided into two types: loss- and gain-of functions.
2.1 Cardiovascular systemPiezo1 can be assembled into a transmembrane triple helix that combines fine force sensing with regulated calcium influx. Accumulating evidence suggests that they have important roles in vascular permeability and remodeling, blood pressure regulation, insulin sensitivity, endothelial shear stress sensing and secretion, nitric oxide production, vascular tone, angiogenesis, and atherosclerosis (Beech and Kalli, 2019b).
First, Piezo1 channels are closely related to hemorheology. In the context of the cardiovascular system, experiments have demonstrated that Piezo1 and Piezo2 are essential for blood pressure regulation as baroreceptors (Ranade et al., 2014). It has been shown that in vascular endothelial cells, mechanical stimuli can activate Piezo1 channels, leading to regulation of blood vessel dilation and contraction (Beech and Kalli, 2019a). With the increase of fluid flow, Piezo1, a non-inactivated non-selective cation channel, can depolarize membrane potential to activate voltage-gated Ca2+ channels in adjacent vascular smooth muscle cells, causing vasoconstriction. Notably, it can raise blood pressure during physical activity by causing vasoconstriction, but this effect is not required during inactivity (Rode et al., 2017). Relevant animal experiments, such as zebrafish experiments, have shown that Piezo1 can be expressed in cardiomyocytes and has a homeostatic effect on cardiac function. The abnormal activity of Piezo1 channels has been implicated in the development and progression of several cardiovascular diseases, such as hypertensive thrombosis (Albarrán-Juárez et al., 2018a; Zhao et al., 2021). The opening of Piezo1 under high hydrostatic pressure has been found to disrupt pulmonary endothelial barrier function, remodel arteries during hypertension, and cause atherosclerosis during turbulence (Douguet et al., 2019). Genetic engineering experiments have shown that overexpression of Piezo1 in cardiomyocytes leads to severe cardiac hypertrophy and arrhythmias (Jiang F. et al., 2021). Similarly, artificial inhibition or activation of Piezo1 expression in mouse heart can lead to the interruption of Ca2+ and ROS signal transduction and promote the development of cardiomyopathy. In addition, Piezo1 expression or activity in the heart was increased under continuous stress and heart failure. Among them, the use of Piezo1 antagonists can inhibit excessive Piezo1 activity, providing therapeutic benefits (Rolland et al., 2023) (Figure 2).
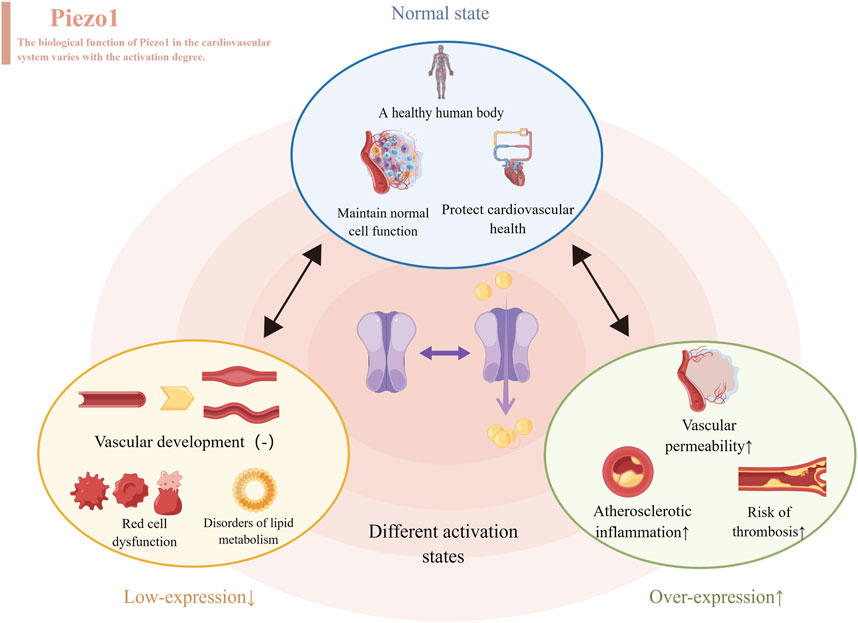
Figure 2. Different levels of Piezo1 expressed produce different effects, represented by the cardiovascular system. When normally activated, Piezo1 maintains normal cell function and protects cardiovascular health. When inhibitors of Piezo1 expression are used, they affect the development of blood vessels, disrupt the function of red blood cells and throw lipid metabolism out of kilter. When Piezo1 was overexpressed with an activator, it led to atherosclerosis, thrombosis and increased permeability of blood vessels.
2.2 Skeletal systemIn the context of the skeletal system, Piezo1 plays a pivotal role in regulating the functions of bones, joints, tendons, and muscles, contributing to posture maintenance and power generation (Dienes et al., 2023). Numerous studies have highlighted the potential of the Piezo1 channel as a significant breakthrough in the treatment of diseases associated with muscular dystrophy and cartilage degeneration (Bagriantsev et al., 2014; Zhu et al., 2021). For instance, E756del is a genetic gain-function variant of Piezo1, and some research results indicate that the E756del variant of Piezo1 is acquired in mice and appears more frequently in sprinters compared to non-athletes. Additionally, animal experiments have demonstrated that mice with the R2482H mutation in Piezo1 exhibit improved jumping and elastic muscle functions (Nakamichi et al., 2022). Hence, it can be inferred that Piezo1 enhances skeletal muscle stretching function by improving tendon compliance. Furthermore, the application of a double-layer membrane loaded with Yoda1 not only prevents fibroblast entry but also, through Piezo1 activation, promotes bone formation and angiogenesis (Yang et al., 2023). Downregulating Piezo1 inhibits macrophage polarization towards the repair type, thereby affecting bone remodeling. Animal experiments have also shown that the downregulation of Piezo1 significantly reduces exercise-induced bone mass in mice (Cai et al., 2023). Notably, cartilage cells can discern and respond to varying levels of mechanical stimulation, potentially due to differences in mechanically sensitive ion channels, such as TRPV4, Piezo1, and Piezo2. TRPV4, Piezo1 and Piezo2 are capable of sensing different levels of repeated mechanical tensile stimuli, and experiments have indicated that tensile strength can influence Piezo’s expression. When cyclic tensile strains were increased to high strain levels of 13% and 18%, Piezo1 expression exhibited a significant increase, whereas this effect was not observed at low strain levels compared to unstretched controls. Unlike Piezo1, the primary function of Piezo2 is to sense harmful levels of repeated cyclic tensile strains loads (Du et al., 2020). It has been suggested that a synergistic interaction between Piezo1 and Piezo2 is essential for maintaining the functionality of joint cartilage (Zhong et al., 2018).
2.3 Red blood cellsIn terms of the function of red blood cells, the activation of Piezo1 can promote the influx of extracellular Ca2+ into cells. Some studies have found that the activation of Piezo1 during platelet driven blood clot contraction may be caused by the compression and deformation of red blood cells. Antagonizing Piezo1 causes a decrease in clot contraction relative to the control group. Therefore, it can be inferred that the activation of Piezo1 by compressed and deformed red blood cells amplifies platelet contractility, forming a positive feedback mechanism during the contraction of blood clots (Evtugina et al., 2023). Piezo1 can not only affect calcium homeostasis but also directly intervene in red blood cell degradation by regulating iron metabolism. Activating Piezo1 can increase iron content in serum and the liver (Hanchard and Wonkam, 2021). Piezo1 was found to be strongly linked to a variety of anemia. For example, studies have found that HBS aggregation can activate Piezo1, thereby increasing intracellular calcium ion concentration, ultimately increasing the number of sickle red blood cells and promoting the progression of sickle cell anemia (Nader et al., 2023). Targeting Piezo1 may be a promising treatment for sickle cell anemia (Gibson and Stewart, 2023). In addition, mutations in the Piezo1 gene are closely related to the occurrence and development of type B marine anemia by affecting red blood cell function (Pinto et al., 2023). Because Piezo1 is the carrier of Er antigen, Piezo1 mutation is closely related to Er red blood cell antigen, which may explain Piezo1’s connection to neonatal hemolytic anemia (Karamatic et al., 2023). Interestingly, Piezo1 is related not only to anemia but also to the prevention of malaria. Polymorphisms and mutations in Piezo1 have been linked to malaria prevention. The reason is that Piezo1 prevents plasmodium falciparum from entering red blood cells, and E756del has been shown to prevent even severe malaria. Meanwhile, activating Piezo1 has been shown in vivo and in vitro to protect humans from malaria (Lohia et al., 2023).
2.4 ImmunityAn increasing number of studies have found that mechanistically gated ion channels, especially Piezo1, play a key role in regulating the immune system (Hamza et al., 2021). In the case of immunity, bacteria cross the cell membrane as a mechanical signal, stretching the membrane to indirectly activate the channel Piezo1, which triggers changes in the body’s immune system (Giron-Ceron and Jaumouillé, 2023). The mechanical stimulation mediated by membrane folding upon entry activates Piezo1, which in turn increases the production of ATP. In addition, mechanically stimulating the physical signal to activate Piezo1 could lead to increased gene expression in immune and barrier pathways, ultimately leading to an immune response (Tadala et al., 2022). Among them, Piezo1 can alter the activity of immune cells such as macrophages, B cells and microglia. For macrophages, the mechanism may be that Piezo1 mediated mechanical sensitive signals can enhance the aerobic glycolysis of macrophages and promote LPS stimulated macrophage immune response (Leng et al., 2022). Many studies have found that Piezo1 not only exerts an influence on regulating macrophage function and polarization in response to various stimuli, such as inflammatory responses and mechanical stimuli (Tang et al., 2023), but is also widely involved in macrophage-mediated inflammatory diseases, such as pneumonia (Wang L. et al., 2020), atherosclerosis (Lin C. et al., 2022), atherosclerosis (Lin C. et al., 2022) and osteoarthritis (Evans et al., 2018; Jebari-Benslaiman et al., 2022). For B cells, their ability to process presenting antigens depends on Piezo1. Inhibiting Piezo1 with inhibitors reduces the ability of B cells to process presenting antigens other than soluble antigens, providing a possible direction for improving vaccine efficacy (Kwak et al., 2023). For microglia, Piezo1 is closely related to the immune response and migration of microglia, as confirmed by the use of Piezo1 inhibitors and activators in separate experiments (Zhu et al., 2023).
2.5 Other functionsFor other organs, Piezo1 signals pulmonary vascular high permeability by promoting internalization and degradation of the endothelial adhesion junction protein VE-cadherin. Piezo1 was found to be related to the regenerative repair of the bladder, and the results suggest that Piezo1 can influence the proliferative ability (Wang X. et al., 2023). In addition, in primary cultured mouse bladder urothelial cells, the downregulation of Piezo1 affects the reception of mechanical stimulation of the bladder, and the high expression of Piezo1 in the bladder indirectly indicates the important role (Peyronnet et al., 2013). For the liver, Piezo1 alleviates acute liver injury caused by acetaminophen by activating nuclear factor erythroid 2-related factor 2 and reducing mitochondrial reactive oxygen species (Wang Q. et al., 2023). In addition, inducing and inhibiting ferroptosis makes a contribution to the treatment of drug-resistant tumors, ischemic organ damage, and other degenerative diseases related to overwhelming lipid peroxidation (Jiang X. et al., 2021; Hirata et al., 2023). For wound healing, the mechanical sensing mediated by Piezo1 drives the transformation of fat cells into fibroblasts that form scars, thereby promoting wound fibrosis. Inhibiting Piezo1, in turn, could target the transformation of fat cells into fibroblasts to prevent and reduce scarring (Griffin et al., 2023). Recent studies have also found that Piezo1 channels play an important role in pain perception. Mechanical stimulation can activate Piezo1 channels, which in turn cause the excitation of neurons and the transmission of pain signals. These findings provide new targets and strategies for pain treatment (Kim et al., 2012).
3 The signal pathways involved by Piezo1 in diseasesIn human diseases, Piezo1 influences the development of disease through a variety of signaling pathways, including the FGF1/FGFR1 signaling pathway, YAP signaling pathway, BMP2/Smad signaling pathway, Akt signaling pathway, NF-κB signaling pathway, ROCK pathway, and JNK1/mTOR signaling pathway (Han et al., 2019; Wang S. et al., 2020; Hasegawa et al., 2021; Li J. et al., 2022; Malko et al., 2023). But Piezo1 mainly works in the following three signaling pathways (Figure 3).
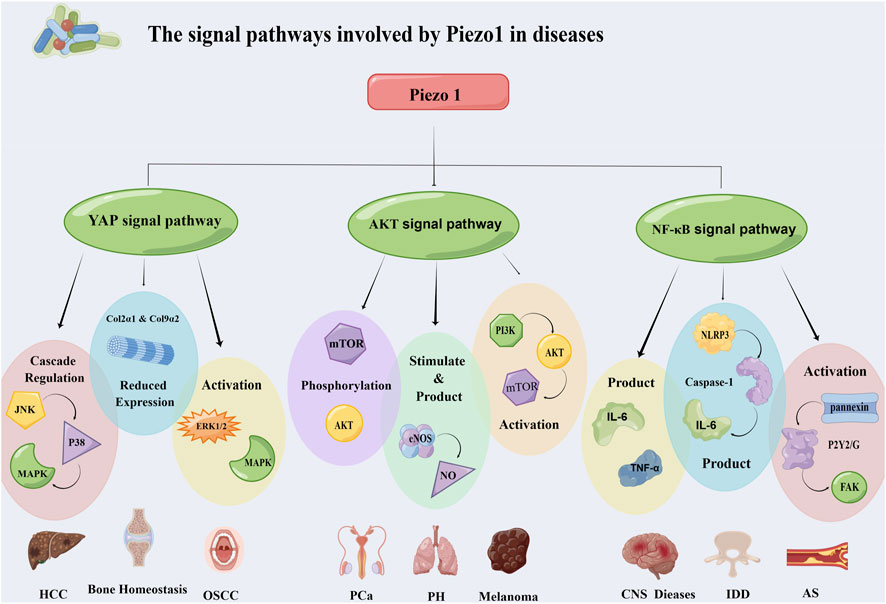
Figure 3. Piezo1 affects the occurrence and development of diseases through multiple signaling pathways. It mainly works through three pathways: YAP signaling pathway, Akt signaling pathway and NF-κB signaling pathway. The three pathways regulate the progression of hepatocellular carcinoma, oral squamous cell carcinoma, prostate cancer, atherosclerosis and other diseases through different mechanisms. Abbreviation: JNK: C-Jun N-terminal kinase; MAPK: Mitogen-activated protein kinases; ERK 1/2: Extracellular signal-regulated kinase 1/2; mTOR: Mammalian target of rapamycin; eNOS: Endothelial nitric oxide synthase; PI3K: Phosphatidylinositol 3-kinase; FAK: Focal adhesion kinase; NLRP3: NOD-like receptor thermal protein domain associated protein 3; HCC: Hepatocellular carcinoma; OSCC: Oral squamous cell carcinoma; PCa: Prostatic carcinoma; PH: Pulmonary hypertension; IDD: Intervertebral disc disease; AS: Atherosclerosis.
3.1 YAP signaling pathwayThe Hippo pathway is a classic tumor-associated highly conserved pathway. Transcriptional coregulatory factor YAP and transcriptional coactivator carrying PDZ-binding motifs (TAZ) are important effectors of the Hippo pathway (Zhao et al., 2008). YAP/TAZ is a sensor and mediator of mechanical signals in the cellular microenvironment (Panciera et al., 2017). Among them, YAP is an important mechanically sensitive transcription activator that can regulate cell proliferation and differentiation, closely related to the occurrence and development of different diseases (Halder et al., 2012). Piezo1 plays a specific role in regulating YAP translocation.
In hepatocellular carcinoma (HCC), the Ca2+ reaction induced by Piezo1 activation can promote YAP phosphorylation, which is mediated by JNK and P38 cascade regulation (Liu S. et al., 2021). Mitogen-activated protein kinases (MAPK) is a group of serine-threonine kinase. The MAPK signaling pathway can be activated through signal transduction of cell surface receptors (Delire and Stärkel, 2015), and improper regulation of this pathway leads to abnormal cell behaviors. After MAPK mediates the phosphorylation of YAP, activated YAP promotes cell proliferation, inhibits cell apoptosis, and ultimately promotes carcinogenesis (Liu S. et al., 2021). Piezo1 can also regulate bone homeostasis through the YAP pathway. Bone homeostasis depends on the overall balance between bone resorption and bone formation (Feng and McDonald, 2011). In addition, promoters of type II collagen-1 (Col2α1) and type IX collagen-2 (Col9α2) are known to be activated by YAP. In this case, Piezo1 acts as a mechanical regulator that directly senses the mechanical load and reduces YAP30 nuclear localization by turning down its expression (Zhang Y. et al., 2022). After the decrease of YAP nuclear localization, the expression of Col2α1 and Col9α2 is further decreased, which leads to the increase of osteoclast differentiation, affects the occurrence of osteoclasts, and thus interferes with bone home (Wang L. et al., 2020).
3.2 Akt signaling pathwayAkt is a serine/threonine kinase that is a central node in many signaling pathways (Revathidevi and Munirajan, 2019). The traditional pathway through which Akt exerts its primary carcinogenic role is the PI3K/Akt signaling pathway (Fresno Vara et al., 2004), with PI3K, Akt, and mTOR as the three major nodes (Kauffmann-Zeh et al., 1997). Overexpression and phosphorylated activation of Akt are two major events widely occurring in human tumors (Cerami et al., 2012) and may be involved in many aspects of tumor development, including proliferation (Zhou et al., 2001), apoptosis (Spokoini et al., 2010), tumor suppressor factor inhibition (Gomes et al., 2014), lipid metabolism (Liu et al., 2016), and angiogenesis (Semenza, 2002).
Prostate cancer is one of the most common cancers in men, and the expression level of the Piezo1 channel in human prostate cancer is significantly higher than that in non-malignant tissues. Moreover, there is experimental evidence that knocking out the Piezo1 channel can lead to reduced proliferation and migration of prostate cancer cells, indicating the anti-proliferative effect of Piezo1 knocking out. When Piezo1 is activated, calcium flow can be increased to further activate the Akt/mTOR signaling pathway, leading to Akt/mTOR phosphorylation, which promotes the proliferation and migration of prostate cancer cells and the growth of prostate tumors (Han et al., 2019).
Similarly, in the case of melanoma, Piezo1 promotes the malignant progression of the disease through Akt/mTOR signaling. Piezo1 ion channel protein can regulate calcium concentration in melanoma cells, further increasing the activation level of the PI3K/Akt/mTOR pathway, thereby regulating the malignant progression of melanoma (Zhang S. et al., 2022).
3.3 NF-κB signaling pathwayNF-κB is an important intracellular nuclear transcription factor (Oeckinghaus et al., 2011). Previous studies have speculated that it can promote tumor development through the following three pathways: Releasing reactive oxygen species leads to DNA damage and gene mutations (Huber et al., 2004; Liou and Storz, 2010); controlling Epithelial-Mesenchymal transition (EMT) and metastasis to promote cancer progression; upregulation of vascular endothelial growth factor and its receptors to control tumor angiogenesis (Xie et al., 2010). NF-κB serves as a key factor connecting immunity and inflammation (Gilmore, 2003), and Piezo1’s role in regulating inflammatory response through this pathway is cell-type specific.
In the occurrence and development of atherosclerosis, when endothelial cells are naturally exposed to interfering and atherogenic blood flow, they will activate endothelial Piezo1, leading to calcium ion influx, and then control the blood flow induced ATP release and subsequent activation of the P2Y2/G protein alpha subunits Galphaq and Galpha11 (Gq/G11) by activating pannexin channels, and then promote endothelial cell NF-κB activation through the mediation of integrin α5β1. This process requires phosphorylation of focal adhesion kinase downstream of integrin (Albarrán-Juárez et al., 2018b).
Intervertebral disc degeneration (IDD) is the main cause of back, neck, and nerve root pain (Risbud and Shapiro, 2014). Inflammatory factors produced by nucleus pulposus cells play an important role in the pathogenesis of IDD (Navone et al., 2017). Piezo1 activates the NOd-like receptor protein 3 (NLRP3) inflammasome in the nucleus pulposus via the Ca2+/NF-κB pathway. The NLRP3 inflammasome is a multiprotein cytoplasmic molecule comprising receptors, junctions, and effector molecules (Gross et al., 2011), and its abnormal activation leads to the occurrence of inflammatory diseases. Piezo1 induces initiation and assembly of the inflammasome via the Ca2+/NF-κB pathway, increasing mechanical-stretching-mediated caspase-1 activation in nucleus pulposus cells. Subsequently, caspase-1 converts pre-IL-1β to IL-1β, and accelerates the production and maturation of IL-1β, thereby leading to the production and development of IDD through pathways such as promoting extracellular matrix degradation, inducing inflammatory cascade reactions, promoting angiogenesis and new nerve innervation, and inducing apoptosis of nucleus pulposus cells (Sun Y. et al., 2020).
In the case of microglia, they are highly mobile under both physiological and pathological conditions and sense mechanical signals in the microenvironment (Ayata and Schaefer, 2020). Abnormal increase of pro-inflammatory signals from microglia is an important pathological factor in brain aging and the pathogenesis of central nervous system damage and neurodegenerative diseases (Bruno et al., 2021). The mechanosensitive Piezo1 channel is functionally expressed in microglia, and the Piezo1 channel activates and inhibits the NF-κB inflammatory signaling pathway by initiating intracellular Ca2+ signaling, thereby down-regulating the proinflammatory function of microglia, especially the production of TNF-α and IL-6, and then it can delay the development of brain aging, central nervous system damage, and neurodegenerative diseases (Malko et al., 2023).
In general, Piezo1 is involved in a variety of signaling pathways for disease progression, including the YAP, Akt, and NF-κB signaling pathways that promote the development of diseases, especially tumors. Exploring Piezo1’s involvement in disease signaling could lead to a clearer understanding of the related diseases and the search for new therapeutic targets. Most of what Piezo1 does in the NF-κB pathway is related to inflammation, and its role in tumors is not fully understood, which could be pursued as a line of inquiry.
4 Association of Piezo1 with tumorsPiezo1 is abnormally expressed in a variety of tumor tissues, and it is related to the malignant degree of some tumors and the survival rate of patients (Table 1). In addition, Piezo1 plays an important role in numerous biological processes in tumors (Table 2). Next, we will describe the specific mechanism by which Piezo1 regulates tumor characteristics in detail.
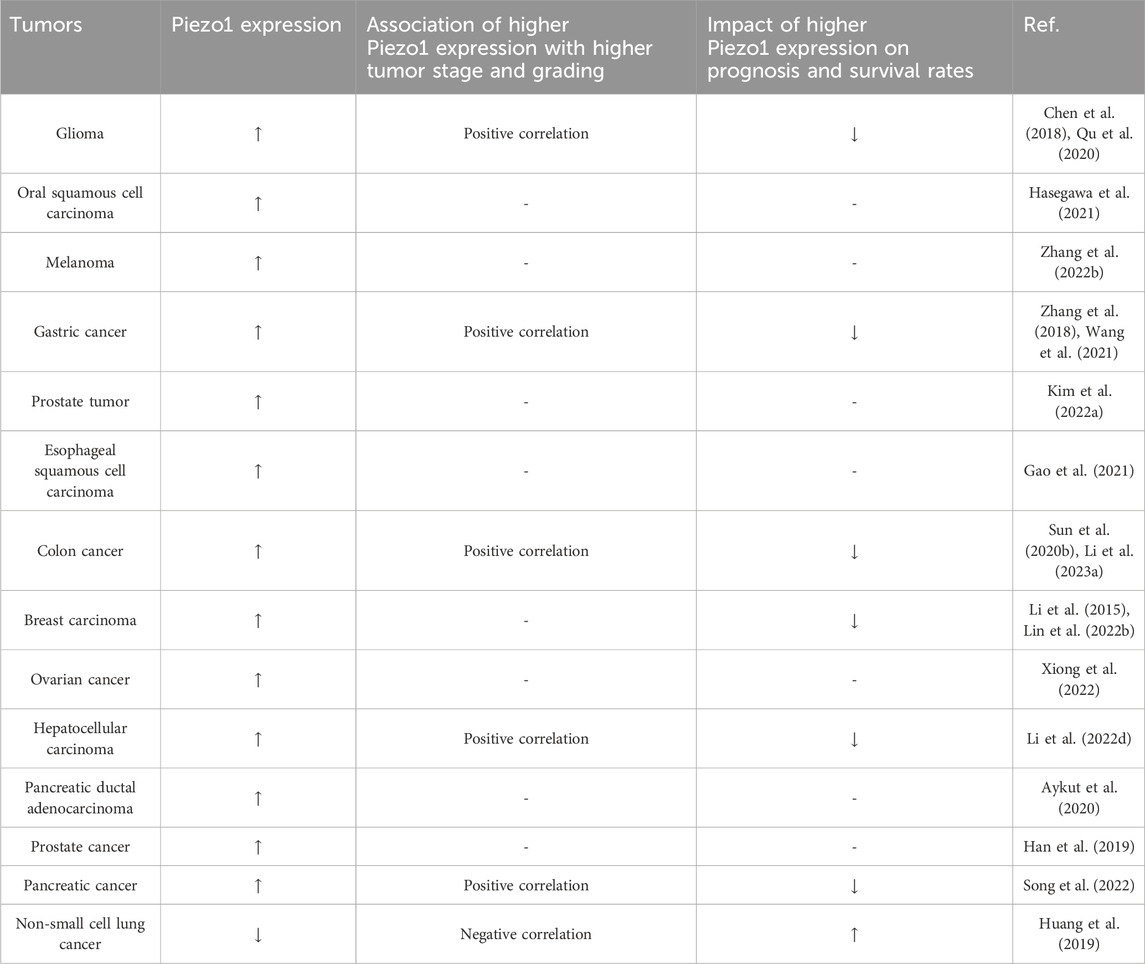
Table 1. The expression of Piezo1 in tumors and its association with tumor stage, grading and patient survival.
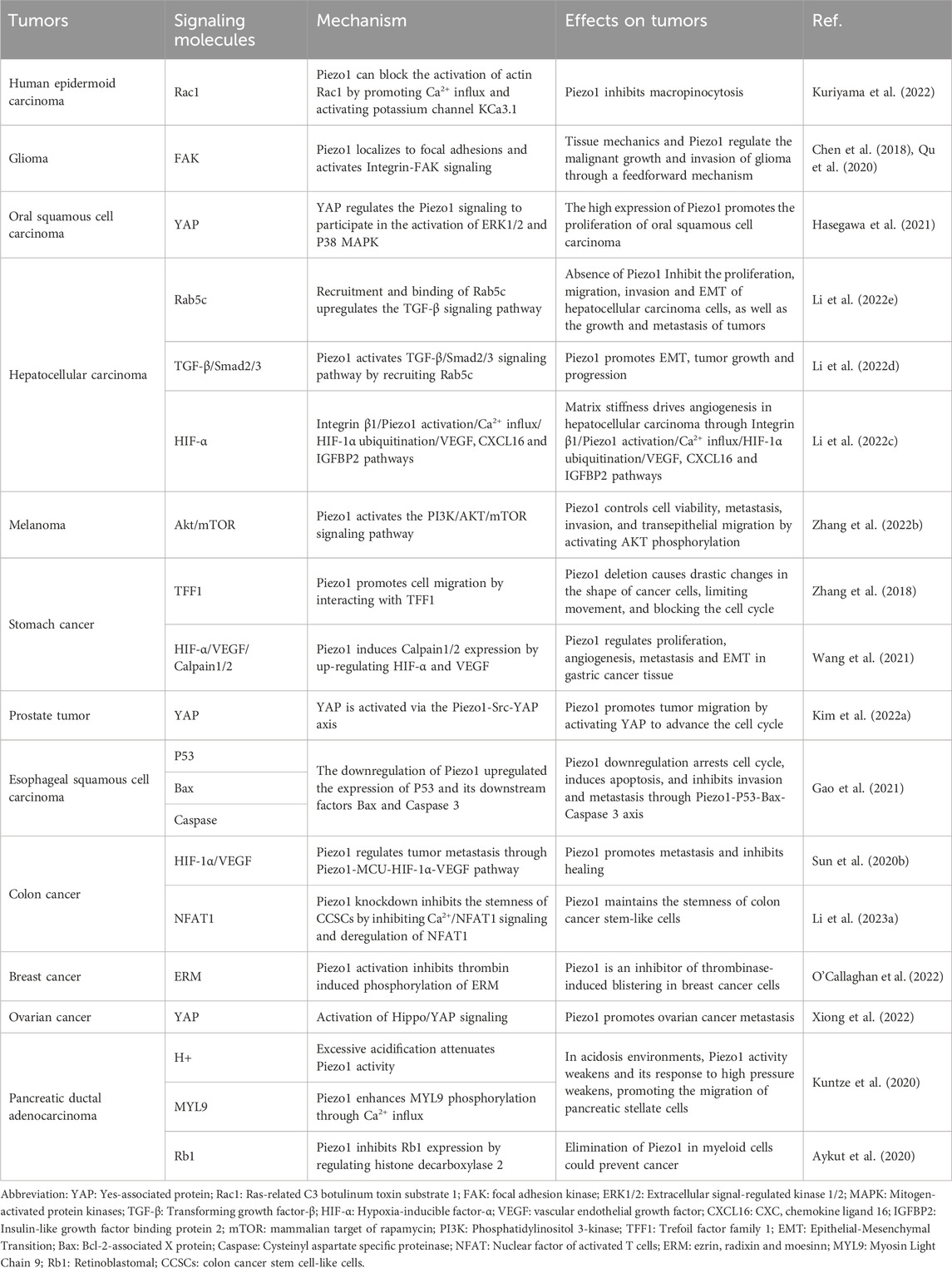
Table 2. Treating tumors by acting on Piezo1-related mechanisms.
4.1 Cell metabolic reprogrammingTumor cells are often in a state of nutrient deficiency due to their continuous proliferation or special location of growth. To adapt to this extreme environment, tumor cells often reprogram their cellular metabolism to take up essential nutrients from the nutritionally deprived environment and adjust intracellular metabolic pathways to supply the more important biological processes (Pavlova Natalya and Thompson, 2016). Macropinocytosis is an important non-selective extracellular amino acid uptake pathway, which has been reported in tumor cells (Del Bello et al., 2022; Xia et al., 2023) and even tumor stromal cells (Zhang et al., 2021). Macropinocytosis is dependent on the formation of peripheral folds, whose formation and migration are regulated by actin (Isogai et al., 2015). Activated Piezo1 can block the activation of actin Rac1 by promoting Ca2+ influx and activating potassium channel KCa3.1, thereby inhibiting macropinocytosis in human epidermoid carcinoma (Kuriyama et al., 2022). The Piezo1 activator Yoda1 inhibits macropinocytosis by interfering with the physiological formation of peripheral folds, and Yoda1 specifically activates the Piezo1 gene without any effect on other endocytic pathways, making it a potential therapeutic option for tumor inhibition by targeting amino acid metabolism.
In addition, purine metabolism, which is inseparable from cell proliferation, was previously found to be enriched in several cancers (Chen X-H. et al., 2019; Chen S. et al., 2022; Liu et al., 2023) and associated with poor efficacy of immunotherapy (Li S. et al., 2022). Through pathway enrichment analysis and correlation detection using KEGG and Wiki databases, it was found that Piezo1 potentially facilitates the growth of breast cancer by modulating the purine metabolism of GUK1, POLD1 and APRT (Chen and Chen, 2022).
4.2 Proliferation and apoptosisTumor cells relieve the regulation of proliferation signals and achieve sustained proliferation, which is the most fundamental characteristic of tumor cells (Hanahan and Weinberg, 2011). The effect of cell proliferation signal may be related to Piezo1 on the cell membrane. For example, in glioma, tumor cells specifically rely on Piezo1 for growth and proliferation, while Piezo1 is not expressed in normal glial cells (Chen et al., 2018). Moreover, knocking out Piezo1 gene has no significant effect on the volume and mitosis of normal glial cells (Chen et al., 2018). In addition, Hasegawa et al. also found that YAP regulates Piezo1 to participate in the proliferation and growth of oral squamous cell carcinoma (OSCC) (Hasegawa et al., 2021). After knocking down Piezo1 gene or using Piezo1 inhibitor GsMTx-4, the proportion of OSCCs and cell proliferation ability decreased (Hasegawa et al., 2021). Among them, Piezo1 is also involved in the activation of agonist-dependent ERK1/2 and p38 MAPK (Hasegawa et al., 2021). Given that ERK1/2 and p38 MAPK are involved in cell proliferation (Nishimoto and Nishida, 2006; Zhang et al., 2019), their activation by Piezo1 may be one of the mechanisms promoting tumor cell proliferation. Besides, fluid shear stress and Yoda1 synergistically activate Piezo1 in prostate cancer cells by increasing the proportion of early S/S/G2/M phase in the cell cycle through the activation of YAP/TAZ, while transplanted Piezo1-silenced prostate cancer cells failed to proliferate in vivo (Kim O-H. et al., 2022). However, the signaling pathway between Piezo1 and YAP/TAZ complex is still unclear, and whether there is a synergistic effect between them in the process of tumor cell proliferation is still a question that needs to be further explored.
Unlimited proliferation of tumor cells depends on continuous cell division in addition to receiving proliferative signals, which is closely linked to cell cycle surveillance. Alterations in Piezo1 expression have been observed in several tumors by regulating key cell cycle regulators and thus affecting tumor cell proliferation. In melanoma cells, knockdown of Piezo1 resulted in alterations in cell cycle-related genes, with a significant decrease in the expression of key effectors CDK2 and CyclinD1 genes and an increase in the expression of classical tumor suppressors P21 and PTEN (Zhang S. et al., 2022). In addition, Piezo1 knockdown induced G1 phase block in gastric cancer cells and downregulated phosphorylated Rb, Cyclin D1, CDK4, and CDK6 (Zhang et al., 2018), suggesting that Piezo1 is required for gastric cancer cell proliferation. Similarly, a study on prostate cancer showed that Piezo1 downregulation inhibited the expression of Cyclin D1 and CDK4, hindering the assembly of the Cyclin D1-CDK4 complex and thus impeding cell cycle progression in prostate cancer cells (Han et al., 2019). Interestingly, the present study demonstrated that Piezo1 regulation of the cell cycle in prostate cancer cells is associated with the Akt/mTOR pathway, but not with the ERK1/2 pathway (Han et al., 2019).
In addition to uncontrolled cell proliferation, inhibition of apoptosis is also a typical feature of tumor cells (Hanahan and Weinberg, 2011). Various key events of apoptosis are concentrated in mitochondria (Green and Reed, 1998). Piezo1 interferes with the apoptosis of tumor cells by inducing Ca2+ influx and mediating mitochondrial dysfunction. In the ultrasonic-targeted microbubble therapy of pancreatic ductal cell carcinoma, Piezo1 blockade reduced Ca2+ influx, decreased cytochrome C and Bax, and increased Bcl-2, which finally assisted microbubble to induce apoptosis of cancer cells (Song et al., 2022). Moreover, the activation of Piezo1 in prostate cancer amplifies the death-induced signal on the apoptosis pathway mediated by TNF-related apoptosis-inducing ligand (TRAIL) via Ca2+, leading to mitochondrial outer membrane permeability and mitochondrial dysfunction, and improving the therapeutic effect of TRAIL through the intrinsic apoptosis pathway (Hope et al., 2019). In addition to Ca2+-related apoptotic pathways, Piezo1 also interacts with P53 in esophageal squamous cell carcinoma cells (ESCCs) (Gao et al., 2021). The expression of P53 downstream factors Bax and Caspase protein was significantly upregulated when Piezo1 was downregulated, and the mouse experiment shows that the growth rate of ESCCs was decreased in association with the upregulation of these factors (Gao et al., 2021). These suggest that Piezo1 downregulation induces ESCCs apoptosis through Piezo1-P53-Bax-Caspase 3 axis.
In summary, Piezo1 has an ingenious role in the activation of tumor cell proliferation signals and cell cycle operation, and has been implicated in multiple tumor apoptotic pathways, which is why the effect of Piezo1 on tumor proliferation and apoptosis is so complex.
4.3 Stem cellsCancer stem cells (CSCs) are a population of cells with the ability to differentiation, proliferation, and self-refinement, which play an important role in tumor progression (Chiba et al., 2007), inflammation (Zhao et al., 2023), drug resistance (Choi et al., 2020), and prognosis (Xu et al., 2023). Abnormal mechanistic signals in TME can affect the proliferation, differentiation, stemness, and other biological behaviors of CSCs (Shen et al., 2021; Nguyen Ho-Bouldoires et al., 2022). Therefore, the regulatory mechanisms acting on the mechanical microenvironment of CSCs have great potential for clinical applications (Tian et al., 2021), and Piezo1 is just related to the maintenance of stemness of CSCs. Compared with low stemness colon cancer stem cell-like cells (CCSCs) and non-CCSCs, Piezo1 is highly expressed in CCSCs with high stemness, and the population with high Piezo1 expression is associated with clinical stage (Li R. et al., 2023). Piezo1 can maintain the stemness of CCSCs through Ca2+/NFAT1 signaling pathway, and the stemness and tumorigenic ability of CCSCs are impaired when Piezo1 is knocked down, while the cloning potential of CCSCs is increased when Piezo1 is overexpressed (Li R. et al., 2023). Furthermore, Piezo1 also affected the stemness and in vivo growth of glioblastoma stem cells (GSCs) (Chen et al., 2018).
In addition to CSCs, the effect of Piezo1 on another pluripotent stem cell, mesenchymal stem cell (MSC), is intriguing. In human dental pulp-derived MSCs, Piezo1 activation stimulates MSC migration by inducing ATP release and activation of the P2 receptor purinergic signaling pathway as well as the downstream PYK2 and MEK/ERK signaling pathways (Mousawi et al., 2020). MSCs are homing to tumor tissues under the regulation of chemokines (Chamberlain et al., 2007), and will transform into tumor-associated fibroblasts upon stimulation by tumor cells (Spaeth et al., 2009). Due to the high affinity of MSCs to tumor cells, an increasing number of studies have used MSCs as a delivery carrier of anti-tumor drugs (Fan et al., 2023). Therefore, the effect of Piezo1 on the homing efficiency of MSCs and tumor progression is an interesting direction to explore.
4.4 AngiogenesisWhile normal vasculature is in a quiescent state and only activates transiently in response to injury and certain physiological conditions, there are “angiogenic switches” in tumor cells that continuously activate the vasculature to generate new blood vessels (Hanahan and Weinberg, 2011). The angiogenic switch is regulated by both pro-angiogenic and anti-angiogenic factors, and Piezo1 promotes tumor angiogenesis by up-regulating pro-angiogenic factors and their upstream and downstream effectors (Han et al., 2019). In HCC, the activation and upregulation of Piezo1 inhibit hypoxia-inducible factor 1 (HIF-1) ubiquitination, which in turn enhances the expression and secretion of downstream pro-angiogenic factors such as vascular endothelial growth factor (VEGF), CXC chemokine ligand 16 (CXCL16) and insulin-like growth factor binding protein 2 (IGFBP2), ultimately accelerating HCC angiogenesis (Li M. et al., 2022). Moreover, in peritoneal metastatic tumor tissues of gastric cancer, Piezo1 also regulates the expression of downstream molecules Calpain1/2 by up-regulating HIF-1 and VEGF, promoting tumor angiogenesis and metastasis (Wang et al., 2021). Among them, Calpain1/2 promote angiogenesis and endothelial motility in response to VEGF signaling through the Ezrin/Calpain/PI3K/AMPK/eNOS signaling axis (Youn et al., 2009). Furthermore, Piezo1 activation in periosteal stem cells also upregulates angiogenic factors such as bone morphogenetic protein-2 (BMP-2) and VEGF-A through the YAP/β-catenin signaling pathway (Liu et al., 2022); however, whether this Piezo1/YAP/β-catenin signaling axis also exists in tumor cells still needs further investigation.
4.5 Invasion and metastasisTumor metastasis is a multi-step cascade process, which can be roughly divided into local invasion of tumors, internal infiltration into the peripheral vasculature and lymphatics, survival in the circulatory system and metastasis, extravasation from the vascular lumen into the parenchymal tissues, and finally adaptation to the microenvironment of the metastasis to complete colonization (Talmadge and Fidler, 2010). A large body of literature shows that Piezo1 plays an important role in tumor migration (Li et al., 2015), extravasation (Zhang S. et al., 2022), and distant metastasis (Lin CY. et al., 2022), and promotes local invasion and distant metastasis of tumors (Figure 4). The Piezo1-HIF-1α-VEGF pathway (Sun YH. et al., 2020; Wang et al., 2021), Piezo1-Src-YAP axis (Kim O-H. et al., 2022), and Piezo1-LAST1/2-YAP axis (Xiong et al., 2022) are all potential mechanisms of tumor metastasis. However, some studies have also proposed opposite conclusions. For example, in human non-small cell lung cancer, especially lung adenocarcinoma patients, high Piezo1 expression correlates with better overall survival, and knockdown of Piezo1 significantly promotes cancer cell migration in vitro and tumor growth in vivo (Huang et al., 2019).
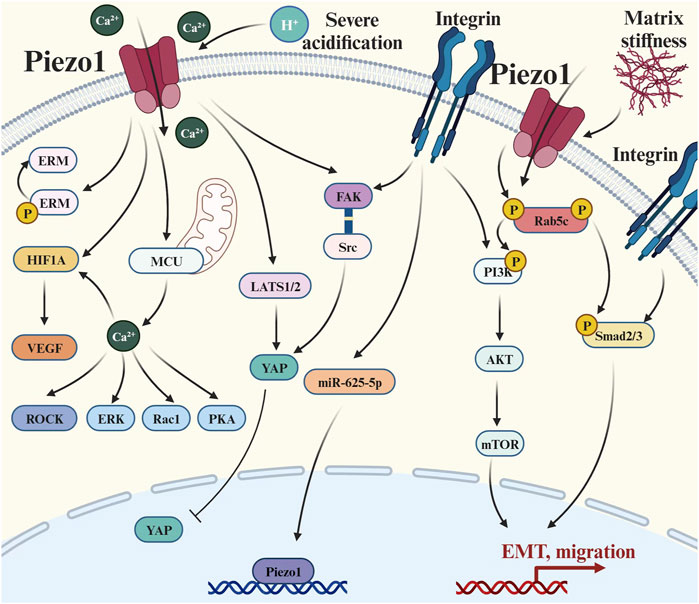
Figure 4. Piezo1 crosstalks with multiple signaling pathways to regulate tumor metastasis: (1) Extracellular matrix signal transduction: there is a positive feedback pathway between Piezo1 and matrix hardness, and also its activity is affected by environmental pH; (2) Integrin-mediated pathways: such as Integrin-Adhesion Spot, Classical Smad2/3 Pathway, and Non-Smad2/3 pathway; (3) Cytoskeletal-related pathways: Causing changes in a series of actin skeleton-related effectors such as ERM, ROCK, and Rac1; (4) Hippo pathways: the LATS1/2-YAP axis and the Piezo1-Src-YAP axis; and (5) Ca2+-mediated pathways: elevated concentrations of Ca2+ caused upregulation of HIF-α and VEGF. Abbreviation: EMT, Epithelial-mesenchymal transition; ERM, Ezrin, radixin and moesinn; ERK1/2, Extracellular signal-regulated kinase 1/2; FAK, Focal adhesion kinase; HIF-1, Hypoxia-inducible factor 1; LATS1/2, Large tumor suppressor 1/2; MCU, Mitochondrial calcium uniporter; mTOR, Mammalian target of rapamycin; P, Phosphorylation; PI3K, Phosphatidylinositol 3-kinase; PKA, Protein kinase A; Rac1, Ras-related C3 botulinum toxin substrate 1; ROCK, Rho-associated protein kinase; Smad2/3, SMAD Family Member 2/3; VEGF, Vascular endothelial growth factor; YAP, Yes-associated protein.
In order to better adapt to the complex and variable microenvironment during metastasis, metastatic tumors often differ from primary tumors in biological and behavioral characteristics, especially the invasive changes induced by EMT (Park et al., 2019; Mao et al., 2020). Several studies have shown that Piezo1 can help tumor cell metastasis by promoting EMT (Gao et al., 2021; Wang et al., 2021). Piezo1 induces EMT by activating the TGF-β/Smad2/3 signaling pathway through the recruitment of Rab5c (Li et al., 2022d). Knockdown of Piezo1 transformes the HCCs from a spindle-like literal morphology to a cobblestone-like epithelial morphology, with a decreased ability of cells to migrate, and the growth of subcutaneous and orthotopic xenograft tumors was also inhibited (Li et al., 2022d). Moreover, Piezo1 also promotes tumor metastasis by activating the non-Smad pathway of TGF-β. Among them, PI3K/AKT/mTOR pathway regulates the invasion and metastasis process of melanoma upon activation by Piezo1, and the expression of EMT-related genes and invasion and metastasis-related genes (MMP2 and MMP9) in cells changes with the alteration of Piezo1 expression (Zhang S. et al., 2022). Similarly, in vitro experiments, Piezo1 downregulation induced reduced migration of prostate cancer cells by inhibiting Akt and mTOR phosphorylation (Han et al., 2019). In addition, Piezo1 has been implicated in apoptosis and senescence resistance of metastatic tumors. A study on colorectal cancer cells showed that compared with metastatic tumors, primary tumors had higher Pizeo1 expression and were more sensitive to TRAIL-mediated apoptosis induced by both mechanical force and shear stress (Greenlee et al., 2022).
During tumor metastasis, tumor cells generally undergo elongated-mesenchymal migratory movements, which are closely related to the integrin-FAK pathway (Wang CH. et al., 2020). The expression of Piezo1 was higher in high invasive gliomas than in low invasive gliomas, and its immunofluorescence images showed that Piezo1 was distributed at the adhesion spots mediated by integrins and FAK activation, indicating that Piezo1 may be related to the integrin-adhesion spot pathway and thus participate in the adhesion and migration of tumors (Chen et al., 2018). However, when integrin functio
留言 (0)